Abstract
The management of hepatocellular carcinoma (HCC) is undergoing transformational changes due to the emergence of various novel immunotherapies and their combination with image-guided locoregional therapies. In this setting, immunotherapy is expected to become one of the standards of care in both neoadjuvant and adjuvant settings across all disease stages of HCC. Currently, more than 50 ongoing prospective clinical trials are investigating various end points for the combination of immunotherapy with both percutaneous and catheter-directed therapies. This review will outline essential tumor microenvironment mechanisms responsible for disease evolution and therapy resistance, discuss the rationale for combining locoregional therapy with immunotherapy, summarize ongoing clinical trials, and report on developing imaging end points and novel biomarkers that are relevant to both diagnostic and interventional radiologists participating in the management of HCC.
© RSNA, 2024
Summary
This review discusses mechanisms behind the immunosuppressive tumor microenvironment, the influence of locoregional therapy on immunotherapy, and the development of imaging and serologic biomarkers in relation to hepatocellular carcinoma.
Essentials
■ The immunosuppressive tumor microenvironment of hepatocellular carcinoma attenuates the potential full efficacy of immunotherapy by hindering the infiltration and activation of antitumor immune cells.
■ Preclinical investigations have demonstrated that locoregional therapy confers immunomodulatory effects to the tumor microenvironment, both proinflammatory and tumorigenic.
■ Clinical trials are underway investigating the potential synergism behind locoregional therapy and immunotherapy across multiple disease stages.
■ There is an unmet need to identify proper radiologic and serologic biomarkers assessing the therapeutic value of locoregional therapy and immunotherapy.
Introduction
Primary liver cancer is the third most common cause of cancer-related death in the world, with hepatocellular carcinoma (HCC) accounting for 80% of all primary liver tumors (1). HCC typically arises from fibrosis and cirrhosis, which may be induced by chronic liver injury of various causes. In North American and European cohorts, cirrhosis is primarily induced by alcoholic steatohepatitis and hepatitis C or metabolic dysfunction–associated fatty liver disease, often called MAFLD. In Asia and sub-Saharan Africa, hepatitis B persists as the key risk factor for HCC (70% of HCC worldwide occurs in Asia) (1). The degree of chronic liver injury in patients with HCC and the propensity for further hepatocarcinogenesis represent a key limiting factor for curative surgical resection, and the availability of donor organs for liver transplant is low.
The Barcelona Clinic Liver Cancer (BCLC) model outlines clinical practice guidelines based on the patient’s tumor burden, liver disease, and performance status to guide management. The BCLC staging system constantly evolves to reflect novel trial data, with the most recent guidelines released in 2022 (2). This update included immunotherapy-based systemic regimens as first-line options in advanced-stage HCC and, for the first time, subdivides the intermediate stage to allow for the allocation of such patients to systemic immunotherapy. However, the most important novelty of the 2022 update is the introduction of stage migration (3). This change reflects a clinical reality in which locoregional therapies are increasingly combined with systemic immunotherapies. Along those lines, the recent BCLC iteration expands indications for ablation and transarterial chemoembolization (TACE) and includes transarterial radioembolization (TARE) with yttrium 90 (90Y) in the algorithm (2).
Despite numerous positive trials, immunotherapy remains limited by objective response rates of 20%–30%, and the majority of patients eventually experience disease progression or failure of their disease to respond (4). The underlying resistance mechanisms to immunotherapy are likely multifactorial and remain poorly understood. The presence of these resistance mechanisms has prompted an in-depth investigation of the tumor microenvironment of HCC (5). The theory is that locoregional therapies target and alter the tumor microenvironment by means of local tumor destruction. Thus, these therapies enhance exposure of tumor antigens to antigen-presenting cells, which prime and convert an immunosuppressive tumor microenvironment toward a more potent T-cell response (6). With many clinical trials currently underway, early evidence suggests that combining immunotherapy with locoregional therapy may improve clinical outcomes (7,8).
This review highlights key targetable molecular mechanisms within the tumor microenvironment of HCC, reviews important clinical trials investigating the combination of interventional oncology and immuno-oncology therapies, and summarizes key predictive biomarkers that can be applied toward monitoring immunotherapy and locoregional therapy.
The Tumor Microenvironment of HCC
Although therapy guidelines include immunotherapy for advanced-stage HCC, clinical response rates remain limited. The HCC tumor microenvironment has multiple mechanisms that contribute to immunotherapy resistance in HCC. This section summarizes the features and clinical implications of the HCC tumor microenvironment. This includes the role of locoregional therapy in converting tumors from “cold” to “hot.” “Cold” tumors rarely respond to immunotherapy, whereas “hot” tumors are more responsive to immunotherapy.
Challenges of Direct Antitumor Treatment of HCC
Considerable efforts have been made to characterize the HCC genome (9). Accordingly, HCC has been classified into a proliferative phenotype (characterized by high heterogeneity, invasiveness, and tumor viability) versus a nonproliferative phenotype (characterized by chromosomal stability and hepatocytic differentiation). These phenotypes include six subtypes (G1–G3 within the proliferative class and G4–G6 within the nonproliferative class) (10). Yet, none of the explored genetic alterations offered targetable driver sequence variations (11). Beyond the lack of targetable variations, both intertumoral and intratumoral heterogeneity represent a challenge for drug development. Specifically, somatic variations account for 90% of molecular alterations in the tumor genome. Thus, substantial intertumoral heterogeneity exists among patients with otherwise similar characteristics. Additionally, HCC displays intratumoral heterogeneity with variable cytologic and genetic features within the same tumor. For example, a genomic analysis of 31 HCCs revealed that nodules as small as 0.6 cm exhibit distinct DNA fingerprints in different sampled sectors of the same tumor (12). Such data challenge the role of tissue biopsy for genomic profiling and outline why targeting the tumor microenvironment has proven more effective than targeting the tumor itself.
The Immunosuppressive Tumor Microenvironment Drives Therapy Resistance
Initially, immune checkpoint inhibitor (ICI) monotherapy failed to achieve meaningful survival benefits in HCC compared with sorafenib (negative trials, such as CheckMate 459 [ClinicalTrials.gov identifier NCT02576509] and Keynote-240 [NCT02702401]). These results were an early indicator for underlying resistance mechanisms within an immunosuppressive tumor microenvironment (Table S1) (13,14). With that in mind, drug combinations using the programmed cell death ligand 1 (PD-L1) inhibitor atezolizumab with the vascular endothelial growth factor (VEGF) inhibitor bevacizumab (IMbrave150 trial [NCT03434379]) and the cytotoxic T-lymphocyte–associated protein 4, or CTLA4, inhibitor tremelimumab with the PD-L1 inhibitor durvalumab (HIMALAYA trial [NCT03298451]) were explored. These drug combinations eventually achieved statistically significant improvement in overall survival in randomized phase 3 clinical trials (15,16). These modest advancements achieved overall response rates of approximately 30%, indicative of resistance mechanisms in the tumor microenvironment, with most phenotypes exhibiting immune evasion.
The efficacy of immunotherapy is dependent on the capability of the intrinsic immune system to both infiltrate the tumor and launch an antitumor response. This is counteracted by metabolic features of the tumor microenvironment driven by dysregulated aerobic glycolysis (ie, Warburg effect) with resulting hypercarbic hypoxia and lactic acidosis. The resulting tumor microenvironment acidosis induces T-cell exhaustion and eventually results in immune cell exclusion from the tumor itself (17,18).
Epithelial-to-mesenchymal transition (a biochemical change in which polarized epithelial cells adopt a more invasive and apoptosis-resistant mesenchymal phenotype) is another critical protumorigenic process facilitated by the tumor microenvironment. Epithelial-to-mesenchymal transition is linked to metastatic invasion and poor prognosis. Stimulated by cytokines and growth factors secreted by the tumor, cancer-associated fibroblasts promote a desmoplastic reaction with excess collagen production, resulting in a dense extracellular matrix along with the secretion of tumor-proliferative cytokines and growth factors (19). A dense extracellular matrix exhibits high affinity for leukocyte-associated immunoglobulin-like receptor 1 and may serve as a physical barrier to infiltration of immune cells (20). These and other factors within the tumor microenvironment therefore determine whether a tumor may be susceptible or resistant to immunotherapy.
Immunologic Phenotypes of HCC: “Cold” Versus “Hot”
The convergence of various innate characteristics of the HCC tumor microenvironment hinders both the adequate infiltration and activation of antitumor immune cells, thus limiting the efficacy of immunotherapy. As illustrated in Figure 1, tumors with an inflamed “hot” phenotype are characterized by high T-cell infiltration; increased interferon gamma, or IFN-γ, secretion; and high expression of PD-L1 and are typically more responsive to immunotherapy, with favorable prognosis. Immune-excluded “cold” tumors, characterized by poor cytotoxic T-cell infiltration, low major histocompatibility complex and PD-L1 expression, and immunosuppressive cell populations, rarely respond to immunotherapy (21,22).
Figure 1:
Chart shows the spectrum of the immune status of the hepatocellular carcinoma (HCC) tumor microenvironment, which can be described as expressing a “hot” or “cold” phenotype. Immuno-evasive tumors are characterized by the presence of immunosuppressive cell populations and genotypic pathways that prevent the infiltration of cytotoxic T cells. While some HCCs indeed demonstrate the presence of cytotoxic T cells, immune-exhausted tumors exhibit molecular drivers such as transforming growth factor beta (TGF-β) and immunosuppressive cell populations, rendering these T cells inactive. The immuno-permissive class, characterized by the presence of activated immune cells and inflammatory cytokines, is often encountered in responders to immunotherapy. IFN-γ = interferon gamma, MDSCs = myelodysplastic stem cells, PD-1 = programmed cell death protein 1, PD-L1 = programmed cell death ligand 1, PTK-2 = protein tyrosine kinase 2, Tregs = regulatory T cells.
