Abstract
Although glycopeptides remain the preferred treatment for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia, the treatment of persistent MRSA bacteremia has been challenging. We investigated real-world treatment strategies for persistent MRSA bacteremia, with a specific emphasis on the use of antimicrobial agents and the frequency of changes during the treatment course. We retrospectively identified patients with persistent MRSA bacteremia in four university-affiliated hospitals between 2017 and 2021. The primary objective of this study was to investigate the patterns of antimicrobial uses for MRSA bacteremia. The secondary objectives were evaluating the associated factors with 1) overall 30-day mortality and 2) changing agents during the treatment course. Time-dependent Cox regression analysis was used to adjust for immortal time bias. Among 116 patients, 37.1% underwent antimicrobials switching, primarily prompted by persistent bacteremia. The 30-day mortality rates of groups with and without antimicrobial switching were 21.4% and 44.2%, respectively (p=0.010 by log-rank test); however, after adjustment for immortal time bias, there was no statistical significance between the two groups (adjusted hazard ratio 0.24, 95% confidence interval 0.03–2.17, p=0.238). Only the Pitt bacteremia score on day 4 and pneumonia were associated with 30-day mortality. Meanwhile, the factors associated with antimicrobial switching were the duration of bacteremia, the initial use of teicoplanin, echocardiogram, and Charlson comorbidity index. This study showed that while over one-third of persistent MRSA bacteremia patients experience changes in antimicrobial agents during treatment, this practice does not significantly improve the 30-day mortality. Our study suggests the need for more effective treatment strategies in managing persistent MRSA bacteremia.
Keywords: Methicillin-resistant Staphylococcus aureus, bacteremia, persistent infection, glycopeptides
Graphical Abstract
Staphylococcus aureus is the most common pathogen for both community and hospital-acquired infections, causing high morbidity and mortality.1 After the emergence of methicillin-resistant S. aureus (MRSA) in 1961,2 this strain has successfully disseminated worldwide, leading to persistent bacteremia and higher mortality rates compared to methicillin-susceptible S. aureus.3,4 Vancomycin has been regarded as the treatment of choice for MRSA infections, but clinicians have been challenged about its use due to its narrow therapeutic range and potential nephrotoxicity.5,6 In addition, there remains a lack of consensus or promising data regarding the treatment strategies for persistent MRSA bacteremia even after securing appropriate serum vancomycin concentrations.6,7 In the present study, we investigated real-world treatment strategies for persistent MRSA bacteremia, with a specific focus on the use of antimicrobial agents and the frequency of changes during the treatment course and its association with 30-day mortality.
This study retrospectively collected data on adult patients (aged 18 years or older) with persistent MRSA bacteremia, from 2017 to December 2021, at four university-affiliated hospitals in the Republic of Korea. It was a post-hoc analysis of a previous study on persistent S. aureus bacteremia (SAB), with a portion of the cohorts from our current study also included in that previous research.8 Persistent bacteremia was defined as bacteremia that lasted for 2 days or more, counted after the initial administration day of active antibiotic therapy. Additionally, a positive follow-up culture within 14 days following a negative conversion was considered to be part of the same bacteremia episode.9 Patients with polymicrobial bacteremia were excluded.
The primary objective of the current study was to evaluate the 30-day in-hospital mortality and its associated clinical factors. Patients were observed until death, loss to follow-up, or 30 days after the initiation of active antibiotic therapy. The secondary study objectives included examining the clinical factors associated with antimicrobial switching during the course of treatment, and identifying the clinical factors associated with 30-day mortality among patients who underwent changes in antimicrobial agents during treatment. Patients’ data were collected from electronic medical records. Bacteremia duration was defined as the period from the first day of initiating antibiotic therapy susceptible to MRSA, following the first positive blood culture, to the day of the last positive follow-up blood culture.9 Disease severity was calculated by Pitt bacteremia score (PBS),10 and underlying disease was evaluated by Charlson comorbidity index (CCI).11 Immunosuppression was defined as the use of steroids (prednisolone >0.5 mg/kg/d or equivalent for >1 month), chemotherapy, or anti-tumor necrosis factor therapy within the past 3 months.8 Surgically removable infections and indwelling foreign bodies, such as intravenous catheters and drainable abscesses, were defined as an eradicable focus.12 Acquisition site of bacteremia was classified as hospital-acquired, healthcare-associated, or community-acquired infection.13 Methicillin resistance was defined as an oxacillin minimum inhibitory concentration ≥4 mcg/mL or positive for cefoxitin disk diffusion test according to the Clinical and Laboratory Standards Institute M100-S16:2021 guidelines.
