Abstract
Objective
The study objective was to examine the disease, demographic, and imaging features associated with different inflammatory phenotypes of calcium pyrophosphate deposition (CPPD) disease, ie, recurrent acute calcium pyrophosphate (CPP) crystal arthritis, chronic CPP crystal inflammatory arthritis, and crowned dens syndrome (CDS).
Methods
Data from an international cohort (assembled from 25 sites in 7 countries for the development and validation of the 2023 CPPD classification criteria from the American College of Rheumatology/EULAR) that met the criteria were included. Three cross‐sectional studies were conducted to determine the phenotypic characteristics of recurrent acute CPP crystal arthritis, chronic CPP crystal inflammatory arthritis, and CDS. Multivariable logistic regression analysis was used to calculate adjusted odds ratio (aOR) and 95% confidence interval (CI) to examine the association between potential risk factors and the inflammatory phenotype.
Results
Among the 618 people included (56% female; mean age [standard deviation] 74.0 [11.9] years), 602 (97.4%) had experienced acute CPP crystal arthritis, 332 (53.7%) had recurrent acute arthritis, 158 (25.6%) had persistent inflammatory arthritis, and 45 (7.3%) had had CDS. Recurrent acute CPP crystal arthritis associated with longer disease duration (aOR 2.88 [95% CI 2.00–4.14]). Chronic CPP crystal inflammatory arthritis was associated with acute wrist arthritis (aOR 2.92 [95% CI 1.81–4.73]), metacarpophalangeal joint osteoarthritis (aOR 1.87 [95% CI 1.17–2.97]), and scapho‐trapezo‐trapezoid (STT) joint osteoarthritis (aOR 1.83 [95% CI 1.15–2.91]), and it was negatively associated with either metabolic or familial risk for CPPD (aOR 0.60 [95% CI 0.37–0.96]). CDS was associated with male sex (aOR 2.35 [95% CI 1.21–4.59]), STT joint osteoarthritis (aOR 2.71 [95% CI 1.22–6.05]), and more joints affected with chondrocalcinosis (aOR 1.46 [95% CI 1.15–1.85]).
Conclusion
CPPD disease encompasses acute and chronic inflammatory phenotypes, each with specific clinical and imaging features that need to be considered in the diagnostic workup.
INTRODUCTION
Calcium pyrophosphate deposition (CPPD) disease occurs as a consequence of the pathological presence of calcium pyrophosphate (CPP) crystals inside joints. 1 , 2 , 3 , 4 It is an umbrella term comprising different acute and chronic phenotypes, which often coexist. 5 , 6 Acute CPP crystal arthritis presents with severe joint pain and swelling, like gout flares, commonly affecting knees, wrists, and ankles and lasting for several days to weeks. 7 , 8 , 9 , 10 These episodes can be recurrent. A specific phenotype of CPPD disease is crowned dens syndrome (CDS), characterized by acute neck pain, elevated markers of systemic inflammation, and imaging evidence of calcification on computed tomography (CT). 7 , 11 Chronic CPP crystal inflammatory arthritis can present with persistent mono, oligo, or polyarthritis. 2 , 12 The relative distribution and the clinical characteristics associated with these different phenotypes are not well understood. 1 , 6 , 13 A recent international initiative led to the development and validation of the American College of Rheumatology (ACR)/EULAR classification criteria of CPPD disease in 2023.5,7,14 Given the relative paucity of literature on the different presentations of CPPD disease, the objectives of this study were to describe the distribution of the main inflammatory phenotypes of CPPD disease in this cohort and to explore the commonalities and differences in demographic, clinical, and imaging features associated across the different CPPD presentations. 7 , 14
METHODS
Participants
Rheumatologists from 25 sites in 7 countries (France, Italy, Ireland, New Zealand, The Netherlands, the United Kingdom, and the United States) submitted deidentified participant profiles for the development of the 2023 ACR/EULAR criteria. Participants were identified retrospectively by investigators using patient lists, with investigators filling in the data collection forms through a review of patient medical records or prospectively during face‐to‐face or remote clinic visits during the data collection period, which occurred during the COVID‐19 pandemic. Data collection occurred between June 2020 and November 2021. The details of this study have been published elsewhere. 7 , 14 The study was approved by the Health Research Authority (Research Ethics Committee reference 20/SC/0243) and the local ethics committees at each participating site as appropriate. People with asymptomatic CPPD were ineligible.
Data collection
A standardized case report form was completed by the participant's rheumatologist. It ascertained information on the following: demographic data (age, sex); CPPD disease (duration of symptoms [≤ or > 2 years], acute inflammatory arthritis and localization, persistent inflammatory arthritis, CDS); family history of CPPD disease; presence of metabolic predisposition (hypercalcemia, primary hyperparathyroidism, hypomagnesemia, genetic hemochromatosis); results of synovial fluid analysis (presence, absence of CPP crystals); imaging evidence of CPPD (collected as part of routine clinical care, not study protocol); and rheumatologist assessment of radiographic osteoarthritis (OA) specifically at the second or third metacarpophalangeal (MCP) joint, wrist, scapho‐trapezo‐trapezoid (STT) joint, or scapholunate advanced collapse (SLAC) wrist (when available). 7 , 11 To standardize data collection, recruiting centers were sent definitions of acute inflammatory arthritis (an episode with acute onset or acute worsening of joint pain with swelling and/or warmth that resolves irrespective of treatment), persistent inflammatory arthritis (an ongoing joint swelling with pain and/or warmth in ≥1 joint), and CDS (defined by the following clinical [A] and imaging [B] features, both needing to be present):
Clinical features: Acute or subacute onset of severe pain localized to the upper neck with elevated inflammatory markers, limited rotation, and often fever. Mimicking conditions such as polymyalgia rheumatica and meningitis should be excluded.
