Abstract
The import of proteins into the nucleus is dependent on cis-acting targeting sequences, nuclear localization signals (NLSs), and members of the nuclear transport receptor (importin-β-like) superfamily. The most extensively characterized import pathway, often termed the classical pathway, is utilized by many basic-type (lysine-rich) NLSs and requires an additional component, importin α, to serve as a bridge between the NLS and the import receptor importin β. More recently, it has become clear that a variety of proteins enter the nucleus via alternative import receptors and that their NLSs bind directly to those receptors. By using the digitonin-permeabilized cell system for protein import in vitro, we have defined the import pathway for the Rex protein of human T-cell leukemia virus type 1. Interestingly, the arginine-rich NLS of Rex uses importin β for import but does so by a mechanism that is importin α independent. Based on the ability of the Rex NLS to inhibit the import of the lysine-rich NLS of T antigen and of both NLSs to be inhibited by the domain of importin α that binds importin β (the IBB domain), we infer that the Rex NLS interacts with importin β directly. In addition, and in keeping with other receptor-mediated nuclear import pathways, Rex import is dependent on the integrity of the Ran GTPase cycle. Based on these results, we suggest that importin β can mediate the nuclear import of arginine-rich NLSs directly, or lysine-rich NLSs through the action of importin α.
The exchange of molecules between the nucleus and the cytoplasm occurs through nuclear pore complexes (NPCs) (15, 24, 41, 48, 49). These large multisubunit complexes span both membranes of the nuclear envelope (NE) and form aqueous channels that can accommodate the passive diffusion of small molecules up to 9 nm in diameter and the active (energy consuming) transport of molecules and complexes with diameters up to ∼25 nm. Thus, proteins with masses that exceed ∼50 kDa, as well as many smaller proteins, are unable to traverse the NE passively and are dependent on active, signal-mediated pathways for translocation.
Many cis-acting peptide sequences that are sufficient to specify nuclear import or nuclear export have now been defined. These are termed nuclear localization signals (NLSs) or nuclear export signals (NESs), respectively. With respect to NLSs, the first sequences to be delineated comprised one or two short stretches of basic amino acids (particularly lysine). NLSs of this class are typified by that of T antigen (proline-lysine-lysine-lysine-arginine-lysine-valine) (32) and are frequently referred to as classical, or basic-type, NLSs (9, 14, 39, 48). More recently, a number of nonconforming NLSs—one example of which is the glycine-rich M9 sequence of the A1 protein—that are not particularly rich in lysine residues have also been defined (31, 45, 48, 61, 64, 68, 70). In contrast to NLSs, the existence of NESs was established relatively recently (20, 44, 73); although fewer such sequences have therefore been identified and characterized, many NESs are marked by an abundance of hydrophobic residues (especially leucine) (4, 21, 48, 54).
Work by many groups has now established that NLSs and NESs function by interacting with members of a family of related ∼100-kDa proteins known as the nuclear transport receptors. These proteins shuttle between the nucleus and the cytoplasm and function by interacting with their substrates in either the cytoplasm or the nucleus, translocating through NPCs via a series of interactions with NPC components (nucleoporins), dissociating from their respective substrates, and then recycling back to their original compartment (2, 24, 31, 38, 41, 48, 49). The first transport receptor to be identified, importin β/karyopherin β1, is essential for the nuclear import of many basic-type NLSs; however, unlike other transport receptors, importin β does not usually bind to its substrates directly but, rather, is bridged via an adapter known as importin α/karyopherin α. Interestingly, importin α’s interaction with importin β is mediated by its amino-terminal 41 amino acids—a region that is also rich in basic amino acids (especially arginine)—and is termed the importin-beta-binding (IBB) domain (25, 72).
Evidence to suggest that the GTPase Ran serves two functions during nuclear transport has been presented (24). (i) The asymmetric distribution of RanGTP (nucleus)-RanGDP (cytoplasm) across the NE provides both import and export with directionality. Specifically, the binding of RanGTP to import receptors triggers the release of NLSs (26, 30, 31, 56), whereas binding to export receptors stimulates their association with NESs (2, 22, 37, 38). Thus, the interaction of RanGTP with transport receptors is thought to terminate nuclear import and to be a prerequisite for nuclear export. This critical asymmetry of Ran’s two nucleotide-bound states is determined by the subcellular localization of its exchange factor, RCC1, which is nuclear, and the GTPase activating protein, RanGAP1, which is cytoplasmic (24). (ii) Ran-mediated GTP hydrolysis appears to be thermodynamically coupled to the import of NLS-bearing substrates (71) and the export of most ribonucleoprotein (RNP) complexes (30). (GTP hydrolysis by Ran has been shown to be dispensable for tRNA export and NES-mediated protein export [30, 58].)
In summary, the nuclear import of a protein carrying a classical, basic-type NLS is therefore thought to involve the following steps (24, 41, 48, 49). The NLS initially binds to importin α in the cytoplasm in the context of an importin α/β heterodimer. The importin β component of this ternary complex interacts with the cytoplasmic face of the NPC and the whole complex then translocates through the pore. Following delivery to the nuclear interior, the binding of RanGTP to importin β results in the disassembly of the ternary complex and the subsequent return of importin α and importin β, but not the NLS-bearing protein, to the cytoplasm.
The analysis of the Rex and Rev posttranscriptional regulatory proteins of the human retroviruses human T-cell leukemia virus type 1 (HTLV-1) and human immunodeficiency virus type 1 (HIV-1), respectively, has contributed much to our current understanding of nuclear transport (10, 11, 16, 54). In particular, Rex and Rev each bind specifically to cis-acting sequences in their substrate unspliced viral mRNAs in the nucleus (5, 13, 75) and then escort those RNAs through NPCs and into the cytoplasm. As such, Rex and Rev are essential for the nuclear export of intron-containing viral transcripts and, therefore, virus replication. These proteins were among the first shown to harbor NESs (4, 20, 34, 43, 51), and it is now known that these leucine-rich domains mediate direct binding to the export receptor exportin 1/Crm1p (22).
