Abstract
This study sought to investigate the demographic and clinical characteristics of Sudanese men diagnosed with prostate cancer (PCa) to highlight differences in diagnosis among the three major ethnolinguistic groups. A total of 532 patients with confirmed PCa diagnosis through biopsy were enrolled from six medical centers in Sudan. The majority of patients, comprising 84.2% (448/532), were diagnosed with advanced-stage disease, with a Grade group above 3. There were no discernible differences in PCa aggressiveness among the ethnolinguistic groups. However, higher levels of prostate-specific antigen (PSA) were observed in the Niger-Congo group, where 55.2% had PSA values exceeding 50 ng/ml. Patients from this group were also diagnosed at a younger age. In contrast, 90.5% of Afro-Asiatic patients are over 60 years old. Further analysis conducted within an age-matched subgroup of patients (n = 273) revealed a higher incidence of perineural invasion in the Afro-Asiatic group. This research represents the first investigation of PCa across different African ethnic groups and associates a higher incidence of perineural invasion with a specific ethnic group. While recent efforts have been made to establish African-relevant risk models to mitigate PCa health disparities, there remains a need for further investigation into genetically distinct populations within the African continent.
Keywords: Perineural invasion, Prostate cancer, Sudan, Niger-Congo, Nilo-Saharan, Afro-Asiatic, Incidence
Subject terms: Cancer epidemiology, Prostate cancer
Background
Prostate cancer (PCa) presents a significant global healthcare issue, constituting the predominant form of cancer found in males1. According to the GLOBOCAN 2020 assessment, PCa emerges as the most frequently detected cancer in regions such as Africa, Europe, and the Americas, and it holds the foremost position in causing cancer-related fatalities among men in 48 nations. These include several countries situated in sub-Saharan Africa, the Caribbean, and Central and South America2.
Despite its prevalent occurrence, there remains a limited understanding of its etiology. Factors that increase the risk of developing this malignancy include advancing age, a family history of the disease, and African ancestry. Numerous investigations have been conducted to gain insight into the various genetic3,4, protein-related5,6, microRNA7, and metabolic8 characteristics of PCa patients with African ancestry. While African ancestry is well-known to play a role in the aggressiveness of PCa, the heterogeneity of African populations is not taken deeply into consideration. The majority of studies focused on the ethnic health disparities are based on the African American population9,10. However, African American genomes are closely related to Niger-Congo (NC)-speaking populations, including non-Bantu such as Igbo and Yoruba from Nigeria11 and Bantu-speaking NC populations12. Although, the recent efforts to establish African-relevant risk models aimed at reducing PCa health disparities13–17, there is still the need for further investigation into other genetically distinct populations in the African continent.
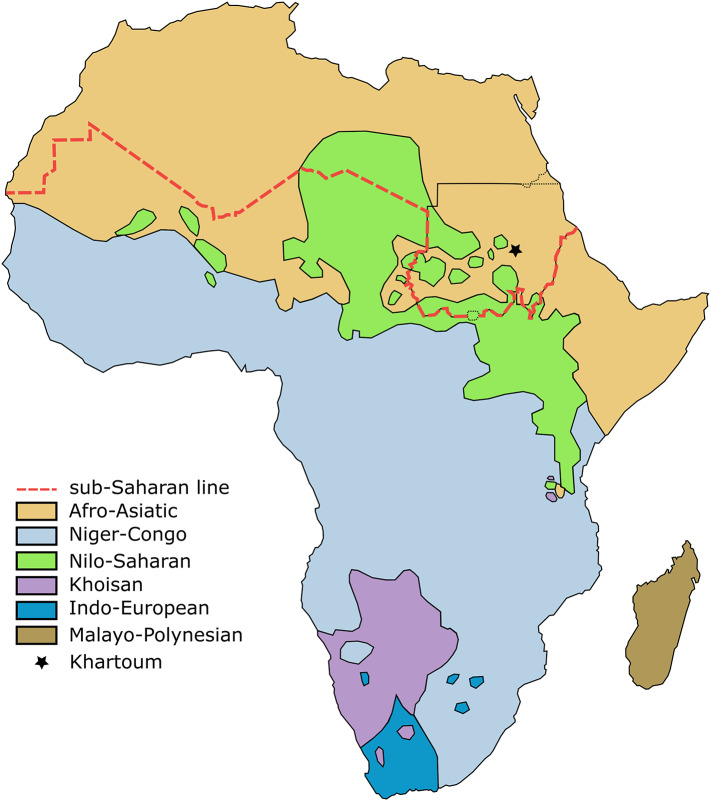
The varied populations of Sudan, boasting both rich cultural traditions and diverse genetic heritage, have an extensive historical presence in northeastern Africa. This area, frequently sought as a sanctuary during extreme climatic conditions, plays a pivotal role in shaping their substantial genetic heterogeneity18. This diversity is exemplified by the prevalence of the major continental African language families in the region (Fig. 1), including the NC, Nilo-Saharan (NS), and Afro-Asiatic (AA) language families19. Populations within these groups exhibit distinct genetic backgrounds20,21 that have evolved under specific environmental pressures22,23, including adaptation to local pathogens24,25 such as malaria. This strong selective pressure has led to population- and region-specific genetic variants, driving the evolution of the human immune system26. Polymorphisms and different expression of immune-related genes could partially explain the different incidences and mortalities of PCa27,28. Compared to the European American population, African American men have increased expression of immune-related genes and increased incidence of inflammation in tumor tissues29,30.