“Cold” liver tumors exhibit a high aggregation of regulatory T cells in the tumor microenvironment and the peripheral blood. Regulatory T cells consequently suppress CD8+ T-cell proliferation and IFN-γ secretion (23). The “cold” HCC tumor microenvironment can also affect the capacity of tumor antigen–presenting cells to induce apoptosis, therefore blunting the antitumor immune response (24). Furthermore, immunosuppressive liver tumors promote the conversion of M1 macrophages (characterized by antitumor activity) into M2 macrophages (characterized by anti-inflammatory and protumor characteristics), therefore facilitating metastatic invasion (25). Additionally, a “cold” tumor microenvironment promotes the transformation of immature myeloid cells into pro-oncogenic myelodysplastic stem cells that may induce cytotoxic T-cell apoptosis, tumor neoangiogenesis, and epithelial-to-mesenchymal transition (26). Interestingly, HCC with chronic infection of hepatitis B or C is also linked with a “cold” tumor microenvironment (27). Achieving sustained virologic response from direct-acting antiviral agents in patients with hepatitis B or C demonstrates a considerable decrease in the incidence of HCC (28). Notably, in patients with advanced cancer with active infection, there lies a theoretical risk of viral reactivation and possible immune-related liver injury upon the initiation of immunotherapy (29). Thus, treatment of hepatitis B– or C–induced HCC calls for a combination of both direct antiviral therapy and immunotherapy.
The majority of HCCs exhibit this “cold” phenotype. Sia et al (30) analyzed HCC samples from 956 patients, which yielded a classification system based on the immune tumor microenvironment. Less than 30% were classified as “immune” (or immunologically “hot”), exhibiting features such as PD-L1/programmed cell death protein 1 (PD-1) expression, IFN-γ activation, the creation of tertiary lymphoid structures, and immune cell infiltration. Furthermore, this analysis found one-third of the “immune” class to be “immune exhausted” (functionally immunologically “cold”), expressing major immunosuppressive drivers, such as transforming growth factor beta, or TGF-β, signaling, which impair cytotoxic T-cell activity. Only the remaining two-thirds exhibited an immunologically active phenotype (which may be dubbed “immune-permissive”) (30). Given that response to immunotherapy is primarily observed in patients with this “immuno-permissive” HCC phenotype (31), the number of immunotherapy responders may be expanded by converting “cold” tumors into “hot” tumors by disrupting the HCC tumor microenvironment.
Locoregional Therapy and the Tumor Microenvironment
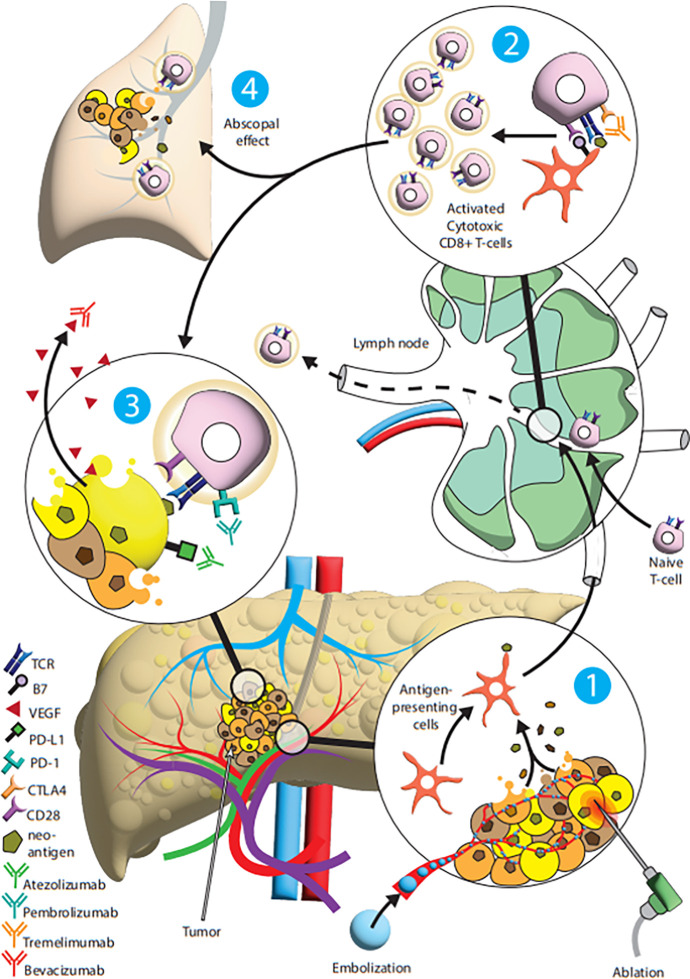
Several preclinical studies have confirmed the use of locoregional therapy as an immunomodulatory instrument to expose tumorigenic antigens, which function as an in situ vaccine for the immune system (6,32,33). This plethora of in situ antigens may aid immune cell infiltration and activation and may even synergize with immunotherapy to aid the immune-mediated tumor destruction (Fig 2) (6).
Figure 2:
Diagram summarizes the synergistic relationship between locoregional therapy and immunotherapy. (1) The tumor undergoes either ablation or chemoembolization, resulting in direct tumor cell death and the subsequent disruption of the tumor microenvironment and the provision of tumor neoantigens for antigen-presenting cells. (2) The antigen-presenting cells travel to lymphatic structures to activate naive cytotoxic T cells. (3) The activated T cells can then attack tumor cells with supplemental immunotherapy to synergistically optimize antitumor response. (4) Furthermore, the combination of immunotherapy with activated T cells may increase the efficacy of immunotherapy on nonablated or nonembolized tumors. CLTA4 = cytotoxic T-lymphocyte–associated protein 4, PD-1 = programmed cell death protein 1, PD-L1 = programmed cell death ligand 1, TCR = T-cell receptor, VEGF = vascular endothelial growth factor.
Ablation and the tumor microenvironment.—Local ablative techniques, including thermal ablation with heat (radiofrequency ablation or microwave ablation), irreversible electroporation, and hypothermal ablation (cryoablation) operate by different mechanisms. However, they all share the goal of inducing tumor necrosis and potentially enhancing the antitumor immune response. Prior studies have shown that thermal ablation yields enhanced antitumor immunogenicity (34). Yet, there is also concern that postablation activation of fibroblasts and heat shock proteins may promote hepatic and systemic tumorigenesis (35). There is evidence that cryoablation may be associated with greater antitumor immune response and synergism with immunomodulation in comparison with radiofrequency ablation (36). It is theorized that cryoablation can induce necrosis at the center of the probe (releasing tumor-associated antigens) while also stimulating apoptosis and the subsequent release of structurally preserved antigen at the margin due to cold-induced mitochondrial injury (rather than hyperthermally induced denaturation in radiofrequency ablation) (32). A study on an HCC murine model treated with incomplete cryoablation plus metalloproteinase inhibition demonstrated increased CD8+ cell infiltration in comparison with tumors treated with either technique alone (37). Irreversible electroporation has also demonstrated immunologic properties (with increased intratumoral and systemic cytotoxic T cells and proinflammatory cytokines, along with decreased regulatory T cells) (38). Irreversible electroporation relies on the induction of nanopores within the tumor cell membrane, resulting in cell necrosis while preserving adjacent structures such as major blood and lymphatic vessels. This results in increased immune cell infiltration along the preserved blood vessels with more robust immunogenic and tumorigenic systemic changes (39). Overall, it remains uncertain which ablative technique confers optimal antitumor efficacy, as each technique likely promotes some proimmunogenic and some protumorigenic pathways. Pairing the appropriate ablative technique with a susceptible tumor microenvironment remains a challenge and will be studied in the future.
Transarterial therapies and their effects on the tumor microenvironment.—TACE has been linked to inducing neoantigenic expansion (an increase in diversity and strength of tumor-specific immune response) (6). Additionally, TACE may reduce intratumoral and peritumoral immune-exhausted T-cell and regulatory T-cell populations while upregulating proinflammatory pathways; these immunologic patterns are associated with improved prognosis in HCC (40). Notably, various TACE regimens (eg, ethiodized oil vs drug-eluting beads) exhibit distinguished histologic and immunologic properties within the embolized tumor. A study in rabbits revealed that TACE with certain drug-eluting beads yielded higher intratumoral T-cell and antigen-presenting cell infiltration compared with conventional TACE with ethiodized oil. However, incorporation of bicarbonate before conventional TACE yielded a significant increase in intratumoral immune cell infiltration (33). Other studies demonstrated that conventional TACE may neutralize intratumoral acidity and decrease hypoxia-inducible factor 1 alpha, or HIF-1α, overexpression (41). Ultimately, it remains unclear which chemoembolic agent will confer the greatest antitumor immunogenicity. However, it appears that means of attenuating embolization-induced hypoxia, acidosis, and angiogenesis may improve patient outcomes, therefore warranting thorough study of TACE regimens with respect to the targeted type of tumor microenvironment.
