Abstract
A 55-year-old male with hemophilia A came to the outpatient clinic with chest pain for several days after overdose injection of coagulation factor. He was a heavy smoker and a chronic alcoholic. An electrocardiogram (ECG) showed no specific change. A coronary computed tomography showed moderate stenosis with soft plaque at the distal segment of right coronary artery. His pain was improved with antianginal and reflux medications. Twenty days later, he ran to the emergency room complaining of squeezing chest pain. ECG showed mild ST segment elevation in inferior territories. Invasive coronary angiography via right radial artery revealed severe thrombotic occlusion at the same lesion. A bare metal stent was deployed and dual antiplatelet therapy including aspirin and clopidogrel had been maintained for 6 months under the conventional hemophilia management. The patient did not develop any coronary events just with single clopidogrel therapy for 5 years until he passed away from pancreatic cancer. Our case implicates that the invasive coronary intervention and post-procedural management could be safely performed with conventional standards of care while maintaining the usual dose of coagulation factors in a hemophilia patient with acute coronary syndrome.
Keywords: Hemophilia, acute myocardial infarction, stent, bleeding, antiplatelet
INTRODUCTION
Hemophilia is an X-linked recessive disease due to factor VIII gene mutations. Advances in treatment technologies have reduced the risk of massive bleeding and improved the life expectancy of hemophilia patients.1 Therefore, hemophilia patients develop atherosclerotic cardiovascular disease (ASCVD) at similar rates to the general population.2 However, the incidence of acute coronary syndrome (ACS) and the rates of thrombotic cardiovascular mortality among patients with hemophilia are notably lower. This discrepancy is associated with reduced thrombin generation and intrinsic hypocoagulable condition of hemophilia patients.3,4 ACS resulting from plaque rupture and thrombotic occlusion may rarely be triggered by an overdose of coagulation factor supplement.5,6 Percutaneous coronary intervention (PCI) for patients with hemophilia carries a higher risk of major bleeding.1 Some experts suggest invasive coronary procedures in hemophiliacs with ACS, lacking established guidelines.7,8 Herein, we share a case of successful PCI and peri-procedural and long-term management of a hemophilia patient who presented with acute myocardial infarction.
CASE REPORT
A 55-year-old male farmer came to our outpatient clinic presenting resting chest pain for several days. He was a current heavy smoker and a chronic alcoholic for 35 years. He was obese (body weight of 76.1 kg and body mass index of 29.0 kg/m2) and had been taking antihypertensive medications for 5 years. After he was diagnosed with hemophilia A during his military service, he had been receiving subcutaneous injections of coagulation factor VIII (Greenmono™, GC Biopharma, Yongin, Korea). He used to inject additional dose of coagulation factor when he felt arthralgia at both the destructed knees and ankles, as he believed the pain would be relieved by supplement. A chest X-ray and initial electrocardiogram (ECG) showed no specific abnormalities. An echocardiography revealed normal left ventricular function without regional wall motion abnormalities. The levels of cardiac enzyme and B-type natriuretic peptide were within the normal range. Low-density lipoprotein-cholesterol level was elevated at 133 mg/dL. The level of factor VIII was 16%.
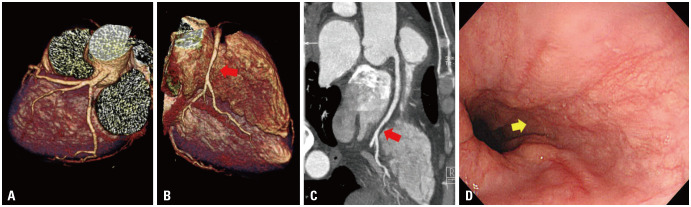
Upon consultation with a hematologist, coronary CT angiography (CCTA) instead of invasive coronary angiography (CAG) was performed to rule out angina. CCTA demonstrated a tubular 50% stenosis with soft plaque at the distal segment of the right coronary artery (RCA) (Fig. 1A to C). An esophagogastroduodenoscopy showed a mucosal break suggesting reflux esophagitis (Fig. 1D). The patient’s pain was improved with antianginal medications, including molsidomine and carvedilol. He was suspected to have intermediate spastic coronary artery disease or reflux esophagitis and was released after a 6-days stay, prescribed statin and pantoprazole, but without any antiplatelet agents. He had been advised against excessive use of the coagulation factor supplement.
Fig. 1. Coronary computed tomography and simultaneous esophagogastroscopy for angina at first admission. (A and B) Coronary computed tomography angiogram with three-dimensional volume rendered, (C) curved muti-planar reconstruction images. Images showed minimal lesion of the distal part of right coronary artery (red arrows) in the hemophilia patient presenting with resting chest pain. (D) Esophagogastroduodenoscopy revealed significant reflux esophagitis (Los Angeles Classification grade B, yellow arrow).