Fig. 1.
Map of Africa showing the distribution of major African linguistic families (adapted from ref. 26). The demarcation line of Sub-Saharan Africa was drawn as indicated by the United Nations Statistics Division.
PCa ranks as the most prevalent cancer among Sudanese men, trailing only behind liver cancer in terms of mortality2. Data indicates that its occurrence remains consistent across various ethnic backgrounds, typically manifesting in individuals aged 72 years or older and often at an advanced clinical stage31. This delayed presentation can be attributed to the limited access to crucial healthcare services32. Shockingly, over 70 percent of healthcare facilities in regions affected by conflicts in Sudan are nonfunctional or closed33. Additionally, the concentration of healthcare centers in urban areas further hampers the ability of patients residing in remote rural areas to access diagnostic and treatment services34,35. This dire situation in Sudan's healthcare system is mirrored by the scarcity of research studies focused on PCa.
In this study, the demographic and clinical features of Sudanese men with PCa were investigated to highlight the difference in the diagnosis among the three major ethnolinguistic groups.
Methods
Approval for this study was obtained from the National Health Research Ethics Committee of the Sudanese Federal Ministry of Health (6–9-2021). The study was performed in accordance with the Declaration of Helsinki. Patients were recruited in the Urology Departments of the Omdurman Teaching Hospital, Kuwaiti Specialized Hospital, Ibn Sina Teaching Hospital, Soba University Hospital, Military Corp, and Gezira Hospital for Renal Disease and Surgery between 2018 and 2023. All males diagnosed with PCa were included in the study. Patients younger than 40 years old were excluded from this study. The ethnicity of the patients was categorized according to the major speaking languages identified by Greenberg19: NC, NS and AA. Two dedicated genitourinary pathologists (Dr Elsadig Ahmed Adam Mohammed and Dr Nemat Mohamed Osman) undertook a systematic histopathologic review of all available hematoxylin and eosin (H&E) slides for each case, blinded to the patient ethnolinguistic group. Pathologic tumor stage (1997 American Joint Committee on Cancer TNM Classification) and perineural invasion (PNI) were reported based on standard H&E staining. PNI was defined as a tumor in close proximity to a nerve and involving any of the three layers of the nerve sheath.
Statistical analysis of the data was generated in the R (version 4.3.0) and R studio ("Ocean Storm" Release) software using functions available in the KODAMA R package36. Wilcoxon rank sum test and Kruskal–Wallis rank sum test were used to compare differences in numerical covariates (e.g., age and metabolite concentration) using the function “continuous.test”. Fisher’s exact test was used to assess differences between categorical variables (e.g., ethnicity) using the function “categorical.test”. P-values less than 0.05 were considered to be significant. Matching patients were selected using the function “frequency_matching”. The geographical map of Africa showing the distribution of major African linguistic families was adapted from ref. 26 using Google Map and refined using Inkscape (version 1.32).