TARE is also being investigated for its abilities to induce immune-mediated antitumor responses by modulating the tumor microenvironment (6). Radiation therapy is associated with immunogenic tumor cell death and may therefore combat an immunosuppressive tumor microenvironment. Conversely, Chew et al (42) demonstrated that while radioembolization induced an increase in antigen-presenting cells, there was also a high percentage of both PD-1 and T-cell immunoglobulin and mucin domain–containing protein 3, or Tim-3, expression (both markers of T-cell exhaustion) after therapy. Additionally, TGF-β is noted to be increased after radiation exposure. However, Vanpouille-Box et al (43) demonstrated that antibody-mediated TGF-β neutralization plus anti–PD-1 blockade after radiation therapy generated cytotoxic antitumor responses in mice with poorly immunogenic carcinoma. Therefore, radioembolization is associated with driving both immunogenic and tumorigenic processes. There may be great benefit from incorporating immunotherapy with radioembolization to exploit the immune-enhancing effects of radiation therapy while potentially counteracting its simultaneous immunosuppressive effects. However, the tumor microenvironment in the setting of radioembolization remains poorly understood.
Clinical Advances Combining Locoregional Therapy and Immunotherapy
A plethora of preclinical evidence supports a synergistic interaction between immunotherapy and locoregional therapy to overcome the immunosuppressive tumor microenvironment. Numerous clinical trials are underway to investigate the clinical impact behind this combination. The Table summarizes recent clinical trials likely to impact the management of HCC in the coming years. The following section highlights important clinical investigations and considerations regarding the combination of locoregional therapy and immunotherapy.
Recent Important Clinical Trials Advocating for the Integration of Immunotherapy and LRT in the Treatment of HCC
Immunotherapy and Percutaneous Ablation
Given the relatively high rates of recurrence of HCC in patients undergoing resection or ablation (5-year recurrence rates as high as 80%), there is great interest in investigating the potential benefit of adjuvant immunotherapy after ablation with curative intent. To date, IMbrave050 (NCT04102098) (while ongoing) is the only phase 3 randomized clinical trial that has published conclusive results. Initial interim analysis (after a median duration of follow-up of 17.4 months) met its primary end point of improved recurrence-free survival with the incorporation of 51 weeks of adjuvant atezolizumab plus bevacizumab in patients with surgically resected or ablated HCC with high risk of recurrence (hazard ratio, 0.72 [95% CI: 0.53, 0.98]). High-risk features included the presence of multiple tumors and tumors larger than 2 cm in the ablation arm (8). However, a second interim analysis (conducted after a median follow-up time of 35.1 months) demonstrated this initial benefit was not sustained (33.2 months vs 36.0 months; hazard ratio, 0.90 [95% CI: 0.72, 1.12]). Although overall survival data remain immature and the safety profile of the adjuvant therapy remained manageable, the current benefit-risk profile does not support the addition of ICI plus VEGF inhibition to the treatment of patients, even in the case high-risk of recurrence (44).
It could be inferred that the addition of adjuvant immunotherapy may counteract recurrence only for the duration of systemic therapy while not exhibiting any long-lasting protection from developing recurrent disease. Additional ongoing clinical trials are underway to investigate other various regimens of immunotherapy after ablation or resection (such as those listed in Table S2) (45). At this point, it is uncertain whether adjuvant immunotherapy will be integrated into the management of very early or early HCC to further reduce risk of recurrence.
Immunotherapy and TACE
Several preclinical studies have established the rationale for pursuing clinical trials combining TACE with immunotherapy (46,47). To date, EMERALD-1 (NCT03778957) is the first and so far only positive phase 3 randomized clinical trial to our knowledge to report a significant increase in progression-free survival when combining TACE with ICI monotherapy plus VEGF inhibitors versus TACE alone in intermediate-stage HCC (BCLC B) (15.0 vs 8.2 months). Notably, the group treated with ICI monotherapy plus TACE without VEGF inhibition did not show statistically longer progression-free survival than patients treated with TACE alone (10.0 months) (7). While not yet mechanistically understood, overall trial evidence strongly suggests that combining VEGF inhibition with ICIs improves clinical outcomes. Notably, the treatment group experienced a greater number of grade 3 or 4 adverse events than the active surveillance group (32.5% vs 13.5%); however, adjuvant therapy was overall well tolerated.
At this time, several additional phase 3 trials aim to establish the combination of TACE with various immunotherapy regimen, such as those listed in Table S3 (48). It is likely that such trial data will be practice-changing for the therapy of select patients with intermediate-stage HCC (BCLC B). Currently, open questions include optimal sequencing of administration of the combination, immunotherapeutic choice, and specific patient selection; however, the optimal pharmacologic treatment regimen has yet to be determined.
The choice of the chemoembolic regimen remains an additional important and currently underinvestigated topic. As such, the readout from EMERALD-1 did not show any differences between conventional TACE and TACE with drug-eluting beads when each was combined with immunotherapy. However, a retrospective study conducted by Ren and Guo et al (49) demonstrated increased progression-free survival in patients who underwent TACE with drug-eluting beads plus camrelizumab compared with conventional TACE plus camrelizumab. More comparative trials investigating immunotherapy combined with TACE are warranted. To isolate and characterize the immunogenic profiles associated with each type of TACE, these future trials should avoid indiscriminate mixing of various chemoembolic regimens within the same patient cohorts.
Immunotherapy and TARE
Ionizing radiation can enhance the effects of immunotherapy in both irradiated and unirradiated tumors (50). Accordingly, multiple clinical trials investigating the synergies between immunotherapy and TARE in HCC are currently underway (such as those listed in Table S4). While no phase 3 randomized controlled trials have been conducted to date, to our knowledge, early phase 2 trial data by De la Torre-Aláez and Matilla et al (51) (NASIR-HCC trial, NCT03380130) revealed that combining adjuvant nivolumab with TARE was safe and effective, with an overall response rate of 41%. However, overall survival as the secondary end point was reported as 20.9 months, which does not exceed comparable outcomes with TARE alone in similar patients with intermediate stage HCC (BCLC B). Numerous prospective phase 2 trials, such as the EMERALD-Y90 trial (NCT06040099), should provide definitive data. As for the BCLC staging system, TARE has already assumed a key role across multiple BCLC stages in clinical practice, particularly addressing shortcomings in the treatment of locally advanced disease with tumor portal venous thrombosis, and is already widely used in combination with immunotherapy.
Proper dosimetry of 90Y remains a major challenge when combining radiation therapy with immunotherapy, particularly for highly potent radiation segmentectomy. As such, ablative segmentectomy doses of more than 400 Gy to a perfused volume may negatively affect the infiltrating immune cells, reducing any combination of 90Y with immunotherapy to additive rather than synergistic effects. Similar to TARE, hypofractionated external beam radiation therapy is also under investigation regarding its potency for increasing the immunogenicity of HCC (52). However, prospective trial data are currently limited to niche cohorts and mostly reported in small phase 1 and 2 clinical trials without sufficiently large control groups to warrant a change in guidelines or clinical practice (53). Alternatively, high-dose-rate brachytherapy, entailing the CT-guided temporary percutaneous insertion of a radioablative iridium 192 source within a tumor. Addressing limitations of other ablative techniques (such as tumor size restrictions, heat sink effect, and proximity of temperature sensitive structures), brachytherapy presents a considerable alternative for the treatment of unresectable HCC. Although a multicenter retrospective cohort study published by Auer et al (54) demonstrated improved overall survival and progression-free survival in comparison with TACE, randomized data further exploring therapeutic superiority (and importantly, its interaction with immunotherapy) remain scarce.
Timing of Locoregional Therapy and Immunotherapy
Optimal sequencing of locoregional therapy with immunotherapy will determine whether the effects of the combination are merely additive or truly synergistic. Various regimen approaches are currently being investigated. Figure 3 highlights some examples (55). Most trials (eg, the EMERALD-1 study) are investigating a “sequential” approach in which patients undergo locoregional therapy to induce initial tumor response that generates tumor antigen and then undergo immunotherapy. This approach is thought to benefit from an altered tumor microenvironment and activated cytotoxic T cells. This approach also emphasizes safety and minimizes risks for adverse events by avoiding overlapping two high-potency therapies. However, the lack of overlap also limits true synergies that are less likely to evolve given the temporal gap between the therapeutic mechanisms.
Figure 3:
Chart shows various locoregional therapy (LRT) plus immunotherapy treatment regimens that may be considered in future trials to optimize the synergistic antitumor activity between both treatment strategies. The sequential schedule (adjuvant) is the initiation of one cycle of locoregional therapy followed by immunotherapy without additional locoregional therapy planned. The interrupted schedule (adjuvant and neoadjuvant) is the administration of a priming dose of immunotherapy followed by locoregional therapy. The patient will then continue systemic therapy without additional locoregional therapy planned. The continuous schedule (overlapping) is the initiation of immunotherapy, and the patient may then be treated with several locoregional therapy sessions depending on recurrence while continuing systemic therapy. IRE = irreversible electroporation, TACE= transarterial chemoembolization, TME = tumor microenvironment. Figure outline adapted, with permission, from reference 55.
An “interrupted” regimen includes “neoadjuvant” immunotherapy before locoregional therapy, followed by “adjuvant” immunotherapy, without any overlap between therapies. The mechanistic principles backing this approach suggest that priming the tumor microenvironment with immunotherapy before locoregional therapy may activate cytotoxic T cells to the released tumor antigen. The consecutive “adjuvant” component is thought to provide therapy completion, helping eradicate persistent microscopic disease after locoregional therapy, even in patients with complete response (55). Evidence from surgical trials supports the administration of immunotherapy within 3–5 days of resection for optimal efficiency (56). The interruption of immunotherapy for the locoregional therapy session is based on safety considerations, similar to the sequential regimen. Accordingly, trials such as NIVOLEP (NCT03630640) are designed to assess the efficacy of adjuvant and neoadjuvant immunotherapy in patients with early-stage HCC (BCLC A) undergoing irreversible electroporation (57).
