Abstract
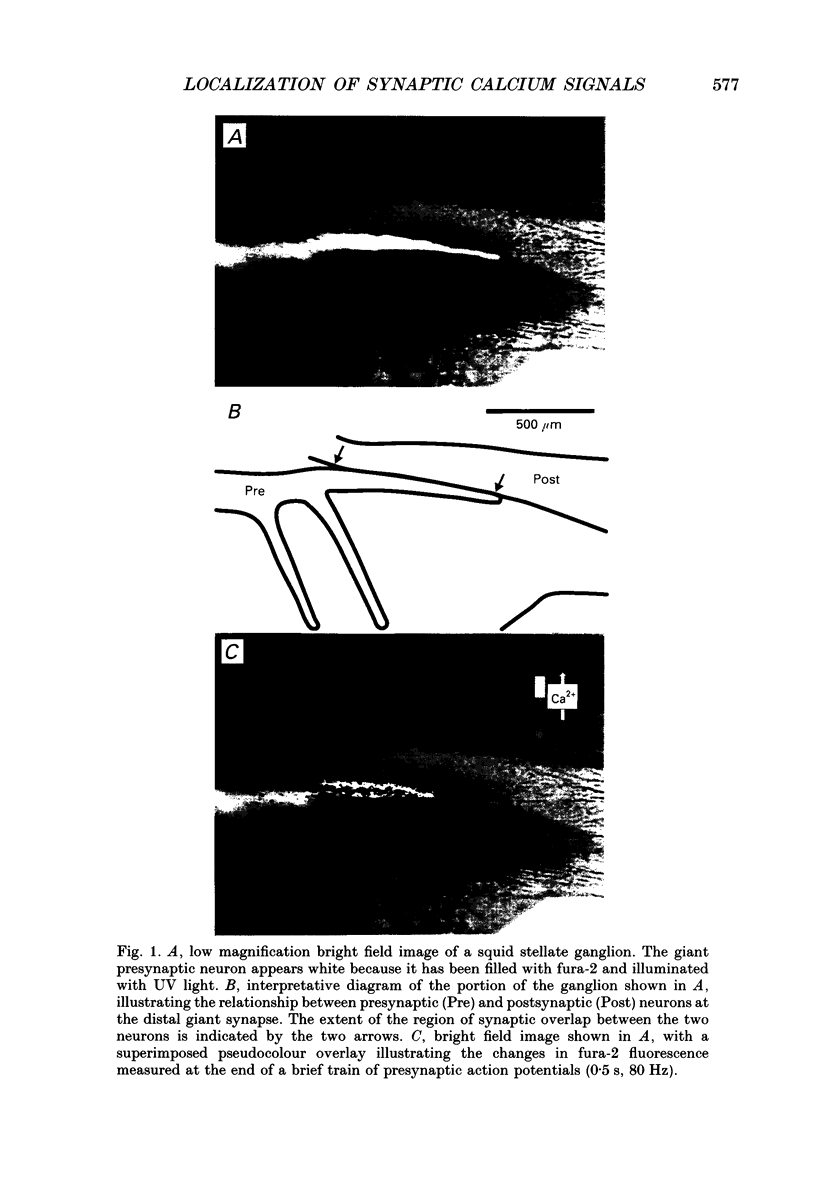
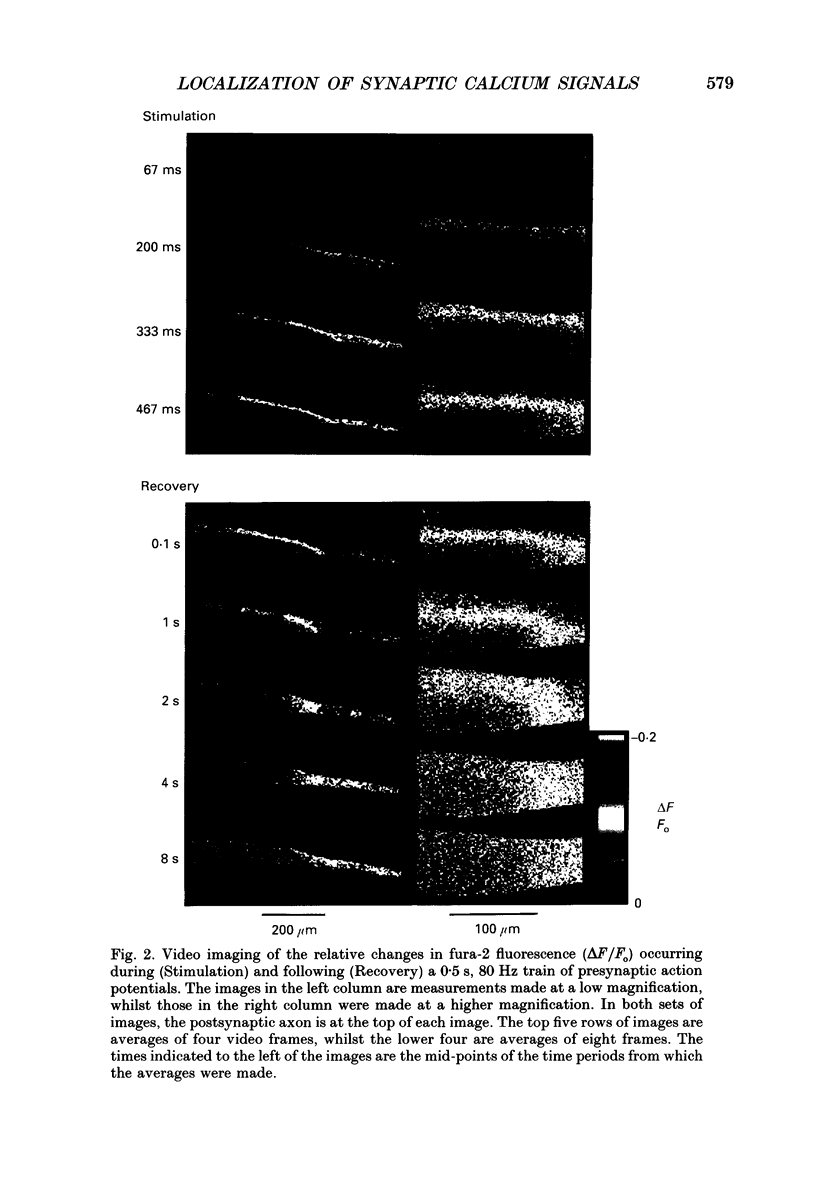
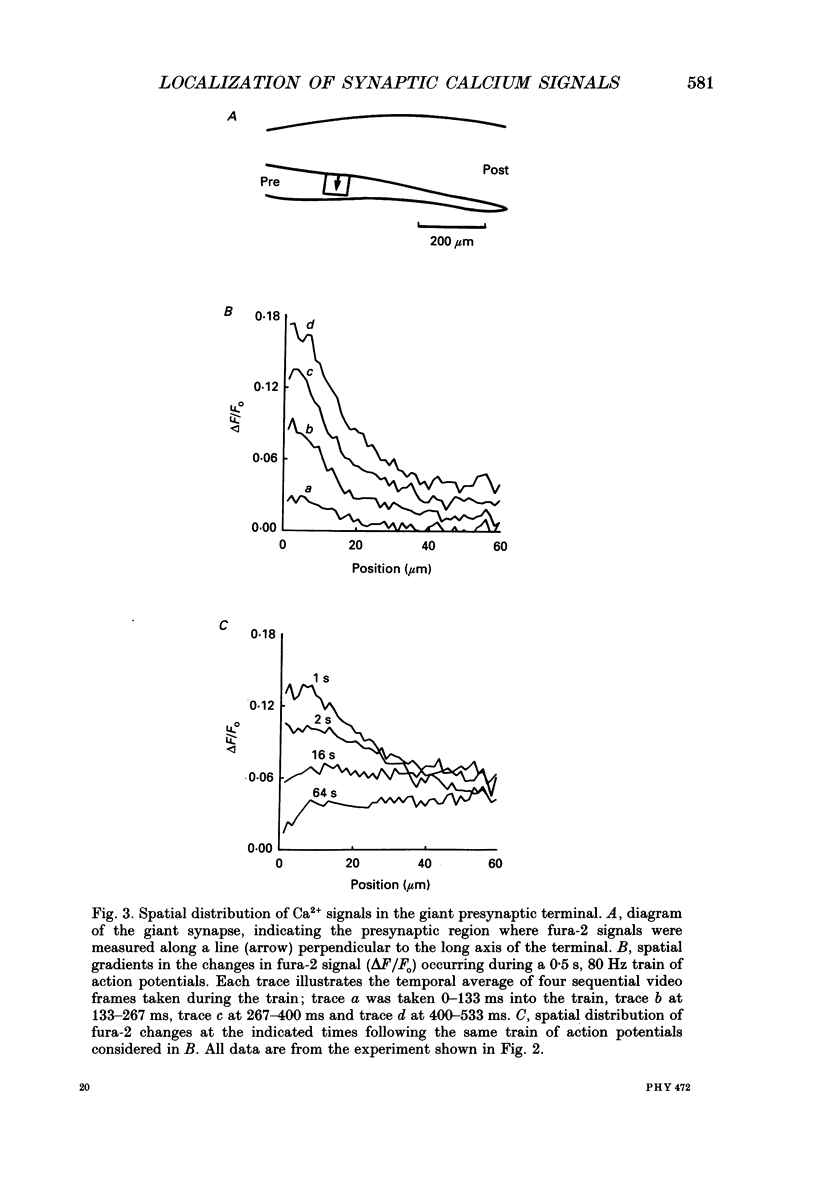
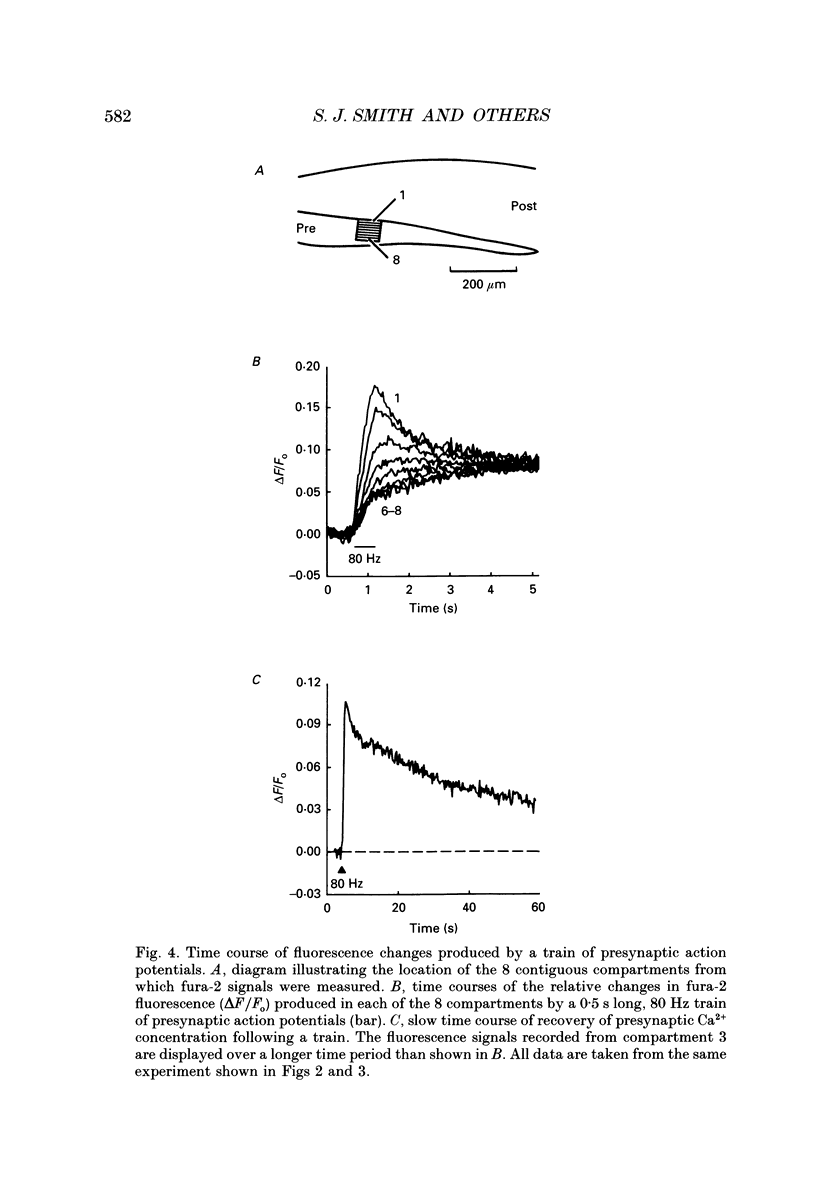
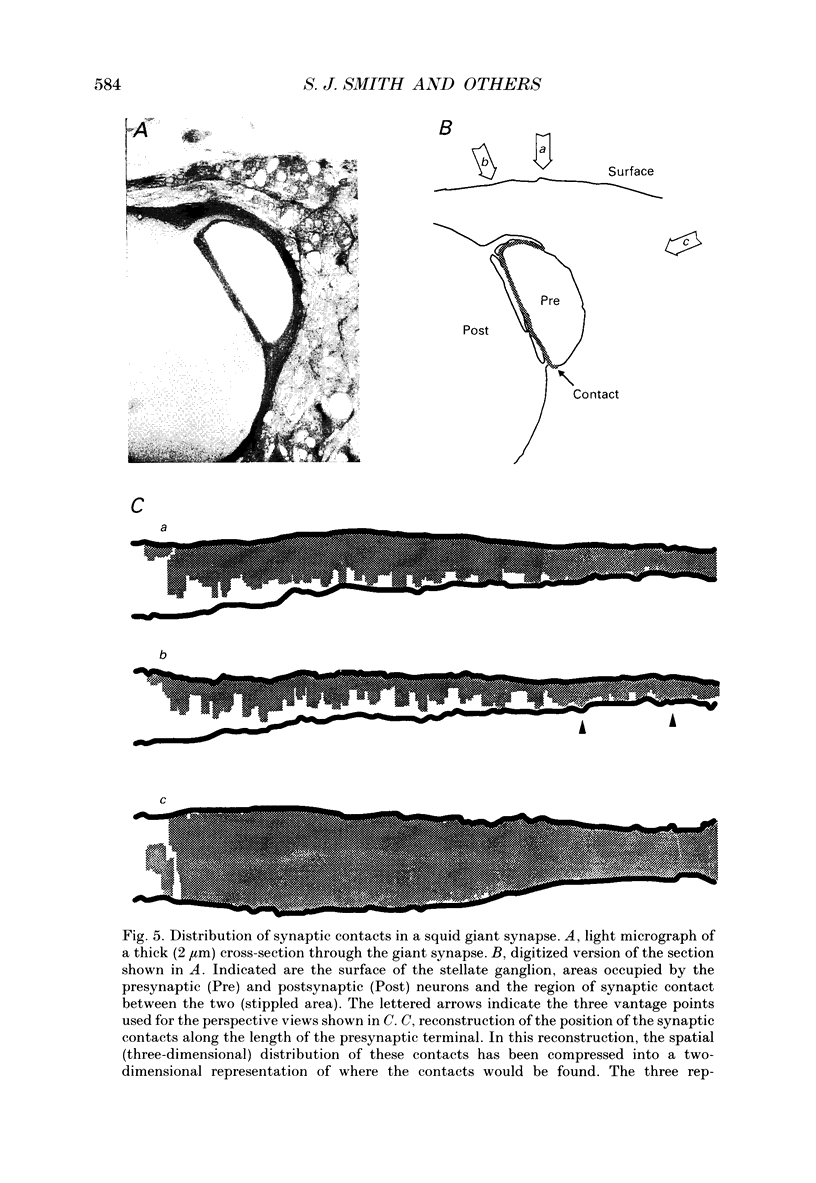
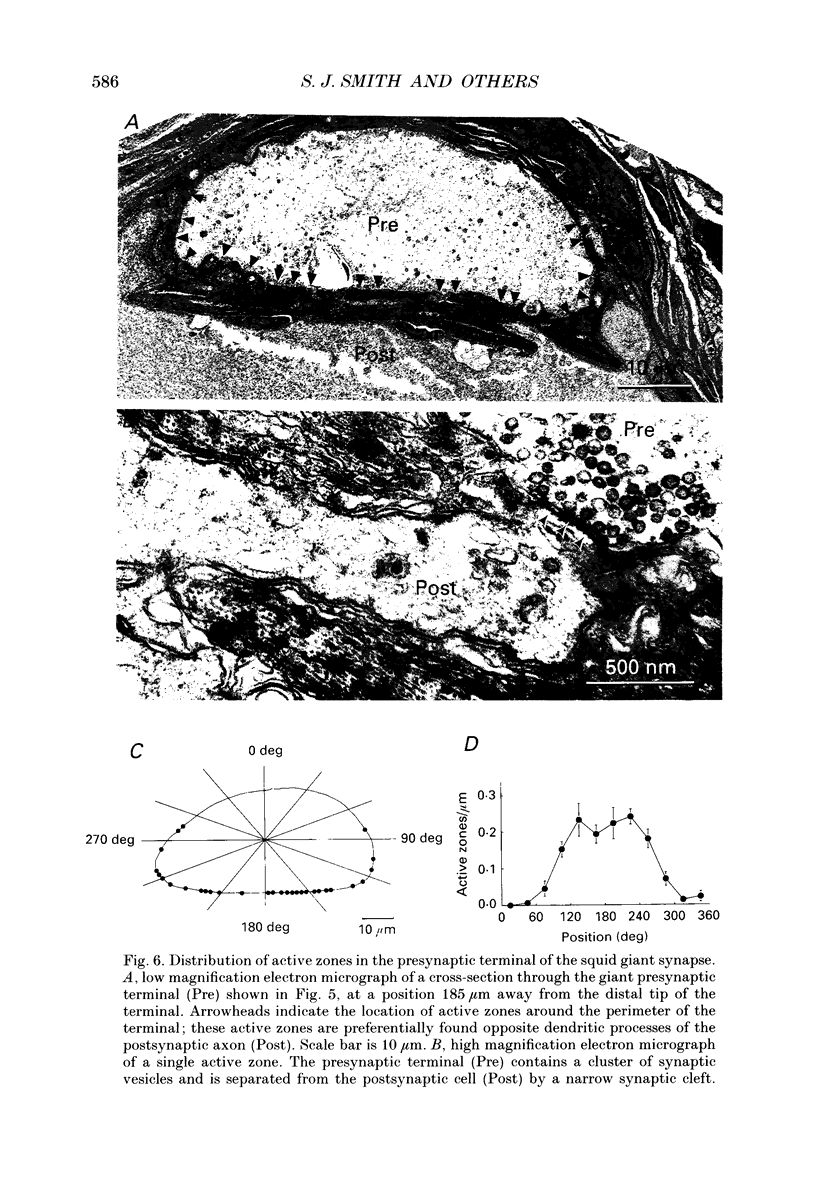
1. The fluorescent Ca2+ indicator dye, fura-2, was used to examine the spatial distribution of intracellular Ca2+ signals in giant presynaptic terminals of squid. Brief trains of presynaptic action potentials were evoked to open Ca2+ channels within the giant presynaptic terminals and elevate presynaptic Ca2+ concentration. 2. Electrical stimulation produced pronounced rises in presynaptic Ca2+ concentration. These rises were much larger in the terminal region than in the adjacent axonal region of the presynaptic neuron, suggesting that Ca2+ channels are most abundant in the terminal. 