All statistical analyses were performed using the Statistical Package for the Social Sciences, version 25.0 for Windows (IBM Corp., Armonk, NY, USA) and R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were compared using Mann-Whitney U test according to the results of the normality test. Fisher’s exact test or chi-square test was used to compare categorical variables. Variables with a p-value less than 0.1 in the univariable logistic regression analysis, along with variables considered potentially clinically meaningful, were included in the multivariable model. The time to death of patients with and without antimicrobial switching during the 30-day follow-up period was calculated using the Kaplan–Meier method and compared using the log-rank test. To evaluate the association between 30-day in-hospital mortality and clinical factors, including antimicrobial switching during the treatment course, a time-dependent multivariable Cox regression analysis was conducted. To adjust for immortal time bias, antimicrobial switching was treated as a time-varying covariate. This approach ensured that patients were considered part of the group without changes in antimicrobial therapy until they received the changes in antimicrobial therapy. Additionally, to assess the association between clinical factors and changes in antimicrobial therapy during the treatment course, binary logistic regression analysis was conducted. All p-values were two-tailed, and p-values less than 0.05 were considered statistically significant.
This study was approved by the institutional review board of Samsung Changwon Hospital (IRB number: SCMC 2023-02-006). The need for informed consent was waived since this was an observational retrospective study, and all patient data were analyzed anonymously.
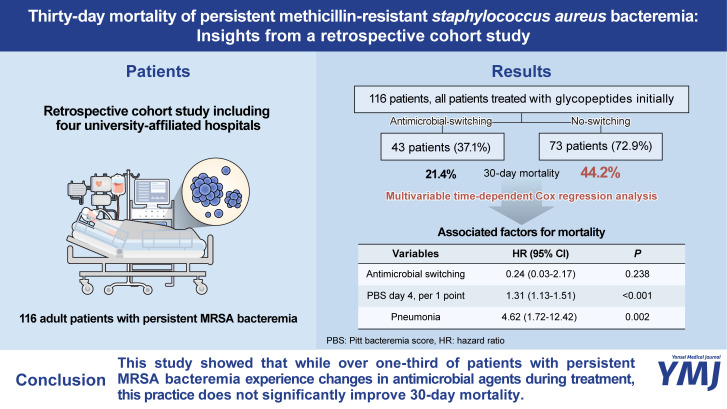
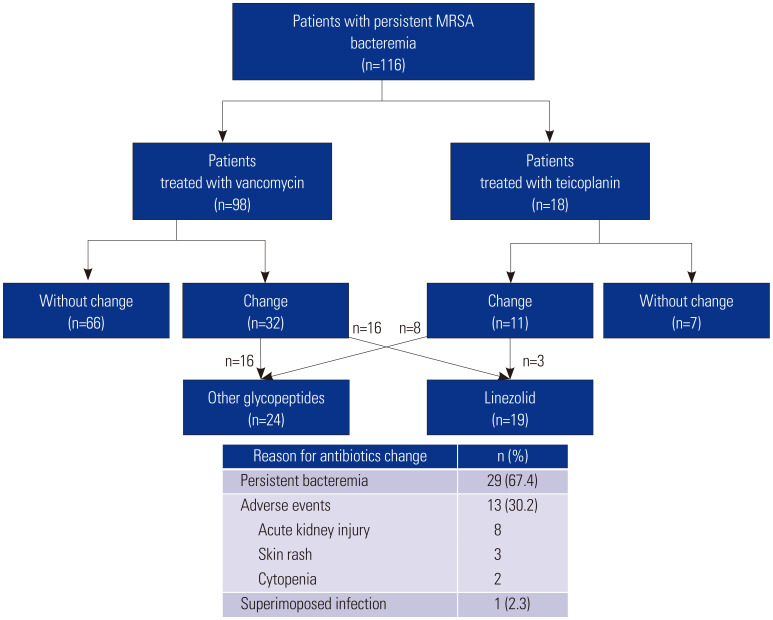
During the study period, a total of 116 patients with persistent MRSA bacteremia were included. Of these, 98 patients (84.5%) were initially treated with vancomycin, and 18 patients (15.5%) were initially treated with teicoplanin. The initial vancomycin trough level (within 7 days) among the group receiving vancomycin was a median of 12.4 mg/L [interquartile range (IQR), 7.0–18.0 mg/L, based on data from 87 patients]. All patients receiving teicoplanin were given an initial loading dose (median 400 mg/dose, IQR 352.5–400mg/dose) and a maintenance dose with a median of 400 mg/dose (IQR 370–400 mg/dose). Furthermore, 43 patients (37.1%) received second-line antimicrobial agents, with the most common reason for changing antibiotics being persistent MRSA bacteremia (29 out of 43 patients, or 67.4%). In addition, antimicrobial switching in 13 cases was due to adverse events, and in one case, it was due to a superimposed infection. These patients were treated with the initial agent for a median duration of 10 days before switching to a second-line agent. Among these patients, 24 received alternative glycopeptides as the second agent, with 16 switching from vancomycin to teicoplanin and eight switching from teicoplanin to vancomycin. Meanwhile, 19 patients were treated with the linezolid (Fig. 1). The overall mortality of the study population was 35.6%.