Imaging features: Conventional CT with calcific deposits, typically linear and less dense than cortical bone, in the transverse retro‐odontoid ligament (transverse ligament of the atlas), often with an appearance of two parallel lines in axial views. Calcifications at the atlanto‐axial joint, alar ligament, and/or in pannus adjacent to the tip of the dens are also characteristic. dual‐energy computed tomography features include a dual‐energy index between 0.016 and 0.036). 14
From the initial cohort, those that met the classification criteria for CPPD disease according to the 2023 ACR/EULAR classification criteria were included in the current study.
Study design
The prevalence of acute CPP crystal arthritis, recurrent acute crystal arthritis, persistent CPP inflammatory arthritis, and CDS were explored.
Cross‐sectional study to describe the prevalence of different inflammatory phenotypes of CPPD
Nested cross‐sectional studies examining the characteristics of inflammatory phenotypes of interest
Three separate nested cross‐sectional studies were conducted. The first nested study compared the aforementioned features for people with recurrent acute inflammatory CPP crystal arthritis (time to maximal pain <24 hours of any joint) compared to those participants with only a single inflammatory episode. The second nested cross‐sectional study examined the features for people with persistent inflammatory CPP crystal arthritis versus those without persistent symptoms. The third nested cross‐sectional study examined the features associated with CDS in people with versus without crowned dens.
Statistical analysis
The prevalence and 95% confidence interval (CI) of acute CPP crystal arthritis, recurrent acute crystal arthritis, persistent CPP inflammatory arthritis, and CDS were calculated. Next, we evaluated the association of clinical features, imaging findings, and metabolic/familial predispositions with each of these three phenotypes using multivariable logistic regression, with adjustment for current age (years) and sex (model 1) and for all covariates (current age [years], gender, symptom duration [>2 or ≤2 years], acute knee arthritis [yes/no], acute wrist arthritis [yes/no], number of acute episodes of acute arthritis [0, 1, or >1], CDS [yes/no], metabolic or family history of CPPD [yes/no], radiographic MCP joint OA [yes/no], radiographic STT joint OA [yes/no], radiographic wrist OA [yes/no], SLAC wrist [yes/no], and number of joints with chondrocalcinosis) (model 2) in separate models. Unadjusted odds ratios (ORs) and adjusted odds ratios (aORs) and their 95% CIs were calculated. Statistical significance was set at P < 0.05. Statistics were performed using Stata software version MP.
RESULTS
Participants
Data for 1,020 participants with different rheumatic musculoskeletal diseases were collected. Among them, 618 fulfilled the ACR/EULAR CPPD classification criteria for CPPD disease and were included in this study. Among them 56% were female, and their mean age was 74.0 (standard deviation 11.9) years (Table 1). Nearly all participants had at least one flare of acute CPP crystal arthritis (97.4% [n = 602] [95% CI 95.8%–98.5%]), whereas persistent CPP crystal inflammatory arthritis was less common (25.6% [n = 158] [95% CI 22.2%–29.2%]), and CDS (7.3% [n = 45] [95% CI 5.4%–9.6%]) was the least common. Of the 602 people with acute CPP crystal arthritis, 332 (55.1%) experienced a recurrent flare. Only 9 participants did not have any inflammatory manifestation (1.4% [95% CI 0.7%–2.7%]). Twenty‐nine percent had features of two or more inflammatory phenotypes (Figure 1). CDS was almost always associated with other inflammatory phenotypes, with CDS being the sole manifestation in only 3 participants. There 6 six people with familial history of CPPD disease (1 with persistent inflammatory arthritis, 1 with persistent inflammatory arthritis and recurrent flares, and 4 with recurrent flares) and 8 people with haemochromatosis (6 with acute arthritis alone and 2 with both acute inflammatory arthritis and persistent inflammatory arthritis). There were 72 people with primary hyperparathyroidism or hypercalcemia (2 without inflammatory symptoms, 1 with CDS and acute inflammatory arthritis and persistent inflammatory arthritis, 1 with CDS and acute inflammatory arthritis, 2 with CDS alone, 10 with acute inflammatory arthritis and persistent inflammatory arthritis, and 56 with acute inflammatory arthritis alone), there were 31 with hypomagnesemia alone (19 with acute inflammatory arthritis alone, 9 with acute inflammatory arthritis and persistent inflammatory arthritis, 2 with CDS and acute inflammatory arthritis, and 1 with CDS and persistent inflammatory arthritis and acute inflammatory arthritis), and there were 15 with both hypercalcemia and hypomagnesemia (14 with acute inflammatory arthritis alone and 1 with persistent inflammatory arthritis and acute inflammatory arthritis).
Table 1.
Participant (N = 618) characteristics*
| Characteristic | Value |
|---|---|
| Current age (y), mean (SD) | 74.0 (11.9) |
| Sex, n (%) | |
| Female | 346 (56.0) |
| Male | 272 (44.0) |
| Ethnicity, n (%) | |
| White | 481 (77.8) |
| Hispanic | 22 (3.6) |
| African/Black | 17 (2.8) |
| East Asian | 7 (1.1) |
| South Asian | 5 (0.8) |
| Pacific Islander | 4 (0.6) |
| Other | 13 (2.1) |
| Missing | 69 (11.2) |
| CPP crystals on synovial fluid analysis a | 302 (48.9) |
| Age at symptom onset, n (%) | |
| ≤50 y | 73 (11.8) |
| 51–60 y | 83 (13.4) |
| 61–70 y | 155 (25.1) |
| 71–80 y | 192 (31.1) |
| ≥81 y | 115 (18.6) |
| Symptom duration, n (%) | |
| ≤2 y | 234 (37.9) |
| >2 y | 384 (62.1) |
| Acute arthritis localization, n (%) | |
| Knee | 415 (67.1) |
| Wrist | 315 (51.0) |
| No. of acute inflammatory arthritis episodes, n (%) | |
| 0 | 16 (2.6) |
| 1 | 270 (43.7) |
| >1 | 332 (53.7) |
| Persistent arthritis, n (%) | 158 (25.6) |
| Noninflammatory joint pain, n (%) | 9 (1.4) |
| Crowned dens syndrome, n (%) | 45 (7.3) |
| Metabolic or familial predisposition, n (%) | 168 (27.2) |
| 2/3 MCPJ OA, n (%) | 165 (26.7) |
| Any STTJ OA, n (%) | 158 (25.6) |
| Any wrist OA, n (%) | 189 (30.6) |
| Any SLAC wrist, n (%) | 46 (7.4) |
| Number of joints with CC, median (IQR) | 2 (1–4) |
CC, chondrocalcinosis; CPP, calcium pyrophosphate; IQR, interquartile range; MCPJ, meta‐carpo‐phalangeal joint; OA, osteoarthritis; SLAC, scapho‐lunate advanced collapse; STTJ, scapho‐trapezo‐trapezoid joint.
