Summary
Background
Adrenomedullin has angiogenic and vasoprotective effects in acute ischemic stroke (AIS). This investigator-initiated trial aimed to evaluate the safety, efficacy, and optimal administration of adrenomedullin in treating AIS.
Methods
In this single-center, multi-cohort, double-blinded, randomized, placebo-controlled, Phase II trial, patients with AIS received pulsed adrenomedullin (9 ng/kg/min for 8 h daily over 7 days) or placebo in the first-half cohort, and continuous-pulsed adrenomedullin (9 ng/kg/min for 72 h during the first 3 days and 8 h daily between Day 4–7) or placebo in the second-half cohort. We included male and female patients aged 20–90 years with newly confirmed ischemic lesions on diffusion-weighted magnetic resonance imaging, and for whom protocol treatment could be initiated within 24 h of symptom onset. The primary safety endpoint was the occurrence of intervention-related severe adverse events. For the primary efficacy endpoint, the least square means and 95% confidence intervals of National Institutes of Health Stroke Scale (NIHSS) scores up to 7 days post-intervention initiation were calculated using generalized estimating equation models. This trial was registered at Japan Registry of Clinical Trials, jRCT2051190092.
Findings
Between January 16, 2020, and November 14, 2021, 60 patients were enrolled (median [interquartile range] age, 75 [66–81] years; NIHSS score, 3 [2–4]; 21 [35.0%] females). Neither intervention-related serious adverse events nor severe adverse events were observed in patients receiving adrenomedullin. No life-threatening adverse events or deaths were reported. The least square means (95% confidence intervals) of the changes in NIHSS scores from pre-treatment to Day 7 were −0.76 (−1.43 to −0.09) in the adrenomedullin group (−1.08 [−2.17 to 0.00] in the pulsed adrenomedullin group and −0.42 [−1.12 to 0.29] in the continuous-pulsed adrenomedullin group) and −1.08 (−2.11 to −0.05) in the placebo group.
Interpretation
Adrenomedullin was well tolerated in patients with non-severe, non-embolic AIS, although its beneficial effects were not demonstrated. It is necessary to show the efficacy of adrenomedullin in further clinical trials.
Funding
Japan Agency for Medical Research and Development.
Keywords: Adrenomedullin, Acute ischemic stroke, Randomized controlled trial, Phase II trial, Safety
Research in context.
Evidence before this study
Adrenomedullin is a bioactive peptide secreted by endothelial cells in the cerebral vasculature. Adrenomedullin can act at multiple sites within the neuro-glial-vascular unit by binding to a heterodimer receptor composed of the calcitonin receptor-like receptor and receptor activity-modifying protein type 2 or 3. Based on these modes of action, adrenomedullin has demonstrated angiogenic, vasodilatory, anti-inflammatory, and anti-oxidative properties, showing promise in mitigating ischemic damage in a mouse model of stroke. However, the safety, efficacy and the optimal administration method of adrenomedullin in patients with acute ischemic stroke remain unknown. We searched MEDLINE from January 1, 1970, to December 31, 2023, for articles published in English, using the terms “adrenomedullin” and “stroke”. No randomized controlled trials of adrenomedullin in patients with ischemic stroke have been published.
Added value of this study
The AdrenoMedullin for Ischemic Stroke (AMFIS) study was a single-center, double-blinded, randomized, placebo-controlled, Phase II trial. This multi-cohort, parallel-group study was the first to administer adrenomedullin or the placebo to patients with acute ischemic stroke. No serious adverse events were observed in patients receiving adrenomedullin. The efficacy of adrenomedullin on National Institutes of Health Stroke Scale scores was not demonstrated.
Implications of all the available evidence
This trial demonstrated that adrenomedullin was well tolerated; however, it did not show clinical improvements.
Introduction
Advances in acute stroke therapies, such as intravenous (IV) tissue plasminogen activator and endovascular treatment, have improved vessel recanalization rate by > 70%,1 benefiting many patients with acute ischemic stroke (AIS). However, <5% of patients with AIS received IV tissue plasminogen activator within the eligible therapeutic time window globally, and <100,000 endovascular treatments were performed worldwide in 2016,2 highlighting a significant gap in access to these therapies.2,3 As a result, stroke remains the second-leading cause of death worldwide, accounting for 11.6% of all deaths3 and the third-leading cause of death and disability combined in 2019,3 because no treatment can significantly reduce post-stroke sequelae, which interfere considerably with daily living. The pressing issues to be addressed in AIS treatment are reducing tissue damage and facilitating vascular regeneration, such as arteriogenesis and angiogenesis.4
Adrenomedullin, a vasoactive peptide, was originally discovered in human pheochromocytoma tissue and is primarily secreted by endothelial cells in the cerebral vasculature.4,5 Adrenomedullin plays a crucial role in the protective response against cerebral ischemia,6, 7, 8, 9 and is upregulated in response to hypoxia or shear stress as a compensatory mechanism.7,10 It exerts its biological effects by binding to a heterodimer receptor composed of the calcitonin receptor-like receptor and receptor activity-modifying protein (RAMP) type 2 or 3 expressed on the plasma membranes of endothelial and mural cells.11,12 These effects are mediated by the activation of specific signaling pathways, including phosphatidylinositol 3-kinase/Akt signaling. Additionally, adrenomedullin exerts anti-inflammatory effects by directly stimulating adrenomedullin receptors in immune cells.13
Adrenomedullin is potentially effective as a therapeutic agent for acute cerebral infarction, as demonstrated in various animal models.6, 7, 8, 9 Its overexpression or administration promotes ischemic tolerance, suppresses neuronal loss, and decreases reactive oxygen species levels, reducing infarct volumes. In a mouse model of cerebral hypoperfusion induced by bilateral common carotid artery stenosis, adrenomedullin upregulated the expression of vascular endothelial growth factor and basic fibroblast growth factor, particularly in hypoperfused brain regions but not in normoperfused regions, which may circumvent the off-target effects of adrenomedullin in normal tissues.7,8 Nevertheless, there is a potential risk of worsened cerebral ischemia, as intracerebroventricular adrenomedullin administration at a high dose exacerbated focal brain ischemic damage in a rat model of ischemic stroke induced by middle cerebral artery occlusion.14 Our Phase I study reported no severe adverse events (AEs) in healthy volunteers undergoing adrenomedullin treatment15; however, the safety and efficacy of adrenomedullin in treating vascular diseases have not been elucidated. Furthermore, the optimal administration method of adrenomedullin remains unknown. In sheep with pacing-induced heart failure, reduced cardiac output gradually improved with continuous administration of adrenomedullin. However, cardiac output began to decrease 6 h after the start of administration.16 Continuous infusion may lead to feedback inhibition and suppress cellular adrenomedullin signaling, as a cell culture study reported that phosphorylation levels of Akt peaked approximately 30 min after the initiation of adrenomedullin treatment but returned to baseline within 24 h.17 Therefore, we planned the AdrenoMedullin for Ischemic Stroke (AMFIS) study. This clinical trial study aimed to explore the safety, efficacy, and optimal administration method of adrenomedullin treatment in patients with AIS. The findings of this trial could provide new strategies for improving treatments for cerebrovascular diseases using adrenomedullin.