Results
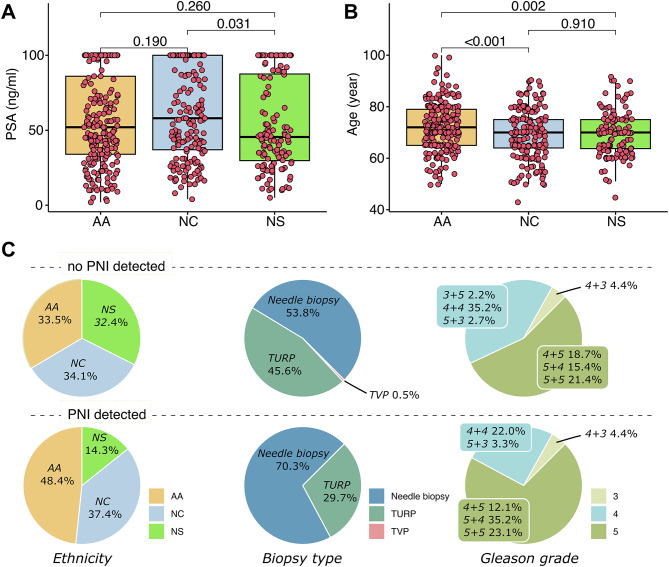
A total of 532 patients were recruited with diagnosed PCa confirmed by biopsy from six Sudanese medical centers, including three in the capital Khartoum (i.e., Kuwaiti Specialized Hospital, Ibn Sina Teaching Hospital, and Soba University Hospital), two in the close city of Omdurman (i.e., Omdurman Teaching Hospital and Military Corp), and one in the city of Wad Madani (i.e., Gezira Hospital for Renal Disease and Surgery). Each ethnolinguistic group was represented with a similar percentage in this study, where the AA group was the largest group of patients. The demographic and clinical features are summarized in Table 1. The majority of the patients, 84.2% (448/532), were diagnosed with an advanced disease with a Grade group above 3. No differences were observed in terms of aggressiveness of PCa among the ethnolinguistic groups. However, higher values of prostate-specific antigen (PSA) were reported in the NC group compared to the NS group. Although the median value of PSA in the NC group was higher than in the AA group, no statistically significant differences were reported (Fig. 2A). AA patients were diagnosed at older age compared to NC and NS patients (Fig. 2B). Except for a small portion of patients (9.5%), AA patients were older than 60 years old.
Table 1.
Demographic and clinical features of the patients recruited in this study.
| Feature | AA (n = 222) | NC (n = 181) | NS (n = 128) | p-value |
|---|---|---|---|---|
| Hospital | 0.166 | |||
| GNH, n (%) | 7 (3.2) | 4 (2.2) | 4 (3.1) | |
| ISH, n (%) | 77 (34.7) | 67 (37.0) | 33 (25.8) | |
| KSH, n (%) | 17 (7.7) | 11 (6.1) | 4 (3.1) | |
| MC, n (%) | 5 (2.3) | 10 (5.5) | 4 (3.1) | |
| OTH, n (%) | 29 (13.1) | 22 (12.2) | 25 (19.5) | |
| SUH, n (%) | 87 (39.2) | 67 (37.0) | 58 (45.3) | |
| Age (year), median [95%CI] | 72 [55 89.475] | 70 [50.5 87.5] | 70 [55 89.65] | < 0.001 |
| Age (≤ 60 years old) | 0.009 | |||
| Negative, n (%) | 201 (90.5) | 145 (80.1) | 107 (83.6) | |
| Positive, n (%) | 21 (9.5) | 36 (19.9) | 21 (16.4) | |
| Hypertension | 0.844 | |||
| Negative, n (%) | 102 (45.9) | 78 (43.1) | 56 (43.8) | |
| Positive, n (%) | 120 (54.1) | 103 (56.9) | 72 (56.2) | |
| T2DM | 0.680 | |||
| Negative, n (%) | 120 (54.1) | 96 (53.0) | 74 (57.8) | |
| Positive, n (%) | 102 (45.9) | 85 (47.0) | 54 (42.2) | |
| PSA (ng/ml), median [95%CI] | 52 [10 115.825] | 58 [15 131] | 45.5 [10.835 100] | 0.077 |
| PSA (> 50 ng/ml) | 0.036 | |||
| Negative, n (%) | 108 (48.6) | 81 (44.8) | 76 (59.4) | |
| Positive, n (%) | 114 (51.4) | 100 (55.2) | 52 (40.6) | |
| Biopsy type | 0.362 | |||
| Needle Biopsy, n (%) | 121 (54.5) | 110 (60.8) | 75 (58.6) | |
| TURP, n (%) | 98 (44.1) | 71 (39.2) | 51 (39.8) | |
| TVP, n (%) | 3 (1.4) | 0 (0.0) | 2 (1.6) | |
| PNI | 0.047 | |||
| Negative, n (%) | 178 (80.2) | 147 (81.2) | 115 (89.8) | |