Presence of calcium pyrophosphate crystals on synovial fluid analysis on polarized light microscopy.
Figure 1.
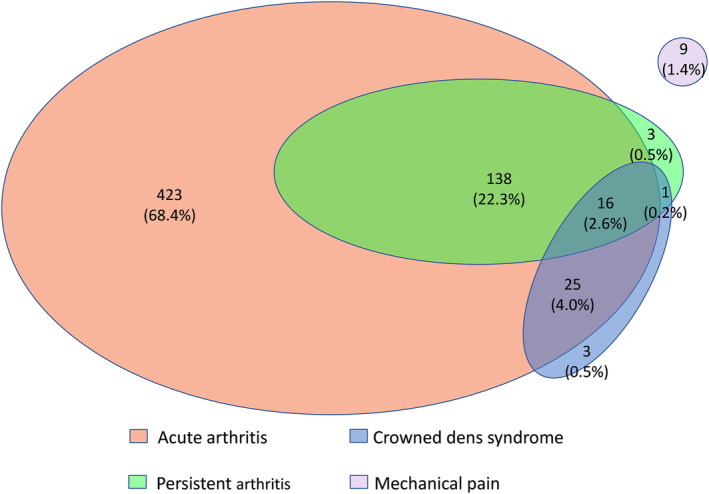
Venn diagram of distribution of acute CPP crystal arthritis, persistent CPP crystal inflammatory arthritis, crowned dens syndrome, and isolated pain without acute inflammatory arthritis in the cohorts assembled for the development and validation of the ACR/EULAR classification criteria for CPPD disease. ACR, American College of Rheumatology; CPP, calcium pyrophosphate; CPPD, calcium pyrophosphate deposition.
Features associated with recurrent acute CPP crystal arthritis
Symptom duration >2 years was significantly associated with recurrent CPP crystal arthritis flares (aOR 2.88 [95% CI 2.00–4.14], model 2). Recurrent acute CPP crystal arthritis was also significantly associated with having experienced an acute arthritis at the knee (aOR 3.65 [95% CI 2.41–5.51], model 2) and wrist (aOR 2.11 [95% CI 1.38–3.22], model 2) (Table 2).
Table 2.
Factors associated with recurrent flares among people classified as having CPPD disease with episodes of acute CPP crystal arthritis (N = 602)*
| Recurrent flares | Odds ratio (95% CI) | ||||
|---|---|---|---|---|---|
| No (n = 270) | Yes (n = 332) | Unadjusted | Model 1 a | Model 2 b | |
| Current age (y), mean (SD) | 74.4 (11.9) | 73.7 (12.0) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) |
| Sex, n (%) | |||||
| Female (ref) | 153 (56.7) | 180 (54.2) | 1 | 1 | 1 |
| Male | 117 (43.3) | 152 (45.8) | 1.10 (0.80–1.52) | 1.09 (0.79–1.51) | 1.29 (0.90–1.84) |
| Symptom duration, n (%) | |||||
| ≤2 y (ref) | 141 (52.2) | 89 (26.8) | 1 | 1 | 1 |
| >2 y | 129 (47.8) | 243 (73.2) | 2.98 (2.12–4.20) | 3.05 (2.16–4.30) | 2.88 (2.00–4.14) |
| Acute arthritis | |||||
| Knee, n (%) | |||||
| No (ref) | 116 (43.0) | 71 (21.4) | 1 | 1 | 1 |
| Yes | 154 (57.0) | 261 (78.6) | 2.77 (1.94–3.95) | 2.77 (1.94–3.95) | 3.65 (2.41–5.51) |
| Wrist, n (%) | |||||
| No (ref) | 140 (51.8) | 147 (44.3) | 1 | 1 | 1 |
| Yes | 130 (48.1) | 185 (55.7) | 1.36 (0.98–1.87) | 1.38 (0.99–1.91) | 2.11 (1.38–3.22) |
| Persistent inflammatory arthritis, n (%) | |||||
| No (ref) | 199 (73.7) | 249 (75.0) | 1 | 1 | 1 |
| Yes | 71 (26.3) | 83 (25.0) | 0.93 (0.65–1.35) | 0.93 (0.64–1.35) | 0.71 (0.46–1.09) |
| CDS, n (%) | |||||
| No (ref) | 254 (94.1) | 307 (92.5) | 1 | 1 | 1 |
| Yes | 16 (5.9) | 25 (7.5) | 1.29 (0.68–2.47) | 1.27 (0.66–2.44) | 1.62 (0.76–3.43) |
| Metabolic or familial predisposition, n (%) | |||||
| No (ref) | 201 (74.4) | 239 (72.0) | 1 | 1 | 1 |
| Yes | 69 (25.6) | 93 (28.0) | 1.14 (0.79–1.63) | 1.14 (0.79–1.63) | 0.97 (0.65–1.46) |
| 2/3 MCPJ OA, n (%) | |||||
| No (ref) | 206 (76.3) | 236 (71.1) | 1 | 1 | 1 |
| Yes | 64 (23.7) | 96 (28.9) | 1.31 (0.91–1.89) | 1.34 (0.93–1.95) | 1.16 (0.73–1.85) |
| Any STTJ OA, n (%) | |||||
| No (ref) | 210 (77.8) | 238 (71.7) | 1 | 1 | 1 |
| Yes | 60 (22.2) | 94 (28.3) | 1.38 (0.95–2.01) | 1.43 (0.98–2.01) | 1.11 (0.69–1.77) |
| Any wrist OA, n (%) | |||||
| No (ref) | 202 (74.8) | 216 (65.1) | 1 | 1 | 1 |
| Yes | 68 (25.2) | 116 (34.9) | 1.59 (1.12–2.28) | 1.66 (1.16–2.39) | 1.42 (0.90–2.24) |
| Any SLAC wrist, n (%) | |||||
| No (ref) | 249 (92.2) | 308 (92.8) | 1 | 1 | 1 |
| Yes | 21 (7.8) | 24 (7.2) | 0.92 (0.50–1.70) | 0.94 (0.51–1.72) | 0.77 (0.38–1.53) |
| Number of joints with CC, median (IQR) | 2 (1–4) | 2 (1–4) | 1.01 (0.90–1.13) | 1.02 (0.91–1.14) | 0.91 (0.80–1.04) |
Statistically significant associations are shown in bold typeface. CC, chondrocalcinosis; CDS, crowned dens syndrome; CI, confidence interval; CPP, calcium pyrophosphate; CPPD, calcium pyrophosphate deposition; IQR, interquartile range; MCPJ, meta‐carpo‐phalangeal joint; OA, osteoarthritis; ref, reference; SLAC, scapho‐lunate advanced collapse; STTJ, scapho‐trapezo‐trapezoid joint.