Methods
Study design and participants
The AMFIS study was an investigator-initiated, single-center, double-blinded, randomized, placebo-controlled, Phase II trial. This study was designed using two cohorts as detailed in Appendix A (eFig. 1, page 4). In both the first-half and second-half cohorts, male and female patients with AIS presenting with neurological symptoms with a National Institutes of Health Stroke Scale (NIHSS) score of ≥1 were enrolled. In each cohort, the first six patients enrolled were aged 20–74 years, and the subsequent patients were aged 20–90 years. We included only patients with new ischemic lesions confirmed by magnetic resonance diffusion-weighted imaging and for whom the protocol treatment could be initiated within 24 h from symptom onset. We excluded patients with ischemic stroke of embolic etiology, which was defined as ischemic lesions in the territory served by one or more cortical branches. Those with active intracranial hemorrhage, or those with an indication of endovascular therapy were also excluded. The details of the inclusion and exclusion criteria are listed in Appendix A (eTable 1, page 7).
Ethics
We followed the principles of the Declaration of Helsinki, International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Guidelines for Good Clinical Practice, and Japan Pharmaceutical Affairs Law. The trial protocol was approved by the National Cerebral and Cardiovascular Center institutional review board (#1110). There were no major amendments to the protocol during the study period.
Randomization and masking
In the first-half cohort, patients were randomly allocated to a pulsed (intermittent) adrenomedullin group or a placebo group, while in the second-half cohort, patients were randomly allocated to a continuous-pulsed adrenomedullin group or a placebo group. Before patient enrollment, the randomization table was uploaded to the electronic data-capture (EDC) system after confirming that the vial of adrenomedullin and that of placebo were indistinguishable by a vendor not involved in data collection or analysis (EPS Corporation, Tokyo, Japan). The vendor retained the randomization table for emergency unblinding purposes. However, unblinding did not occur until after data lock. Patients were assigned unique drug codes and allocated to either the adrenomedullin or placebo groups using a stratified block randomization method with a 2:1 ratio by the PLAN procedure in SAS 9.4 (SAS Institute, Cary, NC). Block sizes of 3 or 6 were randomly assigned. Stratification was based on age (<75 years and 75–90 years) due to concerns about potential distribution imbalances between the adrenomedullin and placebo groups that could influence results in this medium-sized randomized trial.
Procedures
Signed informed consent was obtained from the patients or their legally authorized representatives before the initiation of the study procedures. All patients underwent standard assessments, including demographic characteristics, medical history, laboratory values, and magnetic resonance imaging (MRI) evaluations.
Patients allocated to the pulsed adrenomedullin group or placebo group received adrenomedullin (9 ng/kg/min) or the placebo for 8 h from Day 1 to Day 7 in the first-half cohort. The pulsed adrenomedullin group received a total of 30 μg/kg of adrenomedullin over the seven days. Patients allocated to the continuous-pulsed adrenomedullin or placebo group received adrenomedullin (9 ng/kg/min) or the placebo for 72 h for the first three days and adrenomedullin or the placebo for 8 h from Day 4 to Day 7 in the second-half cohort. The continuous-pulsed adrenomedullin group received a total of 56 μg/kg of adrenomedullin over the seven days.
The investigational drugs were supplied by the University of Miyazaki. The University of Miyazaki contracted a Contract Manufacturing Organization to produce adrenomedullin and the placebo. Adrenomedullin is a peptide containing 52 amino acid residues in a ring structure formed by an intramolecular disulfide bond and an amidated structure formed by a C-terminal tyrosine. Bulk adrenomedullin powder was chemically synthesized and purified to over 95% purity using high-performance liquid chromatography at the Peptide Institute (Osaka, Japan). Subsequently, the active pharmaceutical ingredient was dissolved in water containing D-mannitol and formulated as a freeze-dried material by an established pharmaceutical company (Fuji Yakuhin, Toyama, Japan). D-mannitol was added to improve solubility and as an excipient. A vial of adrenomedullin contains 500 μg adrenomedullin and 50 mg D-mannitol. The placebo vial contained only 50 mg of D-mannitol. Given that D-mannitol is clinically administered in doses of 60–180 g per 60 kg of body weight, the 50 mg of D-mannitol is considered to have no significant impact. Investigators or pharmacists were in charge of the preparation of the investigational drugs. All the procedures for the drug formulation were conducted under the guidelines for good manufacturing practices.