In contrast to the intensive analysis of Rex/Rev nuclear export, the import of these shuttling proteins into the nucleus has received comparatively little attention. Both proteins harbor transferable NLSs that, although highly basic in nature, contain many more arginines than lysines (36, 40, 52, 59, 62). In addition to this variance with many basic-type NLSs, it has also been found that the nuclear import of Rex and Rev is inhibited when cells are treated with transcriptional inhibitors, such as actinomycin D (33, 42, 51, 57, 65, 74). These two observations suggested that there may be differences between the pathways of import used by classical NLSs and Rex/Rev. By using the digitonin-permeabilized cell assay for in vitro nuclear import, we show that the Rex NLS, unlike the lysine-rich NLS of T antigen, utilizes importin β for import with no apparent requirement for importin α. However, and in keeping with previously described nuclear import pathways, the import of Rex remains dependent on the Ran GTPase cycle. These findings therefore demonstrate that importin β can mediate the nuclear import of NLSs either with or without the adapter protein importin α.
MATERIALS AND METHODS
Molecular clones.
The wild-type HTLV-1 Rex expression vector, pcRex, expresses the full-length 189-amino-acid protein under the transcriptional control of the cytomegalovirus immediate-early promoter (60). Its NLS-deficient variant, pcRex(1 + 2), was generated by PCR-mediated site-directed mutagenesis and has the stretches of basic residues at positions 5 to 7 and 13 to 15 exchanged for aspartic acid-leucine (see Fig. 1). The wild-type Rex and 1 + 2 mutant import substrate expression vectors, pGST-Rex-Myc and pGST-Rex(1 + 2)-Myc, are based on the pGEX-2T glutathione S-transferase (GST) expression vector and were constructed by insertion of the sequences that encode the amino-terminal residues of Rex up to the threonine at position 18 between a glycine-glycine hinge at the carboxy terminus of GST and the c-Myc epitope tag (43). The pGST-T vector which expresses the NLS of T antigen fused to the carboxy terminus of GST has been described previously (55). The plasmids used to express recombinant fusions of various cellular nuclear import factors, GST-importin α (NPI-1/hSRP-1), His6-ZZ-IBB, GST-importin β, GST-Ran (wild type), GST-RanT24N, His6-RanQ69L, GST-RanD125N, and GST-p10, have been described elsewhere (26, 55, 68).
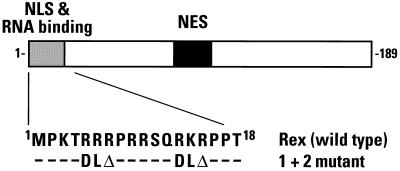
FIG. 1.
Domain organization of the 189-amino-acid Rex protein of HTLV-1. The domains that mediate nuclear localization and RNA binding (residues 1 to 18, gray box), and nuclear export (residues 82 to 93, solid box) are indicated. The amino acid sequence of the wild-type NLS is indicated below together with the changes that were introduced in the 1 + 2 mutant. −, no change; Δ, deletion of one residue.
Transient transfection and indirect immunofluorescence.
Thirty-five-millimeter-diameter subconfluent HeLa cell monolayers were transiently transfected with 5 μg of the Rex expression vectors by using calcium phosphate. At 48 h, the cells were fixed using paraformaldehyde, permeabilized, and incubated first with a Rex-specific antiserum raised in rabbit and then with a Texas red-conjugated secondary antibody (51). Stained samples were viewed by epifluorescence with a Nikon Microphot-SA microscope at a magnification of ×400.
Purification of recombinant proteins from Escherichia coli and synthetic peptides.
All GST- and His6-fusion proteins were purified from exponentially growing E. coli that had been induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) using standard affinity chromatography techniques. For the His6 fusions, purifications were performed under denaturing conditions with 6 M guanidine hydrochloride and the eluted proteins initially dialyzed in phosphate-buffered saline. For the GST fusions, purifications were carried out under nondenaturing conditions, and the import factors were separated from GST by proteolytic cleavage with either factor Xa (wild-type Ran, RanD125N, and p10) or thrombin (importin α, importin β, and RanT24N) for 6 h at 25°C. All import factors as well as the uncleaved import substrates (GST-Rex NLS, GST-Rex[1 + 2], and GST-T NLS), which were eluted from glutathione-Sepharose 4B columns using 20 mM glutathione, were finally dialyzed in 1× import buffer (20 mM HEPES [pH 7.3], 110 mM potassium acetate, 5 mM sodium acetate, 2 mM magnesium acetate, 1 mM EGTA, 2 mM dithiothreitol, and 1-μg/ml concentrations each of the protease inhibitors aprotinin, leupeptin, and pepstatin), concentrated to ∼1 mg/ml, and stored in aliquots at −80°C. The integrity of each protein preparation was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. For the Rex import substrates, standard Western blotting using GST-specific (Santa Cruz Biotechnology, Inc.) or Myc-specific (9E10) (17) monoclonal antibodies was also performed to ensure protein quality (data not shown). Peptides were synthesized using an Advanced Chemtech MPS 396 synthesizer and comprised T antigen (residues 125 to 135 [C-YPKKKRKVEDP]) and the Rex wild type (residues 1 to 18 [C-MPKTRRRPRRSQRKRPPT]).
Permeabilized cell assay for in vitro nuclear import.
Permeabilized cell assays were performed essentially as described previously (1). Adherent HeLa cells were plated onto glass coverslips in 35-mm-diameter culture dishes and maintained until almost confluent. Cells were then washed with cold 1× import buffer, permeabilized by immersion in cold 1× import buffer supplemented with 30 μg of digitonin/ml for 5 min, and rinsed with excess import buffer. Import assays were performed by blotting the coverslips to remove excess liquid, inverting over a drop of import cocktail (factors plus substrate) on a Parafilm sheet in a humidified box, and maintaining at room temperature for 30 min. Nuclear import was assessed by washing the cells with phosphate-buffered saline, paraformaldehyde fixation, and indirect immunofluorescence using the GST-specific monoclonal antibody.