| Positive, n (%) | 44 (19.8) | 34 (18.8) | 13 (10.2) | |
| Grade group | 0.288 | |||
| 1, n (%) | 12 (5.4) | 8 (4.4) | 9 (7.0) | |
| 2, n (%) | 17 (7.7) | 6 (3.3) | 5 (3.9) | |
| 3, n (%) | 7 (3.2) | 12 (6.6) | 7 (5.5) | |
| 4, n (%) | 66 (29.7) | 67 (37.0) | 44 (34.4) | |
| 5, n (%) | 120 (54.1) | 88 (48.6) | 63 (49.2) | |
CI confidence interval, GNH Gezira Hospital for Renal Disease and Surgery, ISHIbn Sina Teaching Hospital, KSH Kuwaiti Specialized Hospital, MC Military Corp, OTH Omdurman Teaching Hospital, SUH Soba University Hospital, T2DM type-2 diabetes mellitus, PSA prostate-specific antigen, TURP transurethral resection of the prostate, TVP transurethral laser vaporization of the prostate, PNI perineural invasion.
Fig. 2.
Boxplots showing the difference of (A) PSA and (B) age among the three ethnolinguistic groups. (C) Pie charts representing the difference in percentage of ethnicity, biopsy type, and Gleason grade between tumor without and with PNI detected.
No differences were observed in the incidence of type-2 diabetes mellitus (T2DM) and hypertension among the ethnolinguistic groups.
An important determinant of tumor behavior is the ability to breach basement membranes and spread outside the confines of the organ of origin. PCa has long been recognized to show a propensity to invade and grow along prostatic nerves, which lead out of the prostate to the pelvic plexus37. This phenomenon, known as PNI, has been associated with the inhibition of apoptosis, thereby allowing for increased proliferation of cancer cells38.
Interestingly, a different incidence of PNI was observed among the three ethnolinguistic groups with the highest value for the AA group. This difference was also confirmed when only the reports from needle biopsy examination were taken into consideration (Table S1). No statistically significant differences in the incidence of PNI were observed in the TURP samples (Table S2).
A further analysis was performed to compare the patients positive to PNI with patients negative to PNI with a grade group higher than 2. PNI-negative patients were randomly selected to match the age of PNI-positive patients in a ratio of 2:1. The demographic and clinical features are summarized in Table 2. As expected, 70.3% of the PNI was diagnosed through needle biopsy inspection, which is primarily peripheral zone cancer. The percentage of PNI diagnosis is higher in the group with needle biopsy, 20.9% (64/306), than TURP, 12% (27/220). The analysis performed in this subgroup of patients (n = 273) confirmed a higher incidence of PNI in the AA group. The Grade group classification was reported to be higher in the PNI-positive patients. These differences are visualized in Fig. 2C. No associations were detected with other clinical features (i.e., PSA, hypertension, and T2DM).