The administration dose was determined based on findings from prior preclinical and clinical studies. Therapeutic benefits of adrenomedullin for ischemic infarcts were previously observed in transgenic mice expressing the human adrenomedullin gene, where blood adrenomedullin levels exceeded 15 pg/mL.6 In a Phase I study, an adrenomedullin dose of 9 ng/kg/min raised blood levels to 10–20 pg/mL.15 Therefore, we administered adrenomedullin at a dose of 9 ng/kg/min for 8 h daily for seven days in the first-half cohort. To investigate whether continuous administration of adrenomedullin is superior to pulsed administration, we chose to continuously administer 9 ng/kg/min of adrenomedullin for 72 h initially, followed by 8 h daily for the next 4 days in the second-half cohort. This approach was chosen for two main reasons. Firstly, prior research in a rodent model demonstrated that continuous adrenomedullin administration significantly reduced infarct volume after a 20-min middle cerebral artery occlusion. However, adrenomedullin was not effective when administration started 72 h after middle cerebral artery occlusion, highlighting the importance of adrenomedullin treatment during the first 72 h of AIS.6 Secondly, the total dose in this regimen in the second-half cohort matched that of other studies, where no serious AEs (SAEs) were observed, indicating a favorable safety profile. In one arm of the Phase I study, participants received 15 ng/kg/min of adrenomedullin for 8 h daily for 7 days (total dose: 50 μg/kg).15 Another study in patients with ulcerative colitis administered adrenomedullin at 9 ng/kg/min for 8 h daily for 14 days (total dose: 60 μg/kg).18 Based on these findings, we opted for continuous administration of 9 ng/kg/min adrenomedullin for 72 h initially, followed by 8 h daily for the subsequent 4 days in the second-half cohort (total administration dose: 56 μg/kg), which falls between the aforementioned 50 and 60 μg/kg.
The safety of adrenomedullin was assessed by monitoring AEs that occurred from the initiation of the intervention to 3 days after the intervention ended, based on findings from our Phase I study showing that the half-life of adrenomedullin is < 60 min.15 In particular, blood pressure was carefully monitored owing to the potential risk of a significant decrease in blood pressure resulting from vasodilation associated with adrenomedullin administration.19 The primary investigator classified the AEs based on four categories: expectedness, seriousness, severity, and causality. SAEs were defined as events that resulted in death, were life-threatening, required prolonged hospitalization, or resulted in persistent or significant disability. Their severity was determined based on whether the events interfered with the participants’ daily activities and was classified into the following five levels: 1. Mild, 2. Moderate, 3. Severe, 4. Life-threatening, 5. Death. The causality of AEs to the intervention was determined as either “not ruled out” or “ruled out” by an independent data-monitoring committee. The AEs determined as “not ruled out” are described as “AEs related to the intervention” in this report.
The Data and Safety Monitoring Board (DSMB) evaluated the safety of the investigational drug under blinded conditions, as required by Japanese regulatory authorities, given that the AMFIS study was the first to assess adrenomedullin's safety in patients with stroke. The DSMB comprised two stroke neurology experts and one basic researcher specializing in stroke, none of whom had any conflicts of interest with the principal investigator. For each DSMB review, case lists and AEs were provided for discussion, and the continuation of the trial and the need for protocol modifications were carefully assessed.
A web-based EDC system was used in this study. All clinical data were verified by independent monitors (CMIC, Tokyo, Japan). For efficacy evaluations, NIHSS scores were recorded at baseline (pre-treatment), and at 8, 24, and 72 h post-intervention, as well as on Day 7. The modified Rankin Scale (mRS) score was recorded on Day 90 to measure the degree of disability. These assessments were conducted by trained stroke neurologists, with efforts made to have the same examiner assess the same patient whenever possible for consistency. Furthermore, brain imaging tests were performed, including non-contrast computed tomography (CT) scans at 24 h and on Day 7, and MRI on Day 90.
Adrenomedullin concentrations were measured using a specific radioimmunoassay, as described previously.15 Briefly, the blood sample was collected in tubes containing 1.5 mg/mL ethylenediaminetetraacetic acid (EDTA-2Na) and 500 U/mL aprotinin. After centrifugation, the supernatant plasma was transferred to polypropylene tubes containing 20% w/v CHAPS PBS solution (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate in phosphate buffered salt solution) and stored at −25 °C until assayed. The concentration of adrenomedullin was quantified using an automated enzyme immunoassay analyzer (AIA-1800, Toso, Tokyo, Japan) at the Bozo Research Center (Tsukuba, Japan). We only measured the mature form of adrenomedullin because exogenous adrenomedullin is composed only of the mature form.20 In the first-half cohort, adrenomedullin concentration in blood was measured three times: pre-treatment, 8 h after initiation of the intervention, and on Day 7. For the second-half cohort, adrenomedullin concentration was assessed at five time points: pre-treatment, and at 8 h, 24 h, 72 h, and Day 7 following the initiation of the intervention. We investigated the associations between adrenomedullin concentrations in blood and the efficacy endpoints.
Outcomes
The primary safety endpoint was SAEs related to the intervention. The secondary safety endpoint was all AEs. The primary efficacy endpoint was the NIHSS scores at 8 h, 24 h, and 72 h after intervention initiation, and the last day of the intervention (Day 7). The secondary efficacy endpoint was the mRS score on Day 90.
Statistics
The statistical methods, other than post-hoc analysis, were predefined in the clinical trial protocol and the statistical analysis plan in the Supplemental Data. Categorical variables are summarized as numbers (percentages). Continuous variables are summarized as medians (interquartile ranges [IQR]).
The sample size was determined based on feasibility and necessary number of participants to demonstrate a statistical difference in the NIHSS scores seven days after stroke onset between the adrenomedullin and placebo groups. For this calculation, we retrospectively evaluated the NIHSS scores of 180 patients with acute lacunar infarcts admitted to the National Cerebral and Cardiovascular Center (NCVC, Suita, Japan) within 24 h of symptom onset between April 1, 2015, and March 31, 2017. The mean change in the NIHSS score from pre-treatment to Day 7 was −1.08, with a standard deviation of 2.84. Assuming a mean NIHSS score change of −3 with a standard deviation of 3 in the adrenomedullin group, a minimum of 19 participants per group was estimated to be necessary for the upper limit of the 95% CI of the intergroup difference in the NIHSS score change from pre-treatment to Day 7 to be less than 0 (95% CI: −3.97 to −0.03).
Safety analyses were performed on the population, excluding participants who did not receive any investigational drug among the participants enrolled and randomized in this study. Efficacy analyses were conducted on the Full Analysis Set, which excluded participants who did not receive any investigational drug or lacked efficacy data after randomization. This approach was adopted because we were initially concerned that some randomized participants might not receive the investigational drug due to various errors, especially since this study was designed to evaluate emergency treatment for AIS. The Per-Protocol Set was defined as the group excluding participants who did not meet the eligibility criteria, which might affect the primary efficacy endpoint.