Adjustment for age and sex.
Adjustment for all covariates: current age (years), gender, symptom duration (>2 or ≤ 2 years), acute knee arthritis (yes/no), acute wrist arthritis (yes/no), only one or recurrent acute episodes, persistent inflammatory arthritis (yes/no), CDS (yes/no), metabolic or familial predisposition (yes/no), MCPJ OA (yes/no), STTJ OA (yes/no), wrist OA (yes/no), SLAC wrist (yes/no), and number of joints with CC.
Features associated with chronic CPP crystal inflammatory arthritis
Chronic CPP crystal inflammatory arthritis was associated with acute CPP crystal arthritis at the wrist (aOR 2.92 [95% CI 1.81–4.73], model 2), MCP joint 2 and/or 3 OA (aOR 1.87 [95% CI 1.17–2.97], model 2), and STT joint OA (aOR 1.83 [95% CI 1.15–2.91], model 2); it was not associated with age (aOR 0.98 [95% CI 0.97–1.00], P = 0.071) or with the presence of either metabolic risk or familial history of CPPD disease (aOR 0.60 [95% CI 0.37–0.96], model 2) (Table 3).
Table 3.
Factors associated with persistent inflammatory arthritis among people classified as having CPPD disease
| Persistent inflammatory arthritis | Odds ratio (95% CI) | ||||
|---|---|---|---|---|---|
| No (n = 460) | Yes (n = 158) | Unadjusted | Model 1 a | Model 2 b | |
| Current age, mean (SD) | 74.2 (12.5) | 73.6 (9.9) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.98 (0.97–1.00) |
| Sex, n (%) | |||||
| Female (ref) | 256 (55.6) | 90 (57.0) | 1 | 1 | 1 |
| Male | 204 (44.3) | 68 (43.0) | 0.95 (0.66–1.36) | 0.94 (0.65–1.35) | 1.00 (0.67–1.50) |
| Symptom duration, n (%) | |||||
| ≤2 y (ref) | 181 (39.3) | 53 (33.5) | 1 | 1 | 1 |
| >2 y | 279 (60.6) | 105 (66.5) | 1.28 (0.88–1.88) | 1.28 (0.88–1.88) | 1.37 (0.89–2.08) |
| Acute arthritis | |||||
| Knee, n (%) | |||||
| No (ref) | 139 (30.2) | 64 (40.5) | 1 | 1 | 1 |
| Yes | 321 (69.8) | 94 (59.5) | 0.64 (0.44–0.93) | 0.63 (0.43–0.92) | 1.04 (0.66–1.63) |
| Wrist, n (%) | |||||
| No (ref) | 260 (56.5) | 43 (27.2) | 1 | 1 | 1 |
| Yes | 200 (43.5) | 115 (72.8) | 3.48 (2.34–5.17) | 3.51 (2.36–5.22) | 2.92 (1.81–4.73) |
| No. of acute inflammatory arthritis episodes, n (%) | |||||
| 0 (ref) | 12 (2.6) | 4 (2.5) | 1 | 1 | 1 |
| 1 | 199 (43.3) | 71 (44.9) | 1.07 (0.33–3.43) | 1.08 (0.34–3.48) | 0.70 (0.19–2.54) |
| >1 | 249 (54.1) | 83 (52.5) | 1.00 (0.31–3.19) | 1.01 (0.32–83.23) | 0.48 (0.13–1.81) |
| CDS, n (%) | |||||
| No (ref) | 432 (93.9) | 141 (89.2) | 1 | 1 | 1 |
| Yes | 28 (6.1) | 17 (10.8) | 1.86 (0.99–3.50) | 1.89 (1.00–3.57) | 1.97 (0.98–3.97) |
| Metabolic or familial predisposition, n (%) | |||||
| No (ref) | 325 (70.6) | 125 (79.1) | 1 | 1 | 1 |
| Yes | 135 (29.3) | 33 (20.9) | 0.64 (0.41–0.98) | 0.63 (0.41–0.97) | 0.60 (0.37–0.96) |
| 2/3 MCPJ OA, n (%) | |||||
| No (ref) | 363 (78.9) | 90 (57.0) | 1 | 1 | 1 |
| Yes | 97 (21.1) | 68 (43.0) | 2.83 (1.92–4.16) | 2.93 (1.98–4.33) | 1.87 (1.17–2.97) |
| Any STTJ OA, n (%) | |||||
| No (ref) | 370 (80.4) | 90 (57.0) | 1 | 1 | 1 |
| Yes | 90 (19.6) | 68 (43.0) | 3.11 (2.10–4.59) | 3.27 (2.20–4.87) | 1.83 (1.15–2.91) |
| Any wrist OA, n (%) | |||||
| No (ref) | 340 (73.9) | 89 (56.3) | 1 | 1 | 1 |
| Yes | 120 (26.1) | 69 (43.7) | 2.20 (1.51–3.20) | 2.29 (1.56–3.37) | 1.21 (0.75–1.94) |
| Any SLAC wrist, n (%) | |||||
| No (ref) | 432 (93.9) | 140 (88.6) | 1 | 1 | 1 |
| Yes | 28 (6.1) | 18 (11.4) | 1.98 (1.06–3.70) | 2.02 (1.08–3.78) | 1.07 (0.53–2.14) |
| Number of joints with CC, median (IQR) | 2 (1–4) | 2 (2–4) | 1.05 (0.93–1.19) | 1.05 (0.93–1.20) | 0.91 (0.79–1.05) |
Statistically significant associations are shown in bold typeface. CC, chondrocalcinosis; CDS, crowned dens syndrome; CI, confidence interval; CPPD, calcium pyrophosphate deposition; IQR, interquartile range; MCPJ, meta‐carpo‐phalangeal joint; OA, osteoarthritis; ref, reference; SLAC, scapho‐lunate advanced collapse; STTJ, scapho‐trapezo‐trapezoid joint.