Table 2.
Demographic and clinical features comparison between PNI-positive patients and age-matched PNI-negative patients.
| Feature | PNI-negative (n = 182) | PNI-positive (n = 91) | p-value |
|---|---|---|---|
| Ethnic group | 0.004 | ||
| AA, n (%) | 61 (33.5) | 44 (48.4) | |
| NC, n (%) | 62 (34.1) | 34 (37.4) | |
| NS, n (%) | 59 (32.4) | 13 (14.3) | |
| Hospital | 0.225 | ||
| GNH, n (%) | 2 (1.1) | 3 (3.3) | |
| ISH, n (%) | 62 (34.1) | 39 (42.9) | |
| KSH, n (%) | 7 (3.8) | 6 (6.6) | |
| MC, n (%) | 7 (3.8) | 4 (4.4) | |
| OTH, n (%) | 21 (11.5) | 9 (9.9) | |
| SUH, n (%) | 83 (45.6) | 30 (33.0) | |
| Age (year), median [95%CI] | 70 [54.525 86.425] | 70 [55 92.25] | 0.735 |
| Age (≤ 60 years old) | 1.000 | ||
| Negative, n (%) | 156 (85.7) | 78 (85.7) | |
| Positive, n (%) | 26 (14.3) | 13 (14.3) | |
| Hypertension | 0.370 | ||
| Negative, n (%) | 89 (48.9) | 39 (42.9) | |
| Positive, n (%) | 93 (51.1) | 52 (57.1) | |
| T2DM | 1.000 | ||
| Negative, n (%) | 97 (53.3) | 48 (52.7) | |
| Positive, n (%) | 85 (46.7) | 43 (47.3) | |
| PSA (ng/ml), median [95%CI] | 49 [12.24 101.425] | 47 [13.1 102.25] | 0.954 |
| PSA (> 50 ng/ml) | 0.898 | ||
| Negative, n (%) | 93 (51.1) | 48 (52.7) | |
| Positive, n (%) | 89 (48.9) | 43 (47.3) | |
| Biopsy type | 0.018 | ||
| Needle Biopsy, n (%) | 98 (53.8) | 64 (70.3) | |
| TURP, n (%) | 83 (45.6) | 27 (29.7) | |
| TVP, n (%) | 1 (0.5) | 0 (0.0) | |
| Grade group | 0.046 | ||
| 3, n (%) | 8 (4.4) | 4 (4.4) | |
| 4, n (%) | 73 (40.1) | 23 (25.3) | |
| 5, n (%) | 101 (55.5) | 64 (70.3) | |
CI confidence interval, GNH Gezira Hospital for Renal Disease and Surgery, ISHIbn Sina Teaching Hospital, KSH Kuwaiti Specialized Hospital, MC Military Corp, OTH Omdurman Teaching Hospital, SUH Soba University Hospital, T2DM type-2 diabetes mellitus, PSA prostate-specific antigen, TURP transurethral resection of the prostate, TVP transurethral laser vaporization of the prostate, PNI perineural invasion.
Discussion
The Republic of Sudan covers a significantly smaller geographical area than the expansive ethnic region in Africa that has been variably referred to as Kush, Nubia, and Bilad-Es-Sudan (Land of the blacks). Sudan boasts a linguistic diversity with over 140 languages spoken39. These languages can be categorized into three of Greenberg's four African language families: Afro-Asiatic, Niger-Congo, and Nilo-Saharan19. There are approximately 570 tribes, which have been organized into 56 ethnic groups based on linguistic, cultural, and other ethnological traits. The capital, Khartoum, has experienced rapid population growth primarily due to migration, rather than local birthrates. Between 1984 and 1995, there was a significant influx of migrants from the western and southern regions of the country, a consequence of civil conflict and natural disasters40.
In this study, we examined the demographic and clinical features of patients diagnosed with PCa in six health institutions in Central Sudan. For the first time, the healthy disparities of PCa were analyzed among three major ethnolinguistic groups in Africa.