Fig. 1. Treatment sequences for persistent MRSA bacteremia in the study population. MRSA, methicillin-resistant Staphylococcus aureus.
In the univariable analysis, patients who had their anti-MRSA agents changed exhibited a lower mortality rate (21.4%) compared to those who did not have their anti-MRSA agents changed (44.2%), demonstrating a difference that was statistically significant (p=0.010 by log rank test) (Fig. 2A). In further survival analysis conducted over the first 10 days, the mortality rate differed between each group (0% vs. 12.5%, p=0.017) (Fig. 2B). However, this difference diminished in another analysis that included only patients who were observed for at least 10 days or more (21.4% vs. 36.2%, p=0.101) (Fig. 2C). Additionally, the factors representing disease severity (indicated by intensive care unit (ICU) admission within 24 hours, initial PBS, and day 4 PBS) and the presence of pneumonia were associated with increased mortality. In contrast, undergoing echocardiography and experiencing a longer duration of bacteremia were factors associated with lower mortality (Table 1). After adjustment using time-dependent Cox regression analysis, with antimicrobial switching as a time-varying covariate, there was no statistical significance between the groups with and without antimicrobial switching [adjusted hazard ratio (HR) 0.24, 95% confidence interval (CI) 0.03–2.17, p=0.238]. This finding suggests that the observed positive effects of antimicrobial switching on 30-day mortality among patients with persistent MRSA bacteremia may be attributed to immortal time bias. Only day 4 PBS (an aHR of 1.31, 95% CI 1.13–1.51, p<0.001) and the presence of pneumonia (aHR 4.62, 95% CI 1.72–12.42, p=0.002) were found to be associated with increased mortality (Table 2). In the subgroup analysis of patients who underwent antimicrobial switching during treatment, those who received the initial antimicrobial for a longer duration had higher survival rates (Supplementary Table 1, only online); however, after adjusting for other clinical factors, there was no factor associated with 30-day mortality (Supplementary Table 2, only online).
Fig. 2. Kaplan–Meier curve showing the probability of survival in patients with persistent MRSA bacteremia. (A) The probability of survival in groups with and without antimicrobial switching over 30 days after the onset of bacteremia. (B) Survival analysis in groups with and without antimicrobial switching conducted over the first 10 days after the onset of bacteremia. (C) Survival analysis in subgroups with and without antimicrobial switching, including only patients who were observed for at least 10 days, conducted over 30 days after the onset of bacteremia. MRSA, methicillin-resistant Staphylococcus aureus.