Adjustment for age and sex.
Adjustment for all covariates: current age (years), gender, symptom duration (>2 or ≤2 years), acute knee arthritis (yes/no), acute wrist arthritis (yes/no), only one or recurrent acute episodes, CDS (yes/no), metabolic or familial predisposition (yes/no), MCPJ OA (yes/no), STTJ OA (yes/no), wrist OA (yes/no), SLAC wrist (yes/no), and number of joints with CC.
Features associated with CDS
CDS was statistically significantly associated with male sex (aOR 2.35 [95% CI 1.21–4.59], model 2), having STT joint OA (aOR 2.71 [95% CI 1.22–6.05], model 2), and having more joints affected with chondrocalcinosis (aOR 1.46 [95% CI 1.15–1.85], model 2) (Table 4).
Table 4.
Factors associated with crown dens syndrome among people classified as having CPPD disease
| Crowned dens syndrome | Odds ratio (95% CI) | ||||
|---|---|---|---|---|---|
| No (n = 573) | Yes (n = 45) | Unadjusted | Model 1 a | Model 2 b | |
| Current age (y), mean (SD) | 74.1 (12 .0) | 73.5 (10.0) | 1.00 (0.97–1.02) | 1.00 (0.97–1.02) | 0.99 (0.97–1.02) |
| Sex, n (%) | |||||
| Female (ref) | 328 (57.2) | 18 (40.0) | 1 | 1 | 1 |
| Male | 245 (42.8) | 27 (60) | 2.01 (1.08–3.73) | 2.00 (1.07–3.73) | 2.35 (1.21–4.59) |
| Symptom duration, n (%) | |||||
| ≤2 y (ref) | 217 (37.9) | 17 (37.8) | 1 | 1 | 1 |
| >2 y | 356 (62.1) | 28 (62.2) | 1.00 (0.54–1.88) | 1.06 (0.56–1.99) | 0.84 (0.42–1.68) |
| Acute arthritis | |||||
| Knee, n (%) | |||||
| No (ref) | 183 (31.9) | 20 (44.4) | 1 | 1 | 1 |
| Yes | 390 (68.0) | 25 (55.6) | 0.59 (0.32–1.08) | 0.58 (0.31–1.08) | 0.60 (0.29–1.24) |
| Wrist, n (%) | |||||
| No (ref) | 280 (48.9) | 23 (51.1) | 1 | 1 | 1 |
| Yes | 293 (51.1) | 22 (48.9) | 0.91 (0.50–1.68) | 0.96 (0.52–1.78) | 0.60 (0.28–1.28) |
| No. of acute inflammatory arthritis episodes, n (%) | |||||
| 0 (ref) | 12 (2.1) | 4 (8.9) | 1 | 1 | 1 |
| 1 | 254 (44.3) | 16 (35.6) | 0.19 (0.05–0.65) | 0.15 (0.04–0.53) | 0.22 (0.05–0.94) |
| >1 | 307 (53.6) | 25 (55.6) | 0.24 (0.07–0.81) | 0.19 (0.05–0.65) | 0.33 (0.07–1.49) |
| Persistent inflammatory arthritis, n (%) | |||||
| No (ref) | 280 (48.9) | 23 (51.1) | 1 | 1 | 1 |
| Yes | 293 (51.1) | 22 (48.9) | 1.86 (0.99–3.50) | 1.89 (1.00–3.57) | 1.91 (0.95–3.85) |
| Metabolic or familial predisposition, n (%) | |||||
| No (ref) | 415 (72.4) | 35 (77.8) | 1 | 1 | 1 |
| Yes | 158 (27.6) | 10 (22.2) | 0.75 (0.36–1.55) | 0.79 (0.38–1.63) | 0.92 (0.42–1.99) |
| 2/3 MCPJ OA, n (%) | |||||
| No (ref) | 419 (73.1) | 34 (75.6) | 1 | 1 | 1 |
| Yes | 154 (26.7) | 11 (24.4) | 0.88 (0.44–1.78) | 0.91 (0.45–1.85) | 0.56 (0.24–1.32) |
| Any STTJ OA, n (%) | |||||
| No (ref) | 433 (75.6) | 27 (60.0) | 1 | 1 | 1 |
| Yes | 140 (24.4) | 158 (25.6) | 2.06 (1.10–3.86) | 2.23 (1.18–4.24) | 2.71 (1.22–6.05) |
| Any wrist OA, n (%) | |||||
| No (ref) | 398 (69.5) | 31 (68.9) | 1 | 1 | 1 |
| Yes | 140 (24.4) | 14 (31.1) | 1.03 (0.53–1.98) | 1.08 (0.55–2.11) | 0.63 (0.27–1.46) |
| Any SLAC wrist, n (%) | |||||
| No (ref) | 532 (92.8) | 572 (92.6) | 1 | 1 | 1 |
| Yes | 41 (7.2) | 46 (7.4) | 1.62 (0.61–4.33) | 1.62 (0.60–4.36) | 1.46 (0.49–4.37) |
| Number of joints with CC, mean (SD) | 2 (1–4) | 3 (2–4) | 1.33 (1.07–1.65) | 1.37 (1.10–1.71) | 1.46 (1.15–1.85) |
Statistically significant associations are shown in bold typeface. CC, chondrocalcinosis; CI, confidence interval; CPPD, calcium pyrophosphate deposition; MCPJ, meta‐carpo‐phalangeal joint; OA, osteoarthritis; ref, reference; SLAC, scapho‐lunate advanced collapse; STTJ, scapho‐trapezo‐trapezoid joint.