For the primary efficacy endpoint, we calculated the least square mean (LSM) and 95% confidence interval (CI) of the NIHSS score at each time point among participants receiving adrenomedullin or placebo in the first or second cohort. Generalized estimating equations were applied, which included the trial treatments, time points, and interaction of trial treatments with time points as fixed effects, with the baseline NIHSS score included as a covariate. The same analysis was conducted between the pulsed adrenomedullin group in the first-half cohort and continuous-pulsed adrenomedullin group in the second-half cohort. For the secondary efficacy endpoint, we calculated the mean and 95% CI of the mRS on Day 90 in the pulsed adrenomedullin, continuous-pulsed adrenomedullin, and placebo groups. Statistics for other analyses, including post-hoc analyses, are presented in Appendix A (Supplementary Methods, page 2). All the analyses were performed using SAS version 9.4 (SAS Institute). This clinical trial was registered with a Japanese regulatory agency and the Japan Registry of Clinical Trials (jRCT2051190092).
Role of the funding source
This study was funded by the Intramural Research Fund for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center and the Japan Agency for Medical Research and Development. The Japan Agency for Medical Research and Development had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Results
Study population
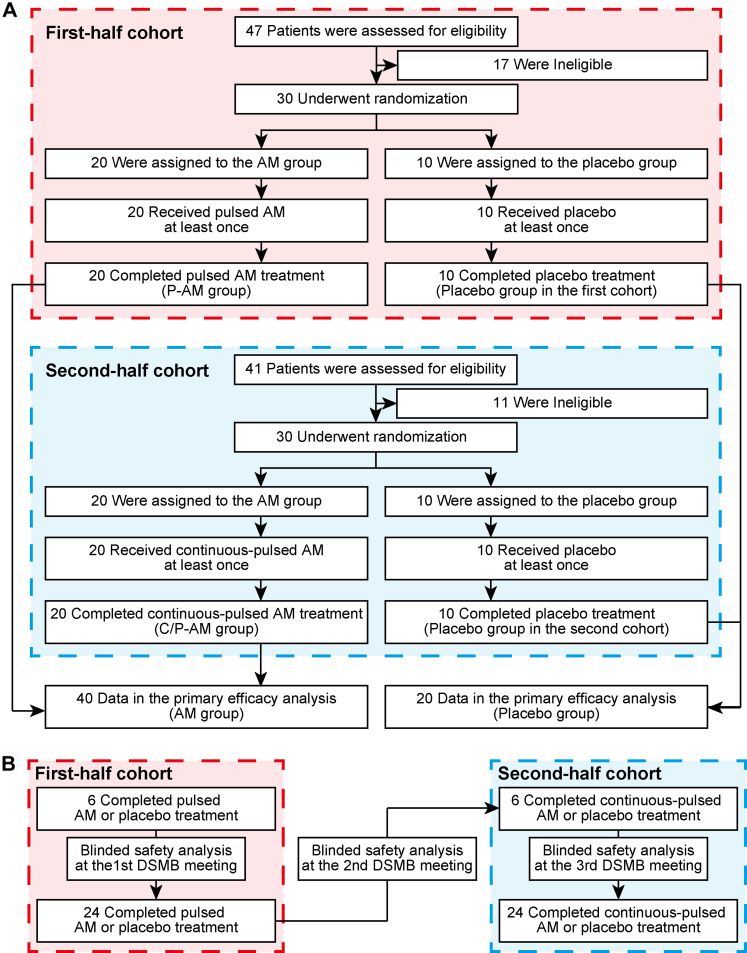
At the NCVC in Japan, between January 16, 2020, and November 14, 2021, 60 patients were enrolled, as per the initial plan (Fig. 1). Twenty-one (35.0%) were female individuals. The median (IQR) age was 75 (66–81) years, NIHSS score was 3 (2–4), and time from onset/last known well to investigational drug administration was 19.1 (12.1–22.3) hours. Participants were randomly assigned to the pulsed adrenomedullin or placebo group in the first-half cohort and the continuous-pulsed adrenomedullin or placebo group in the second-half cohort. The demographic characteristics were nearly equivalent between the adrenomedullin and placebo groups (Table 1). The characteristics in each cohort are presented in Appendix A (first-half cohort: eTable 2, page 9, second-half cohort: eTable 3, page 10). All the participants received and completed the allocated intervention without early termination, implying that the populations defined by intention-to-treat, full analysis set, per-protocol set, and safety analysis populations were identical. There were no major protocol deviations. The records for concomitant drugs (eTable 4, page 11) and investigational drug administration (eTable 5, page 12) are shown in Appendix A.
Fig. 1.
CONSORT flow diagram. A. In the first cohort, 30 patients were randomly assigned to either the pulsed AM or the placebo group between January and October 2020. AM or placebo was administered for 8 h daily, followed by saline (not placebo) for 16 h from days 1–3. From days 4–7, AM or placebo was administered for 8 h daily, with no infusion for the remaining 16 h. In the second cohort, another 30 patients were randomly assigned to either the continuous-pulsed AM or the placebo group between January and November 2021. AM or placebo was administered for the first 72 h from days 1–3. From days 4–7, AM or placebo was administered for 8 h daily. For the primary efficacy endpoint, the AM group (n = 40) were compared with the placebo group (n = 20). In addition, comparisons among the pulsed AM group in the first cohort (n = 20), the continuous-pulsed AM group in the second cohort (n = 20) and the placebo group in the first and second cohorts (n = 20) were conducted. B. Initially, only patients aged 20–74 years were enrolled as the first six cases of the first cohort. After reviewing these cases, the 1st DSMB meeting discussed the safety of the investigational drugs under blinded conditions and approved the enrolment of the remaining 24 patients, whose ages ranged from 20 to 90 years. A blinded safety analysis by the 2nd DSMB meeting was conducted after the final follow-up visit of the last patient in the first-half cohort, leading to the approval to initiate the second cohort. Similarly, only patients aged 20–74 years were enrolled in the first six cases of the second cohort. The 3rd DSMB meeting then discussed the safety of the investigational drugs in these six cases under blinded conditions and approved the enrolment of the remaining 24 patients, ranging in age from 20 to 90 years. AM, adrenomedullin; CONSORT, Consolidated Standards of Reporting Trials; C/P-AM, continuous-pulsed adrenomedullin; DSMB, Data and Safety Monitoring Board; P-AM, pulsed adrenomedullin.