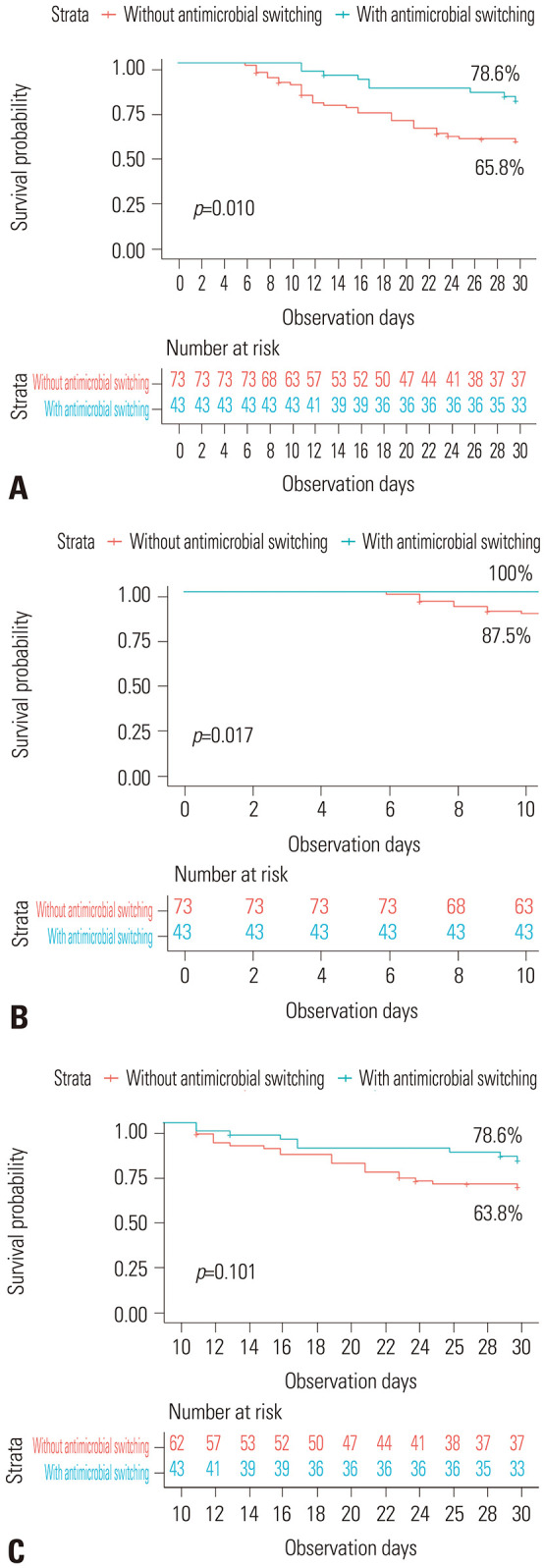
Table 1. Factors Associated with 30-Day Mortality in Patients with Persistent MRSA Bacteremia.
| Variable | Overall (n=116) | Survived (n=76) | Deceased (n=40) | HR (95% CI) | p value | ||
|---|---|---|---|---|---|---|---|
| Sex, male | 60 (51.7) | 44 (57.9) | 16 (40.0) | 1.76 (0.94–3.32) | 0.080 | ||
| Age (yr) | 69 (58.25–81) | 67.5 (58–80) | 74 (60.25–81) | 1.02 (0.99–1.05) | 0.113 | ||
| Vancomycin MIC ≥1 mg/L | 73 (62.9) | 51 (67.1) | 22 (55.0) | 1.20 (0.88–1.64) | 0.249 | ||
| Initial treatment | |||||||
| Definitive antimicrobial agents | 0.801 | ||||||
| Vancomycin | 98 (84.5) | 64 (84.2) | 34 (85.0) | 0.95 (0.61–1.46) | |||
| Teicoplanin | 18 (15.5) | 12 (15.8) | 6 (15.0) | Ref | |||
| Beta-lactams co-administration | 71 (61.2) | 40 (52.6) | 31 (77.5) | 2.80 (1.33–5.89) | 0.007 | ||
| Switching to other anti-MRSA agents (other glycopeptides and linezolid) | 43 (37.1) | 34 (44.7) | 9 (22.5) | 0.39 (0.19–0.83) | 0.014 | ||
| Switching in treatment course | 0.031 | ||||||
| No switching | 73 (62.9) | 42 (55.3) | 31 (77.5) | Ref. | |||
| Switching to other glycopeptides | 24 (20.7) | 19 (25.0) | 5 (12.5) | 0.39 (0.15–1.00) | |||
| Switching to linezolid | 19 (16.4) | 15 (19.7) | 4 (10.0) | 0.40 (0.14–1.14) | |||
| Time to active antibiotics (days)* | 2 (1–3) | 1.5 (0.25–3) | 2 (1–3) | 1.00 (0.89–1.12) | 0.986 | ||
| Initial laboratory findings | |||||||
| White blood cell count (/µL) | 12940 (10570–18080) | 12840 (10780–17420) | 13130 (9640–19850) | 1.02 (0.99–1.05) | 0.281 | ||
| C-reactive protein (mg/dL) | 19.38 (6.62–26.75) | 19.16 (5.72–26.75) | 20.18 (7.63–27.43) | 1.01 (0.98–1.04) | 0.455 | ||
| Disease severity | |||||||
| ICU admission within 24 hours* | 50 (43.1) | 26 (34.2) | 24 (60.0) | 2.40 (1.27–4.51) | 0.007 | ||
| Pitt bacteremia score (initial) | 2 (1–3) | 1 (0–2.75) | 3 (1–5) | 1.22 (1.07–1.40) | 0.003 | ||
| Pitt bacteremia score (day 4)* | 1 (0–3) | 0.5 (0–2) | 3.5 (0.25–5) | 1.32 (1.19–1.47) | <0.001 | ||
| Focus of infection | |||||||
| Endocarditis | 14 (12.1) | 7 (9.2) | 7 (17.5) | 1.76 (0.78–3.99) | 0.173 | ||