In contrast, “continuous” trial designs initiate therapy with immunotherapy followed by multiple treatments based on degree and change in tumor burden (eg, NCT01853618, combining TACE with dual-ICI therapy followed by additional as-needed TACE sessions in the event of residual tumor). This strategy includes “on-demand” rather than scheduled locoregional therapy procedures in the context of uninterrupted immunotherapy. This approach focuses on maximizing tumor response by enhancing continuous immunotherapy with the synergistic effects of repeated locoregional therapy as clinically indicated. This high-potency approach may carry potential risks of additive adverse events (58).
Therapy Combinations in Advanced-Stage HCC
Currently, BCLC guidelines do not endorse locoregional therapy alone for advanced HCC, particularly in the setting of macrovascular invasion. However, some clinical studies are investigating the inclusion of locoregional therapy for debulking as well as increasing tumor antigen presentation to enhance the effects of systemic immunotherapy. This is supported by occasionally encountered abscopal effects in which nontargeted lesions demonstrate response to locoregional therapy (Fig 4). Accordingly, Duffy et al (59) conducted a trial in 32 patients with advanced HCC (BCLC C) that demonstrated that combining ablation with tremelimumab increased the level of intratumoral CD8+ T cells and boosted objective response beyond the ablated tumor (NCT01853618). Lyu, Kong, and Li et al (60) demonstrated that heat-based thermal ablation in addition to nivolumab or pembrolizumab for sorafenib-resistant advanced HCC increases response rates and improves overall survival (NCT03939975). For embolotherapies, the phase 3 LAUNCH trial (NCT03905967) demonstrated that TACE combined with lenvatinib (a tyrosine kinase inhibitor) can improve clinical outcomes in advanced HCC (61). Accordingly, combining TACE with immunotherapy in advanced HCC is being considered. A retrospective analysis comparing patients with advanced HCC who underwent TACE with ICI monotherapy versus ICI monotherapy alone demonstrated an increased progression-free survival and median overall survival (62). Another retrospective review of 142 patients with advanced HCC who underwent TARE combined with immunotherapy versus 1522 patients who underwent immunotherapy alone collected from the National Cancer Database demonstrated significantly increased median overall survival (19.8 vs 9.5 months) (63). Overall, it is likely that future BCLC updates will include a component that allows for patients with advanced HCC to undergo stage migration toward combinations of locoregional therapy with systemic immunotherapy.
Figure 4:
(A) Coronal and (B) axial contrast-enhanced MRI scans of the abdomen in a 74-year-old male patient show left liver–predominant biopsy-proven poorly differentiated hepatocellular carcinoma. Portal venous thrombus and a left retroperitoneal nodule concerning for metastasis (arrow) are also seen. The patient underwent transarterial radioembolization with yttrium 90. (C) Coronal and (D) axial follow-up MRI scans demonstrate near-complete resolution of the hepatic primary along with resolution of the metastatic retroperitoneal nodule, indicative of abscopal reaction (arrow). The patient became a candidate for and successfully underwent left hepatectomy for residual disease.
Advanced Therapeutic Considerations
Local delivery of novel immunotherapeutic agents is also under investigation (example trials are listed in Table S5). Chimeric antigen receptor, or CAR, T-cell therapy has emerged as a promising treatment for HCC. CAR T cells are not major histocompatibility complex restricted and can bind to tumor-associated antigens and enhance immune responses (64). Glypican-3, a proteoglycan cell surface anchor, has demonstrated overexpression in 90% of poorly differentiated HCC, making it an ideal immune target for CAR T cells. Two trials investigating the local intra-arterial delivery of anti–glypican-3 CAR T cells are currently underway (NCT02715362 and NCT02959151), with initial results expected in 2026 (65). Similarly, adjuvant administration of cytokine-induced CD8+ T cells in resected or ablated HCC increased recurrence-free survival in a small cohort (66). Additionally, oncolytic viruses have been investigated as in situ vaccines, selectively infecting tumor cells and replicating within them, eventually inducing cell lysis and the consequent release of tumor-associated antigens (67). While early clinical trials investigating a high dose of the oncolytic virus Pexa-Vec (pexastimogene devacirepvec or JX-594) demonstrated some antitumor efficacy, the phase 3 trial (NCT02562755) was terminated early due to lack of tumor response (68). Ongoing trials such as MASTERKEY-318 (NCT02509507) are investigating the utility of direct intratumoral injection of oncolytic viruses in combination with immunotherapy (69).
Future advances such as bispecific antibodies, which can potentiate unique strong immune-enhancing effects by means of either tumor-targeted immunomodulation (specific to tumor antigen and CD40) or dual immunomodulation (specific to PD-1 and cytotoxic T-lymphocyte–associated protein 4), are under early clinical investigation and may be combined with established ICIs, anti-VEGF therapies, and even locoregional therapy to enhance antitumor efficacy while minimizing toxicity (70).
Tumor Response Assessment and Predictive Biomarkers
To date, the Liver Imaging Reporting and Data System (LI-RADS) is the only internationally unified diagnostic reporting system for HCC. Standardized criteria such as Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 are mandated by the U.S. Food and Drug Administration for the assessment of imaging outcomes in clinical trials (71,72). However, most assessment criteria do not currently consider response patterns characteristic of immunotherapy or locoregional therapy. This section outlines existing concepts and biomarkers for the assessment of response and progression of HCC treated with combination therapies.
Imaging Characteristics of HCC Treated With Locoregional Therapy
The majority of HCC can be diagnosed using multiphasic contrast-enhanced imaging according to the primary features of arterial phase hyperenhancement and portal venous or delayed phase washout, as codified in LI-RADS for eligible patients. In some cases, auxiliary features such as T2 signal appearance and threshold growth allow the upgrade of suspicious lesions to be diagnosed as HCC even if some primary features are absent. While diagnosis with imaging alone has benefitted patient safety, the lack of biopsy samples makes HCC histologically poorly understood, with only very few biomarkers available for radiologic-pathologic validation. Auxiliary imaging features such as nonsmooth tumor margins, peritumoral hyperenhancement, no intratumoral fat, hepatobiliary hypointensity, and tumor in vein favor poor prognosis and high risk of recurrence or metastatic spread (73). However, they remain poorly correlated with the underlying histopathologic phenotypes. Findings indicative of tumor response include decrease in tumor size, T1 hyperintensity (indicative of hemorrhagic necrosis), reduction of signal at diffusion imaging, and loss of arterial phase hyperenhancement in the locoregional therapy treatment zone. Although a thin rim of enhancement along the ablation or embolization margin is representative of expected transient posttherapy hyperemia, foci of nodular enhancement should be concerning for residual or recurrent tumor (74). The timing of tumor response assessment after locoregional therapy is of central importance. Traditional response criteria, such as RECIST (which primarily depends on lesion size reduction) and modified RECIST, or mRECIST (which aims to specifically measure viable disease), may demonstrate visible changes as early as 1 month after therapy with TACE or ablation; however, radiation-induced tumor necrosis and shrinkage is often delayed. This delay makes size measurements within 3 months of TARE often unreliable. In fact, radiologists should be aware that transient increase in size and prolonged arterial enhancement are often seen on the first set of follow-up images (75). Examples of patients with LI-RADS 5 lesions who underwent therapy and experienced both response and recurrence are provided in Figures 5–7.
Figure 5:
(A) Contrast-enhanced abdominal MRI scan in a 72-year-old male patient with hepatitis C and alcohol-induced cirrhosis shows two arterially enhancing lesions (arrows) on axial section with portal venous washout (not shown), concordant with Liver Imaging Reporting and Data System 5 (LR 5) lesions smaller than 3.0 cm. (B) Axial CT image acquired during CT-guided microwave ablation shows the ablation probes positioned within the tumors (arrows). (C) Follow-up axial abdominal MRI scan demonstrates absence of enhancement within the ablation zone (arrows) consistent with nonviable tumor; (D) however, surveillance MRI scan shows that the patient developed two new arterially enhancing lesions (arrows) with portal venous washout (not shown) larger than 3.0 cm, both indicative of LR 5 lesions. The patient underwent transarterial chemoembolization of both new lesions, with (E) transcatheter subtraction angiogram demonstrating arterial blush of the tumor in comparison with the liver background (arrow). (F) Subsequent abdominal axial MRI scan demonstrates absence of enhancement within the tumor (arrows), indicative of nonviable tumor. The patient eventually developed additional lesions requiring initiation of bevacizumab and atezolizumab. The patient’s disease remained stable up until his death, likely from sequelae of decompensated cirrhosis.
Figure 7:
(A, B) Initial multiphase axial CT images in a 65-year-old male patient with presumed metabolic dysfunction–associated fatty liver disease–induced cirrhosis decompensated by biopsy-proven moderately differentiated hepatocellular carcinoma show a central hepatic mass with (A) heterogeneous arterial enhancement and (B) portal venous washout consistent with Liver Imaging Reporting and Data System 5 (arrows). The patient underwent repeated conventional transarterial chemoembolization procedures with ethiodized oil; however, despite multiple procedures, (C, D) axial sections from a follow-up abdominal MRI examination show persistent arterial enhancement and tumor size indicative of viable tumor with extension into the portal veins, hepatic veins, and inferior vena cava (arrows). The patient was initiated on atezolizumab and bevacizumab. (E, F) Axial sections from subsequent MRI examinations demonstrate decrease in size of tumor burden along with decreased arterial enhancement indicative of tumor response (arrows). The tumor venous thrombus eventually progressed, and the patient is currently undergoing a new clinical trial treatment regimen.