Table 1.
Baseline characteristics of the patients.
| AM group, n = 40 | Placebo group, n = 20 | |
|---|---|---|
| Women | 10 (25.0) | 11 (55.0) |
| Age, years | 75 (68–79) | 75 (58–81) |
| Body weight, kg | 64 (56–71) | 57 (51–66) |
| Lifestyle and medical history | ||
| Current smoking | 9 (23.0) | 3 (15.0) |
| Atrial fibrillation | 1 (2.5) | 0 (0.0) |
| Chronic heart failure | 0 (0.0) | 0 (0.0) |
| Hypertension | 37 (92.5) | 20 (100.0) |
| Diabetes mellitus | 11 (27.5) | 5 (25.0) |
| Dyslipidemia | 26 (65.0) | 16 (80.0) |
| Chronic kidney disease | 11 (27.5) | 3 (15.0) |
| Stroke/TIA prior to index stroke | 9 (22.5) | 2 (10.0) |
| Ischemic heart disease | 4 (10.0) | 2 (10.0) |
| Pre-morbid mRS | ||
| 0 | 31 (77.5) | 18 (90.0) |
| 1 | 6 (15.0) | 0 (0.0) |
| 2 | 3 (7.5) | 2 (10.0) |
| Laboratory data | ||
| White blood cell count,/μL | 6275 (5310–7755) | 6190 (5685–7305) |
| Hemoglobin, g/dL | 14.0 (13.3–14.8) | 14.3 (12.8–14.9) |
| Blood glucose, mg/dL | 122 (103–148) | 125 (110–170) |
| D-dimer,/μL | 0.9 (0.6–1.1) | 1.0 (0.6–1.4) |
| C-reactive protein, mg/dL | 0.09 (0.07–0.15) | 0.08 (0.04–0.14) |
| Time from onset/last known well, hour | ||
| To randomization | 9.2 (4.4–17.9) | 11.7 (7.3–19.0) |
| To investigational drug administration | 18.6 (9.6–22.2) | 20.4 (17.0–22.9) |
| Treatment | ||
| IV thrombolysis | 7 (18.0) | 3 (15.0) |
Data are presented as medians (interquartile ranges) or numbers (percentages).
AM, adrenomedullin; IV, intravenous; mRS, modified Rankin Scale; TIA, transient ischemic attack.
Safety endpoints
No life-threatening AEs or death were reported in the first-half and second-half cohorts, and no “SAEs related to the intervention” were reported. Severe AEs (grade 3) were observed only in the placebo group: aspiration pneumonia, respiratory failure and delirium. These three AEs were observed in one patient. AEs reported in 10% or more of patients in either group are listed in Table 2. The pulsed and continuous-pulsed adrenomedullin groups experienced 16 and 26 AEs in 11 (55%, 95% CI: 31.5–76.9%) and 14 (70%, 95% CI: 45.7–88.1%) patients, respectively, and the placebo group experienced 21 AEs in eight (40%, 95% CI: 19.1–63.9%) patients. Details of the AEs (eTable 6, page 13) and treatment-emergent AEs (eTable 7, page 16) are shown in Appendix A.
Table 2.
Adverse events reported in ≥10% of patients in either treatment group.
| Events | P-AM group, n = 20 | C/P-AM group, n = 20 | Placebo group, n = 20 |
|---|---|---|---|
| Atrial fibrillation | |||
| Mild | 1 (5.0) | 2 (10.0) | 1 (5.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Aspiration pneumonia | |||
| Mild | 0 (0.0) | 1 (5.0) | 1 (5.0) |
| Moderate | 1 (5.0) | 2 (10.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Pneumonia | |||
| Mild | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Calcium pyrophosphate deposition disease | |||
| Mild | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 2 (10.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Drop in blood pressure | |||
| Mild | 1 (5.0) | 3 (15.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Headache | |||
| Mild | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | |||
| Mild | 2 (10.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Data are presented as numbers (percentages).
C/P-AM, continuous-pulsed adrenomedullin; P-AM, pulsed adrenomedullin.
In the adrenomedullin group, there were four cases (10.0%) of “drop in blood pressure” and one case (2.5%) of “low blood pressure”. Changes in the blood pressure during the trial period are detailed in Appendix A (eTable 8, page 19). Since adrenomedullin can induce angiogenesis,21 intracerebral hemorrhage has been a concern. However, no intracerebral hemorrhage was observed on CT and MRI from Day 1 to Day 90, as detailed in Appendix A (eTable 9, page 21). Laboratory tests showed no clinically significant changes.
Primary efficacy endpoints
The mean (median) change in the NIHSS score from pre-treatment to Day 7 was −0.75 (−1.00) in patients receiving adrenomedullin (−1.05 [−1] in the pulsed adrenomedullin group, −0.45 [−0.5] in the continuous-pulsed adrenomedullin group), and −1.10 (−1) in those receiving the placebo. In the generalized estimating equation models, the LSM (95% CI) of the change in the NIHSS score from pre-treatment to Day 7 was −0.76 (−1.43 to −0.09) in patients receiving adrenomedullin (−1.08 [−2.17–0.00] in the pulsed adrenomedullin group and −0.42 [−1.12–0.29] in the continuous-pulsed adrenomedullin group) and −1.08 (−2.11 to −0.05) in those receiving the placebo. The intergroup difference in the changes in the LSMs (95% CI) of the NIHSS scores from the pre-treatment scores between patients receiving adrenomedullin and those receiving placebo (adrenomedullin group minus placebo group) were 0.25 (−0.24–0.74) (8 h after intervention initiation), 0.15 (−0.46–0.75) (24 h after), 0.35 (−0.93–1.63) (72 h after), and 0.32 (−0.91–1.56) (Day 7) (Table 3). We also applied the generalized estimating equation model to each cohort (eTable 10, page 22, Appendix A). However, the results remained unchanged.
Table 3.