| Osteoarticular focus | 29 (25.0) | 22 (28.9) | 7 (17.5) | 0.61 (0.27–1.38) | 0.237 | ||
| Pneumonia | 10 (8.6) | 2 (2.6) | 8 (20.0) | 4.83 (2.21–10.60) | <0.001* | ||
| Surgical wound infection | 2 (1.7) | 2 (2.6) | 0 (0.0) | N/A | |||
| Skin and soft tissue infection | 12 (10.3) | 12 (15.8) | 0 (0.0) | N/A | |||
| Intravascular catheter | 24 (20.7) | 16 (21.1) | 8 (20.0) | 0.84 (0.39–1.83) | 0.668 | ||
| Unknown focus | 21 (18.1) | 12 (15.8) | 9 (22.5) | 1.46 (0.70–3.07) | 0.316 | ||
| Metastatic infection | 33 (28.4) | 19 (25.0) | 14 (35.0) | 1.36 (0.71–2.61) | 0.351 | ||
| Echocardiography | 83 (71.6) | 61 (80.3) | 22 (55.0) | 0.38 (0.20–0.70) | 0.002* | ||
| Onset of bacteremia | 0.903 | ||||||
| Hospital-acquired | 63 (54.3) | 40 (52.6) | 23 (57.5) | Reference | |||
| Healthcare-associated | 28 (24.1) | 19 (25.0) | 9 (22.5) | 1.10 (0.72–1.67) | |||
| Community-acquired | 25 (21.6) | 17 (22.4) | 8 (20.0) | 0.92 (0.58–1.63) | |||
| Bacteremia duration (days) | 6 (3–12) | 7.5 (3–15) | 6 (3–9.75) | 0.94 (0.89–1.00) | 0.034* | ||
| Prostheses | |||||||
| Orthopedic device | 21 (18.1) | 15 (19.7) | 6 (15.0) | 0.77 (0.32–1.83) | 0.552 | ||
| Cardiovascular device | 4 (3.4) | 2 (2.6) | 2 (5.0) | 1.59 (0.39–6.57) | 0.526 | ||
| Long-term CVC | 25 (21.6) | 15 (19.7) | 10 (26.0) | 1.15 (0.56–2.36) | 0.697 | ||
| Removal of eradicable focus | 0.306 | ||||||
| No removal | 31 (26.7) | 21 (27.6) | 10 (25.0) | Reference | |||
| Non-eradicable | 49 (42.2) | 28 (36.8) | 21 (52.5) | 1.54 (0.73–3.28) | |||
| Removal of focus after day 3* | 12 (10.3) | 9 (11.8) | 3 (7.5) | 0.76 (0.21–2.77) | |||
| Removal of focus before day 3* | 24 (20.7) | 18 (23.7) | 6 (15.0) | 0.75 (0.27–2.05) | |||
| Charlson comorbidity index | 2 (1–4) | 2 (0.25–4) | 3 (1–5) | 1.07 (0.96–1.20) | 0.228 | ||
| Immunosuppression | 21 (18.1) | 11 (14.5) | 10 (25.0) | 1.53 (0.75–3.13) | 0.247 | ||
| ID consultation within 7 days* | 75 (64.7) | 51 (67.1) | 24 (60.0) | 0.81 (0.43–1.53) | 0.522 | ||
HR, hazard ratio; CI, confidence interval; MIC, minimal inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; ICU, intensive care unit; CVC, central venous catheter; ID, infectious diseases.