Our data confirms how the higher risk of PCa associated with African ancestry could be linked as we hypothesize to the NC-speaking population. The higher PSA and the younger age at the diagnosis of NC patients corroborate the data regarding the higher PCa aggressiveness of African American patients. Our data are aligned with those recently reported on the South Africa population41 where Bantu-speaking NC population showed a significant risk for PCa diagnosis and aggressive disease presentation.
There is a lack of comparative studies between NS and AA population and others. No statistical significant difference have been reported for the PSA screening between European and Arab ancestry in US patients42. The major source of information on PCa relies on the national registries indicating a large disparities between North Africa dominated by AA population, such as Arabs, and sub-Saharan countries excluding those from the horn of Africa43. Indeed, in Ethiopia where approximately three-quarters of its population speak AA languages, the PCa incidence and mortality are lower than in other sub-Saharan nations, with a majority of NC-speaking populations2.
Our research has unveiled a novel finding, marking the first instance where the incidence of PNI in prostatic tissue is notably elevated among patients of AA population. Tumor cells interact with nerve components within the tumor microenvironment, giving rise to perineural hollows that provide a conducive environment for their survival and subsequent invasion of nerve cells44. PNI tends to be more prevalent in advanced-stage tumors and it is often associated with unfavorable outcomes45. Recent evidence links PNI to an approximately 50% heightened risk of biochemical recurrence46 and the development of bone metastasis47. Further studies are needed to exploit the possibility of a higher incidence of biochemical recurrence and bone metastasis in the AA population.
While Sudanese patients tend to be diagnosed with advanced-stage disease, it is important to consider that the biology of PCa among the three ethnic groups may be different. Given the significant genetic diversity within African populations, it becomes evident that there is a compelling need to refine the term “African ancestry” when assessing PCa risk. It is currently accepted the association of higher risk of PCa in men with African ancestry, a connection that has been consistently supported by numerous studies within the U.S. population48,49. These studies result in several organizations have recommended adjusting screening criteria based on ethnicity to better reflect these risks. However, the terminology often used in guidelines, such as “African descent”, “Black ancestry”, or “African ancestry”, fails to accurately capture the genetic similarities and differences among populations. Our findings highlight the importance of refining ethnic terminology in screening guidelines to more precisely reflect the complex genetic diversity within African ancestry groups, thereby improving the effectiveness of PCa screening and prevention efforts.
Limitations and strengths
The recurrent conflicts in Sudan during this five-year render instability of the fragile healthcare system, less prioritizing of clinical research. The increasing number of patients with cancer has intensified the actual critical situation in terms of cancer care in Sudan32. This study is limited by the possibility to provide statistical analysis on clinical outcome measures, such as biochemical failure rates, progression free survival and overall survival. To our knowledge, we cannot exclude that different clinical features of PCa could be due to the use of cyanide in mining practices and pesticides by farmers in some areas of the country dominated by an ethnolinguistic group.
Conclusions
Given the persistent issue of health disparities in PCa, it is imperative that we adopt fresh perspectives for the study of PCa in individuals with African ancestry, focusing on a more precise genetic basis for the heightened risk rather than merely associating it with a specific geographical region.
Supplementary Information
Acknowledgements
We would like to express out deep and sincere gratitude and appreciation to all urology and histopathology surgeons, clinicians and technical staff for their efforts in performing this study. A particular thanks to Prof. Adil Mergani Babikir Hassan, Prof. Dafalla Omer Abuidris, Prof. Sami Mahjoub Taha and Mr Hussam ALnaieem for their suggestions, and guidance throughout the study period.
Abbreviations
- AA
Afro-asiatic
- CI
Confidence interval
- GNH
Gezira hospital for renal disease and surgery
- ISH
Ibn sina teaching hospital
- KSH
Kuwaiti specialized hospital
- MC
Military corp
- NC
Niger-congo
- NS
Nilo-saharan
- OTH
Omdurman teaching hospital
- PCa
Prostate cancer
- PNI
Perineural invasion
- PSA
Prostate-specific antigen
- SUH
Soba university hospital
- T2DM
Type-2 diabetes mellitus
- TURP
Transurethral resection of the prostate
- TVP
Transurethral laser vaporization of the prostate
- UN
United nations
Funding
The research was funded by International Centre for Genetic Engineering and Biotechnology (L.F.Z. and S.C.), and the UN Technology Bank for Least Developed Countries, TWAS and ICGEB under South-North and South-South Fellowships (D.A.).