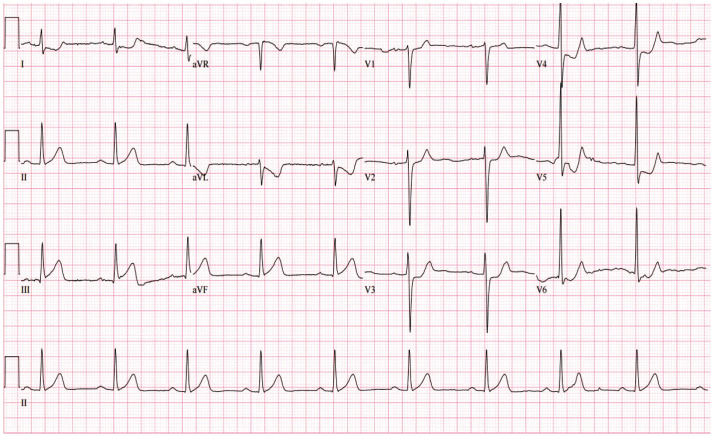
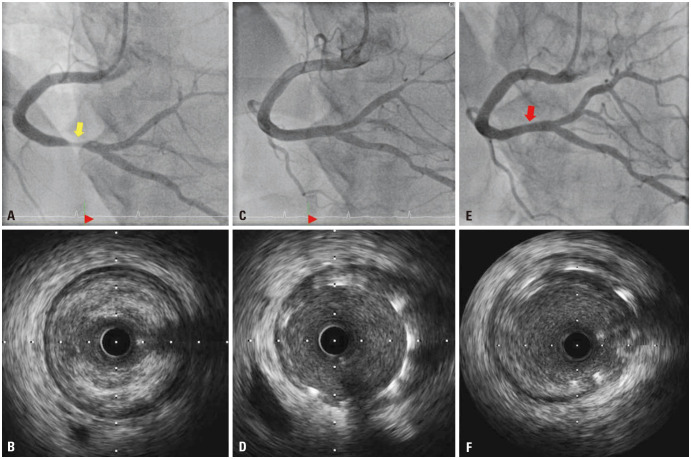
Twenty days later, the patient was brought to our emergency room due to severe squeezing chest pain. He continued to smoke and drink due to heavy farming work, and was administered factor VIII injections up to three times a day to alleviate severe arthralgia. ECG demonstrated ST-segment elevation in inferior leads and reciprocal depression (Fig. 2). Due to the patient’s persistent chest pain despite medical management including nitroglycerin, heparin infusion (loading dose of 5000 IU with maintaining dose of 18 IU/hr) and dual antiplatelet therapy (DAPT) (aspirin 300 mg, clopidogrel 600 mg) were loaded. The minimally invasive CAG intending PCI was performed after a shared decision-making with the patient. Diagnostic CAG via the right radial artery with 4 French sheaths and Judkin catheters showed more than 80% luminal narrowing at the same segment of RCA seen on the prior CCTA. For revascularization, 5 French sheaths and Judkin right guiding catheters were used. Intravascular ultrasound confirmed 90% area stenosis with thrombotic occlusion (Fig. 3A and B). After pre-dilatation with a Genoss balloon™ (3.0×15 mm, Genoss, Suwon, Korea), a bare metal stent (BMS), Integrity™ (4.0×22 mm, Medtronic, Minneapolis, MN, USA) was deployed (Fig. 3C and D). At the end of the procedure, the activated clotting time was 180 msec, and 5000 IU of heparin was additionally infused. Six days after index PCI, the patient was discharged without any complications. Blood quantification of coagulation factor VIII at admission was high (94 IU/dL).
Fig. 2. Electrocardiogram showing acute myocardial infarction at second admission. Electrocardiogram taken when the patient came to the emergency room complaining of severe squeezing chest pain, demonstrating mild ST segment elevation in lead II, III, and aVF with reciprocal depression in lead V2–6.
Fig. 3. Serial coronary angiography with intravascular imaging for acute myocardial infarction at second admission and 6 months later. (A) Initial coronary angiography via right radial artery with 4 French sheath and catheter showed 80% luminal narrowing of diameter with haziness (yellow arrow). (B) Intravascular ultrasound image showed approximately 90% area stenosis with a fibro-fatty plaque with thrombotic occlusion (external elastic membrane 5.5 mm, minimal luminal diameter 2.2 mm). (C) Final coronary angiography after successful percutaneous coronary intervention via 5 French sheath and catheter demonstrated well-dilated stenotic lesion. (D) A simultaneous intravascular ultrasound image also demonstrated that the struts of bare metal stent were well-apposed to the vessel wall. (E and F) After 6 months, follow-up coronary angiography and intravascular ultrasound with 4 French sheath and catheter via left radial artery showed patent previous stent (red arrow) with minimal late loss.
Six months later, follow-up angiography and intravascular ultrasound showed patent BMS with minimal late loss (Fig. 3E and F). Under the conventional supplement, the level of factor VIII was usually varied, ranging between 6% and 49%. Unfortunately, more than 5 years later, the patient passed away from pancreatic cancer. He had been remaining stable without any clinical events while adhering to a single antiplatelet agent (clopidogrel 75 mg) and conventional hemophilia management with proper arthralgia control. The informed consent for the case report was received from the guardian and the institute review board approved it (No. 202311015-HE001).