3. Stimulation also produced gradients in Ca2+ concentration across the width of the presynaptic terminal. During stimulation, Ca2+ concentration was highest in the compartment of the presynaptic terminal closest to the postsynaptic neuron. This suggests that the Ca2+ channels are localized to this region of the presynaptic terminal. 4. Following the end of action potential trains, the rises in Ca2+ concentration became uniform across the width of the terminal. The redistribution of Ca2+ presumably is due to diffusion of Ca2+ throughout the presynaptic cytoplasm. Stimulus-evoked rises in Ca2+ declined slowly over several tens of seconds. 5. Histological examination of a giant presynaptic terminal used for imaging experiments revealed that the spatial compartments where stimulus-induced rises in Ca2+ concentration were highest were also enriched in active zones, the presynaptic sites of transmitter secretion. The co-localization of Ca2+ transients and active zones strongly suggests that neurons cluster Ca2+ channels selectively at active zones and that they do so to enhance the magnitude of Ca2+ signals in the vicinity of the active zone. 6. Longitudinal gradients in Ca2+ concentration also occur within presynaptic terminals and can be quantitatively accounted for by gradients in surface/volume ratio and density of active zones along the length of the presynaptic terminal.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Buchanan J. A., Charlton M. P., Osses L. R., Smith S. J. Fingering the trigger for neurotransmitter secretion: studies on the calcium channels of squid giant presynaptic terminals. Soc Gen Physiol Ser. 1989;44:203–223. [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Augustine G. J. Classification of presynaptic calcium channels at the squid giant synapse: neither T-, L- nor N-type. Brain Res. 1990 Aug 13;525(1):133–139. doi: 10.1016/0006-8993(90)91328-e. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. W., Jones O. T., Angelides K. J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991 Apr;11(4):1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C. The submembrane machinery for nicotinic acetylcholine receptor clustering. J Cell Biol. 1991 Jul;114(1):1–7. doi: 10.1083/jcb.114.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga H., Engel A. G., Lang B., Newsom-Davis J., Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Wallace B. G. Molecules in basal lamina that direct the formation of synaptic specializations at neuromuscular junctions. Dev Neurosci. 1989;11(4-5):227–247. doi: 10.1159/000111903. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Calcium transients recorded with arsenazo III in the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):197–211. doi: 10.1098/rspb.1981.0034. [DOI] [PubMed] [Google Scholar]
- Pappas G. D., Kriho V. Fine structural localization of Ca2+-ATPase activity at the frog neuromuscular junction. J Neurocytol. 1988 Aug;17(4):417–423. doi: 10.1007/BF01189799. [DOI] [PubMed] [Google Scholar]
- Poo M. M. Mobility and localization of proteins in excitable membranes. Annu Rev Neurosci. 1985;8:369–406. doi: 10.1146/annurev.ne.08.030185.002101. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M. E., Nuño C. M., Buchanan J., Augustine G. J. Contractions of the squid stellate ganglion. J Exp Biol. 1990 Sep;152:369–387. doi: 10.1242/jeb.152.1.369. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels on a cholinergic presynaptic nerve terminal. Neuron. 1991 Oct;7(4):585–591. doi: 10.1016/0896-6273(91)90371-6. [DOI] [PubMed] [Google Scholar]
- Stockbridge N., Ross W. N. Localized Ca2+ and calcium-activated potassium conductances in terminals of a barnacle photoreceptor. Nature. 1984 May 17;309(5965):266–268. doi: 10.1038/309266a0. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Hans M., Zipser K., Augustine G. J. Role of residual calcium in synaptic depression and posttetanic potentiation: fast and slow calcium signaling in nerve terminals. Neuron. 1991 Dec;7(6):915–926. doi: 10.1016/0896-6273(91)90337-y. [DOI] [PubMed] [Google Scholar]
- Torri-Tarelli F., Grohovaz F., Fesce R., Ceccarelli B. Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine. J Cell Biol. 1985 Oct;101(4):1386–1399. doi: 10.1083/jcb.101.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Ritchie J. M. Organization of ion channels in the myelinated nerve fiber. Science. 1985 Jun 28;228(4707):1502–1507. doi: 10.1126/science.2409596. [DOI] [PubMed] [Google Scholar]
- Westenbroek R. E., Ahlijanian M. K., Catterall W. A. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990 Sep 20;347(6290):281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- Young J. Z. The giant fibre synapse of Loligo. Brain Res. 1973 Jul 27;57(2):457–460. doi: 10.1016/0006-8993(73)90149-2. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]