Data availability
The authors declare the availability of the data used in the research to obtain the results reported in the manuscript upon reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Specimen collection and research contents were examined and approved by the National Health Research Ethics Committee of the Sudanese Federal Ministry of Health (6–9-2021). The study was performed in accordance with the Declaration of Helsinki. Informed consent was taken from all the participants.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors equally contributed: Stefano Cacciatore and Luiz Fernando Zerbini.
Contributor Information
Stefano Cacciatore, Email: stefano.cacciatore@icgeb.org.
Luiz Fernando Zerbini, Email: luiz.zerbini@icgeb.org.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77475-7.
References
- 1.Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. Ca Cancer J. Clin.73, 17–48 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians71, 209–249 (2021). [DOI] [PubMed]
- 3.Jaratlerdsiri, W. et al. African-specific molecular taxonomy of prostate cancer. Nature609, 552–559 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temilola, D. O. et al. Detection of Cancer-Associated Gene Mutations in Urinary Cell-Free DNA among Prostate Cancer Patients in South Africa. Genes14, 1884 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeola, H. A. et al. Discovery of novel candidate urinary protein biomarkers for prostate cancer in a multiethnic cohort of South African patients via label-free mass spectrometry. Proteomics-Clin. Appl.9, 597–609 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Adeola, H. A., Smith, M., Kaestner, L., Blackburn, J. M. & Zerbini, L. F. Novel potential serological prostate cancer biomarkers using CT100+ cancer antigen microarray platform in a multi-cultural South African cohort. Oncotarget7, 13945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temilola, D. O. et al. Potential of miRNAs in Plasma Extracellular Vesicle for the Stratification of Prostate Cancer in a South African Population. Cancers15, 3968 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cacciatore, S. et al. Inflammatory metabolic profile of South African patients with prostate cancer. Cancer Metab.9, 29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherer, M. V., Qiao, E. M., Kotha, N. V., Qian, A. S. & Rose, B. S. Association Between Prostate-Specific Antigen Screening and Prostate Cancer Mortality Among Non-Hispanic Black and Non-Hispanic White US Veterans. JAMA Oncol.8, 1471–1476 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basourakos, S. P. et al. Harm-to-benefit of three decades of prostate cancer screening in Black men. NEJM evidence1, EVIDoa2200031 (2022). [DOI] [PMC free article] [PubMed]
- 11.Bryc, K. et al. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc. Natl. Acad. Sci.107, 786–791 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zakharia, F. et al. Characterizing the admixed African ancestry of African Americans. Genome Biol.10, 1–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong, T. et al. Genome-wide interrogation of structural variation reveals novel African-specific prostate cancer oncogenic drivers. Genome Med.14, 100 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, F. W. et al. Exome sequencing of African-American prostate cancer reveals loss-of-function ERF mutations. Cancer Disc.7, 973–983 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blattner, M. et al. SPOP mutation drives prostate tumorigenesis in vivo through coordinate regulation of PI3K/mTOR and AR signaling. Cancer Cell31, 436–451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand, K. A. et al. Whole-exome sequencing of over 4100 men of African ancestry and prostate cancer risk. Hum. Mol. Genet.25, 371–381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soh, P. X. et al. Prostate cancer genetic risk and associated aggressive disease in men of African ancestry. Nat. Commun.14, 8037 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim, M. E. Genetic diversity of the Sudanese: insights on origin and implications for health. Hum. Mol. Genet.30, R37–R41 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg, J. H. The languages of Africa. International journal of American linguistics (1963).