Data are presented as numbers (%) or median (interquartile range) unless otherwise indicated.
*Time points were calculated from the onset of bacteremia.
Table 2. Multivariable Analysis for 30-Day Mortality in Patients with Persistent MRSA Bacteremia.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| Sex, male | 0.57 (0.30–1.07) | 0.080 | 0.58 (0.29–1.16) | 0.124 |
| Age, per 1-year | 1.02 (0.99–1.05) | 0.113 | 1.02 (0.99–1.04) | 0.268 |
| Beta-lactams co-administration | 2.80 (1.33–5.89) | 0.007 | 1.07 (0.44–2.67) | 0.859 |
| Switching to other anti-MRSA agents | 0.39 (0.19–0.83) | 0.014 | 0.24 (0.03–2.17) | 0.238 |
| ICU admission within 24 hours* | 2.40 (1.27–4.51) | 0.007 | 1.81 (0.74–4.41) | 0.195 |
| Pitt bacteremia score, initial, per 1-point | 1.22 (1.07–1.40) | 0.003 | 0.86 (0.69–1.08) | 0.187 |
| Pitt bacteremia score, day 4, per 1-point* | 1.32 (1.19–1.47) | <0.001 | 1.31 (1.13–1.51) | <0.001 |
| Pneumonia | 4.83 (2.21–10.60) | <0.001 | 4.62 (1.72–12.42) | 0.002 |
| Echocardiography | 0.38 (0.20–0.70) | 0.002 | 0.53 (0.25–1.14) | 0.104 |
| Bacteremia duration, days, per 1-day | 0.94 (0.89–1.00) | 0.034 | 0.98 (0.92–1.04) | 0.477 |
MRSA, methicillin-resistant Staphylococcus aureus; HR, hazard ratio; CI, confidence interval; ICU, intensive care unit.
*Time points were calculated from the onset of bacteremia.
In our analysis of clinical factors associated with antimicrobial switching, patients who received teicoplanin as the definitive agent, underwent echocardiography, had prolonged MRSA bacteremia, and had a higher CCI were more likely to experience changes in their antimicrobial treatment during therapy, according to univariate analyses (Supplementary Table 3, only online).
Our study revealed that around one-third of patients with persistent MRSA bacteremia passed away. Although physicians frequently switch antimicrobial agents to treat persistent MRSA bacteremia, our analysis, which accounts for immortal time bias, indicates that these changes in antimicrobial strategy do not significantly impact mortality reduction. Analyses using Kaplan–Meier curves showed that the difference in raw 30-day mortality between patients with and without antimicrobial switching originated from the mortality difference in the initial period of MRSA bacteremia. This suggests that the lower mortality observed in the antimicrobial switching group resulted because these patients survived longer, allowing them the opportunity to receive an alternative agent, compared to those without antimicrobial switching.