Figure 6:
(A, B) Axial abdominal MRI scans in a 65-year-old female patient with hepatitis C–induced cirrhosis decompensated by hepatocellular carcinoma show (A) an arterially enhancing lesion with portal venous washout with (B) pseudocapsule appearance consistent with Liver Imaging Reporting and Data System 5 lesion larger than 3.0 cm in segment IVb (arrows). (C–E) After the patient underwent conventional transarterial chemoembolization (with ethiodized oil) of this lesion, axial sections from a subsequent MRI examination demonstrate (C) involution of the lesion, along with lack of enhancement (arrow) indicative of no residual tumor; however, (D) a new arterially enhancing lesion with (E) restricted diffusion smaller than 0.3 cm was discovered, concerning for recurrent viable tumor (arrows). (F) Axial CT image shows gas formation during active hyperthermal ablation at the distal end of the probe (arrow) when the patient underwent CT-guided microwave ablation. (G–I) Axial sections from a subsequent MRI examination demonstrate lack of enhancement within the ablation zone. Notably, the presence of postprocedural hemorrhagic products at noncontrast T1-weighted MRI (not shown) make it critical to confirm on enhancement subtraction images that any signal on the (G) contrast-enhanced MRI scan is attributable to true enhancement within the ablation zone (arrow), which could reflect recurrence. Further follow-up images show (H) a new large central arterially enhancing lesion (arrow) with (I) washout overlying the portal vein (arrow), consistent with tumor-in-vein or macrovascular invasion, a poor prognostic indicator requiring the patient to undergo atezolizumab and bevacizumab. The patient eventually died of ascending cholangitis complicated by Klebsiella bacteremia likely attributable to biliary obstruction.
Preclinical efforts to characterize the effects of locoregional therapy on the tumor microenvironment with molecular imaging techniques have also proven feasible. These include noninvasive tumor pH and extracellular matrix mapping (41) and immune cell molecular probes (such as superparamagnetic iron oxide nanoparticles and gadolinium-labeled CD68 antibodies) (76). Yet, such methods serve niche experimental applications with limited value for clinical practice.
Emerging Imaging Concepts of Immunotherapy in HCC
The Food and Drug Administration continues to mandate the use of methods such as RECIST for reporting of clinical trial outcomes and therefore only accepts the reduction in tumor size as a reliable metric for tumor response. Classic response patterns to immunotherapy are illustrated in Figures 8 and 9. RECIST and mRECIST, however, may not initially reflect some less common response patterns observed in patients treated with immunotherapy (77). Noteworthy treatment changes such as pseudoprogression, dissociated response, and hyperprogression are detailed in Figure 10 (78,79). Accordingly, modifications such as immune-related RECIST (or irRECIST) (2013) and immune RECIST (or iRECIST) (2017) have been developed to include such immune-related response patterns; although similar, subtle differences between these two methods are present. Most notably, iRECIST incorporated a designation of unconfirmed progression in new lesions (specifically lymph nodes) and even nontarget lesions to account for phenomena such as pseudoprogression (80). In consideration of these encountered response phenomena, experts concluded that immunotherapy surveillance should be conducted every 6–12 weeks upon using iRECIST (as conducted with RECIST 1.1); however, upon encounter of unconfirmed disease progression, subsequent follow-up imaging may be repeated within 4–8 weeks to confirm progression (81). Yet, no individual method has been validated with the combination of locoregional therapy and immunotherapy in mind. Therefore, this makes the assessment of tumor response outcomes in trials such as EMERALD-1 very challenging (6).
Figure 8:
(A, B) Baseline axial abdominal MRI scans in a 76-year-old male patient with hepatitis C–induced cirrhosis complicated by hepatocellular carcinoma show (A) an arterially enhancing lesion in segment VIII (arrow), with (B) washout and pseudocapsule on portal venous phase image (arrow) consistent with a Liver Imaging Reporting and Data System 5 (LR 5) lesion. (C) Axial chest CT image shows pulmonary nodules concerning for metastatic disease (arrowheads). The patient underwent systemic therapy with atezolizumab and bevacizumab. The patient’s initial LR 5 lesion (arrows) decreased in size and degree of enhancement over the course of (D, E, G, H) two subsequent surveillance abdominal MRI examinations. (F, I) Tandem surveillance chest CT examinations demonstrate resolution of the patient’s initial pulmonary nodules (arrowheads). (Findings are consistent with partial response as per immune Response Evaluation Criteria in Solid Tumors.)
Figure 9:
Axial images in a 61-year-old female patient without history of underlying liver disease presented with multifocal hepatocellular carcinoma with peritoneal metastases at abdominal CT (not shown) with a serum α-fetoprotein level of 404 584 ng/mL. The patient was started on sorafenib; however, due to bowel perforation, systemic chemotherapy was halted. The patient was started on the programmed cell death protein 1, or PD-1, inhibitor nivolumab. (A) Baseline abdominal MRI scan acquired before first dose of nivolumab shows multiple arterial-enhancing lesions (arrows). Subsequent follow-up images from (B) CT at 6 months, (C) CT at 12 months, (D) MRI at 24 months, (E) MRI at 3 years, and (F) MRI at 5 years show decrease in size of the intrahepatic lesions along with complete loss of arterial enhancement (arrows), representing nonviable tumor. The patient’s peritoneal metastases also resolved (not shown), consistent with complete response per modified Response Evaluation Criteria in Solid Tumors, or mRECIST. The patient currently remains on immunotherapy and maintains negligible α-fetoprotein levels.
Figure 10:
Diagram shows various tumor response types observed in patients undergoing immunotherapy. In addition to the classic appearance of decrease in tumor size indicative of tumor response, additional atypical response patterns can be observed upon the initiation of immunotherapy. To avoid inaccurate conclusions and improper treatment regimen changes, radiologists must be aware of these infrequent phenomena and the clinical implications behind them.
While CT remains the most widely used imaging modality for the assessment of tumor response to immunotherapy, most novel developments in response assessment are MRI-based and have shown potential in the prediction of therapy outcome. For instance, blood oxygen level–dependent, dynamic contrast-enhanced, and diffusion-weighted MRI may be used to detect signs of tumor microenvironment hypoxia and angiogenesis (82). Stable or increasing apparent diffusion coefficients at follow-up imaging predict therapeutic benefit from immunotherapy (83). Furthermore, hepatocyte-specific contrast agents, such as gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (ie, Gd-EOB-DTPA), have been successfully used to assess density of tumor-infiltrating T cells, facilitating the development of predictive immunoscores (84).
PET/CT may also provide valuable information during immunotherapy. While fluorodeoxyglucose (or FDG) uptake can be used to assess metabolic response, particularly in the setting of poorly differentiated HCC with unchanged tumor size, and help identify immunotherapy-related adverse events (85), wide variability in glucose metabolism of HCC (with many HCCs exhibiting low FDG uptake) limits its diagnostic accuracy (86). Due to the limitations of FDG PET/CT for assessing HCC, other tracers are currently under consideration. Using gallium 68 (68Ga) prostate-specific membrane antigen PET/CT to assess HCC-associated neovascularization can improve diagnostic accuracy and even be used to evaluate degree of angiogenesis (86). 68Ga-labeled inhibitors targeting cancer-associated fibroblast activation protein have demonstrated superior sensitivity and accuracy in the detection of viable HCC in comparison with FDG. The uptake of these fibroblast activation protein inhibitors is not associated with tumor differentiation but rather with aggressive fibroblastic behavior of the tumor microenvironment (87). Zirconium 89–conjugated antibodies against glypican-3 are also under investigation; however, suboptimal imaging pharmacokinetics and poor tumor penetration limit their ability to overcome background liver activity (88). Molecular probes targeting PD-1, PD-L1, and CD8 are under investigation for assessing the immune cell infiltration into the tumor microenvironment after immunotherapy (79).
Artificial Intelligence in the Detection and Characterization of HCC
The integration of artificial intelligence is projected to enhance the clinical management of HCC by being applied to tasks including tumor detection, lesion annotation, diagnosis, and response assessment. Preliminary deep learning studies have shown that artificial neural networks can accurately distinguish HCC from various other liver tumor types with accuracy as high as 92% (89). Additionally, they may be trained to perform automated segmentation of tumors versus background liver to not only assist in treatment planning, but even assess features such as shape and texture to predict responders to locoregional therapy (90). These models can also receive input on baseline demographic characteristics, liver function parameters, and relevant comorbidities in addition to tumor size to predict patient survival after locoregional therapy (91). While incorporation into real-world clinical practice remains a challenge, the ability of artificial intelligence to provide insightful information (often beyond human capacity) would greatly assist in guiding treatment.
Cellular and Molecular Biomarkers of Immunotherapy Response
The relative paucity of diagnostic tissue samples in HCC has delayed the development of reliable tissue-derived biomarkers of immunotherapy response. Despite our ability to diagnose HCC with use of LI-RADS, biopsy sampling is now increasingly pursued at tertiary care centers and is critical in the context of clinical trials that involve novel methodologic developments in digital pathology. Specifically, conventional histopathologic analysis can now be supplemented with deep learning models and automated computer-generated architectural phenotyping to facilitate tumor microenvironment characterization. Automated interpretation of tissue has the capacity to recognize complex patterns linked to specific sequence variations and disease prognosis (92). In addition, artificial intelligence models can be applied to detect immune or inflammatory gene signatures and conduct spatial analysis of noncancerous immune cells in the tumor microenvironment such as intratumoral lymphocytes and endothelial cells to predict positive responders to immunotherapy (93,94). This degree of histologic analysis may provide valuable prognostic and treatment-guiding data in the management of HCC.
