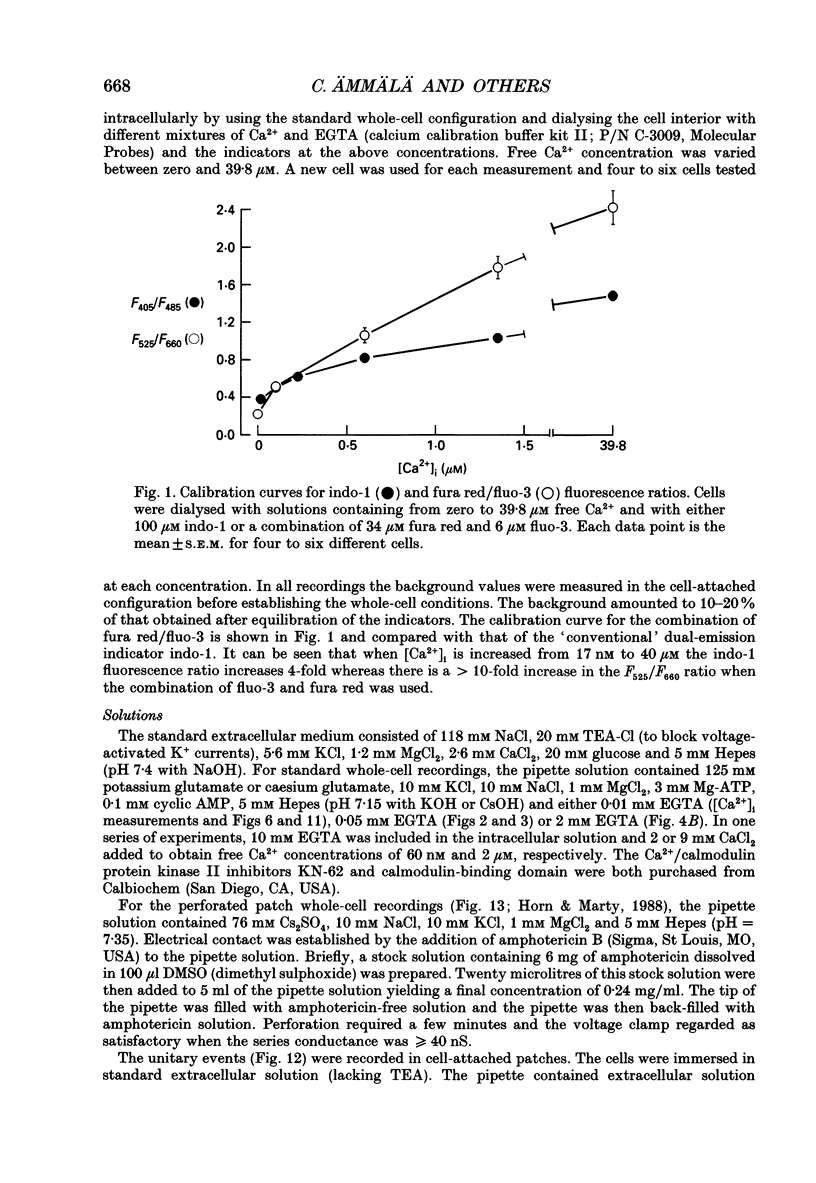
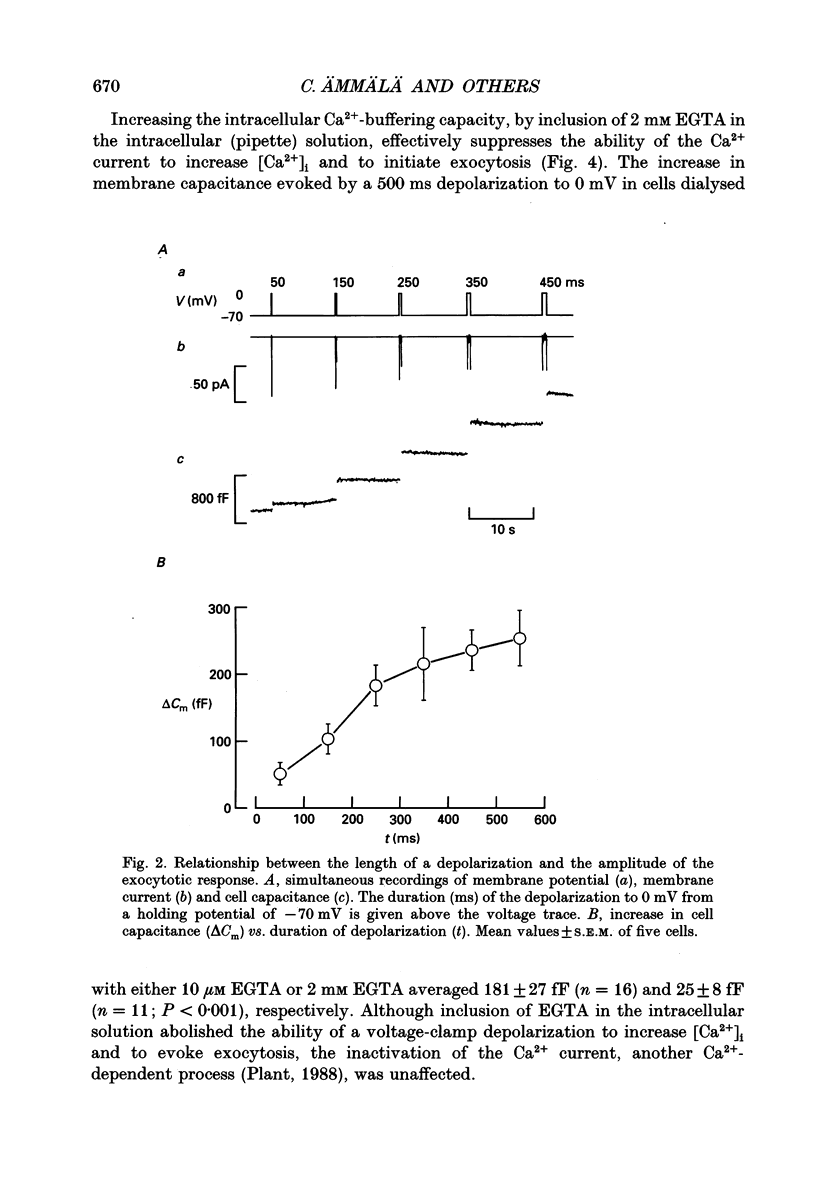
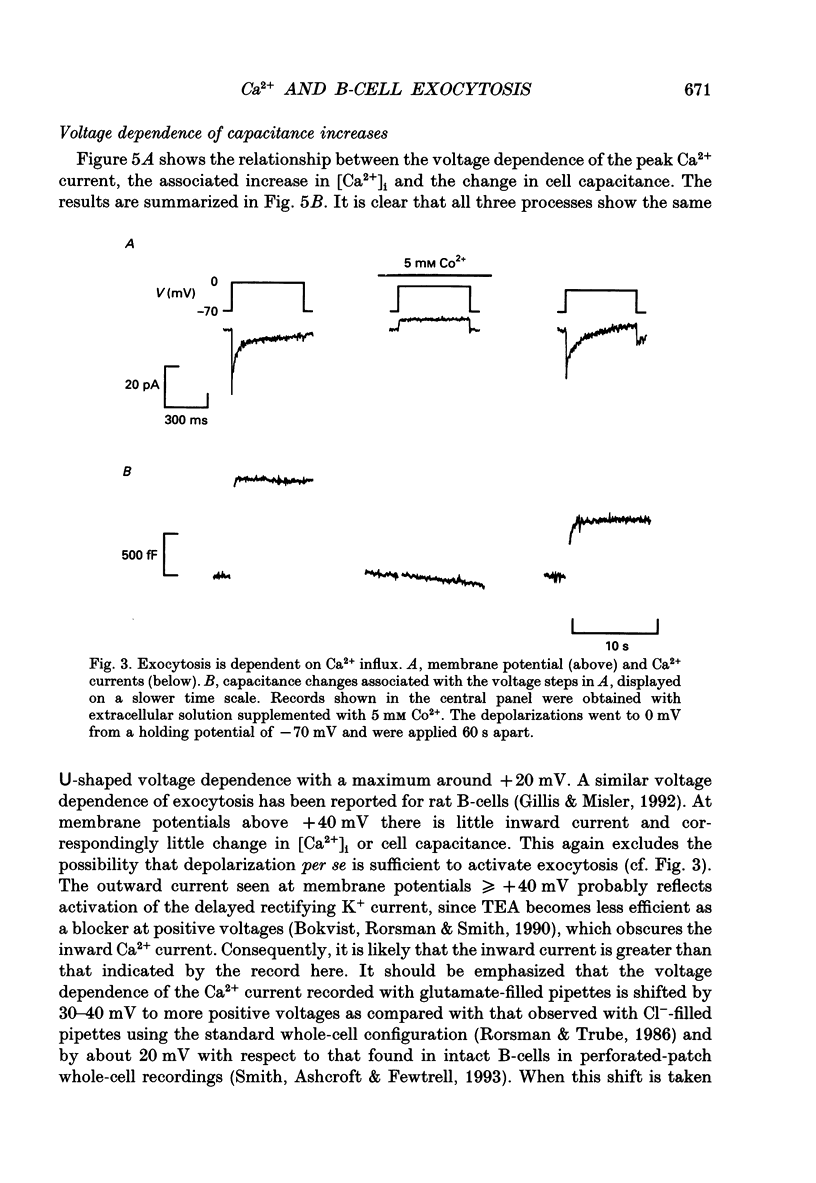
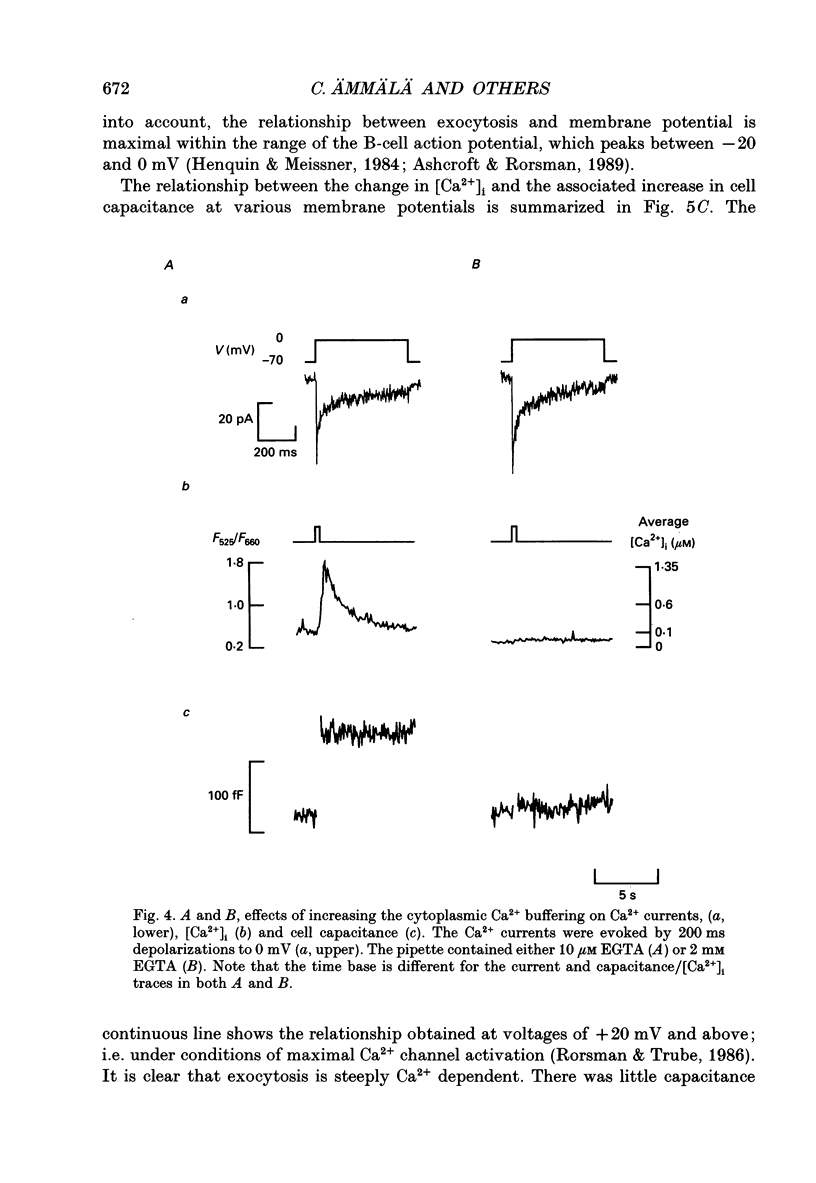
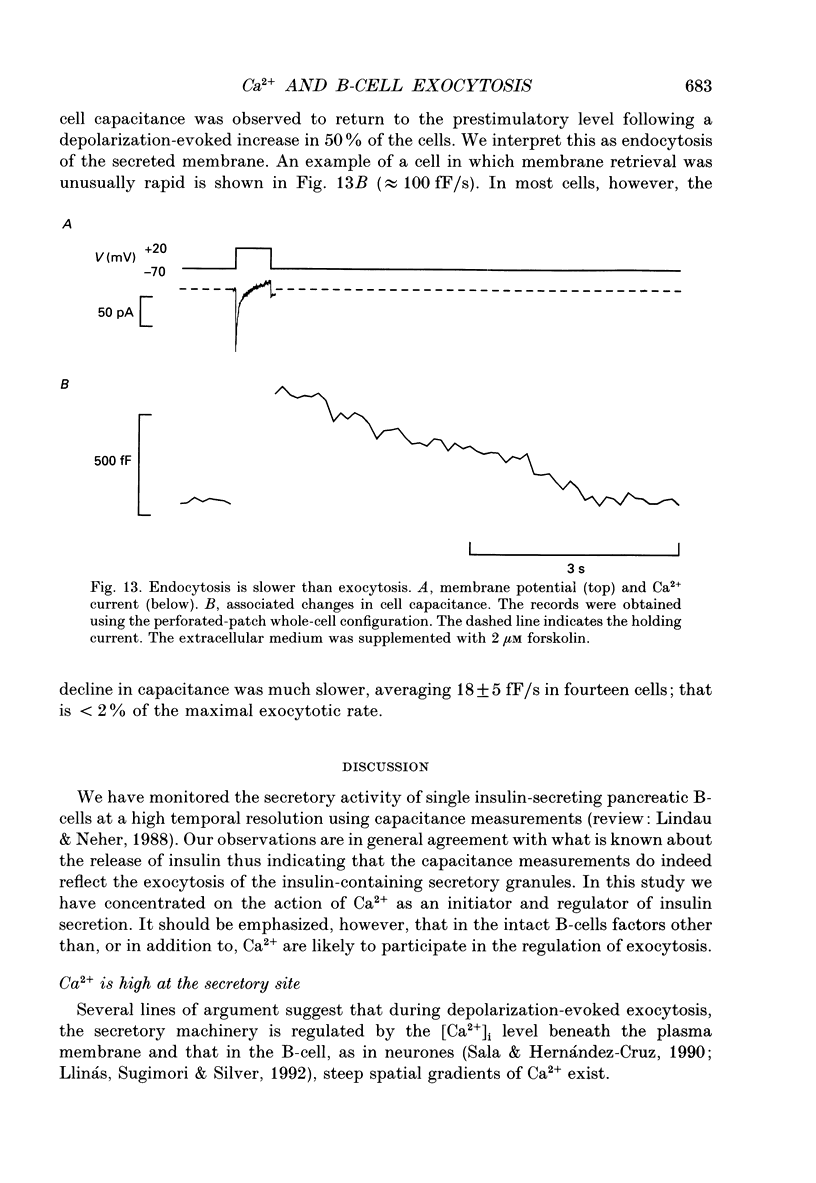
Abstract
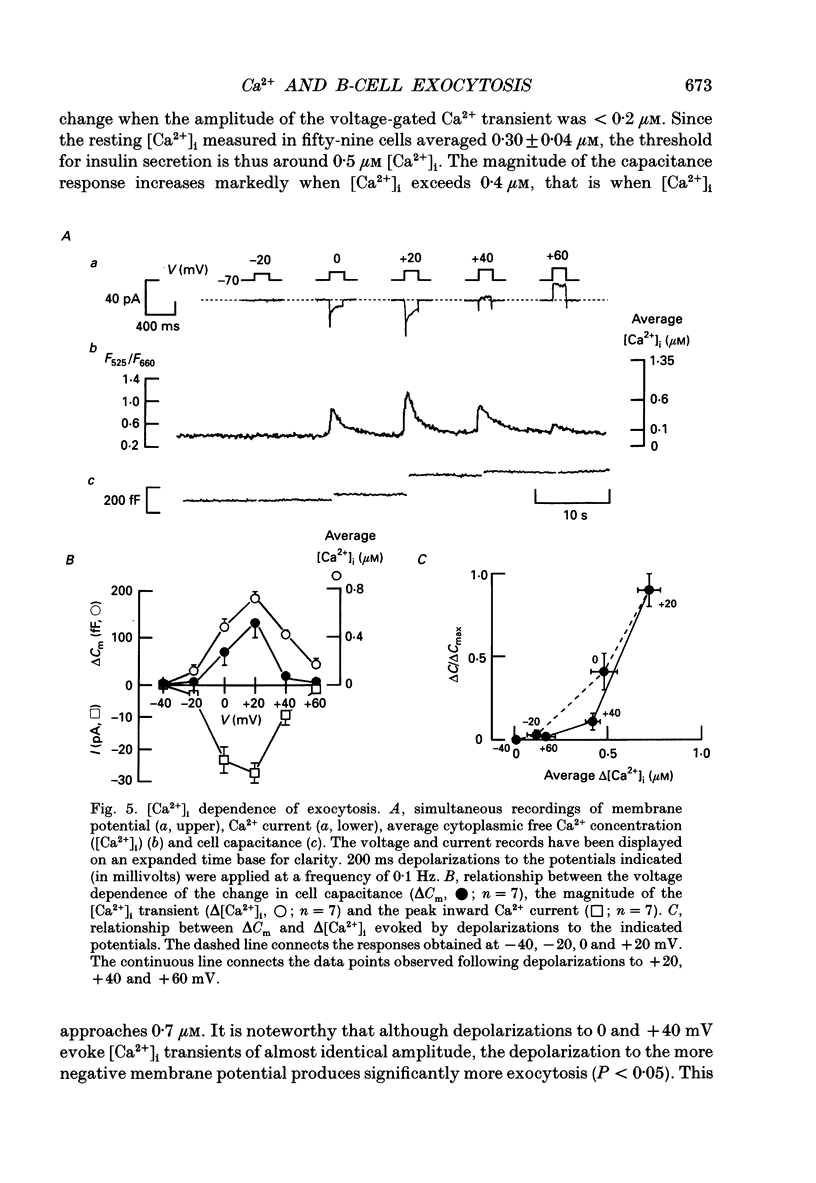
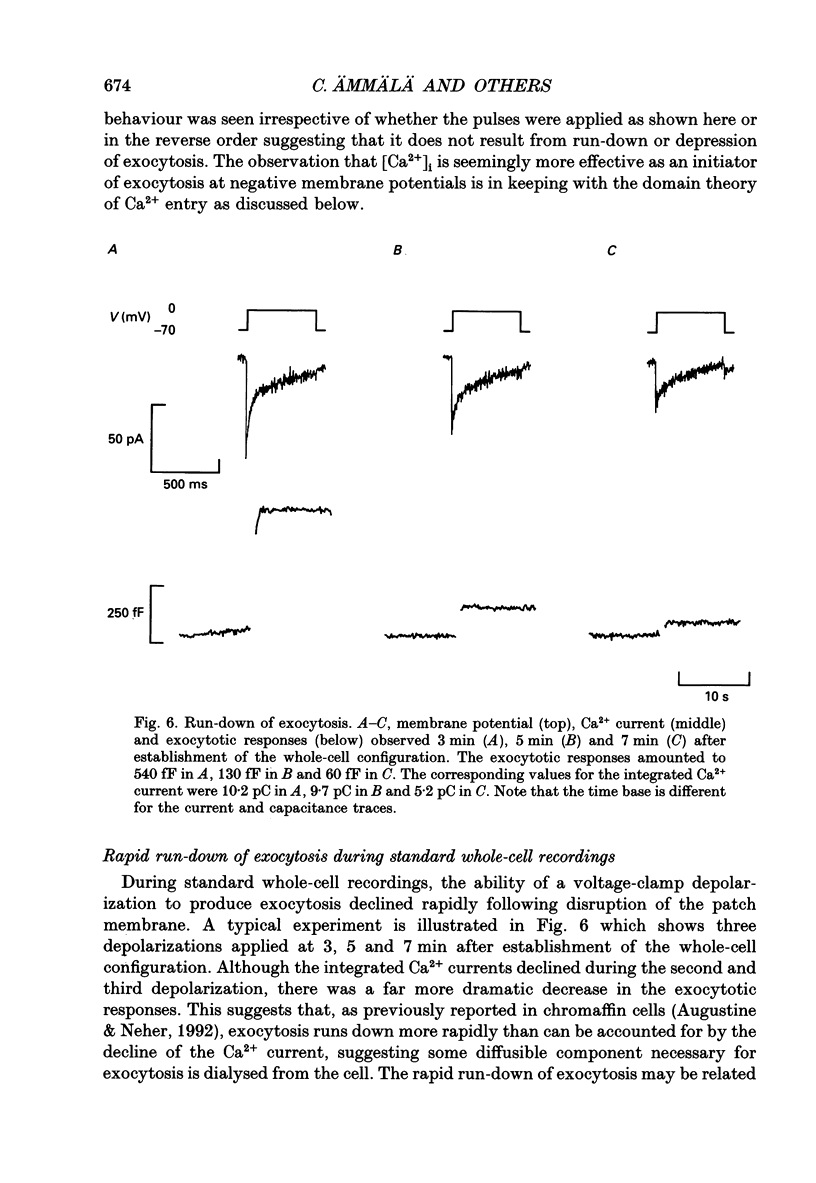
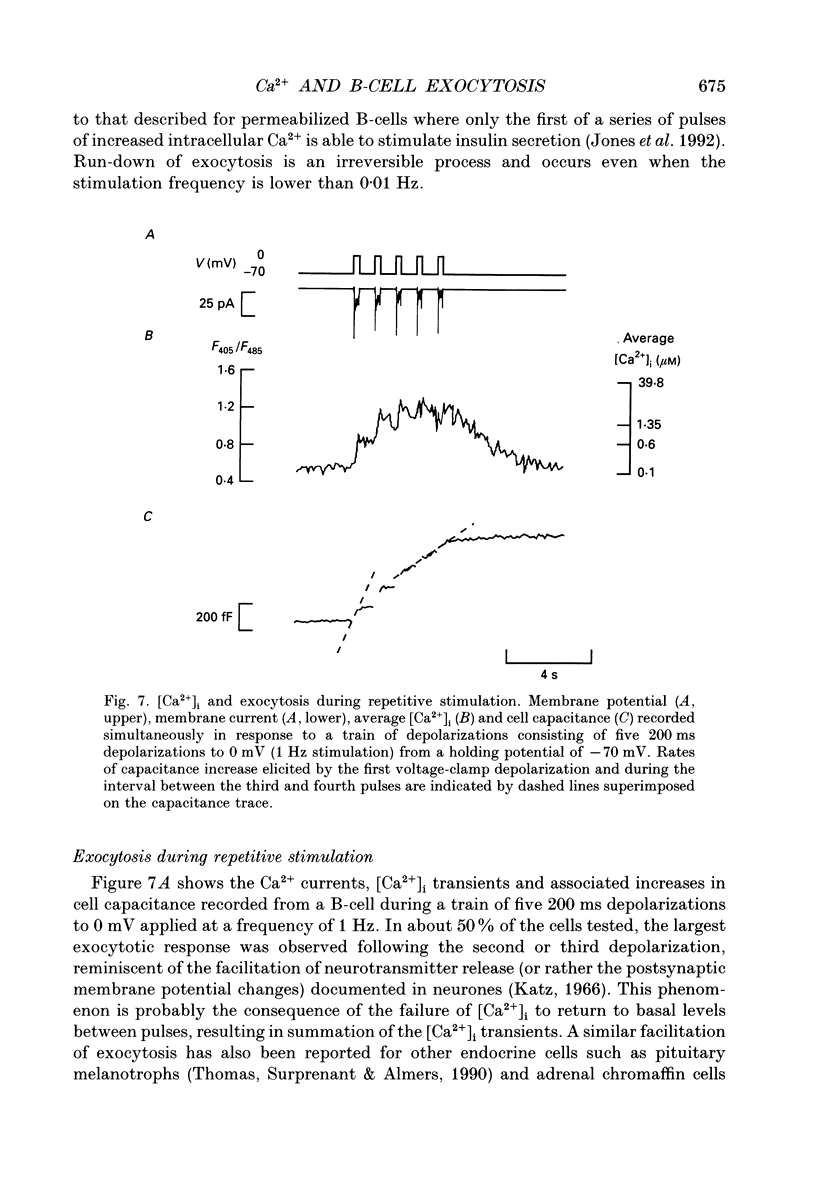
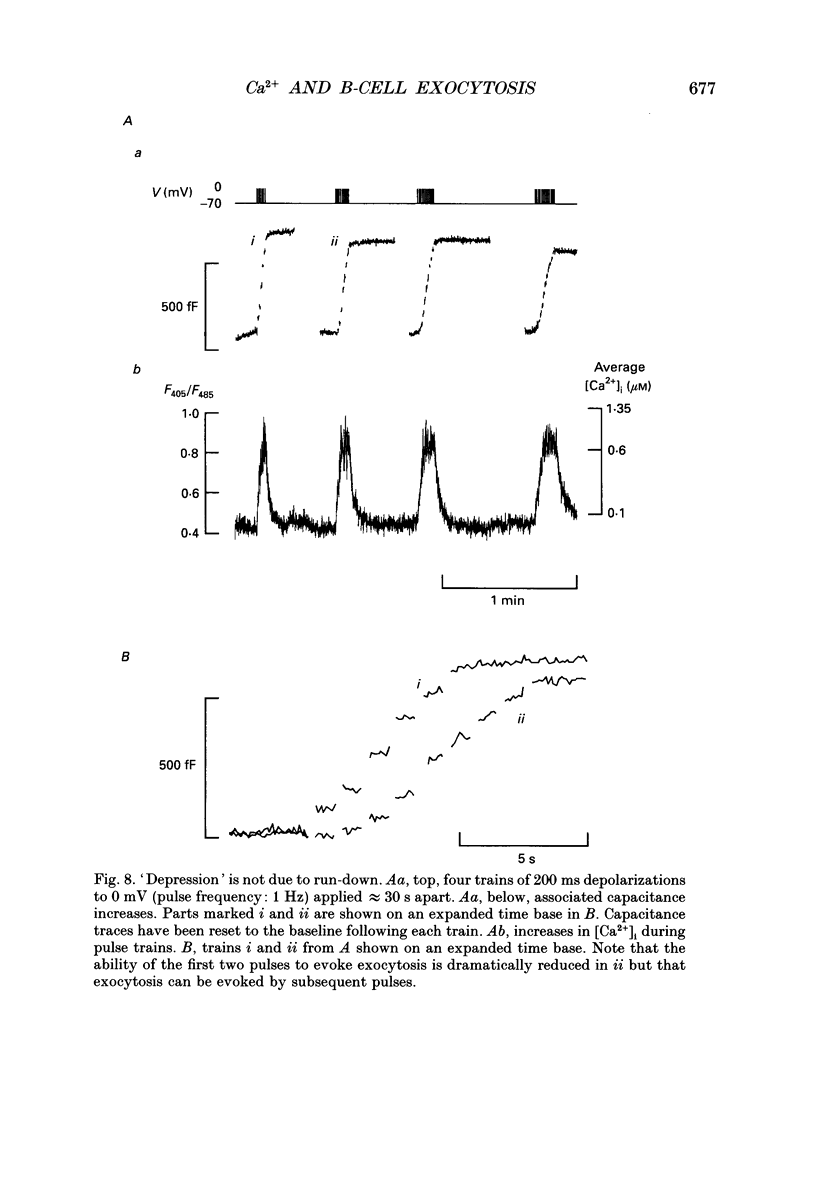
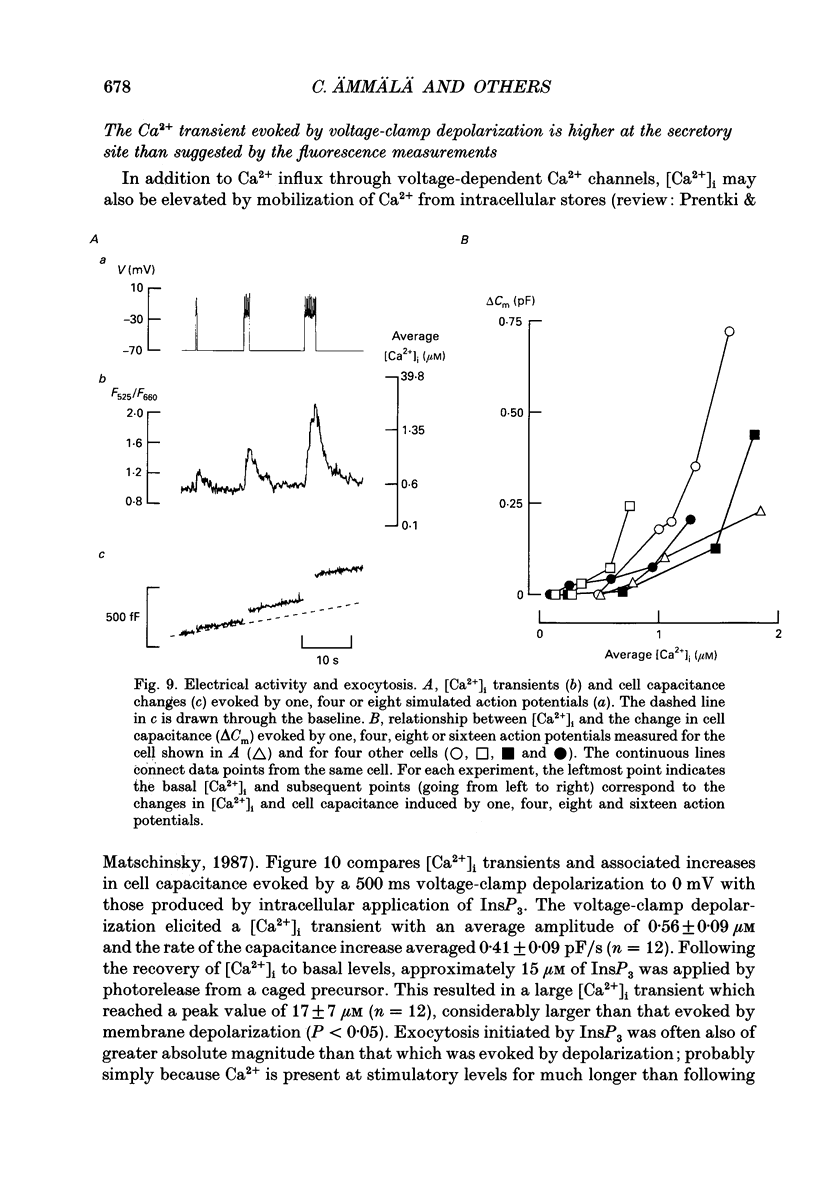
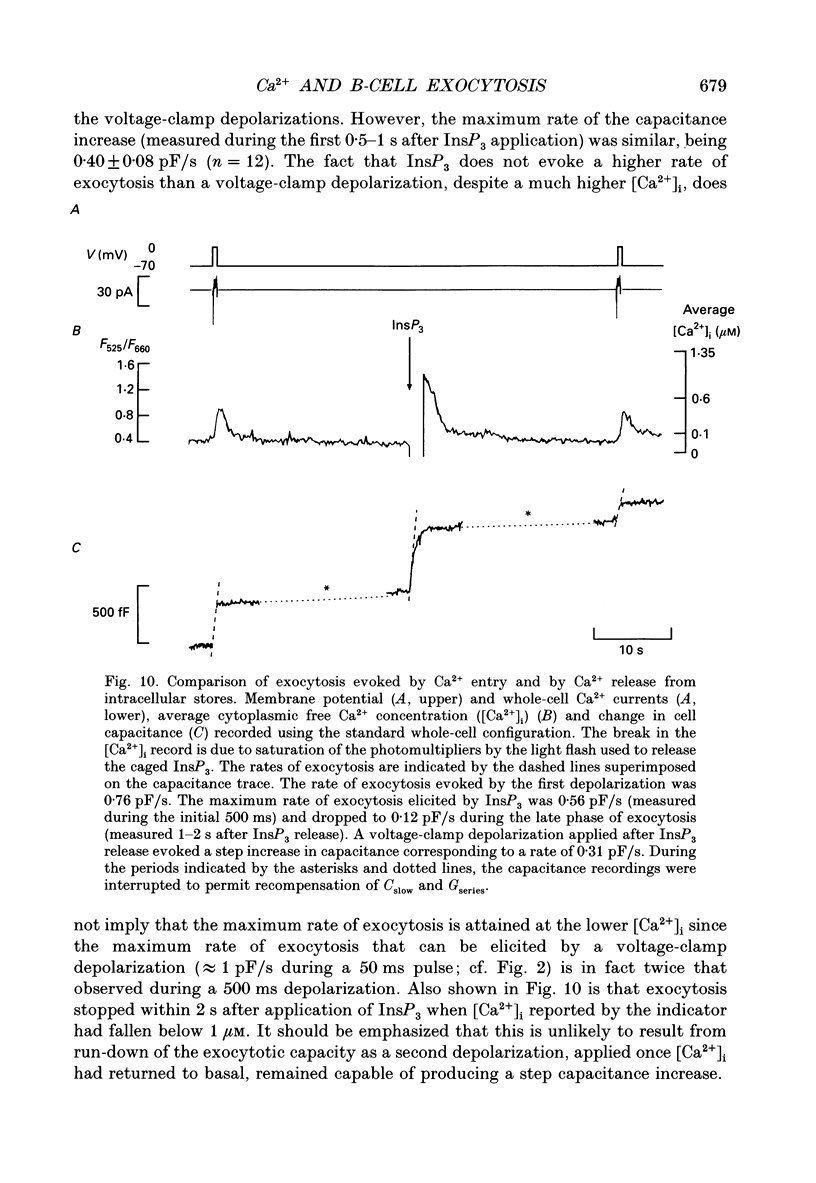
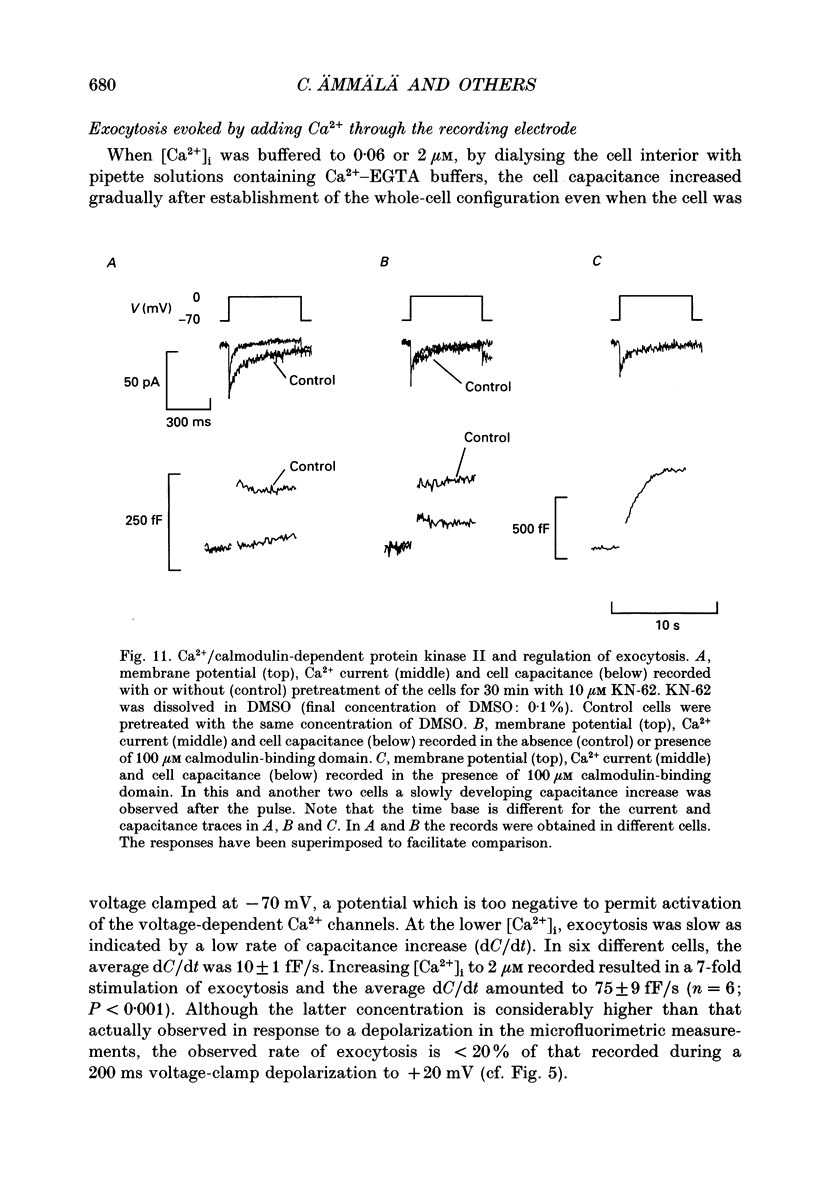
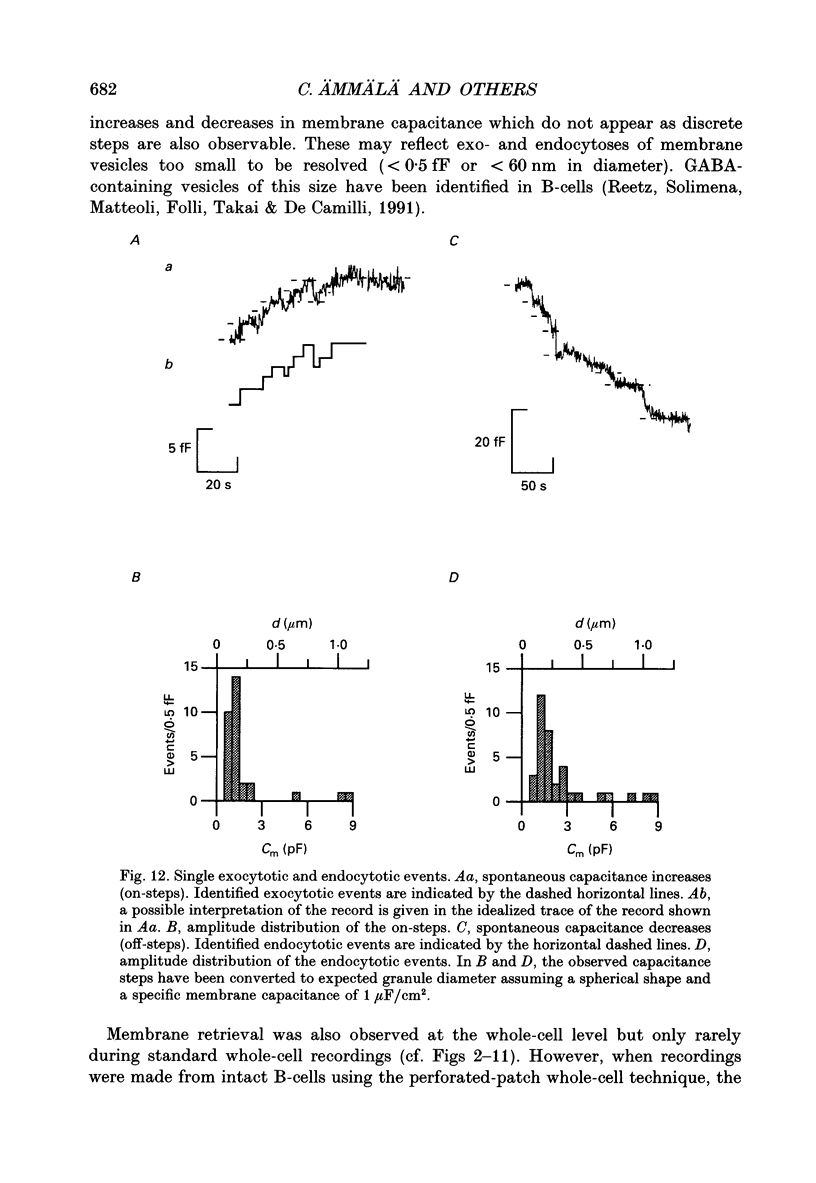
1. Measurements of membrane capacitance, as an indicator of exocytosis, and intracellular Ca2+ concentration ([Ca2+]i) were used to determine the Ca2+ dependence of secretion in single pancreatic B-cells. 2. Exocytosis was dependent on a rise in [Ca2+]i and could be evoked by activation of voltage-dependent Ca2+ currents. The threshold for depolarization-induced release was 0.5 microM [Ca2+]i. Once the [Ca2+]i threshold was exceeded, exocytosis was rapidly (< 50 ms) initiated. When individual pulses were applied, exocytosis stopped immediately upon repolarization and the Ca2+ channels closed, although [Ca2+]i remained elevated for several seconds. 