ABSTRACT
Serum sickness is rare; however, there is a significant hypersensitivity reaction to streptokinase used in different cardiac problems. Treatment often involves discontinuing the offending agent and administering corticosteroids. This case underscores the complexities of managing prosthetic valve thrombosis and highlights the importance of monitoring and addressing complications of thrombolytic therapy.
Keywords: hypersensitivity, serum sickness, streptokinase, thrombolysis
Summary.
Serum sickness is rare; however, there is a significant hypersensitivity reaction to streptokinase used in different cardiac problems.
Treatment often involves discontinuing the offending agent and administering corticosteroids.
This case underscores the complexities of managing prosthetic valve thrombosis and highlights the importance of monitoring and addressing complications of thrombolytic therapy.
1. Introduction
Streptokinase is a widely used fibrinolytic agent for treating cardiovascular diseases, particularly in developing countries due to its cost‐effectiveness [1]. Isolated from hemolytic streptococci, streptokinase forms complexes with plasminogen to activate it into plasmin, which then dissolves blood clots and helps in improving reperfusion and left ventricular function in different cardiovascular diseases [2]. Being derived from bacterial protein, it can result in allergic reactions and bleeding, which are common adverse events. Patients might also have bradycardia and hypotension, along with fever, shivering, and rashes, as well as anaphylactic reactions [3]. Few case reports have reported serum sickness as a complication of streptokinase therapy. Serum sickness is a Type III immune complex mediated hypersensitivity reaction, which was first recognized in a patient who received heterologous antisera in the early 1990s. The symptoms usually occur after 1–2 weeks after exposure to offending agents and is a self‐limited disease [4].
This is the first study to show the association in the 21st century. In this, we report a rare case from a tertiary teaching hospital of a 36‐year‐old female, with a known history of mitral valve stenosis secondary to rheumatic heart disease, who underwent streptokinase treatment and presented with serum sickness. This case underscores the complexities of managing prosthetic valve thrombosis and highlights the importance of monitoring and addressing potential complications of thrombolytic therapy.
2. Case History/Examination
A 36‐year‐old female, with a known history of mitral valve stenosis secondary to rheumatic heart disease, underwent thrombolysis with streptokinase 3 months ago and presented with the primary complaints of shortness of breath on exertion for 4 days, orthopnea, and paroxysmal nocturnal dyspnea. She had a significant past medical history of mitral valve stenosis for which mitral valvotomy, double valve repair, and redo mitral valve repair were done. The patient had recurring symptoms for which thrombolysis with streptokinase started 2 weeks ago. Following streptokinase treatment, the patient now presents with shortness of breath, fever, and bilateral knee pain for the past 3 days, with no history of photosensitivity, skin rashes, myalgia, malaise, and lymphadenopathy (Figure 1). Her bowel and bladder habits were normal.
FIGURE 1.
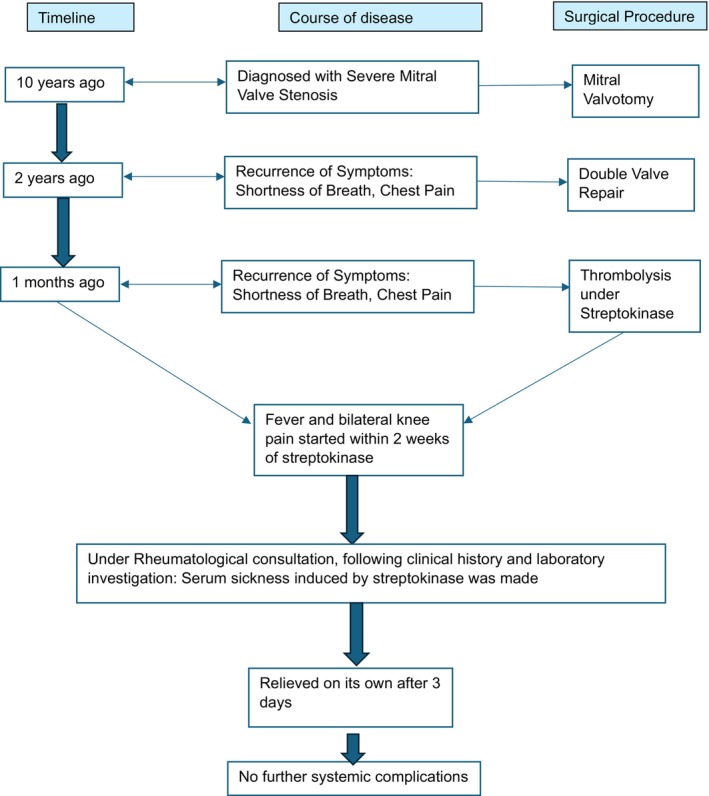
Diagnostic workup of a 36‐year‐old female with serum sickness.