Primary efficacy endpoint.
| Timing | Group | N | NIHSS score, LSM (SE) | NIHSS changes from pre-treatment, LSM (SE) | Intergroup difference in NIHSS changes, LSM (95% CI) |
|---|---|---|---|---|---|
| Overall analysis | |||||
| 8-h | AM | 40 | 3.32 (0.17) | −0.03 (0.17) | 0.25 (−0.24−0.74) |
| Placebo | 20 | 3.07 (0.18) | −0.28 (0.18) | – | |
| 24-h | AM | 40 | 2.92 (0.24) | −0.43 (0.24) | 0.15 (−0.46−0.75) |
| Placebo | 20 | 2.77 (0.18) | −0.58 (0.18) | – | |
| 72-h | AM | 40 | 2.97 (0.36) | −0.38 (0.36) | 0.35 (−0.93−1.63) |
| Placebo | 20 | 2.62 (0.54) | −0.73 (0.54) | – | |
| 7-day | AM | 40 | 2.59 (0.34) | −0.76 (0.34) | 0.32 (−0.91−1.56) |
| Placebo | 20 | 2.27 (0.53) | −1.08 (0.53) | – | |
| Dose-finding analysis | |||||
| 8-h | C/P-AM | 20 | 3.41 (0.25) | 0.23 (0.25) | 0.52 (−0.12−1.16) |
| P-AM | 20 | 2.89 (0.21) | −0.28 (0.21) | – | |
| 24-h | C/P-AM | 20 | 3.06 (0.30) | −0.12 (0.30) | 0.62 (−0.29−1.53) |
| P-AM | 20 | 2.44 (0.34) | −0.73 (0.34) | – | |
| 72-h | C/P-AM | 20 | 2.96 (0.37) | −0.22 (0.37) | 0.32 (−1.09−1.72) |
| P-AM | 20 | 2.64 (0.61) | −0.53 (0.61) | – | |
| 7-day | C/P-AM | 20 | 2.76 (0.36) | −0.42 (0.36) | 0.67 (−0.64−1.98) |
| P-AM | 20 | 2.09 (0.55) | −1.08 (0.55) | – | |
Generalized estimating equations were applied, which included trial treatments, time points, and interaction of trial treatments with time points as fixed effects, with the pre-treatment NIHSS score included as a covariate.
AM, adrenomedullin; C/P-AM, continuous-pulsed adrenomedullin; LSM, least square mean; NIHSS, National Institutes of Health Stroke Scale; P-AM, pulsed adrenomedullin; SE, standard error.
To explore the optimal dosage of adrenomedullin for AIS treatment, we once again applied the generalized estimating equation models and calculated the intergroup difference in the changes in the LSMs (95% CI) of the NIHSS scores from the pre-treatment scores between the pulsed adrenomedullin group in the first-half cohort and continuous-pulsed adrenomedullin group in the second-half cohort (continuous-pulsed adrenomedullin group minus pulsed adrenomedullin group): 0.52 (−0.12–1.16) (8 h after intervention initiation), 0.62 (−0.29–1.53) (24 h after), 0.32 (−1.09–1.72) (72 h after), and 0.67 (−0.64–1.98) (Day 7) (Table 3).
Secondary and other efficacy endpoints
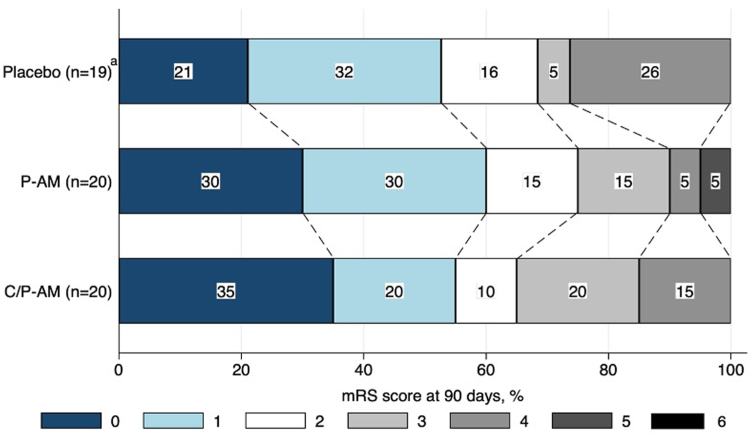
The distribution of the mRS scores on Day 90 in each group is shown in Fig. 2. The median (IQR) mRS scores on Day 90 were 1.0 (0–2.5) in the pulsed adrenomedullin, 1.0 (0–3.0) in the continuous-pulsed adrenomedullin group, and 1.0 (1.0–4.0) in the placebo group (Table 4). Several participants in both the adrenomedullin and placebo groups experienced a decline in the activities of daily living by Day 90. The details are summarized in Appendix A (eTable 11, page 23). Results for other analyses, including post-hoc analyses, are presented in Appendix A (Supplementary Results, page 2, eFig. 2, page 5, eTable 12–14, page 24). All efficacy summaries were described in Appendix B.
Fig. 2.
Distribution of mRS score at 90 days. a In the placebo group, mRS score at 90 days was missing in one participant. AM, adrenomedullin; C/P-AM, continuous-pulsed adrenomedullin; mRS, modified Rankin Scale; P-AM, pulsed adrenomedullin.
Table 4.
Secondary efficacy endpoint.
| Group | N | mRS score at 90 days | Changes from pre-morbid mRS |
|---|---|---|---|
| P-AM | 20 | 1.0 (0–2.5) | 1.0 (0–2.0) |
| C/P-AM | 20 | 1.0 (0–3.0) | 1.0 (0–2.0) |
| Placebo | 19 | 1.0 (1.0–4.0) | 1.0 (0–2.0) |
Data are presented as medians (interquartile ranges). In the placebo group, mRS at 90 days was missing in one participant.
C/P-AM, continuous-pulsed adrenomedullin; mRS, modified Rankin Scale; P-AM, pulsed adrenomedullin.
Pharmacokinetics
Adrenomedullin concentrations in the blood were elevated in patients with adrenomedullin treatment (eTable 15, page 27, Appendix A). Adrenomedullin concentration with peak levels (Cmax) in the pulsed adrenomedullin group was 44.21 pg/mL, and that in the continuous-pulsed adrenomedullin group was 61.01 pg/mL. Neither the changes in the NIHSS score from pre-treatment to Day 7 (eFig. 3A, page 6, Appendix A) nor the mRS score on Day 90 (eFig. 3B, page 6, Appendix A) were associated with the Cmax of adrenomedullin.