For assays in which HeLa cell cytosol was used as a source of import factors, the import cocktail comprised 1× import buffer containing 40 to 50% cytosol, ∼25 μg of import substrate/ml, 1 mM ATP, 5 mM creatine phosphate, 20 U of creatine phosphokinase/ml, 0.1 mM GTP, and 1 μg each of aprotinin, leupeptin, and pepstatin/ml. The cytosolic extracts were prepared in advance from HeLa S3 cells by hypotonic lysis, Dounce homogenization, centrifugation, and dialysis in 1× import buffer and were stored in aliquots at −80°C (1). For the competition assays, either a 200-fold molar excess of synthetic peptide (relative to substrate) or 30 μM IBB was added to the import cocktails. In experiments which required recombinant import factors, these were added to a final concentration of ∼5 μM. Importin β was specifically depleted from 500 μl of cytosol by two independent 3-h incubations at 4°C with 100 μg of purified importin-β-specific monoclonal antibody (3E9) (8) that had been prebound to 100 μl of protein G-agarose. The agarose beads were removed by centrifugation at 1,000 × g for 2 min, and the loss of importin β from the extract was confirmed by Western blotting.
RESULTS
The principal goal of this study was to define the pathway of nuclear import used by the Rex protein of HTLV-1, and to compare and contrast this with the classical pathway. As discussed above, there were already hints that Rex nuclear import may differ from that of basic-type NLSs, such as the T antigen NLS. Specifically, Rex’s NLS is arginine rich rather than lysine rich (59, 62), and the nuclear import of Rex in living cells is sensitive to the addition of inhibitors of cellular transcription (51, 65). Interestingly, nuclear import that is mediated by NLSs found in a number of the heterogeneous ribonucleoprotein (RNP) particle (hnRNP) proteins—for example, A1 and K—can also be inhibited by treatment with transcriptional inhibitors (45, 53, 63). Moreover, the transcriptionally sensitive NLSs of both A1 and K function by accessing pathways of nuclear import that are clearly distinct from the classical pathway (45, 55); in the case of A1, import is mediated by direct interaction with the import receptor transportin/karyopherin β2 (7, 23, 55, 63).
Sequence-specific nuclear import of Rex.
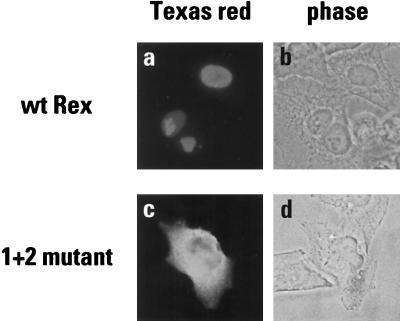
The domain structure of the HTLV-1 Rex protein is illustrated in Fig. 1. Towards the middle of the linear sequence is the leucine-rich NES that mediates Rex’s interaction with the export receptor exportin 1 (6, 28, 69). At the amino terminus is a highly basic region (9 out of the first 15 amino acids) that mediates both nuclear localization and binding to the Rex response element (RxRE) in Rex-responsive viral mRNAs (5, 59, 62). To confirm that this arginine-rich region is indeed the Rex NLS, site-directed mutagenesis was performed on a wild-type rex gene such that six of the basic residues were eliminated from this region (1 + 2 mutation). The wild-type and mutated expression vectors were transiently transfected into HeLa cells, and the ensuing patterns of protein expression were determined by indirect immunofluorescence using a rabbit Rex-specific antiserum (Fig. 2). As expected, the wild-type Rex protein efficiently accumulated in the nuclei of expressing cells (panels a and b), whereas the 1 + 2 mutant protein failed to localize to the nucleus and was found predominantly in the cytoplasm (panels c and d).
FIG. 2.
Subcellular localization of Rex proteins in transfected HeLa cells. Monolayers were transiently transfected with the wild-type vector pcRex (a and b) or its mutated derivative pcRex (1 + 2) (c and d), maintained for 48 h and subjected to indirect immunofluorescence using a Rex-specific antiserum raised in rabbit and a Texas red-conjugated secondary antibody. Samples were viewed by epifluorescence (a and c) or phase contrast (b and d).
The standard approach for studying nuclear import pathways in vitro is the digitonin-permeabilized cell system, as originally developed by Adam et al. (1). Here, cells are treated with the nonionic detergent digitonin such that the plasma membrane, but not the NE, is permeabilized and many cytosolic factors are released; nuclear import and accumulation of added substrates in the presence of various extracts, recombinant factors and compounds can then be used to analyze a given nuclear import pathway. To examine Rex import, we therefore constructed and purified fusion proteins that comprised the wild-type NLS of Rex, or the 1 + 2 mutant derivative, sandwiched between GST and the c-Myc epitope tag. The integrity of each purified protein was confirmed by Western blotting using both GST- and Myc-specific antibodies. Each detected single species, which indicated that both protein preparations were full length and essentially homogeneous (data not shown). As a control for the following experiments, we used the well-characterized fusion of GST to the NLS of T antigen (55).
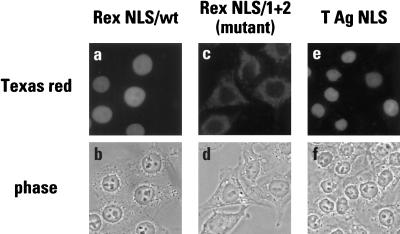
All three fusion proteins were then used as import substrates in a permeabilized cell assay in which the import factors were provided by a HeLa cell cytosolic extract. Following a reaction time of 30 min, the substrates were detected by indirect immunofluorescence using the GST-specific antibody (Fig. 3). Both wild-type NLS-containing proteins, GST-Rex NLS and GST-T NLS, were imported into the nucleus (panels a, b, e, and f) whereas the fusion carrying the inactive NLS, GST-Rex (1 + 2), was not (panels c and d). Importantly, the same results were obtained for the GST-Rex proteins when the Myc-specific antibody was used for detection, a finding which confirmed that the full-length input proteins were being detected in these assays (data not shown). These results are, therefore, consistent with the transfection-based studies (Fig. 2) and establish these GST fusions as legitimate substrates with which to analyze the mechanism of Rex nuclear import.
FIG. 3.