Adjustment for age and sex.
Adjustment for all covariates: current age (years), gender, symptom duration (>2 or ≤2 years), acute knee arthritis (yes/no), acute wrist arthritis (yes/no), only one or recurrent acute episodes, persistent inflammatory arthritis (yes/no), metabolic or familial predisposition (yes/no), MCPJ OA (yes/no), STTJ OA (yes/no), wrist OA (yes/no), SLAC wrist (yes/no), and number of joints with CC.
DISCUSSION
This is the first study to evaluate the clinical, imaging, and demographic factors associated with different inflammatory phenotypes of CPPD disease in a set of people who met the 2023 ACR/EULAR classification criteria for CPPD disease. The most frequent inflammatory phenotype was acute CPP crystal arthritis, which was recurrent in half of the cases. Recurrence was associated with increasing disease duration. A quarter also experienced persistent CPP crystal inflammatory arthritis, usually at a younger age, and this phenotype was associated with a history of acute wrist arthritis and radiographic features of OA in the second or third MCP and/or STT joint. CDS was an uncommon phenotype, rarely occurred as the sole manifestation, and was associated with more extensive chondrocalcinosis in peripheral joints as well as hand OA in specific joints. There were very few participants with CPPD and OA alone potentially due to hospital‐based recruitment resulting in selection bias.
Two prior, single‐center cohort studies reported that recurrent flares occurred in approximately 25% of people with an initial episode of acute CPP crystal arthritis. 15 , 16 In those cohort studies, cancer/chemotherapy and chronic kidney disease were significantly associated with recurrent flares. Our prevalence of recurrent flares was double that of the prior studies, which may be explained by the way the current cohort was assembled; experts were asked to provide deidentified patient profiles of those with a high probability of having CPPD disease, which may have created selection bias favoring people with more severe or extreme phenotypes of CPPD disease. We also found an association of recurrent acute CPP crystal arthritis with longer disease duration, which was expected because more time after the first flare allows for greater opportunity of recurrent episodes to be experienced.
Approximatively a quarter of people experiencing acute CPP crystal arthritis had chronic CPP crystal inflammatory arthritis (commonly mistaken for seronegative rheumatoid arthritis 17 , 18 ). Isolated persistent inflammatory arthritis was an uncommon finding in this cohort, potentially reflecting that this phenotype of CPPD disease is underrecognized in the absence of a previous acute episode, or that acute flares are also common in those with the chronic inflammatory phenotype, a possibility that is plausible on theoretical grounds. However, this needs to be evaluated in future studies. A previous study from a large German tertiary center showed that one‐third of people identified as having seronegative rheumatoid arthritis (RA; ie, being negative for the rheumatoid factor and anti‐citrullinated protein anitbodies) were eventually diagnosed with CPPD disease with a “pseudo‐RA” phenotype. 17 , 19 In a European cohort of 129 people with chronic symptoms of CPPD disease recruited in referral centers for CPPD disease, the persistent CPP crystal arthritis phenotype was as frequent as the recurrent acute CPP crystal arthritis phenotype. 12 In our study, the persistent CPP crystal arthritis phenotype was associated with radiographic OA in particular hand joints. Specifically, it was associated with OA in the second or third MCP joints and/or the STT joint in best adjusted models. The associations with these specific sites of structural damage were also noted in the German study, and they were more prevalent than in seropositive RA. 17 Persistent CPP crystal arthritis was commonly associated with acute wrist arthritis, which may further explain why this phenotype is commonly misdiagnosed for RA, as such episodes could resemble RA flares. From a diagnostic perspective, these unusual features of hand OA are particularly suggestive of the diagnosis of CPPD disease, especially as people with the persistent CPP crystal arthritis phenotype did not exhibit extensive chondrocalcinosis compared to the other phenotypes. Known metabolic conditions involved in CPPD disease include primary hyperparathyroidism, hereditary haemochromatosis, hypomagnesemia (in particular due to renal magnesium wasting such as in Gitelman disease), familial hypocalciuric hypercalcemia, and hypophosphatasia. 20 , 21 , 22 , 23 , 24 They often cause extensive CPPD through enhanced CPP crystal formation. Hereditary causes are associated with early and severe cases of familial CPPD disease and are linked to ANKH (and ATP transporter) and osteoprotegerin polymorphisms. 25 , 26 In our cohorts, persistent CPP crystal arthritis was negatively associated with known metabolic or genetic (familial) risk factors for CPPD disease, suggesting that these conditions are more commonly responsible for episodes of acute arthritis.