- 20.Atkinson, E. G. et al. Genetic structure correlates with ethnolinguistic diversity in eastern and southern Africa. Am. J. Hum. Genetics109, 1667–1679 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan, S. et al. African evolutionary history inferred from whole genome sequence data of 44 indigenous African populations. Genome Biol.20, 1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan, S., Hansen, M. E., Lo, Y. & Tishkoff, S. A. Going global by adapting local: A review of recent human adaptation. Science354, 54–59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell, M. C. & Tishkoff, S. A. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu. Rev. Genomics Hum. Genet.9, 403–433 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nédélec, Y. et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell167, 657–669 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Quach, H. et al. Genetic adaptation and neandertal admixture shaped the immune system of human populations. Cell167, 643–656 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez, F., Hirbo, J. & Tishkoff, S. A. Genetic variation and adaptation in Africa: implications for human evolution and disease. Cold Spring Harbor Perspect. Biol.6, a008524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel, D. A. et al. Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: the Bogalusa Heart Study. Metabolism55, 699–705 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Batai, K., Murphy, A. B., Nonn, L. & Kittles, R. A. Vitamin D and immune response: implications for prostate cancer in African Americans. Front. Immunol.7, 53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastham, J. A. et al. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. JNCI90, 756–760 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Wallace, T. A. et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res.68, 927–936 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Taha, S. M. et al. Prostate cancer clinical characteristics and outcomes in Central Sudan. ecancermedicalscience14 (2020). [DOI] [PMC free article] [PubMed]
- 32.Das, M. Sudan’s cancer care in crisis. Lancet Oncol.23, e405 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Committee, I. R. Crisis in Sudan: What is happening and how to help, 2024.
- 34.Maki, H. A. H. Cancer in the Arab World 251–264 (Springer, 2022). [Google Scholar]
- 35.Abuidri, D. et al. Cancer management in Sudan: current status and future perspectives. Sudan Journal of Medical Sciences 4, 189–193 (2009).
- 36.Cacciatore, S., Tenori, L., Luchinat, C., Bennett, P. R. & MacIntyre, D. A. KODAMA: an R package for knowledge discovery and data mining. Bioinformatics 33, 621–623 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayala, G. E. et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res.64, 6082–6090 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Lubig, S. et al. Quantitative perineural invasion is a prognostic marker in prostate cancer. Pathology50, 298–304 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Gordon Jr, R. G. Ethnologue, languages of the world. 15th Edition, SIL International, Dallas. http://www.ethnologue.com/ (2005).
- 40.Mohammed, A. O. et al. Relationship of the sickle cell gene to the ethnic and geographic groups populating the Sudan. Commun. Genetics9, 113–120 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Gheybi, K. et al. Linking African ancestral substructure to prostate cancer health disparities. Sci. Reports13, 20909 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dallo, F. J. & Kindratt, T. B. Disparities in preventive health behaviors among non-Hispanic White men: heterogeneity among foreign-born Arab and European Americans. Am. J. Men’s Health9, 124–131 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians74, 229–263 (2024). [DOI] [PubMed]
- 44.Niu, Y., Förster, S. & Muders, M. The Role of Perineural Invasion in Prostate Cancer and Its Prognostic Significance. Cancers14, 4065 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer 2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol.79, 243–262 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Ström, P. et al. Prognostic value of perineural invasion in prostate needle biopsies: a population-based study of patients treated by radical prostatectomy. Journal of clinical pathology (2020). [DOI] [PMC free article] [PubMed]
- 47.Delahunt, B. et al. Perineural invasion by prostate adenocarcinoma in needle biopsies predicts bone metastasis: Ten year data from the TROG 03.04 RADAR Trial. Histopathology77, 284–292 (2020). [DOI] [PubMed]
- 48.Chornokur, G., Dalton, K., Borysova, M. E. & Kumar, N. B. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate71, 985–997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moul, J. W. et al. Prostate-specific antigen values at the time of prostate cancer diagnosis in African-American men. Jama274, 1277–1281 (1995). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare the availability of the data used in the research to obtain the results reported in the manuscript upon reasonable request from the corresponding author.