Sequence specific nuclear import of Rex in digitonin-permeabilized HeLa cells. In vitro import assays were performed using fusions of the wild-type Rex NLS (a and b), the 1 + 2 mutant variant of the Rex NLS (c and d) or the wild-type T antigen NLS (e and f) to the carboxy terminus of GST as substrates. Import factors were supplied using HeLa cell cytosol and reactions were run for 30 min in the presence of an energy regenerating system. Samples were analyzed by indirect immunofluorescence using a GST-specific monoclonal antibody followed by a Texas red-conjugated secondary antibody and epifluorescence (a, c, and e). The corresponding phase-contrast analyses are also shown (b, d, and f).
Sensitivity of nuclear import to WGA.
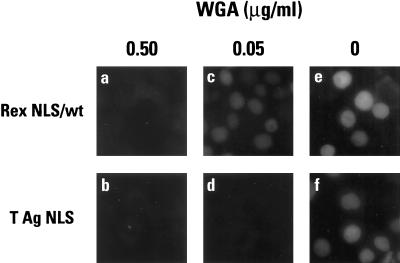
The lectin wheat germ agglutinin (WGA) inhibits nuclear import both in vivo and in vitro and has been shown to bind to a number of glycosylated nucleoporins (12, 18). To evaluate the effects of WGA on Rex import, two concentrations of WGA were added to import reactions in which GST-Rex NLS (wild type) or GST-T NLS were the substrates and import factors were supplied using HeLa cytosol (Fig. 4). Although both NLSs were imported with similar efficiencies in the absence of lectin (panels e and f), the Rex NLS was less sensitive to inhibition than the T NLS since significant import was still observed in the presence of 0.05 μg of WGA/ml (compare panels c and d). In contrast, the nuclear import of both NLSs was blocked at the higher dose of 0.5 μg of WGA/ml (panels a and b). This differential susceptibility of the Rex and T NLSs to lectin-mediated inhibition further suggested that their modes of nuclear import may differ.
FIG. 4.
Inhibition of nuclear import by wheat germ agglutinin. In vitro import assays using GST-Rex NLS (wild type) (a, c, and e) or GST-T NLS (b, d, and f) as substrate and HeLa cell cytosol were carried out in the presence of 0.5 mg of WGA/ml (a and b) or 0.05 mg of WGA/ml (c and d), or in the absence of WGA (e and f). All samples were analyzed by indirect immunofluorescence as described in the legend for Fig. 3.
Saturation of nuclear import pathways.
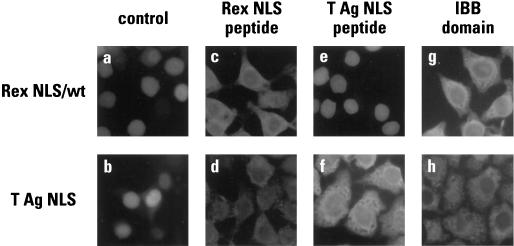
One of the defining features of signal-mediated nuclear transport is that it is saturable in that inhibition is observed in the presence of excess cognate competitor. To investigate this aspect of Rex NLS function, import assays were performed using GST-Rex NLS or GST-T NLS as substrate and synthetic NLS peptides as competitors (Fig. 5). The IBB domain of importin α was used as a third competitor since appending this 41-amino-acid sequence to a heterologous substrate has been shown to confer nuclear import by mediating direct binding to importin β (25, 72); in other words, this domain can also be regarded as having an NLS function.
FIG. 5.
The nuclear import of Rex is a saturable process. In vitro import assays were performed using GST-Rex NLS (wild type) (a, c, e, and g) or GST-T NLS (b, d, f, and h) as a substrate, HeLa cell cytosol as a source of import factors, and synthetic peptides corresponding to the Rex NLS (c and d) or the T NLS (e and f), or purified His6-IBB (g and h) as competitors. Samples were analyzed as described in the legend for Fig. 3.
As predicted for sequence specific import, the nuclear uptake of both substrates was inhibited by addition of their matching NLS peptide competitors (panels c and f). In contrast, whereas the Rex peptide was also able to block the import of the T NLS (panel d), the T antigen peptide had no discernible effect on the efficiency of Rex NLS import (panel e). Interestingly, addition of the IBB domain resulted in the same pattern of interference as was seen with the Rex competitor in that the import of the Rex and T NLSs was inhibited (panels c, d, g, and h). The most likely explanation for these observations is that the Rex NLS and the IBB domain interact with overlapping sites (or the same site) on importin β such that the import of any substrate that requires access to that site—including the T NLS via importin α bridging—would be inhibited. This hypothesis suggests, therefore, that Rex nuclear import occurs independently of importin α and that the addition of T NLS competitor is inconsequential because this peptide acts “upstream” by disrupting interactions between NLSs and importin α.
Rex nuclear import is mediated by importin β independently of importin α.
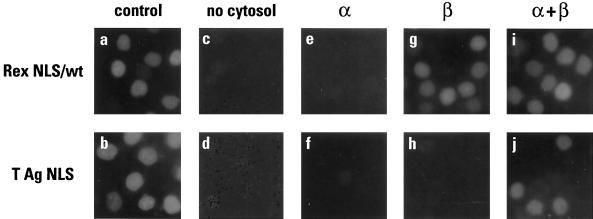
To test the aforementioned model for Rex NLS function more directly, we conducted a series of assays in which the import factors were supplied as purified recombinant proteins rather than in the context of HeLa cytosol (Fig. 6). Specifically, once the cells had been permeabilized with digitonin, no cytosol, importin α alone, importin β alone, or importin α plus importin β were added together with the Rex or T antigen substrates. Of note, we found that the addition of Ran and/or p10/NTF2 to these reaction cocktails had no effect on the efficiency of nuclear import in any of the samples (data not shown). We presume that this reflects the incomplete depletion of these factors during permeabilization (the role of Ran will be discussed further below).
FIG. 6.
Reconstitution of nuclear import using purified recombinant proteins. GST-Rex NLS (wild type) (a, c, e, g, and i) and GST-T NLS (b, d, f, h, and j) were used as in vitro import substrates in the presence of HeLa cytosol (a and b), no cytosol or added factors (c and d), importin α (e and f), importin β (g and h), or importin α plus importin β (i and j). Samples were analyzed as described in the legend for Fig. 3.