On examination, the patient was afebrile, pulse was 70/min regular, respiratory rate was 20/min, blood pressure was 100/70 mmHg. General examination revealed no pallor, icterus, cyanosis, edema, or increased jugular venous pressure. Dermatological, cardiovascular, and respiratory system examinations were done and found to be normal. On joint examination, there was no swelling, redness, or warmth.
3. Methods
Blood investigation revealed elevated lymphocyte level with 43% (normal: 20%–40%); however, her hemoglobin level, neutrophil count, and platelet count were normal. Her PT count was high and revealed 32.8 s (normal: 11–13.5 s), and INR was also high and was 2.5 (normal: 0.8–1.2). Her biochemical profile such as blood urea nitrogen, creatinine level, sodium and potassium level were normal (Table 1). Blood culture and urine culture were sterile. However, hematology and serology tests suggested increased ESR and positive CRP Latex. The renal function test was also normal. Further, urinalysis was found to be normal (Table 1).
TABLE 1.
Laboratory, serological and biochemical investigations.
| S. no. | Laboratory investigations | Findings | |
|---|---|---|---|
| 1 | Erythrocyte sedimentation rate (ESR) | Increased | References |
| 2 | C‐reactive protein (CRP) latex | Positive | |
| 3 | Hemoglobin | 9.65 g% | 12–16 g% |
| 4 | Neutrophil | 41% | 40%–60% |
| 5 | Lymphocyte | 43% | |
| 6 | Platelet count | 4,456,000 | 150,000–450,000 cells/cumm |
| 7 | Prothrombin time (PT) | 32.8 s | 11–13.5 s |
| 8 | INR | 2.5 | 0.8–1.2 |
| 9 | Blood urea nitrogen (BUN) | 11 mg/dL | 7–20 mg/dL |
| 10 | Serum creatinine | 0.7 mg/dL | 0.6–1.2 mg/dL |
| 11 | Sodium | 137 mmol/L | 135–145 mmol/L |
| 12 | Potassium | 3.7 mmol/L | 3.5–5.0 mmol/L |
| 13 | Urine analysis | Normal | |
| 13 | Blood culture | Sterile | |
| 14 | Urine culture | Sterile | |
| 15 | Hepatitis antigen | Negative | |
| 16 | Antinuclear antibody | Negative | |
| 17 | Anti‐cyclic citrullinated peptide (anti‐CCP) | Negative | |
| 18 | Lactate dehydrogenase | Negative |
Based on the history, examination, and investigations, serum sickness, hepatitis, and viral illness, including dengue, acute rheumatic fever, and subacute bacterial endocarditis, were considered for differential diagnosis. For further confirmation and to rule out other conditions, hepatitis B antigen serology, antinuclear antibody, ELISA, direct Coombs, rheumatic factor, anti‐CCP, and LDH tests were done to rule out other conditions and came back negative. The absence of fever, thrombocytopenia, malaise, and weakness ruled out other differential diagnoses such as rheumatic fever and dengue, except for serum sickness and infective endocarditis. Having a temporal association with streptokinase, absence of infective endocarditis symptoms (petechiae, Osler node, Janeway node, splinter hemorrhage, sign of sepsis), sterile blood culture, normal cardiac examination (including inspection, palpation, percussion and auscultation), a diagnosis of serum sickness was made. Further rheumatological consultation was done, and a clinical diagnosis of serum sickness secondary to streptokinase therapy was made, considering the clinical history and laboratory investigations.
4. Conclusion and Results
For serum sickness as a complication of streptokinase therapy, pain management and steroids were considered. Pain was managed by using Ibuprofen 400 mg orally every 6 h, and prednisolone was used at 1 mg/kg/day orally for 3 days and tapered. Supportive care involved continuous monitoring of thrombolysis‐related complications and symptomatic treatment for heart failure, including potential diuretics and oxygen therapy. The symptoms of serum sickness had subsided after 4 days of NSAID and steroid management. So further complement testing, which included C3 and C4 for diagnosis, was not sent.
Echocardiography was done, which suggested a high‐pressure gradient prosthetic mitral valve of 11 mmHg, and a diagnosis of a stuck mitral valve was made, for which streptokinase thrombolysis was started. Though streptokinase was started again, the patient had developed a headache for which neurology consultation was done; however, the patient had not developed joint pain, fever, and rashes. Post‐surgery echocardiography suggested a normal prosthetic valve with 4.4 mmHg mean gradient across the valve and normal left ventricular systolic function. The patient was ambulatory, tolerating oral feeds, and stable. The patient was discharged after 18 days of hospital stay and given oral furosemide 40 mg BD, oral warfarin 9 mg OD, oral metoprolol 37.5 mg OD, and oral aspirin 75 mg OD. On regular follow‐up, the patient was normal, and her shortness of breath had also resolved. Following streptokinase, she had not developed symptoms consistent with serum sickness like the previous one.