Reputed to be a rare but specific manifestation of the disease when present, 7 , 11 the CDS phenotype was experienced by 7.3% of participants with CPPD disease in our cohort, which was assembled to aid in classification criteria development and therefore may overrepresent rare but highly specific manifestations of CPPD disease. Prevalence of crowned dens features on CT (calcification of the transverse ligament of the atlas) varied between 25% and 60% in case series of people with CPPD disease, but the prevalence of symptoms related to these deposits is unknown. 27 , 28 , 29 In the COLCHICORT trial, 5% of participants with acute episodes of CPPD disease had CDS, 8 and in the European cohort of people with chronic phenotypes of CPPD, 35 (27%) of 129 had cervical pain, although the exact proportion of participants fulfilling the strict definition of the CDS is unknown. 7 , 11 , 12 The association of CDS with male sex in our study is not consistent with previous case series, which found either no sex ratio imbalance or a female predominance. 27 , 28 In a previous small case series of 12 people with CDS, peripheral imaging evidence of CPPD was inconsistent, 27 whereas in our cohorts, the CDS phenotype was associated with more extensive chondrocalcinosis than the other phenotypes. A French case series of 37 participants 28 found the same result and showed that the CDS phenotype exhibited some association with radiographic OA (of STT joints), but this association was less marked than the one with persistent arthritis. However, this more extensive CPPD observed in CDS was not associated with metabolic causes that are known to be associated with extensive chondrocalcinosis, including of the spine like in Gitelman disease. 23 However, this could be due to small sample size.
The study has inherent limitations. First, the cohorts were not designed for epidemiological purposes, and selection and reporting biases may have occurred. The reported people therefore do not reflect the whole population of people with CPPD disease in the community. Because some of these patient profiles were used to develop the CPPD classification criteria, the frequency of particular clinical and imaging findings that are highly weighted in the CPPD classification criteria may be particularly high in this cohort. This limitation may have affected the prevalence of certain phenotypes such as OA and CPPD. 7 Noninflammatory arthritis or mechanical joint pain was not an item collected in the case report form and could only be deduced when all inflammatory features were absent. This cohort represents the largest population of people with CPPD meeting the ACR/EULAR CPPD classification criteria. Another limitation is that the investigators did not receive any specific training to standardize readings of imaging techniques, and no centralized reading was organized, which may have induced a variability in reporting the presence of chondrocalcinosis, particularly with advanced techniques such as ultrasound, which is operator dependent. 30 The consensus definitions of imaging evidence of CPP crystal deposition, however, demonstrated good performance characteristics for conventional radiography. 11 , 31 Finally, only a preselected group of clinical, imaging, and laboratory variables were collected on these participants, which precludes the identification of a broader range of associations with clinical phenotypes.
This large international cohort, which informed and validated the 2023 ACR/EULAR classification criteria for CPPD disease, provides new insights into the clinical phenotypes encompassed by the disease. Clinical phenotypes overlapped, underscoring the importance of considering how to assess outcomes in future clinical trials that may enroll people with more than one manifestation. Future studies looking into the genetics of the disease, still only superficially explored, 26 , 32 should take into account the heterogeneity and specific clinical associations to better understand the pathophysiology of the disease. Clinical trials in CPPD disease will also need to take this heterogeneity of phenotypes into account since some treatments and outcome measures will certainly be more appropriate for some phenotypes than others. 8 , 12 , 33 , 34
AUTHOR CONTRIBUTIONS
All authors contributed to at least one of the following manuscript preparation roles: conceptualization AND/OR methodology, software, investigation, formal analysis, data curation, visualization, and validation AND drafting or reviewing/editing the final draft. As corresponding author, Dr Pascart confirms that all authors have provided the final approval of the version to be published, and takes responsibility for the affirmations regarding article submission (eg, not under consideration by another journal), the integrity of the data presented, and the statements regarding compliance with institutional review board/Helsinki Declaration requirements.
Supporting information
Disclosure Form
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/art.42962.
REFERENCES
- 1. Abhishek A, Neogi T, Choi H, et al. Unmet needs and the path forward in joint disease associated with calcium pyrophosphate crystal deposition. Arthritis Rheumatol 2018;70:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenthal AK, Ryan LM. Calcium pyrophosphate deposition disease. N Engl J Med 2016;374:2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis 2011;70:563–570. [DOI] [PubMed] [Google Scholar]
- 4. Latourte A, Ea H‐K, Richette P. Calcium pyrophosphate and basic calcium phosphate crystal arthritis: 2023 in review. Gout, Urate, and Crystal Deposition Disease 2024;2:101–107. [Google Scholar]
- 5. Tedeschi SK. A new era for calcium pyrophosphate deposition disease research: the first‐ever calcium pyrophosphate deposition disease classification criteria and considerations for measuring outcomes in calcium pyrophosphate deposition disease. Gout, Urate, and Crystal Deposition Disease 2024;2:52–59. [Google Scholar]
- 6. McCarthy GM, Dunne A. Calcium crystal deposition diseases—beyond gout. Nat Rev Rheumatol 2018;14:592–602. [DOI] [PubMed] [Google Scholar]