As expected, neither Rex nor T antigen was imported when no factors were added (panels c and d) or when only importin α was added (panels e and f). Most significantly, however, when importin β was added in the absence of importin α efficient import of the Rex NLS (panel g) but not the T NLS (panel h) was observed; indeed, the efficiency of reconstituted import closely paralleled that seen with whole cytosol (panel a). Consistent with the current definition of the classical import pathway, further supplementation with importin α restored import capacity to the T NLS (panel j). Of note, it might have been expected that addition of importin α to the Rex NLS reaction would have reduced import efficiency in the same manner as the IBB domain (Fig. 5); that this was not seen (panel i) is likely to be a reflection of the lower concentration of importin α used in this experiment, as well as the higher concentration of importin β. In summary, the most parsimonious interpretation of these data is that the Rex NLS binds to importin β directly and is imported into the nucleus independently of importin α.
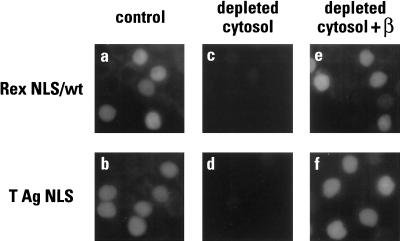
Recent data have indicated that there is marked redundancy in the nuclear import of certain proteins. In particular, the ribosomal proteins L23a, S7 and L5 can each be imported into the nucleus by the action of four distinct import receptors: importin β acting independently of importin α, transportin, Ran binding protein 5 (RanBP5) and RanBP7 (31). Thus, although our data reveal that the nuclear import of Rex can be mediated by importin β in the absence of importin α, it remains formally possible that additional receptors could also direct Rex import (though this seemed unlikely given the sensitivity of Rex import to competition by the IBB domain). Nevertheless, to address this point using a different approach, importin β was specifically removed from HeLa cytosol by two serial rounds of immunodepletion with the 3E9 monoclonal antibody (8), and the resulting extract was then used in import assays in which the NLSs of Rex or T antigen served as substrates (Fig. 7). In each case, import was abolished by the removal of importin β (panels c and d) but not by mock depletion of cytosol with an irrelevant antibody (data not shown). Importantly, the addition of recombinant importin β to the depleted extract restored efficient import to both substrates (compare panels e and f with a and b). Based on these findings, we conclude that importin β is the sole member of the import receptor superfamily that can mediate the nuclear uptake of Rex.
FIG. 7.
Importin β is required for Rex and T antigen nuclear import. GST-Rex NLS (wild type) (a, c, and e) and GST-T NLS (b, d, and f) were used as in vitro import substrates in the presence of HeLa cytosol (a and b), cytosol that had been immunodepleted of importin β (c and d), or depleted cytosol supplemented with purified importin β (e and f). Samples were analyzed as described in the legend for Fig. 3.
Rex nuclear import is dependent on the Ran GTPase cycle.
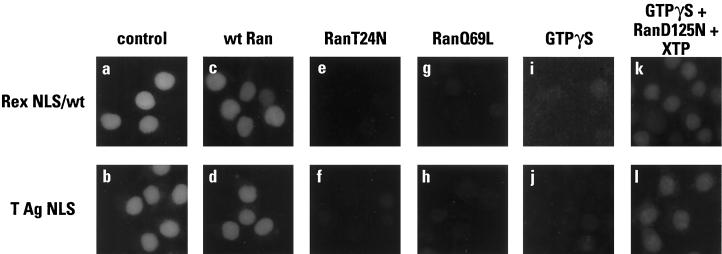
As discussed previously, protein nuclear import is thought to be Ran dependent for two reasons: (i) Ran-mediated hydrolysis of GTP provides energy that is critical to import, and (ii) the asymmetry of Ran’s two nucleotide-bound states provides directionality to import. To this point, however, the dependence of Rex NLS function on the integrity of the Ran GTPase cycle has not been established. For instance, under the permeabilization conditions used here, purified Ran did not have to be included in our reconstitution experiments (Fig. 6). Previous analyses have established that the Ran dependence of nuclear transport can be revealed through the use of mutant Ran proteins. Accordingly, the effects of three different Ran mutants on Rex nuclear import were determined (Fig. 8). RanT24N is unable to bind GTP and has a very low affinity for GDP, and is therefore a competitive inhibitor of RCC1-mediated nucleotide exchange (35); RanQ69L is deficient in GTPase function and is therefore “locked” in the GTP-bound state (3, 35); and RanD125N hydrolyzes xanthosine 5′-triphosphate (XTP) rather than GTP (71).
FIG. 8.
The importance of Ran for nuclear import. GST-Rex NLS (wild type) (a, c, e, g, i, and k) and GST-T NLS (b, d, f, h, j, and l) were used as in vitro import substrates in the presence of HeLa cytosol (a and b), or cytosol supplemented with wild-type Ran (c and d), RanT24N (e and f), RanQ69L (g and h), the nonhydrolyzable GTP analog GTPγS (2.5 mM) (i and j), or GTPγS plus RanD125N plus XTP (0.75 mM) (k and l). Samples were analyzed as described in the legend for Fig. 3.
In all these experiments, the effects of the different Ran proteins were indistinguishable for the NLSs of Rex or T antigen (Fig. 8). Not surprisingly, the addition of wild-type Ran to whole cytosol had no evident consequence for import (panels a to d), presumably because these cells had retained sufficient Ran during digitonin treatment. In contrast, the addition of the T24N or Q69L mutant protein efficiently inhibited import (panels e to h). The addition of excess levels of these mutant proteins to import reactions collapses the RanGTP (nucleus)-RanGDP (cytoplasm) gradient and eliminates directionality: RanT24N increases the RanGDP concentration in the nucleus and presumably prevents the release of import substrates from import receptors, whereas RanQ69L increases cytoplasmic RanGTP levels and dissociates import substrates from receptors prior to translocation.