“Liquid biopsy” or biomarkers in the peripheral blood are of growing interest for assessing and predicting response to immunotherapy. For instance, a decrease in neutrophil to lymphocyte ratio has been associated with improved patient outcomes in various cancers treated with immunotherapy, including in HCC (95). Winograd et al (96) found that circulating PD-L1+ tumor cells were associated with favorable objective response to immunotherapy. Conversely, their study also described a lower overall survival in such patients when compared with those without circulating PD-L1+ tumor cells. These data confirm that a high concentration of circulating tumor cells is an overall negative predictor and that patients with PD-L1+ circulating tumor cells will benefit from targeted systemic immunotherapy. Furthermore, given its association with tumor proliferation and immune cell exhaustion, TGF-β may serve as a predictive biomarker of response to immunotherapy and overall survival (97). Additionally, a study investigating circulating tumor DNA demonstrated that increased levels were associated with greater tumor burdens, and patients who developed undetectable levels after treatment demonstrated longer progression-free survival (98). Other serologic biomarkers that may be associated with increased tumorigenic response after radiofrequency ablation include Ki-67, hepatocyte growth factor, VEGF, angiopoietin, and miR-21 (99,100). Despite these discoveries, no robust tissue- or blood-based biomarker capable of predicting response to immunotherapy has been identified. Therefore, there is an unmet clinical need to establish a means of identifying responders to immunotherapy, given the high number of patients with HCC with “cold” tumors.
Conclusion and Future Outlook
Although the advent of immunotherapy has demonstrated promising prospects for the treatment of hepatocellular carcinoma (HCC), clinical data indicate that efficacy remains modest, with limited response in many patients. Specific mechanisms that confer therapeutic resistance are not well understood due to the substantial tumor heterogeneity and the complexity of its tumorigenesis. However, the ongoing characterization of the immunosuppressive components of the tumor microenvironment provides multiple mechanisms that contribute to immunotherapy resistance. Early mechanistic evidence supports that the integration of locoregional therapy with immunotherapy may modulate the immunosuppressive tumor microenvironment and consequently convert immunologically “cold” tumors into “hot” tumors. At this point, pivotal clinical trials are underway that will provide clinical data regarding the impact of combining various locoregional therapies with immunotherapy across several stages of HCC. Critical considerations hold precedent for future investigations on this potential synergistic combination. For ablation, choice of technique will be key in light of the different immunomodulating and tumorigenic effects on tumor microenvironment, along with the clinical application of either neoadjuvant or adjuvant combinations. For transarterial chemoembolization, trials discerning identification of proper embolic material will be necessary. Moreover, combination with immunotherapy will likely become standard and will require the selection of a proper sequencing of the combination. For radioembolization, the establishment of optimal dosimetry will be essential to optimize the benefits of incorporating immunotherapy. Finally, to properly guide the assessment of the therapeutic value of locoregional therapy and immunotherapy, future methods to address the unmet and urgent need for applicable biomarkers and appropriate imaging protocols for response prediction are warranted. Ultimately, while much is to be understood regarding achieving ideal synergism of immunotherapy and locoregional therapy, current data and investigations suggest that the combination of interventional oncology and immuno-oncology across all Barcelona Clinic Liver Cancer stages of HCC is here to stay.
Acknowledgments
Acknowledgments
Mishal Mendiratta-Lala, MD, is an abdominal radiologist at the University of Michigan with numerous contributions toward researching liver tumor ablation and imaging liver tumor response. Dr Mendiratta-Lala has graciously provided images from a case at her institution that demonstrate imaging features of response of hepatocellular carcinoma to immunotherapy. William Baker, BS, is a 1st-year medical student at the Yale School of Medicine. Having prior experience with medical illustrations, Mr Baker was recruited to assist in the construction of artist-rendered illustrations for the submitted manuscript.
Disclosures of conflicts of interest: R.B. No relevant relationships. R.S. Consulting fees from AstraZeneca, Boston Scientific, Cook Medical, Bard, Genentech, Eisai, Siemens, and Merck. R.F. Grants to institution from Adaptimmune, Bristol Myers Squibb, Eisai, Eli Lilly, Pfizer, Novartis, Merck, and Roche/Genentech; consulting fees and support for travel from AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Exelixis, Eli Lilly, Merck, Pfizer, Novartis, and Roche/Genentech; payment for lectures from Genentech; participation on a data safety monitoring board or advisory board for AstraZeneca. T.F.G. No relevant relationships. S.N.G. Grant to institution from the Israel Science Foundation; consulting fees from Cambridge Interventional and consulting fees to institution from CAPS Medical and XACT Robotics. J.C. Grants from the National Institutes of Health, Society of Interventional Oncology, Philips, and Boston Scientific; consulting fees from AstraZeneca, Bayer, Guerbet, Eisai, and Genentech.
Abbreviations:
- BCLC
- Barcelona Clinic Liver Cancer
- HCC
- hepatocellular carcinoma
- ICI
- immune checkpoint inhibitor
- LI-RADS
- Liver Imaging Reporting and Data System
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed cell death ligand 1
- RECIST
- Response Evaluation Criteria in Solid Tumors
- TACE
- transarterial chemoembolization
- TARE
- transarterial radioembolization
- VEGF
- vascular endothelial growth factor
References
- 1. Singal AG , Kanwal F , Llovet JM . Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy . Nat Rev Clin Oncol 2023. ; 20 ( 12 ): 864 – 884 . [DOI] [PubMed] [Google Scholar]
- 2. Reig M , Forner A , Rimola J , et al . BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update . J Hepatol 2022. ; 76 ( 3 ): 681 – 693 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vitale A , Trevisani F , Farinati F , Cillo U . Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy . Hepatology 2020. ; 72 ( 6 ): 2206 – 2218 . [DOI] [PubMed] [Google Scholar]
- 4. Ruf B , Heinrich B , Greten TF . Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells . Cell Mol Immunol 2021. ; 18 ( 1 ): 112 – 127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llovet JM , Castet F , Heikenwalder M , et al . Immunotherapies for hepatocellular carcinoma . Nat Rev Clin Oncol 2022. ; 19 ( 3 ): 151 – 172 . [DOI] [PubMed] [Google Scholar]
- 6. Erinjeri JP , Fine GC , Adema GJ , et al . Immunotherapy and the interventional oncologist: challenges and opportunities—a Society of Interventional Oncology White Paper . Radiology 2019. ; 292 ( 1 ): 25 – 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lencioni R , Kudo M , Erinjeri J , et al . EMERALD-1: a phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization . J Clin Oncol 2024. ; 42 ( 3 suppl ): LBA432 . [Google Scholar]
- 8. Qin S , Chen M , Cheng AL , et al . Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial . Lancet 2023. ; 402 ( 10415 ): 1835 – 1847 . [DOI] [PubMed] [Google Scholar]
- 9. Hytiroglou P , Bioulac-Sage P , Theise ND , Sempoux C . Etiology, pathogenesis, diagnosis, and practical implications of hepatocellular neoplasms . Cancers (Basel) 2022. ; 14 ( 15 ): 3670 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calderaro J , Ziol M , Paradis V , Zucman-Rossi J . Molecular and histological correlations in liver cancer . J Hepatol 2019. ; 71 ( 3 ): 616 – 630 . [DOI] [PubMed] [Google Scholar]
- 11. Llovet JM , Ricci S , Mazzaferro V , et al. ; SHARP Investigators Study Group . Sorafenib in advanced hepatocellular carcinoma . N Engl J Med 2008. ; 359 ( 4 ): 378 – 390 . [DOI] [PubMed] [Google Scholar]
- 12. Sirivatanauksorn Y , Sirivatanauksorn V , Bhattacharya S , et al . Evolution of genetic abnormalities in hepatocellular carcinomas demonstrated by DNA fingerprinting . J Pathol 1999. ; 189 ( 3 ): 344 – 350 . [DOI] [PubMed] [Google Scholar]
- 13. Yau T , Park JW , Finn RS , et al . Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial . Lancet Oncol 2022. ; 23 ( 1 ): 77 – 90 . [DOI] [PubMed] [Google Scholar]
- 14. Finn RS , Ryoo BY , Merle P , et al. ; KEYNOTE-240 investigators . Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial . J Clin Oncol 2020. ; 38 ( 3 ): 193 – 202 . [DOI] [PubMed] [Google Scholar]
- 15. Finn RS , Qin S , Ikeda M , et al. ; Imbrave150 Investigators . Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma . N Engl J Med 2020. ; 382 ( 20 ): 1894 – 1905 . [DOI] [PubMed] [Google Scholar]
- 16. Abou-Alfa GK , Chan SL , Kudo M , et al . Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA . J Clin Oncol 2022. ; 40 ( 4 suppl ): 379 . [Google Scholar]
- 17. Calcinotto A , Filipazzi P , Grioni M , et al . Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes . Cancer Res 2012. ; 72 ( 11 ): 2746 – 2756 . [DOI] [PubMed] [Google Scholar]
- 18. Huber V , Camisaschi C , Berzi A , et al . Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation . Semin Cancer Biol 2017. ; 43 : 74 – 89 . [DOI] [PubMed] [Google Scholar]