5. Discussion
Serum sickness is a delayed immune reaction characterized by fever, rash, arthralgias, lymphadenopathy, and polyarthritis [5]. The main etiology behind this is immune complex formation and medications, which consist of heterologous antigen as a component, are the most common cause of it [6]. Streptokinase used as a thrombolytic agent has serum sickness, which is a rare but significant side effect where patients present with mild to severe symptoms causing significant morbidity. Serum sickness can occur regardless of the dose or route of streptokinase administration, including intracoronary therapy [4]. Mohsenzadeh et al. found serum sickness to be prevalent in the Iranian population due to antibiotic use, especially penicillin and the cephalosporin group of drugs [7]. Streptokinase has been used in various cardiovascular problems including myocardial infarction, arrhythmia and valve stenosis [8]. Few studies have shown an association between serum sickness and streptokinase therapy in the 20th century. Alexopoulos et al. have reported serum sickness as a complication of intravenous streptokinase when used for acute myocardial infaraction [9].
The correlation between detailed history and examination is required for suspected serum sickness. For the diagnosis, exposure to the offending agent within 2 weeks should be present; however, in case of repeat exposure, within a few days before presentation. The physical findings include arthralgia in the hands, feet, ankles, knees, and shoulders [4]. Rashes can also be present, which can be urticarial, maculopapular, or vasculitis eruptions. The rashes might take weeks to resolve once the offending agent is resolved. Less commonly, there can be associated edema, lymphadenopathy, headache, and splenomegaly [7]. Our case presented with a history of streptokinase therapy within 2 weeks and presented with fever and arthralgia of bilateral knee joints.
To assess potential additional etiologies and multi‐organ system involvement, the clinician ought to consider the subsequent laboratory tests: erythrocyte sedimentation rate, C‐reactive protein, total hemolytic complement, C3, C4, basic metabolic panel, liver transaminases, antinuclear antibody, rheumatoid factor, and complete blood count with differential [10]. These findings suggest the role of immune complexes in pathophysiology of serum sickness. In our case as well, the patient had elevated ESR and CRP level suggesting infectious pathology; however, renal function test and urinalysis were normal. To rule out other rheumatological conditions, antinuclear antibody, ELISA, direct Coombs, rheumatic factor, anti‐CCP, and LDH tests were done and were negative. Treatment typically involves withdrawal of streptokinase, but systemic corticosteroids may be necessary in severe cases with end‐organ damage [6, 11]. In this case, the patient had symptoms of fever and arthralgia for 3 days, which relieved on withdrawing the offending agent streptokinase. Though less common, early recognition and prompt treatment in severe cases is crucial otherwise it will lead to morbidity of the patient [12]. The prognosis of serum sickness is quite good and used to resolve within 1–2 weeks if the offending agent is discontinued. However, repeated exposure to a causative agent have also led to renal failure and even death [4]. Long‐term follow‐up for patients with serum sickness should include regular clinical evaluations for late‐onset symptoms, such as chronic arthralgia or immune complex‐mediated complications. Regular monitoring of inflammatory markers such as ESR and CRP, as well as periodic echocardiography to assess prosthetic valve function, is recommended. In this case, since the symptoms were resided within 4 days of conservative treatment and ESR, CRP level subsided on its own. In patients with a known history of hypersensitivity to streptokinase, alternative thrombolytic agents such as tissue plasminogen activator (tPA) or tenecteplase can be considered. Tenecteplase showed comparable efficacy to SK in treating mitral prosthetic valve thrombosis, with a faster onset of action within 12 h [13]. Although costlier, tenecteplase offer a safer profile in patients predisposed to allergic reactions, reducing the risk of complications like serum sickness [14].
6. Conclusion
The study highlights the potential risk of serum sickness following streptokinase administration, a rare but significant complication of thrombolytic therapy. Early recognition and appropriate management, primarily the cessation of streptokinase and corticosteroid treatment, can prevent severe outcomes. The case emphasizes the importance of monitoring for hypersensitivity reactions during thrombolytic treatment, especially in patients with a history of cardiovascular conditions. Despite the complication, the patient's symptoms resolved with conservative treatment, demonstrating the effectiveness of early intervention in serum sickness cases.
Author Contributions
Bibek Shrestha: conceptualization, data curation, formal analysis, methodology, project administration, visualization, writing – original draft, writing – review and editing. Rebicca Pradhan: investigation, supervision, validation. Pradeep Shrestha: conceptualization, data curation, investigation, resources, supervision, validation. Sudip Bastakoti: investigation, supervision, validation.
Disclosure
The authors have nothing to report.