Given that GTP hydrolysis is important for receptor-mediated nuclear import, it was expected that addition of the nonhydrolyzable GTP analog GTPγS would inhibit import (panels i and j). That import could then be rescued to a substantial degree by the addition of XTP and RanD125 (panels k and l) supports the idea that the Ran-mediated hydrolysis of GTP is coupled to the nuclear import of the Rex and T antigen NLSs. We therefore conclude that the requirement for Ran in the importin-α/β-mediated import of basic-type NLSs appears to be conserved for the importin-β-mediated import of the arginine-rich NLS of Rex.
DISCUSSION
In this article, we describe in vitro experiments which help define the nuclear import pathway used by the HTLV-1 posttranscriptional trans-activator Rex. Using a permeabilized cell system, it was confirmed that Rex harbors an arginine-rich NLS (Fig. 1) that is sufficient to confer nuclear import on a heterologous substrate (Fig. 3). A combination of competition (Fig. 5), reconstitution (Fig. 6), and immunodepletion (Fig. 7) experiments revealed that importin β is accessed directly by the Rex NLS to achieve nuclear import and that importin α plays no role in this process. Finally, and consistent with previously described pathways of receptor-mediated nuclear import, Rex NLS function was shown to be dependent on the asymmetry of RanGTP-RanGDP across the NE and to be coupled to Ran-mediated nucleoside triphosphate hydrolysis (Fig. 8).
There are two principal reasons for considering it most likely that the Rex NLS binds to importin β directly. One, even though the reconstitution studies (Fig. 6) do not by themselves eliminate the possibility that an adapter protein could bridge Rex to importin β, the ability of the Rex peptide to block T NLS import (Fig. 5) shows that a protein-protein interaction that is critical to the classical import pathway, yet is distinct from the binding of the T NLS to importin α, must be impeded by this competitor. Given the dependence of Rex import on importin β (Fig. 6 and 7) and its sensitivity to competition by the IBB domain (Fig. 5), the only remaining target for Rex binding is, presumably, importin β. Two, a number of other NLSs have now been shown to bind to importin β directly and/or to be imported into the nucleus by a mechanism that is dependent on importin β, but independent of importin α. Interestingly, these NLSs—which include those found in HIV-1 Rev (27, 67), HIV-1 Tat (67), L23a (31), the yeast RNA binding protein Nab2 (68), and the T-cell protein tyrosine phosphatase (66), as well as the IBB domain of importin α (25, 72)—tend, like Rex, to be rich in arginine residues rather than in the lysines that are more commonly found in classical/basic-type NLSs. The recent elucidation of the structure of a 50-kDa proteolytic fragment of yeast importin α by crystallographic approaches helps explain why arginine-rich NLSs that utilize importin β for import appear to do so by bypassing importin α (9). Specifically, the asparagine, tryptophan, and negatively charged residues that help form the NLS binding sites appear to be better positioned to participate in multiple hydrogen bonding and hydrophobic, van der Waals, and electrostatic interactions with the side chains of lysines than with the side chains of arginines.
In summary, we propose that the nucleocytoplasmic shuttling cycle of Rex includes, but is not necessarily restricted to, the following interactions. Following initial synthesis in the cytoplasm, the arginine-rich NLS binds to importin β and Rex is transported through the NPC. Once in the nucleus, RanGTP interacts with importin β and releases Rex into the nucleoplasm. Rex can then bind and multimerize on the RxRE of Rex-responsive viral mRNAs, and, together with RanGTP, interact with the export receptor exportin 1. This complex is subsequently exported to the cytoplasm and exportin 1 is dissociated following the hydrolysis of Ran-bound GTP. The presumed release of Rex from the RxRE would then allow Rex to rebind importin β and to be reimported into the nucleus, and the RNA to be translated or packaged into progeny virions. The fact that the arginine-rich NLS of Rex also serves as the RNA binding domain likely means that these two activities are mutually exclusive (this has been shown to be the case for the arginine-rich NLS of Rev [27, 67] and ensures that Rex cannot carry exported viral transcripts back into the nucleus.
During the course of these experiments, it was noted that the nuclear import of Rex was less sensitive to the inhibitory effects of WGA than was the import of the classical NLS of T antigen (Fig. 4). Interestingly, it has previously been demonstrated that the import of U-rich small nuclear RNPs (U snRNPs) is also not as sensitive to inhibition by WGA as is classical NLS import (19, 46). Consistent with our delineation of the Rex import pathway, snRNP import is also importin α independent but importin β dependent (50). However, and in contrast to Rex which does not require a bridging factor to interact with importin β, the bipartite NLS of snRNPs that comprises the m3-5′ cap and the Sm core appears to bind to the importin-α-related receptor snurportin 1 as well as to an unidentified receptor, rather than to importin β directly (29). Taken together, these findings raise the possibility that WGA’s inhibitory effects on signal-mediated nuclear import may not be exerted entirely through interference with import receptor-NPC interactions. In particular, it seems likely that importin α function is also sensitive to the presence of WGA, perhaps as a consequence of direct interactions between the NPC and importin α (47).
ACKNOWLEDGMENTS
We thank Steve Adam, Bryan Cullen, Gideon Dreyfuss, Matt Michael, and Ray Truant for sharing reagents; Paul Eder, Ron Fouchier, Sara Nakielny, and Vicki Pollard for helpful discussions; and Laurie Zimmerman for excellent secretarial support.
This work was supported by the Howard Hughes Medical Institute, a U.S. Public Service grant AI41933 (to M.H.M.) from NIAID, and a Minority Predoctoral Fellowship (5-F31-HG-00065) from NIGMS (to D.P.).