Discussion
The AMFIS study demonstrated the safety of 9 ng/kg/min of adrenomedullin treatment in patients with AIS within 24 h of symptom onset. No SAEs were observed in patients who received pulsed or continuous-pulsed adrenomedullin. However, no beneficial effects of adrenomedullin were demonstrated.
Adrenomedullin is a biosynthesized peptide which exerts various physiological effects, including vasodilation, regulation of vascular permeability, inhibition of vascular endothelial apoptosis and oxidative stress, as well as the regulation of vascular smooth muscle growth and angiogenesis.19,21 Several naturally occurring peptides or peptidomimetics have been approved and used clinically as safe components of pre-existing drugs that the human body has created through evolution.22 These include tissue plasminogen activator for AIS and anti-natriuretic peptide for chronic heart failure. These endogenous substances generally have a higher chance of passing clinical trial testing because of their low antigenicity and high safety profile. Given its various physiological activities, adrenomedullin is expected to have clinical applications for various diseases. In this AMFIS study, adrenomedullin was administered to patients with AIS. Phase II trials targeting patients with ulcerative colitis23 and Crohn's disease24 were previously conducted as well. Furthermore, a new clinical trial, the AMCAD study, is ongoing, in which adrenomedullin is administered to patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.25 The growing body of evidence from these clinical trials supports the safety profile of adrenomedullin.
This study was planned in 2019 and conducted from 2020. During this period, mechanical thrombectomy for acute large vessel occlusion (LVO) was evolving. The efficacy of mechanical thrombectomy for acute LVO within 6 h of onset was reported in 2015, and the efficacy for acute LVO between 6 and 24 h of onset was first reported in 2018.26 As the indication for mechanical thrombectomy in clinical practice was just expanding, it was anticipated that the standard therapy would significantly change during the trial period if we included LVO cases in this trial. Therefore, this trial excluded cases of stroke due to LVO or those indicated for mechanical thrombectomy. As a result, relatively mild strokes were predominantly registered in this trial, as reflected by the relatively low median baseline NIHSS score of 3 (IQR: 2−4). However, the favorable efficacy of adrenomedullin has been demonstrated in rats subjected to permanent middle cerebral artery occlusion.27 Although the angiogenic effect of adrenomedullin does pose a temporary risk of inducing an impaired blood–brain barrier, many reports have shown the protective effects of adrenomedullin on maintaining endothelial permeability28 and blood–brain barrier integrity.6,29 Given that the safety of adrenomedullin for patients with ischemic stroke has been shown in this trial, more severe strokes including those with LVO can be recruited for the next trial.
Our study has several limitations. First, this study was conducted at a single hospital, and multicenter studies are required for validating results across different populations and clinical settings. Second, there was imbalance in baseline characteristics among the three groups such as age, gender, body weight, vascular risk factors, chronic kidney diseases, and IV thrombolysis, which might introduce bias. We conducted a post-hoc analysis by adjusting for these factors and confirmed that the results remained unchanged. However, not all imbalances could be completely adjusted in this study. A previous study reported that high body weight was inversely associated with unfavorable outcomes in AIS.30 Future research, as a sub-study or through additional clinical studies, is warranted. Third, patients with embolic stroke were excluded. This is because embolic stroke is often accompanied by acute LVO. In addition, embolic stroke occasionally induces severe hemorrhagic infarction. We were concerned that the angiogenic effect of adrenomedullin might induce intracranial hemorrhage. Generalizability to embolic stroke should be investigated in future study.
In conclusion, adrenomedullin was well tolerated in patients with non-severe, non-embolic AIS, although its beneficial effects were not demonstrated.
Contributors
Conceptualization: Ihara, Saito, and Yoshimoto. Data curation: Yoshimoto, Saito, Fukuma, Washida, Abe, Ishiyama, Yamaguchi, Hattori, Tanaka T, and Ihara. Formal Analysis: Omae, and Tanaka K. Funding acquisition: Ihara. Investigation: Kita and Kitamura. Methodology: Ihara, Saito, Omae, and Tanaka K. Project administration: Ihara. Resources: Yoshimoto, Saito, Fukuma, Washida, Abe, Ishiyama, Yamaguchi, Hattori, Tanaka T, and Ihara. Software: None. Supervision: Ihara. Validation: None. Visualization: None. Writing—original draft: Yoshimoto and Saito. Writing—review & editing: All authors contributed equally. All authors read and approved the final version of the manuscript. Yoshimoto, Saito, Omae, and Tanaka K accessed and verified the data. Ihara was responsible for the decision to submit the manuscript.
Data sharing statement
Dr. Ihara had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study protocol and statistical analysis plan are shared in the appendix. It is not permitted to make secondary use of data from this study.
Declaration of interests
Kitamura K holds a patent for methods of producing adrenomedullin lyophilised formulations (P6781974, Japan Patent Office). Yamagami H, Nagatsuka K, and Tsuji M were the members of the Data Safety Monitoring Board of the AMFIS study. Furthermore, Yamagami H reports lecturer fees from Boston Scientific, Daiichi-Sankyo, Stryker, Otsuka Pharmaceutical, Abbott Medical, Bristol-Myers Squibb, and Medtronic, and grant support from Bristol-Myers Squibb. Tanaka T reports lecture fees from Eisai, Daiichi Sankyo, and UCB Japan and grant support from PDRadiopharma and Nihon Medi-Physics Corporation. Ihara M reports lecturer fees from Daiichi Sankyo and Eisai and grant support from Panasonic, GE Precision Healthcare LLC, Bristol-Myers Squibb, and Shimadzu Corporation. The other authors declare no conflicts of interest.
Acknowledgements
This study was funded by the Intramural Research Fund (24-A-1) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center and the Japan Agency for Medical Research and Development (grant number 19lk0201096h0001). We are grateful to the patients and their families who agreed to participate in the study. We would like to acknowledge the role of paramedics, nurses, and other investigators for their dedication; their role is important for any study conducted in emergency settings, but the combination with the COVID-19 pandemic made their role crucial.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102901.