- 19. Sas Z , Cendrowicz E , Weinhäuser I , Rygiel TP . Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for new treatment options . Int J Mol Sci 2022. ; 23 ( 7 ): 3778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy AM , Iyer R , Chakraborty S . The extracellular matrix in hepatocellular carcinoma: mechanisms and therapeutic vulnerability . Cell Rep Med 2023. ; 4 ( 9 ): 101170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu YT , Sun ZJ . Turning cold tumors into hot tumors by improving T-cell infiltration . Theranostics 2021. ; 11 ( 11 ): 5365 – 5386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao Q , Qiu SJ , Fan J , et al . Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection . J Clin Oncol 2007. ; 25 ( 18 ): 2586 – 2593 . [DOI] [PubMed] [Google Scholar]
- 23. Chen KJ , Lin SZ , Zhou L , et al . Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis . PloS One 2011. ; 6 ( 9 ): e24671 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kotsari M , Dimopoulou V , Koskinas J , Armakolas A . Immune system and hepatocellular carcinoma (HCC): new insights into HCC progression . Int J Mol Sci 2023. ; 24 ( 14 ): 11471 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hao X , Sun G , Zhang Y , et al . Targeting immune cells in the tumor microenvironment of HCC: new opportunities and challenges . Front Cell Dev Biol 2021. ; 9 : 775462 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan S , Kuo N , Kryczek I , Zou W , Welling TH . Myeloid cells in hepatocellular carcinoma . Hepatology 2015. ; 62 ( 4 ): 1304 – 1312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonilla CM , McGrath NA , Fu J , Xie C . Immunotherapy of hepatocellular carcinoma with infection of hepatitis B or C virus . Hepatoma Res 2020. ; 6 : 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanwal F , Kramer J , Asch SM , Chayanupatkul M , Cao Y , El-Serag HB . Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents . Gastroenterology 2017. ; 153 ( 4 ): 996 – 1005.e1 . [DOI] [PubMed] [Google Scholar]
- 29. Pu D , Yin L , Zhou Y , et al . Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review . Medicine (Baltimore) 2020. ; 99 ( 5 ): e19013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sia D , Jiao Y , Martinez-Quetglas I , et al . Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features . Gastroenterology 2017. ; 153 ( 3 ): 812 – 826 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montironi C , Castet F , Haber PK , et al . Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification . Gut 2023. ; 72 ( 1 ): 129 – 140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie L , Meng Z . Immunomodulatory effect of locoregional therapy in the tumor microenvironment . Mol Ther 2023. ; 31 ( 4 ): 951 – 969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berz AM , Santana JG , Iseke S , et al . Impact of chemoembolic regimen on immune cell recruitment and immune checkpoint marker expression following transcatheter arterial chemoembolization in a VX2 rabbit liver tumor model . J Vasc Interv Radiol 2022. ; 33 ( 7 ): 764 – 774.e4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ali MY , Grimm CF , Ritter M , et al . Activation of dendritic cells by local ablation of hepatocellular carcinoma . J Hepatol 2005. ; 43 ( 5 ): 817 – 822 . [DOI] [PubMed] [Google Scholar]
- 35. Ahmed M , Kumar G , Gourevitch S , et al . Radiofrequency ablation (RFA)-induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins . Int J Hyperthermia 2018. ; 34 ( 7 ): 934 – 942 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jansen MC , van Hillegersberg R , Schoots IG , et al . Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model . Surgery 2010. ; 147 ( 5 ): 686 – 695 . [DOI] [PubMed] [Google Scholar]
- 37. Shewarega A , Santana JG , Nam D , et al . Effect of incomplete cryoablation and matrix metalloproteinase inhibition on intratumoral CD8+ T-Cell infiltration in murine hepatocellular carcinoma . Radiology 2024. ; 310 ( 2 ): e232365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dai Z , Wang Z , Lei K , et al . Irreversible electroporation induces CD8+ T cell immune response against post-ablation hepatocellular carcinoma growth . Cancer Lett 2021. ; 503 : 1 – 10 . [DOI] [PubMed] [Google Scholar]
- 39. Bulvik BE , Rozenblum N , Gourevich S , et al . Irreversible electroporation versus radiofrequency ablation: a comparison of local and systemic effects in a small-animal model . Radiology 2016. ; 280 ( 2 ): 413 – 424 . [DOI] [PubMed] [Google Scholar]
- 40. Pinato DJ , Murray SM , Forner A , et al . Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy . J Immunother Cancer 2021. ; 9 ( 9 ): e003311 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Savic LJ , Schobert IT , Peters D , et al . Molecular imaging of extracellular tumor pH to reveal effects of locoregional therapy on liver cancer microenvironment . Clin Cancer Res 2020. ; 26 ( 2 ): 428 – 438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chew V , Lee YH , Pan L , et al . Immune activation underlies a sustained clinical response to yttrium-90 radioembolisation in hepatocellular carcinoma . Gut 2019. ; 68 ( 2 ): 335 – 346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vanpouille-Box C , Diamond JM , Pilones KA , et al . TGFβ is a master regulator of radiation therapy-induced antitumor immunity . Cancer Res 2015. ; 75 ( 11 ): 2232 – 2242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Llovet JM , De Baere T , Kulik L , et al . Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma . Nat Rev Gastroenterol Hepatol 2021. ; 18 ( 5 ): 293 – 313 . [DOI] [PubMed] [Google Scholar]
- 45. Zhu A , Kudo M , Vogel A , et al . Abstract CT284: phase 3 KEYNOTE-937: adjuvant pembrolizumab versus placebo in patients with hepatocellular carcinoma and complete radiologic response after surgical resection or local ablation . Cancer Res 2020. ; 80 ( 16 Supplement ): CT284 . [Google Scholar]
- 46. IMMUTACE . IMMUTACE: a phase 2 single-arm, open-label study of transarterial chemoembolization in combination with nivolumab performed for intermediate-stage hepatocellular carcinoma . Gastroenterol Hepatol (N Y) 2021. ; 17 ( 11 Suppl 6 ): 16 – 17 . [PMC free article] [PubMed] [Google Scholar]
- 47. Lu J , Zhao M , Arai Y , et al. ; International Society of Multidisciplinary Interventional Oncology (ISMIO) . Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO) . Hepatobiliary Surg Nutr 2021. ; 10 ( 5 ): 661 – 671 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sangro B , Harding JJ , Johnson M , et al . A phase III, double-blind, randomized study of nivolumab (NIVO) and ipilimumab (IPI), nivo monotherapy or placebo plus transarterial chemoembolization (TACE) in patients with intermediate-stage hepatocellular carcinoma (HCC) . J Clin Oncol 2021. ; 39 ( 3 suppl ): TPS349 . [Google Scholar]
- 49. Ren Y , Guo Y , Chen L , et al . Efficacy of drug-eluting beads transarterial chemoembolization plus camrelizumab compared with conventional transarterial chemoembolization plus camrelizumab for unresectable hepatocellular carcinoma . Cancer Contr 2022. ; 29 : 10732748221076806 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Twyman-Saint Victor C , Rech AJ , Maity A , et al . Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer . Nature 2015. ; 520 ( 7547 ): 373 – 377 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De la Torre-Aláez M , Matilla A , Varela M , et al . Nivolumab after selective internal radiation therapy for the treatment of hepatocellular carcinoma: a phase 2, single-arm study . J Immunother Cancer 2022. ; 10 ( 11 ): e005457 . [Published correction appears in J Immunother Cancer 2023;11(3):e005457corr1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lubas MJ , Kumar SS . The combined use of SBRT and immunotherapy—a literature review . Curr Oncol Rep 2020. ; 22 ( 12 ): 117 . [DOI] [PubMed] [Google Scholar]
- 53. Juloori A , Katipally RR , Lemons JM , et al . Phase 1 randomized trial of stereotactic body radiation therapy followed by nivolumab plus ipilimumab or nivolumab alone in advanced/unresectable hepatocellular carcinoma . Int J Radiat Oncol Biol Phys 2023. ; 115 ( 1 ): 202 – 213 . [DOI] [PubMed] [Google Scholar]
- 54. Auer TA , Müller L , Schulze D , et al . CT-guided high-dose-rate brachytherapy versus transarterial chemoembolization in patients with unresectable hepatocellular carcinoma . Radiology 2024. ; 310 ( 2 ): e232044 . [DOI] [PubMed] [Google Scholar]
- 55. Strebel BM , Dufour JF . Combined approach to hepatocellular carcinoma: a new treatment concept for nonresectable disease . Expert Rev Anticancer Ther 2008. ; 8 ( 11 ): 1743 – 1749 . [DOI] [PubMed] [Google Scholar]
- 56. Liu J , O’Donnell JS , Yan J , et al . Timing of neoadjuvant immunotherapy in relation to surgery is crucial for outcome . OncoImmunology 2019. ; 8 ( 5 ): e1581530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Foerster F , Galle PR . The current landscape of clinical trials for systemic treatment of HCC . Cancers (Basel) 2021. ; 13 ( 8 ): 1962 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang S , Wang WS , Zhong BY , Ni CF . Subsequent treatment after transarterial chemoembolization failure/refractoriness: a review based on published evidence . J Clin Transl Hepatol 2022. ; 10 ( 4 ): 740 – 747 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Duffy AG , Ulahannan SV , Makorova-Rusher O , et al . Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma . J Hepatol 2017. ; 66 ( 3 ): 545 – 551 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lyu N , Kong Y , Li X , et al . Ablation reboots the response in advanced hepatocellular carcinoma with stable or atypical response during PD-1 therapy: a proof-of-concept study . Front Oncol 2020. ; 10 : 580241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peng Z , Fan W , Zhu B , Li J , Kuang M . Lenvatinib combined with transarterial chemoembolization as first-line treatment of advanced hepatocellular carcinoma: a phase 3, multicenter, randomized controlled trial . J Clin Oncol 2022. ; 40 ( 4 suppl ): 380 . [DOI] [PubMed] [Google Scholar]