REFERENCES
- 1.Adam S A, Marr R S, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arts G J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff F R, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc Natl Acad Sci USA. 1994;91:2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogerd H P, Fridell R A, Benson R E, Hua J, Cullen B R. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogerd H P, Huckaby G L, Ahmed Y F, Hanly S M, Greene W C. The type I human T-cell leukemia virus (HTLV-I) Rex trans-activator binds directly to the HTLV-I Rex and the type 1 human immunodeficiency virus Rev RNA response elements. Proc Natl Acad Sci USA. 1991;88:5704–5708. doi: 10.1073/pnas.88.13.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Böhnlein S, Pirker F P, Hoger L, Zimmermann K, Bachmayer H, Böhnlein E, Hauber J. Transdominant repressors for human T-cell leukemia virus type I Rex and human immunodeficiency virus type 1 Rev function. J Virol. 1991;65:81–88. doi: 10.1128/jvi.65.1.81-88.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonifaci N, Moroianu J, Radu A, Blobel G. Karyopherin β2 mediates nuclear import of a mRNA binding protein. Proc Natl Acad Sci USA. 1997;94:5055–5060. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi N C, Adam J H, Adam S A. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conti E, Uy M, Leighton L, Blobel G, Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin α. Cell. 1998;94:193–204. doi: 10.1016/s0092-8674(00)81419-1. [DOI] [PubMed] [Google Scholar]
- 10.Cullen B R. Mechanism of action of regulatory proteins encoded by complex retroviruses. Microbiol Rev. 1992;56:375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen B R. HIV-1 auxiliary proteins: making connections in a dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 12.Dabauvalle M C, Schulz B, Scheer U, Peters R. Inhibition of nuclear accumulation of karyophilic proteins in living cells by microinjection of the lectin wheat germ agglutinin. Exp Cell Res. 1988;174:291–296. doi: 10.1016/0014-4827(88)90163-2. [DOI] [PubMed] [Google Scholar]
- 13.Daly T J, Cook K S, Gray G S, Maione T E, Rusche J R. Specific binding of HIV-1 recombinant Rev protein to the Rev-responsive element in vitro. Nature. 1989;342:816–819. doi: 10.1038/342816a0. [DOI] [PubMed] [Google Scholar]
- 14.Dingwall C, Laskey R A. Nuclear targeting sequences—a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 15.Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 16.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 17.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finlay D R, Newmeyer D D, Price T M, Forbes D J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987;104:189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer U, Darzynkiewicz E, Tahara S M, Dathan N A, Luhrmann R, Mattaj I W. Diversity in the signals required for nuclear accumulation of U snRNPs and variety in the pathways of nuclear transport. J Cell Biol. 1991;113:705–714. doi: 10.1083/jcb.113.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer U, Huber J, Boelens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 21.Fischer U, Michael W M, Lührmann R, Dreyfuss G. Signal-mediated nuclear export pathways of proteins and RNAs. Trends Cell Biol. 1996;6:290–293. doi: 10.1016/0962-8924(96)20030-3. [DOI] [PubMed] [Google Scholar]
- 22.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 23.Fridell R A, Truant R, Thorne L, Benson R E, Cullen B R. Nuclear import of hnRNP A1 is mediated by a novel cellular cofactor related to karyopherin-beta. J Cell Sci. 1997;110:1325–1331. doi: 10.1242/jcs.110.11.1325. [DOI] [PubMed] [Google Scholar]
- 24.Görlich D. Nuclear protein import. Curr Opin Cell Biol. 1997;9:412–419. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 25.Görlich D, Henklein P, Laskey R A, Hartmann E. A 41 amino acid motif in importin-α confers binding to importin-β and hence transit into the nucleus. EMBO J. 1996;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- 26.Görlich D, Panté N, Kutay U, Aebi U, Bischoff F R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson B R, Percipalle P. Interactions between HIV Rev and nuclear import and export factors: the Rev nuclear localisation signal mediates specific binding to human importin-beta. J Mol Biol. 1997;274:693–707. doi: 10.1006/jmbi.1997.1420. [DOI] [PubMed] [Google Scholar]
- 28.Hope T J, Bond B L, McDonald D, Klein N P, Parslow T G. Effector domains of human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type I Rex are functionally interchangeable and share an essential peptide motif. J Virol. 1991;65:6001–6007. doi: 10.1128/jvi.65.11.6001-6007.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber J, Cronshagen U, Kadokura M, Marshallsay C, Wada T, Sekine M, Lührmann R. Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. EMBO J. 1998;17:4114–4126. doi: 10.1093/emboj/17.14.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izaurralde E, Kutay U, von Kobbe C, Mattaj I W, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jäkel S, Görlich D. Importin β, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–4502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalderon D, Richardson W D, Markham A F, Smith A F. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984;311:33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 33.Kalland K-H, Szilvay A M, Brokstad K A, Sætrevik W, Haukenes G. The human immunodeficiency virus type 1 Rev protein shuttles between the cytoplasm and nuclear compartments. Mol Cell Biol. 1994;14:7436–7444. doi: 10.1128/mcb.14.11.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim F J, Beeche A A, Hunter J J, Chin D J, Hope T J. Characterization of the nuclear export signal of human T-cell lymphotropic virus type 1 Rex reveals that nuclear export is mediated by position-variable hydrophobic interactions. Mol Cell Biol. 1996;16:5147–5155. doi: 10.1128/mcb.16.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klebe C, Bischoff F R, Ponstingl H, Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995;34:639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- 36.Kubota S, Siomi H, Satoh T, Endo S-I, Maki M, Hatanaka M. Functional similarity of HIV-1 rev and HTLV-1 rex proteins: identification of a new nucleolar-targeting signal in rev protein. Biochem Biophys Res Commun. 1989;162:963–970. doi: 10.1016/0006-291x(89)90767-5. [DOI] [PubMed] [Google Scholar]