3. During repetitive stimulation (1 Hz), when [Ca2+]i attained micromolar levels, exocytosis also took place during the interpulse intervals albeit at a slower rate than during the depolarizations. 4. Exocytosis could be initiated by simulated action potentials. Whereas a single action potential only produced a small capacitance increase, and in some cells even failed to stimulate release, larger and more consistent responses were obtained with > or = four action potentials. 5. Comparison of the rates of exocytosis measured in response to depolarization, mobilization of Ca2+ from intracellular stores or infusion of Ca2+ through the patch pipette suggests that [Ca2+]i at the secretory sites attains a concentration of several micromolar. This is much higher than the average [Ca2+]i detected by microfluorimetry suggesting the existence of steep spatial gradients of [Ca2+]i within the B-cell. 6. Inclusion of inhibitors of Ca2+/calmodulin-dependent protein kinase II in the intracellular solution reduced the depolarization-induced exocytotic responses suggesting this enzyme may be involved in the coupling between elevation of [Ca2+]i to stimulation of the secretory machinery. 7. The size of the unitary exocytotic event was 2 fF, corresponding to a secretory granule diameter of 250 nm. 8. Over short periods, exocytosis may be extremely fast (1 pF/s or 500 granules/s), which is much higher than the rate of endocytosis (18 fF/s or 9 granules/s). Since the latter is in better agreement with the maximum rate of insulin secretion from islets (approximately 2 granules/s), we suggest that membrane retrieval may set an upper limit on the rate of exocytosis during extended periods of secretion.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammälä C., Bokvist K., Galt S., Rorsman P. Inhibition of ATP-regulated K(+)-channels by a photoactivatable ATP-analogue in mouse pancreatic beta-cells. Biochim Biophys Acta. 1991 May 17;1092(3):347–349. doi: 10.1016/s0167-4889(97)90011-2. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokvist K., Rorsman P., Smith P. A. Effects of external tetraethylammonium ions and quinine on delayed rectifying K+ channels in mouse pancreatic beta-cells. J Physiol. 1990 Apr;423:311–325. doi: 10.1113/jphysiol.1990.sp018024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca J. R., Brooks C. L., Landt M., McDaniel M. L. Correlation of Ca2+-and calmodulin-dependent protein kinase activity with secretion of insulin from islets of Langerhans. Biochem J. 1983 Jun 15;212(3):819–827. doi: 10.1042/bj2120819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M. Ultrastructural morphometry of the pancreatic -cell. Diabetologia. 1973 Apr;9(2):115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- Gillis K. D., Misler S. Single cell assay of exocytosis from pancreatic islet B cells. Pflugers Arch. 1992 Jan;420(1):121–123. doi: 10.1007/BF00378654. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Ashcroft S. J. Effects of Ca2+, calmodulin and cyclic AMP on the phosphorylation of endogenous proteins by homogenates of rt islets of langerhans. Biochim Biophys Acta. 1982 Feb 2;714(2):313–319. doi: 10.1016/0304-4165(82)90339-7. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Poje M., Rocic B., Ashcroft S. J. Effects of dehydrouramil on protein phosphorylation and insulin secretion in rat islets of Langerhans. Biochem J. 1986 Jul 1;237(1):191–196. doi: 10.1042/bj2370191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C. The insulin secretory granule. Diabetologia. 1989 May;32(5):271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Fyles J. M., Howell S. L. Regulation of insulin secretion by cAMP in rat islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1986 Sep 15;205(2):205–209. doi: 10.1016/0014-5793(86)80898-5. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Howell S. L. Ca2(+)-induced insulin secretion from electrically permeabilized islets. Loss of the Ca2(+)-induced secretory response is accompanied by loss of Ca2(+)-induced protein phosphorylation. Biochem J. 1992 Aug 1;285(Pt 3):973–978. doi: 