Appendix A. Supplementary data
References
- 1.Goyal M., Menon B.K., van Zwam W.H., et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 2.Saini V., Guada L., Yavagal D.R. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97:S6–S16. doi: 10.1212/WNL.0000000000012781. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ihara M., Washida K., Yoshimoto T., Saito S. Adrenomedullin: a vasoactive agent for sporadic and hereditary vascular cognitive impairment. Cereb Circ Cogn Behav. 2021;2 doi: 10.1016/j.cccb.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura K., Kangawa K., Kawamoto M., et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 6.Miyashita K., Itoh H., Arai H., et al. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology. 2006;147:1642–1653. doi: 10.1210/en.2005-1038. [DOI] [PubMed] [Google Scholar]
- 7.Maki T., Ihara M., Fujita Y., et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke. 2011;42:1122–1128. doi: 10.1161/STROKEAHA.110.603399. [DOI] [PubMed] [Google Scholar]
- 8.Maki T., Ihara M., Fujita Y., et al. Angiogenic roles of adrenomedullin through vascular endothelial growth factor induction. Neuroreport. 2011;22:442–447. doi: 10.1097/WNR.0b013e32834757e4. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K., Takayasu M., Noda A., et al. Adrenomedullin reduces ischemic brain injury after transient middle cerebral artery occlusion in rats. Acta Neurochir. 2001;143:1157–1161. doi: 10.1007/s007010100007. [DOI] [PubMed] [Google Scholar]
- 10.Serrano J., Alonso D., Encinas J.M., et al. Adrenomedullin expression is up-regulated by ischemia-reperfusion in the cerebral cortex of the adult rat. Neuroscience. 2002;109:717–731. doi: 10.1016/s0306-4522(01)00532-2. [DOI] [PubMed] [Google Scholar]
- 11.Husmann K., Born W., Fischer J.A., Muff R. Three receptor-activity-modifying proteins define calcitonin gene-related peptide or adrenomedullin selectivity of the mouse calcitonin-like receptor in COS-7 cells. Biochem Pharmacol. 2003;66:2107–2115. doi: 10.1016/j.bcp.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Sauze S., Delfino C., Mabrouk K., et al. Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int J Cancer. 2004;108:797–804. doi: 10.1002/ijc.11663. [DOI] [PubMed] [Google Scholar]
- 13.Geven C., Kox M., Pickkers P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Front Immunol. 2018;9:292. doi: 10.3389/fimmu.2018.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Yue T.L., Barone F.C., et al. Discovery of adrenomedullin in rat ischemic cortex and evidence for its role in exacerbating focal brain ischemic damage. Proc Natl Acad Sci U S A. 1995;92:11480–11484. doi: 10.1073/pnas.92.25.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kita T., Kaji Y., Kitamura K. Safety, tolerability, and pharmacokinetics of adrenomedullin in healthy males: a randomized, double-blind, Phase 1 clinical trial. Drug Des Devel Ther. 2020;14:1–11. doi: 10.2147/DDDT.S225220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rademaker M.T., Charles C.J., Espiner E.A., et al. Long-term adrenomedullin administration in experimental heart failure. Hypertension. 2002;40:667–672. doi: 10.1161/01.hyp.0000037132.90640.26. [DOI] [PubMed] [Google Scholar]
- 17.Maki T., Takahashi Y., Miyamoto N., et al. Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-basic-protein expressing oligodendrocytes under pathological conditions in vitro. Stem Cell Res. 2015;15:68–74. doi: 10.1016/j.scr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashizuka S., Inatsu H., Kita T., et al. Adrenomedullin therapy in patients with refractory ulcerative colitis: a case series. Dig Dis Sci. 2016;61:872–880. doi: 10.1007/s10620-015-3917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaya N., Satoh T., Nishikimi T., et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101:498–503. doi: 10.1161/01.cir.101.5.498. [DOI] [PubMed] [Google Scholar]
- 20.Ohta H., Tsuji T., Asai S., et al. One-step direct assay for mature-type adrenomedullin with monoclonal antibodies. Clin Chem. 1999;45:244–251. [PubMed] [Google Scholar]
- 21.Nagaya N., Mori H., Murakami S., Kangawa K., Kitamura S. Adrenomedullin: angiogenesis and gene therapy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1432–R1437. doi: 10.1152/ajpregu.00662.2004. [DOI] [PubMed] [Google Scholar]
- 22.Qvit N., Rubin S.J.S., Urban T.J., Mochly-Rosen D., Gross E.R. Peptidomimetic therapeutics: scientific approaches and opportunities. Drug Discov Today. 2017;22:454–462. doi: 10.1016/j.drudis.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kita T., Ashizuka S., Ohmiya N., et al. Adrenomedullin for steroid-resistant ulcerative colitis: a randomized, double-blind, placebo-controlled phase-2a clinical trial. J Gastroenterol. 2021;56:147–157. doi: 10.1007/s00535-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kita T., Ashizuka S., Takeda T., et al. Adrenomedullin for biologic-resistant Crohn's disease: a randomized, double-blind, placebo-controlled phase 2a clinical trial. J Gastroenterol Hepatol. 2022;37:2051–2059. doi: 10.1111/jgh.15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Washida K., Saito S., Tanaka T., et al. A multicenter, single-arm, phase II clinical trial of adrenomedullin in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Cereb Circ Cogn Behav. 2024;6 doi: 10.1016/j.cccb.2024.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogueira R.G., Jadhav A.P., Haussen D.C., et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 27.Dogan A., Suzuki Y., Koketsu N., et al. Intravenous infusion of adrenomedullin and increase in regional cerebral blood flow and prevention of ischemic brain injury after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1997;17:19–25. doi: 10.1097/00004647-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Hippenstiel S., Witzenrath M., Schmeck B., et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91:618–625. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- 29.Kondoh T., Ueta Y., Torii K. Pre-treatment of adrenomedullin suppresses cerebral edema caused by transient focal cerebral ischemia in rats detected by magnetic resonance imaging. Brain Res Bull. 2011;84:69–74. doi: 10.1016/j.brainresbull.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.H., Jung J.M., Park M.H. Obesity paradox and stroke outcomes according to stroke subtype: a propensity score-matched analysis. Int J Obes. 2023;47:669–676. doi: 10.1038/s41366-023-01318-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.