- 37.Kutay U, Bischoff F R, Kostka S, Kraft R, Görlich D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 38.Kutay U, Lipowsky G, Izaurralde E, Bischoff F R, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 39.LaCasse E C, Lefebvre Y A. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 1995;23:1647–1656. doi: 10.1093/nar/23.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malim M H, Böhnlein S, Hauber J, Cullen B R. Functional dissection of the HIV-1 Rev trans-activator-derivation of a trans-dominant repressor of Rev function. Cell. 1989;58:205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 41.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 42.Meyer B E, Malim M H. The HIV-1 Rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 43.Meyer B E, Meinkoth J L, Malim M H. Nuclear transport of the human immunodeficiency virus type 1, visna virus, and equine infectious anemia virus Rev proteins: identification of a family of transferable nuclear export signals. J Virol. 1996;70:2350–2359. doi: 10.1128/jvi.70.4.2350-2359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michael W M, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 45.Michael W M, Eder P S, Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michaud N, Goldfarb D. Microinjected U snRNAs are imported to oocyte nuclei via the nuclear pore complex by three distinguishable targeting pathways. J Cell Biol. 1992;116:851–861. doi: 10.1083/jcb.116.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moroianu J, Blobel G, Radu A. RanGTP-mediated nuclear export of karyopherin α involves its interaction with the nucleoporin Nup153. Proc Natl Acad Sci USA. 1997;94:9699–9704. doi: 10.1073/pnas.94.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 49.Ohno M, Fornerod M, Mattaj I W. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- 50.Palacios I, Hetzer M, Adam S A, Mattaj I W. Nuclear import of U snRNPs requires importin β. EMBO J. 1997;16:6783–6792. doi: 10.1093/emboj/16.22.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmeri D, Malim M H. The human T-cell leukemia virus type 1 posttranscriptional trans-activator Rex contains a nuclear export signal. J Virol. 1996;70:6442–6445. doi: 10.1128/jvi.70.9.6442-6445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkins A, Cochrane A W, Ruben S M, Rosen C A. Structural and functional characterization of the human immunodeficiency virus rev protein. J Acquired Immune Defic Syndr. 1989;2:256–263. [PubMed] [Google Scholar]
- 53.Piñol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- 54.Pollard V W, Malim M H. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 55.Pollard V W, Michael W M, Nakielny S, Siomi M C, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 56.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 57.Richard N, Iacampo S, Cochrane A. HIV-1 Rev is capable of shuttling between the nucleus and cytoplasm. Virology. 1994;204:123–131. doi: 10.1006/viro.1994.1516. [DOI] [PubMed] [Google Scholar]
- 58.Richards S A, Carey K L, Macara I G. Requirement of guanosine triphosphate-bound Ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1847. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- 59.Rimsky L, Duc Dodon M, Dixon E P, Greene W C. Trans-dominant inactivation of HTLV-I and HIV-1 gene expression by mutation of the HTLV-I Rex transactivator. Nature. 1989;341:453–456. doi: 10.1038/341453a0. [DOI] [PubMed] [Google Scholar]
- 60.Rimsky L, Hauber J, Dukovich M, Malim M H, Langlois A, Cullen B R, Greene W C. Functional replacement of the HIV-1 Rev protein by the HTLV-1 Rex protein. Nature. 1988;335:738–740. doi: 10.1038/335738a0. [DOI] [PubMed] [Google Scholar]
- 61.Siomi H, Dreyfuss G. A nuclear localization domain in the hnRNP A1 protein. J Cell Biol. 1995;129:551–560. doi: 10.1083/jcb.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siomi H, Shida H, Nam S H, Nosaka T, Maki M, Hatanaka M. Sequence requirements for nucleolar localization of human T cell leukemia virus type I pX protein, which regulates viral RNA processing. Cell. 1988;55:197–209. doi: 10.1016/0092-8674(88)90043-8. [DOI] [PubMed] [Google Scholar]
- 63.Siomi M C, Eder P S, Kataoka N, Wan L, Liu Q, Dreyfuss G. Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J Cell Biol. 1997;138:1181–1192. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siomi M C, Fromont M, Rain J C, Wan L, Wang F, Legrain P, Dreyfuss G. Functional conservation of the transportin nuclear import pathway in divergent organisms. Mol Cell Biol. 1998;18:4141–4148. doi: 10.1128/mcb.18.7.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stauber R, Gaitanaris G A, Pavlakis G N. Analysis of trafficking of Rev and transdominant Rev proteins in living cells using green fluorescent protein fusions: transdominant Rev blocks the export of Rev from the nucleus to the cytoplasm. Virology. 1995;213:439–449. doi: 10.1006/viro.1995.0016. [DOI] [PubMed] [Google Scholar]
- 66.Tiganis T, Flint A J, Adam S A, Tonks N K. Association of the T-cell protein tyrosine phosphatase with nuclear import factor p97. J Biol Chem. 1997;272:21548–21557. doi: 10.1074/jbc.272.34.21548. [DOI] [PubMed] [Google Scholar]
- 67.Truant R, Cullen B R. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin β-dependent nuclear localization signals. Mol Cell Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Truant R, Fridell R A, Benson R E, Bogerd H, Cullen B R. Identification and functional characterization of a novel nuclear localization signal present in the yeast Nab2 poly(A)+ RNA binding protein. Mol Cell Biol. 1998;18:1449–1458. doi: 10.1128/mcb.18.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weichselbraun I, Farrington G K, Rusche J R, Böhnlein E, Hauber J. Definition of the human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type I Rex protein activation domain by functional exchange. J Virol. 1992;66:2583–2587. doi: 10.1128/jvi.66.4.2583-2587.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weighardt F, Biamonti G, Riva S. Nucleo-cytoplasmic distribution of human hnRNP proteins: a search for the targeting domains in hnRNP A1. J Cell Sci. 1996;108:545–555. doi: 10.1242/jcs.108.2.545. [DOI] [PubMed] [Google Scholar]
- 71.Weis K, Dingwall C, Lamond A I. Characterization of the nuclear protein import mechanism using Ran mutants with altered nucleotide binding specificities. EMBO J. 1996;15:7120–7128. [PMC free article] [PubMed] [Google Scholar]
- 72.Weis K, Ryder U, Lamond A I. The conserved amino-terminal domain of hSRP1 alpha is essential for nuclear protein import. EMBO J. 1996;15:1818–1825. [PMC free article] [PubMed] [Google Scholar]
- 73.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 74.Wolff B, Cohen G, Hauber J, Meshcheryakova D, Rabeck C. Nucleocytoplasmic transport of the Rev protein of human immunodeficiency virus type 1 is dependent on the activation domain of the protein. Exp Cell Res. 1995;217:31–41. doi: 10.1006/excr.1995.1060. [DOI] [PubMed] [Google Scholar]
- 75.Zapp M L, Green M R. Sequence-specific RNA binding by the HIV-1 protein. Nature. 1989;342:714–716. doi: 10.1038/342714a0. [DOI] [PubMed] [Google Scholar]