10.1042/bj2850973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Howell S. L. Time-course of Ca2+-induced insulin secretion from perifused, electrically permeabilised islets of Langerhans: effects of cAMP and a phorbol ester. Biochem Biophys Res Commun. 1989 Aug 15;162(3):998–1003. doi: 10.1016/0006-291x(89)90772-9. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Hidaka H., Wollheim C. B. Inhibition of voltage-gated Ca2+ channels and insulin secretion in HIT cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-62: comparison with antagonists of calmodulin and L-type Ca2+ channels. Mol Pharmacol. 1992 Sep;42(3):489–488. [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Plant T. D. Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J Physiol. 1988 Oct;404:731–747. doi: 10.1113/jphysiol.1988.sp017316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991 May;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Ammälä C., Berggren P. O., Bokvist K., Larsson O. Cytoplasmic calcium transients due to single action potentials and voltage-clamp depolarizations in mouse pancreatic B-cells. EMBO J. 1992 Aug;11(8):2877–2884. doi: 10.1002/j.1460-2075.1992.tb05356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Ashcroft F. M., Trube G. Single Ca channel currents in mouse pancreatic B-cells. Pflugers Arch. 1988 Oct;412(6):597–603. doi: 10.1007/BF00583760. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario L. M., Atwater I., Scott A. M. Pulsatile insulin release and electrical activity from single ob/ob mouse islets of Langerhans. Adv Exp Med Biol. 1986;211:413–425. doi: 10.1007/978-1-4684-5314-0_40. [DOI] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R. M., Rosario L. M., Nadal A., Garcia-Sancho J., Soria B., Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991 May;418(4):417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Aschroft F. M., Fewtrell C. M. Permeation and gating properties of the L-type calcium channel in mouse pancreatic beta cells. J Gen Physiol. 1993 May;101(5):767–797. doi: 10.1085/jgp.101.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Christie M. R., Ashcroft S. J. Presence and possible role of calcium-dependent regulator (calmodulin) in rat islets of Langerhans. FEBS Lett. 1979 Sep 1;105(1):95–100. doi: 10.1016/0014-5793(79)80894-7. [DOI] [PubMed] [Google Scholar]
- Theler J. M., Mollard P., Guérineau N., Vacher P., Pralong W. F., Schlegel W., Wollheim C. B. Video imaging of cytosolic Ca2+ in pancreatic beta-cells stimulated by glucose, carbachol, and ATP. J Biol Chem. 1992 Sep 5;267(25):18110–18117. [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990 Mar 15;265(8):4315–4320. [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Valverde I., Vandermeers A., Anjaneyulu R., Malaisse W. J. Calmodulin activation of adenylate cyclase in pancreatic islets. Science. 1979 Oct 12;206(4415):225–227. doi: 10.1126/science.225798. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Ullrich S., Meda P., Vallar L. Regulation of exocytosis in electrically permeabilized insulin-secreting cells. Evidence for Ca2+ dependent and independent secretion. Biosci Rep. 1987 May;7(5):443–454. doi: 10.1007/BF01362507. [DOI] [PubMed] [Google Scholar]