DISCUSSION
With increased life expectancy among hemophilia patients, they exhibit multiple risk factors for ASCVD.1 PCI with DAPT for hemophilia patients with ACS would increase the bleeding risk and presents a therapeutic dilemma due to the lack of experience and evidence.8
Our patient presented several risk factors for ASCVD and received an overdose of coagulation factor supplement, resulting in ACS. BMS facilitated a shorter duration of DAPT, and no bleeding or thrombotic complications occurred under clopidogrel monotherapy for the rest of his life.
According to the major consensus, invasive interventions and medical therapy in hemophilia patients with ACS should not be deferred, as delayed reperfusion can lead to poor cardiovascular outcomes.1,7 Although the peak level of clotting factor should be maintained above 80% before PCI,1 confirming the levels cannot justify delaying interventions for ACS.8
Following PCI, the proper maintenance of antiplatelet drugs holds significant importance due to stent thrombosis concern.9,10 Since hemophilia does not involve any platelet abnormalities, DAPT would be utilized without definitive guidance.8 One month of DAPT was suggested following the placement of BMS, as well as 12 months after implanting a drug-eluting stent (DES).7 In the DES era, advances in technologies, including very thin-strut, mitigate the duration of DAPT.11
In conclusion, ASCVD affects hemophilia patients just as it does the general population. It is possible to perform CAG, PCI, and post-procedural management with standards of care while maintaining the usual dose of coagulation factors. Further research to develop specific guidelines concerning PCI in hemophilia patients with ACS would be warranted.
ACKNOWLEDGEMENTS
The authors sincerely respect the patient’s struggle life trajectory against the disease.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sang Min Park.
- Data curation: Sang Min Park and Dong Woo Suh.
- Formal analysis: Sang Min Park and Kyung Soon Hong.
- Funding acquisition: Sang Min Park.
- Investigation: Sang Min Park and Dong Woo Suh.
- Methodology: Sang Min Park and Soo Jung Gong.
- Project administration: Sang Min Park.
- Resources: Sang Min Park and Kyung Soon Hong.
- Software: Sang Min Park.
- Supervision: Sang Min Park.
- Validation: Sang Min Park.
- Visualization: Sang Min Park and Kyung Soon Hong.
- Writing—original draft: Sang Min Park and Dong Woo Suh.
- Writing—review & editing: Sang Min Park, Christopher Y Kim, and Soo Jung Gong.
- Approval of final manuscript: all authors.
References
- 1.Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158. doi: 10.1111/hae.14046. [DOI] [PubMed] [Google Scholar]
- 2.Biere-Rafi S, Tuinenburg A, Haak BW, Peters M, Huijgen R, De Groot E, et al. Factor VIII deficiency does not protect against atherosclerosis. J Thromb Haemost. 2012;10:30–37. doi: 10.1111/j.1538-7836.2011.04499.x. [DOI] [PubMed] [Google Scholar]
- 3.Biere-Rafi S, Zwiers M, Peters M, van der Meer J, Rosendaal FR, Büller HR, et al. The effect of haemophilia and von Willebrand disease on arterial thrombosis: a systematic review. Neth J Med. 2010;68:207–214. [PubMed] [Google Scholar]
- 4.Kamphuisen PW, ten Cate H. Cardiovascular risk in patients with hemophilia. Blood. 2014;123:1297–1301. doi: 10.1182/blood-2013-11-453159. [DOI] [PubMed] [Google Scholar]
- 5.Reddy SA, Hoole SP, Besser MW. Primary percutaneous coronary intervention in a patient with haemophilia A. Case Rep Med. 2013;2013:189796. doi: 10.1155/2013/189796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond L, Bevan D. Myocardial infarction in a patient with hemophilia treated with DDAVP. N Engl J Med. 1988;318:121. [PubMed] [Google Scholar]
- 7.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 8.Staritz P, de Moerloose P, Schutgens R, Dolan G. Applicability of the European Society of Cardiology guidelines on management of acute coronary syndromes to people with haemophilia – an assessment by the ADVANCE Working Group. Haemophilia. 2013;19:833–840. doi: 10.1111/hae.12189. [DOI] [PubMed] [Google Scholar]
- 9.Bovenzi F, De Luca L, Signore N, Fusco F, de Luca I. Abciximab for the treatment of an acute thrombotic coronary occlusion during stent implantation in a patient with severe hemophilia B. Ital Heart J. 2003;4:728–730. [PubMed] [Google Scholar]
- 10.Jabbar AY, Baydoun H, Janbain M, Ferdinand KC. Current concepts in the management of stable ischemic heart disease and acute coronary syndrome in patients with hemophilia. Ann Transl Med. 2018;6:299. doi: 10.21037/atm.2018.05.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. 2020;382:1208–1218. doi: 10.1056/NEJMoa1910021. [DOI] [PubMed] [Google Scholar]