Ethics Statement
The Institutional Review Board of the Institute of Medicine, Nepal, does not mandate ethical approval for the writing or publication of case reports, and patient consent was obtained. Informed written consent was obtained from the patient before writing this case report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images, complying with the requirements as mentioned in Wiley's CCR Consent Form. Informed written consent was obtained from the patient for publication of this case report in a scientific journal.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors have nothing to report.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Zia M. A., “Streptokinase: An Efficient Enzyme in Cardiac Medicine,” Protein and Peptide Letters 27, no. 2 (2019): 111–119, 10.2174/0929866526666191014150408. [DOI] [PubMed] [Google Scholar]
- 2. Bendary A., Tawfik W., Mahrous M., and Salem M., “Fibrinolytic Therapy in Patients With ST‐Segment Elevation Myocardial Infarction: Accelerated Versus Standard Streptokinase Infusion Regimen,” Journal of Cardiovascular and Thoracic Research 9, no. 4 (2017): 209–214, 10.15171/jcvtr.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edwards Z. and Nagalli S., Streptokinase (Archived) (Treasure Island, FL: StatPearls—NCBI Bookshelf, 2023), https://www.ncbi.nlm.nih.gov/books/NBK553215/. [PubMed] [Google Scholar]
- 4. Rixe N. and Tavarez M. M., Serum Sickness (Treasure Island, FL: StatPearls—NCBI Bookshelf, 2023), https://www.ncbi.nlm.nih.gov/books/NBK538312/. [Google Scholar]
- 5. Koransky R., Ferastraoaru D., and Jerschow E., “Single Nonsteroidal Anti‐Inflammatory Drug Induced Serum Sickness‐Like Reaction to Naproxen in a Patient Able to Tolerate Both Aspirin and Ibuprofen,” Journal of Allergy and Clinical Immunology in Practice 4, no. 1 (2015): 160–161, 10.1016/j.jaip.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 6. Ryan N. M., Downes M. A., and Isbister G. K., “Clinical Features of Serum Sickness After Australian Snake Antivenom,” Toxicon 25, no. 108 (2015): 181–183, 10.1016/j.toxicon.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 7. Mohsenzadeh A., Movahedi M., Saatchi M., et al., “Serum Sickness‐Like Reactions in Iranian Children: A Registry‐Based Study in a Referral Center,” Allergologia et Immunopathologia 48, no. 5 (2020): 424–429, 10.1016/j.aller.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 8. Shameem M., Sivakumar R., Komala M., and Bargavi B. H., “Observational Study on the Use of Streptokinase in Patients With Acute Myocardial Infarction and Its Outcome at Discharge,” Saudi Journal of Medicine 6, no. 4 (2021): 65–69, 10.36348/sjm.2021.v06i04.002. [DOI] [Google Scholar]
- 9. Alexopoulos D., Raine A. E. G., and Cobbe S. M., “Serum Sickness Complicating Intravenous Streptokinase Therapy in Acute Myocardial Infarction,” European Heart Journal 5, no. 12 (1984): 1010–1012, 10.1093/oxfordjournals.eurheartj.a061602. [DOI] [PubMed] [Google Scholar]
- 10. Joint Task Force on Practice Parameters , American Academy of Allergy , Asthma and Immunology , American College of Allergy , Asthma and Immunology , and Joint Council of Allergy, Asthma and Immunology , “Drug Allergy: An Updated Practice Parameter,” Annals of Allergy, Asthma & Immunology 105, no. 4 (2010): 259–273, 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 11. Proctor B. D. and Joondeph B. C., “Bilateral Anterior Uveitis: A Feature of Streptokinase‐Induced Serum Sickness,” New England Journal of Medicine 330, no. 8 (1994): 576–577, 10.1056/nejm199402243300818. [DOI] [PubMed] [Google Scholar]
- 12. Lundquist A. L., Chari R. S., Wood J. H., et al., “Serum Sickness Following Rabbit Antithymocyte‐Globulin Induction in a Liver Transplant Recipient: Case Report and Literature Review,” Liver Transplantation 13, no. 5 (2007): 647–650, 10.1002/lt.21098. [DOI] [PubMed] [Google Scholar]
- 13. Kathirvel D., Paul G. J., Kumar G. P., et al., “Tenecteplase Versus Streptokinase Thrombolytic Therapy in Patients With Mitral Prosthetic Valve Thrombosis,” Indian Heart Journal 70, no. 4 (2017): 506–510, 10.1016/j.ihj.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vishandasani J. K., Choudhary A., Chouhan J., and Gulati S., “Assessment of Management Approach for Myocardial Infarction by Family Physicians in Central India: A Cross‐Sectional Survey,” International Journal of Research in Medical Sciences 9, no. 5 (2021): 1346, 10.18203/2320-6012.ijrms20211867. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