Although newer agents for drug-resistant gram-positive coccus infection have been developed, vancomycin is still considered as the drug of choice for invasive MRSA infection.5 However, one meta-analysis indicated that, although the 30-day mortality rate from SAB has decreased over the decades, it remains high at approximately 20%. Furthermore, MRSA bacteremia is associated with higher mortality compared to methicillin-susceptible SAB, with an adjusted odds ratio of 1.04 (95% CI, 1.02–1.06) for every 10% increase in the proportion of MRSA cases.14 In the Republic of Korea, an 11-year longitudinal study reported that the 30-day mortality rate for adult SAB bacteremia was 15.8%, and that it did not decrease over the study period.3 In our study, all patients with persistent MRSA bacteremia initially received glycopeptides; however, the mortality rate was 35.6%, and switching antimicrobials to conventional MRSA agents was not associated with 30-day mortality. Therefore, the need for new agents to treat MRSA infections has been recognized. To date, daptomycin is the only agent, other than vancomycin, that has been approved by the Food and Drug Administration for the treatment of MRSA bacteremia.6 However, the limited indication of daptomycin by the Korean national insurance system—specifically, its approval only for cases of MRSA bacteremia after glycopeptide treatment failure or due to glycopeptide side effects—restricts the use of daptomycin as an initial treatment or for early intervention in persistent MRSA bacteremia.15 One retrospective study focusing on patients with MRSA bacteremia revealed that only those who switched from vancomycin to daptomycin within 3 days exhibited a significant association with lower 30-day mortality (HR, 0.48; 95% CI, 0.25–0.92). In addition, recent data support the effectiveness of newer anti-MRSA agent combinations, such as daptomycin and ceftaroline, for treating persistent MRSA infections.6,16 In our study population, none of the patients received daptomycin. Additionally, among those who were treated with second-line antimicrobial agents, a median of 10 days elapsed before the switch from the initial treatment. Therefore, it is essential to not only encourage the use of newer anti-MRSA agents, but also to ensure their application in the early phase of MRSA bacteremia. This should be supported by appropriate national insurance policies and active interest from physicians.
Our study had several limitations. First, our entire study population was treated with glycopeptides as the first-line agent; however, we could not analyze appropriate glycopeptide exposure due to incomplete data on serum vancomycin concentration and teicoplanin dosage. Since securing appropriate serum concentrations of vancomycin or teicoplanin is critical,17,18,19 data on therapeutic drug levels may be necessary to accurately evaluate the efficacy of glycopeptides. For example, it was known that a loading strategy and a sufficient teicoplanin dosage (≥9 mg/kg per dose) were associated with improved patient outcomes.19 Second, the heterogeneous use of second-line agents and the varied timing of switching to these agents made it difficult to evaluate the efficacy of antimicrobial strategies for MRSA bacteremia. Since the time point of antimicrobial switching was delayed (median 10 days) in our study population, our data may not be applicable to patients who received alternative agents early on after the onset of persistent MRSA bacteremia.
In conclusion, although the strategy of switching antimicrobials appears to be associated with improved 30-day mortality of patients, this could simply be a result of immortal time bias. Therefore, our data suggest that switching antimicrobials to conventional anti-MRSA agents, such as other glycopeptides or linezolid, does not improve mortality in patients with persistent MRSA bacteremia. In this regard, there is an urgent need for both a standardized treatment strategy for persistent MRSA bacteremia and the introduction of new anti-MRSA agents.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Si-Ho Kim and Seok Jun Mun.
- Data curation: Minji Jeon, Sukbin Jang, and Seok Jun Mun.
- Formal analysis: Si-Ho Kim.
- Investigation: all authors.
- Methodology: Si-Ho Kim and Seok Jun Mun.
- Project administration: Seok Jun Mun.
- Resources: Seok Jun Mun and Si-Ho Kim.
- Software: Si-Ho Kim.
- Supervision: Seok Jun Mun and Si-Ho Kim.
- Validation: Minji Jeon and Sukbin Jang.
- Visualization: Seok Jun Mun and Si-Ho Kim.