- 62. Marinelli B , Kim E , D’Alessio A , et al . Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study . J Immunother Cancer 2022. ; 10 ( 6 ): e004205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yeo YH , Liang J , Lauzon M , et al . Immunotherapy and transarterial radioembolization combination treatment for advanced hepatocellular carcinoma . Am J Gastroenterol 2023. ; 118 ( 12 ): 2201 – 2211 . [DOI] [PubMed] [Google Scholar]
- 64. Valery M , Cervantes B , Samaha R , et al . Immunotherapy and hepatocellular cancer: where are we now? Cancers (Basel) 2022. ; 14 ( 18 ): 4523 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sagnella SM , White AL , Yeo D , Saxena P , van Zandwijk N , Rasko JEJ . Locoregional delivery of CAR-T cells in the clinic . Pharmacol Res 2022. ; 182 : 106329 . [DOI] [PubMed] [Google Scholar]
- 66. Lee JH , Lee JH , Lim YS , et al . Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma . Gastroenterology 2015. ; 148 ( 7 ): 1383 – 91.e6 . [DOI] [PubMed] [Google Scholar]
- 67. Lurje I , Werner W , Mohr R , Roderburg C , Tacke F , Hammerich L . In situ vaccination as a strategy to modulate the immune microenvironment of hepatocellular carcinoma . Front Immunol 2021. ; 12 : 650486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cook M , Chauhan A . Clinical application of oncolytic viruses: a systematic review . Int J Mol Sci 2020. ; 21 ( 20 ): 7505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hecht JR , Prat A , Pless M , et al . A phase 1b/2, multicenter, open-label trial to evaluate the safety of talimogene laherparepvec (T-VEC) injected into primary and metastatic liver tumors alone and in combination with pembrolizumab (pembro) (MASTERKEY-318) . J Clin Oncol 2018. ; 36 ( 15 suppl ): TPS3105 . [Google Scholar]
- 70. Yang C , Zhang H , Zhang L , et al . Evolving therapeutic landscape of advanced hepatocellular carcinoma . Nat Rev Gastroenterol Hepatol 2023. ; 20 ( 4 ): 203 – 222 . [DOI] [PubMed] [Google Scholar]
- 71. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics: Guidance for Industry . U.S. Food & Drug Administration . https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics. Published December 2018. Updated May 7, 2020. Accessed May 2024 . [Google Scholar]
- 72. Dioguardi Burgio M , Garzelli L , Cannella R , Ronot M , Vilgrain V . Hepatocellular carcinoma: optimal radiological evaluation before liver transplantation . Life (Basel) 2023. ; 13 ( 12 ): 2267 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ronot M , Chernyak V , Burgoyne A , et al . Imaging to predict prognosis in hepatocellular carcinoma: current and future perspectives . Radiology 2023. ; 307 ( 3 ): e221429 . [DOI] [PubMed] [Google Scholar]
- 74. Yaghmai V , Besa C , Kim E , Gatlin JL , Siddiqui NA , Taouli B . Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy . AJR Am J Roentgenol 2013. ; 201 ( 1 ): 80 – 96 . [DOI] [PubMed] [Google Scholar]
- 75. Mendiratta-Lala M , Masch WR , Shampain K , et al . MRI assessment of hepatocellular carcinoma after local-regional therapy: a comprehensive review . Radiol Imaging Cancer 2020. ; 2 ( 1 ): e190024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Santana JG , Petukhova-Greenstein A , Gross M , et al . MR imaging-based in vivo macrophage imaging to monitor immune response after radiofrequency ablation of the liver . J Vasc Interv Radiol 2023. ; 34 ( 3 ): 395 – 403.e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nishino M , Hatabu H , Hodi FS . Imaging of cancer immunotherapy: current approaches and future directions . Radiology 2019. ; 290 ( 1 ): 9 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ferrara R , Mezquita L , Texier M , et al . Comparison of fast-progression, hyperprogressive disease, and early deaths in advanced non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors or chemotherapy . JCO Precis Oncol 2020. ; 4 ( 4 ): 829 – 840 . [DOI] [PubMed] [Google Scholar]
- 79. Dercle L , Sun S , Seban RD , et al . Emerging and evolving concepts in cancer immunotherapy imaging . Radiology 2023. ; 306 ( 1 ): 32 – 46 . [DOI] [PubMed] [Google Scholar]
- 80. Dromain C , Beigelman C , Pozzessere C , Duran R , Digklia A . Imaging of tumour response to immunotherapy . Eur Radiol Exp 2020. ; 4 ( 1 ): 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seymour L , Bogaerts J , Perrone A , et al. ; RECIST working group . iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics . Lancet Oncol 2017. ; 18 ( 3 ): e143 – e152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen Q , Chen AZ , Jia G , Li J , Zheng C , Chen K . Molecular imaging of tumor microenvironment to assess the effects of locoregional treatment for hepatocellular carcinoma . Hepatol Commun 2022. ; 6 ( 4 ): 652 – 664 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. García-Figueiras R , Baleato-González S , Luna A , et al . Assessing immunotherapy with functional and molecular imaging and radiomics . RadioGraphics 2020. ; 40 ( 7 ): 1987 – 2010 . [DOI] [PubMed] [Google Scholar]
- 84. Chen S , Feng S , Wei J , et al . Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging . Eur Radiol 2019. ; 29 ( 8 ): 4177 – 4187 . [DOI] [PubMed] [Google Scholar]
- 85. Costa LB , Queiroz MA , Barbosa FG , et al . Reassessing patterns of response to immunotherapy with PET: from morphology to metabolism . RadioGraphics 2021. ; 41 ( 1 ): 120 – 143 . [DOI] [PubMed] [Google Scholar]
- 86. Kesler M , Levine C , Hershkovitz D , et al . 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: a prospective pilot study . J Nucl Med 2019. ; 60 ( 2 ): 185 – 191 . [DOI] [PubMed] [Google Scholar]
- 87. Wang H , Zhu W , Ren S , et al . 68Ga-FAPI-04 versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma . Front Oncol 2021. ; 11 : 693640 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lu RC , She B , Gao WT , et al . Positron-emission tomography for hepatocellular carcinoma: current status and future prospects . World J Gastroenterol 2019. ; 25 ( 32 ): 4682 – 4695 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hamm CA , Wang CJ , Savic LJ , et al . Deep learning for liver tumor diagnosis part I: development of a convolutional neural network classifier for multi-phasic MRI . Eur Radiol 2019. ; 29 ( 7 ): 3338 – 3347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Morshid A , Elsayes KM , Khalaf AM , et al . A machine learning model to predict hepatocellular carcinoma response to transcatheter arterial chemoembolization . Radiol Artif Intell 2019. ; 1 ( 5 ): e180021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mähringer-Kunz A , Wagner F , Hahn F , et al . Predicting survival after transarterial chemoembolization for hepatocellular carcinoma using a neural network: a pilot study . Liver Int 2020. ; 40 ( 3 ): 694 – 703 . [DOI] [PubMed] [Google Scholar]
- 92. Ahn JC , Qureshi TA , Singal AG , Li D , Yang JD . Deep learning in hepatocellular carcinoma: current status and future perspectives . World J Hepatol 2021. ; 13 ( 12 ): 2039 – 2051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chon HJ , Kim G , Kang B , et al . Artificial intelligence (AI)-powered tumor microenvironment (TME) analysis to identify potential biomarkers for ICIs with or without bevacizumab in hepatocellular carcinoma (HCC) . J Clin Oncol 2024. ; 42 ( 3 suppl ): 549 . [Google Scholar]
- 94. Zeng Q , Klein C , Caruso S , et al . Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology . J Hepatol 2022. ; 77 ( 1 ): 116 – 127 . [DOI] [PubMed] [Google Scholar]
- 95. Greten TF , Villanueva A , Korangy F , et al . Biomarkers for immunotherapy of hepatocellular carcinoma . Nat Rev Clin Oncol 2023. ; 20 ( 11 ): 780 – 798 . [DOI] [PubMed] [Google Scholar]
- 96. Winograd P , Hou S , Court CM , et al . Hepatocellular carcinoma-circulating tumor cells expressing PD-L1 are prognostic and potentially associated with response to checkpoint inhibitors . Hepatol Commun 2020. ; 4 ( 10 ): 1527 – 1540 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. He Y , Lu M , Che J , Chu Q , Zhang P , Chen Y . Biomarkers and future perspectives for hepatocellular carcinoma immunotherapy . Front Oncol 2021. ; 11 : 716844 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hsu C-H , Lu S , Abbas A , et al . Longitudinal and personalized detection of circulating tumor DNA (ctDNA) for monitoring efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma (HCC) . J Clin Oncol 2020. ; 38 ( 15 suppl ): 3531 . [Google Scholar]
- 99. Stechele M , Wildgruber M , Markezana A , et al . Prediction of protumorigenic effects after image-guided radiofrequency ablation of hepatocellular carcinoma using biomarkers . J Vasc Interv Radiol 2023. ; 34 ( 9 ): 1528 – 1537.e1 . [DOI] [PubMed] [Google Scholar]
- 100. Stechele M , Link H , Hirner-Eppeneder H , et al . Circulating miR-21 as a prognostic biomarker in HCC treated by CT-guided high-dose rate brachytherapy . Radiat Oncol 2023. ; 18 ( 1 ): 125 . [DOI] [PMC free article] [PubMed] [Google Scholar]