- 7. Abhishek A, Tedeschi SK, Pascart T, et al. The 2023 ACR/EULAR classification criteria for calcium pyrophosphate deposition disease. Ann Rheum Dis 2023;82:1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pascart T, Robinet P, Ottaviani S, et al. Evaluating the safety and short‐term equivalence of colchicine versus prednisone in older patients with acute calcium pyrophosphate crystal arthritis (COLCHICORT): an open‐label, multicentre, randomised trial. Lancet Rheumatol 2023;5:e523–e531. [DOI] [PubMed] [Google Scholar]
- 9. Maravic M, Ea H‐K. Hospital burden of gout, pseudogout and other crystal arthropathies in France. Joint Bone Spine 2015;82:326–329. [DOI] [PubMed] [Google Scholar]
- 10. Ramonda R, Oliviero F, Galozzi P, et al. Molecular mechanisms of pain in crystal‐induced arthritis. Best Pract Res Clin Rheumatol 2015;29:98–110. [DOI] [PubMed] [Google Scholar]
- 11. Tedeschi SK, Becce F, Pascart T, et al. Imaging features of calcium pyrophosphate deposition disease: consensus definitions from an international multidisciplinary working group. Arthritis Care Res (Hoboken) 2023;75:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damart J, Filippou G, Andres M, et al. Retention, safety and efficacy of off‐label conventional treatments and biologics for chronic calcium pyrophosphate crystal inflammatory arthritis. Rheumatology (Oxford) 2024;63:446–455. [DOI] [PubMed] [Google Scholar]
- 13. Abhishek A. Calcium pyrophosphate deposition disease: a review of epidemiologic findings. Curr Opin Rheumatol 2016;28:133–139. [DOI] [PubMed] [Google Scholar]
- 14. Abhishek A, Tedeschi SK, Pascart T, et al. The 2023 ACR/EULAR classification criteria for calcium pyrophosphate deposition disease. Arthritis Rheumatol 2023;75:1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yates KA, Yoshida K, Xu C, et al. Acute calcium pyrophosphate crystal arthritis flare rate and risk factors for recurrence. J Rheumatol 2020;47:1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JS, Hong S, Kwon OC, et al. Clinical features and risk of recurrence of acute calcium pyrophosphate crystal arthritis. Clin Exp Rheumatol 2019;37:254–259. [PubMed] [Google Scholar]
- 17. Krekeler M, Baraliakos X, Tsiami S, et al. High prevalence of chondrocalcinosis and frequent comorbidity with calcium pyrophosphate deposition disease in patients with seronegative rheumatoid arthritis. RMD Open 2022;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Codes‐Méndez H, Sainz L, Park HS, et al. Application of the 2023 ACR/EULAR classification criteria for calcium pyrophosphate deposition disease in a seronegative rheumatoid arthritis cohort. RMD Open 2024;10:e004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paalanen K, Rannio K, Rannio T, et al. Prevalence of calcium pyrophosphate deposition disease in a cohort of patients diagnosed with seronegative rheumatoid arthritis. Clin Exp Rheumatol 2020;38:99–106. [PubMed] [Google Scholar]
- 20. Jones AC, Chuck AJ, Arie EA, et al. Diseases associated with calcium pyrophosphate deposition disease. Semin Arthritis Rheum 1992;22:188–202. [DOI] [PubMed] [Google Scholar]
- 21. Volpe A, Guerriero A, Marchetta A, et al. Familial hypocalciuric hypercalcemia revealed by chondrocalcinosis. Joint Bone Spine 2009;76:708–710. [DOI] [PubMed] [Google Scholar]
- 22. Richette P, Ayoub G, Lahalle S, et al. Hypomagnesemia associated with chondrocalcinosis: a cross‐sectional study. Arthritis Rheum 2007;57:1496–1501. [DOI] [PubMed] [Google Scholar]
- 23. Chotard E, Blanchard A, Ostertag A, et al. Calcium pyrophosphate crystal deposition in a cohort of 57 patients with Gitelman syndrome. Rheumatology (Oxford) 2022;61:2494–2503. [DOI] [PubMed] [Google Scholar]
- 24. Mouly C, Vargas‐Poussou R, Lienhardt A, et al. Clinical characteristics of familial hypocalciuric hypercalcaemia type 1: a multicentre study of 77 adult patients. Clin Endocrinol (Oxf) 2020;93:248–260. [DOI] [PubMed] [Google Scholar]
- 25. Abhishek A, Doherty M. Pathophysiology of articular chondrocalcinosis—role of ANKH. Nat Rev Rheumatol 2011;7:96–104. [DOI] [PubMed] [Google Scholar]
- 26. Williams CJ, Qazi U, Bernstein M, et al. Mutations in osteoprotegerin account for the CCAL1 locus in calcium pyrophosphate deposition disease. Osteoarthritis Cartilage 2018;26:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haikal A, Everist BM, Jetanalin P, et al. Cervical CT‐dependent diagnosis of crowned dens syndrome in calcium pyrophosphate dihydrate crystal deposition disease. Am J Med 2020;133:e32–e37. [DOI] [PubMed] [Google Scholar]
- 28. Moshrif A, Laredo JD, Bassiouni H, et al. Spinal involvement with calcium pyrophosphate deposition disease in an academic rheumatology center: a series of 37 patients. Semin Arthritis Rheum 2019;48:1113–1126. [DOI] [PubMed] [Google Scholar]
- 29. Salaffi F, Carotti M, Guglielmi G, et al. The crowned dens syndrome as a cause of neck pain: clinical and computed tomography study in patients with calcium pyrophosphate dihydrate deposition disease. Clin Exp Rheumatol 2008;26:1040–1046. [PubMed] [Google Scholar]
- 30. Filippou G, Sirotti S, Cipolletta E, et al. Optimizing the use of ultrasound in calcium pyrophosphate deposition (CPPD): a review from the ground up. Gout, Urate, and Crystal Deposition Disease 2024;2:17–33. [Google Scholar]
- 31. Sirotti S, Becce F, Sconfienza LM, et al. Reliability and diagnostic accuracy of radiography for the diagnosis of calcium pyrophosphate deposition: performance of the novel definitions developed by an international multidisciplinary working group. Arthritis Rheumatol 2023;75:630–638. [DOI] [PubMed] [Google Scholar]
- 32. Abhishek A, Doherty S, Maciewicz R, et al. The association between ANKH promoter polymorphism and chondrocalcinosis is independent of age and osteoarthritis: results of a case‐control study. Arthritis Res Ther 2014;16:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cai K, Fuller A, Zhang Y, et al. Towards development of core domain sets for short term and long term studies of calcium pyrophosphate crystal deposition (CPPD) disease: a framework paper by the OMERACT CPPD working group. Semin Arthritis Rheum 2021;51:946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuller A, Cai K, Filippou G, et al. Experience and impact of crystal pyrophosphate deposition (CPPD) from a patient and caregiver perspective: a qualitative exploration from the OMERACT CPPD working group. Semin Arthritis Rheum 2021;51:655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