- Writing—original draft: Seok Jun Mun and Si-Ho Kim.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
AVAILABILITY OF DATA AND MATERIAL
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Factors Associated with 30-Day Mortality in Patients with Persistent MRSA Bacteremia Who Underwent Antimicrobial Switching during Treatment
Multivariable Analysis for Factors Associated with 30-Day Mortality in Patients with Persistent MRSA Bacteremia Who Underwent Antimicrobials Switching during Treatment
Clinical Characteristics of Patients with Persistent MRSA Bacteremia Who Underwent Antimicrobials Switching during the Course of Therapy and Those who Did Not
References
- 1.Howden BP, Giulieri SG, Wong Fok Lung T, Baines SL, Sharkey LK, Lee JYH, et al. Staphylococcus aureus host interactions and adaptation. Nat Rev Microbiol. 2023;21:380–395. doi: 10.1038/s41579-023-00852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jevons MP. “Celbenin”-resistant staphylococci. Br Med J. 1961;1:124. [Google Scholar]
- 3.Choi SH, Lee J, Jung J, Kim ES, Kim MJ, Chong YP, et al. A longitudinal study of adult patients with Staphylococcus aureus bacteremia over 11 years in Korea. J Korean Med Sci. 2021;36:e104. doi: 10.3346/jkms.2021.36.e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joo EJ, Park DA, Kang CI, Chung DR, Song JH, Lee SM, et al. Reevaluation of the impact of methicillin-resistance on outcomes in patients with Staphylococcus aureus bacteremia and endocarditis. Korean J Intern Med. 2019;34:1347–1362. doi: 10.3904/kjim.2017.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 6.Minter DJ, Appa A, Chambers HF, Doernberg SB. Contemporary management of Staphylococcus aureus bacteremia-controversies in clinical practice. Clin Infect Dis. 2023;77:e57–e68. doi: 10.1093/cid/ciad500. [DOI] [PubMed] [Google Scholar]
- 7.Holland TL, Bayer AS, Fowler VG. Persistent methicilin-resistant Staphylococcus aureus bacteremia: resetting the clock for optimal management. Clin Infect Dis. 2022;75:1668–1674. doi: 10.1093/cid/ciac364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, Jeon M, Jang S, Mun SJ. Factors for mortality in patients with persistent Staphylococcus aureus bacteremia: the importance of treatment response rather than bacteremia duration. J Microbiol Immunol Infect. 2023;56:1007–1015. doi: 10.1016/j.jmii.2023.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Kuehl R, Morata L, Boeing C, Subirana I, Seifert H, Rieg S, et al. Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis. 2020;20:1409–1417. doi: 10.1016/S1473-3099(20)30447-3. [DOI] [PubMed] [Google Scholar]
- 10.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Chung H, Kim E, Yang E, Lee YW, Park JH, Bae S, et al. C-reactive protein predicts persistent bacteremia caused by community-acquired methicillin-resistant Staphylococcus aureus strain. Eur J Clin Microbiol Infect Dis. 2021;40:2497–2504. doi: 10.1007/s10096-021-04303-5. [DOI] [PubMed] [Google Scholar]
- 13.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 14.Bai AD, Lo CKL, Komorowski AS, Suresh M, Guo K, Garg A, et al. Staphylococcus aureus bacteraemia mortality: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:1076–1084. doi: 10.1016/j.cmi.2022.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Korea Insurance Charge Review Association. Ministry of Health and Welfare Notice No. 2021-161. 2024. [accessed on 2024 January 9]. Available at: http://www.hicra.or.kr/sub_asp/04_data03.html?mode=read&read_no=6722&now_page=1. Korean.
- 16.McCreary EK, Kullar R, Geriak M, Zasowski EJ, Rizvi K, Schulz LT, et al. Multicenter cohort of patients with methicillin-resistant Staphylococcus aureus bacteremia receiving daptomycin plus ceftaroline compared with other MRSA treatments. Open Forum Infect Dis. 2019;7:ofz538. doi: 10.1093/ofid/ofz538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, Kang CI, Lee SH, Choi JS, Huh K, Cho SY, et al. Weight-based vancomycin loading strategy may not improve achievement of optimal vancomycin concentration in patients with preserved renal function. J Chemother. 2021;33:56–61. doi: 10.1080/1120009X.2020.1755590. [DOI] [PubMed] [Google Scholar]
- 18.Choi JS, Kim JM, Kim D, Kim SH, Cho H, Park HD, et al. Therapeutic drug level monitoring of teicoplanin in Korean pediatric patients with normal versus impaired renal function. J Korean Med Sci. 2020;35:e376. doi: 10.3346/jkms.2020.35.e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH, Kang CI, Huh K, Cho SY, Chung DR, Lee SY, et al. Evaluating the optimal dose of teicoplanin with therapeutic drug monitoring: not too high for adverse event, not too low for treatment efficacy. Eur J Clin Microbiol Infect Dis. 2019;38:2113–2120. doi: 10.1007/s10096-019-03652-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Factors Associated with 30-Day Mortality in Patients with Persistent MRSA Bacteremia Who Underwent Antimicrobial Switching during Treatment
Multivariable Analysis for Factors Associated with 30-Day Mortality in Patients with Persistent MRSA Bacteremia Who Underwent Antimicrobials Switching during Treatment
Clinical Characteristics of Patients with Persistent MRSA Bacteremia Who Underwent Antimicrobials Switching during the Course of Therapy and Those who Did Not
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.