Abstract
Riboflavin kinase (RFK) is essential in riboflavin metabolism, converting riboflavin to flavin mononucleotide (FMN), which is further processed to flavin adenine dinucleotide (FAD). While RFK enhances macrophage phagocytosis of Listeria monocytogenes, its role in macrophage polarization is not well understood. Our study reveals that RFK deficiency impairs M(IFN-γ) and promotes M(IL-4) polarization, both in vitro and in vivo. Mechanistically, RFK interacts with inducible nitric oxide (NO) synthase (iNOS), which requires FMN and FAD as cofactors for activation, leading to increased NO production that alters energy metabolism by inhibiting the tricarboxylic acid cycle and mitochondrial electron transport chain. Exogenous FAD reverses the metabolic and polarization changes caused by RFK deficiency. Furthermore, bone marrow adoptive transfer from high-riboflavin-fed mice into wild-type tumor-bearing mice reprograms tumor-associated macrophage polarization and inhibits tumor growth. These results suggest that targeting RFK-iNOS or modulating riboflavin metabolism could be potential therapies for macrophage-related immune diseases.
Keywords: Riboflavin kinase, Inducible nitric oxide synthase, Macrophage polarization
1. Introduction
As important innate immune cells, macrophages play a critical role in regulating immune balance and inflammatory responses [1,2]. In response to diverse stimuli in the local microenvironment, they regulate host immune responses by differentiating into a multi-dimensional phenotypic spectrum [3]. Interferon γ (IFN-γ) and/or bacterial lipopolysaccharide (LPS) induces classical activation of macrophages (M1), which produce inducible nitric oxide synthase (iNOS) and proinflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin-1 β (IL-1β); meanwhile, IL-4 or IL-13 induces alternative activation of macrophages (M2), expressing anti-inflammatory factors such as IL-10 and transforming growth factor-β (TGF-β) as well as producing arginase 1 (ARG1), resistin like alpha (Retnla), and macrophage galactose-type lectin 1 (MGL1) [2,4]. Accordingly, among tumor-associated macrophages (TAMs), M1 macrophages exert anti-tumor effects, while M2 macrophages display a tumor-promoting function. Therefore, unraveling and resolving the regulatory mechanisms of TAM phenotypes may provide clues for reprogramming TAMs into antitumor effector cells [2,4,5].
Cellular metabolism affects the activation and function of macrophages by modulating energy and biosynthetic demands, signal transduction, and other regulatory processes [[6], [7], [8], [9], [10], [11], [12]]. Riboflavin, also known as vitamin B2, is one of the essential trace vitamins for the normal activities of the body. Riboflavin deficiency can cause oral keratitis, glossitis, conjunctivitis and scrotitis, muscle and nervous system diseases, and even cancer [[13], [14], [15]]. Riboflavin kinase (RFK), a rate-limiting enzyme in flavin adenine dinucleotide (FAD) synthesis, catalyzes the phosphorylation of riboflavin to form flavin mononucleotide (FMN), which is further metabolized by FAD synthetase to FAD. FMN and FAD are cofactors of flavoproteins such as dehydrogenases, reductases, and oxidases [15]. RFK deficiency inhibits TNF-induced activation of NADPH oxidase, which uses FAD as a cofactor, and the production of reactive oxygen species (ROS), thus reducing TNF-mediated cell death or defense against Listeria monocytogenes (L.m.) [16,17]. RFK-deficient macrophages also show markedly decreased NO production in response to stimulation by L.m., IFN-γ, or combinations of TNF/IFN-γ, TNF/LPS, or IFN-γ/LPS, indicating that RFK also affects the enzyme activity of iNOS [[16], [17], [18]]. However, the effects of RFK and riboflavin metabolism on macrophage polarization and the underlying mechanism have not been reported.
iNOS (NOS2) functions as a homodimer and synthesizes NO through a two-step conversion of l-arginine, NADPH, and O2 to l-citrulline, NADP+, and NO [19]. FAD, FMN, tetrahydrobiopterin (BH4) and heme b have been shown to be essential cofactors for iNOS function [19]. The electrons of NADPH are transferred from FAD to FMN in the reductase domain and then to the heme oxygenase domain containing BH4 and iron ions. Unlike other types of NOS, such as NOS1 and NOS3, which require calcium ions for their activity, iNOS functions independently of calcium ions [[20], [21], [22]]. iNOS expression is typically induced by inflammatory factors, such as cytokines and pathogens. Once induced, iNOS continuously produces nitric oxide without the need for calcium ions as an activating factor. This characteristic allows iNOS to sustain high levels of NO production during the inflammatory response, regardless of fluctuations in intracellular calcium levels. However, the post-translational regulation of iNOS is still largely unknown.
Here, we report that RFK modulates macrophage polarization by interacting with and activating iNOS. We found that genetic ablation of RFK significantly inhibits M(IFN-γ) macrophages, but enhances M(IL-4) polarization in vitro and in vivo. Mechanistically, RFK interacts with iNOS and enhances iNOS catalytic activity and NO production, which modulate macrophage polarization by reprogramming energy metabolism. Moreover, exogenous FAD or riboflavin supplementation could regulate macrophage polarization in vitro and in vivo. Taken together, these results not only reveal the previously unknown role of RFK-mediated riboflavin metabolism in macrophage polarization, but also suggest that interfering with the RFK–iNOS interaction or modulating riboflavin metabolism could be potential therapies for macrophage-related immune diseases.
2. Material and methods
2.1. Animals
Rfkfl/fl mice were generated by Cyagen Biosciences (Guangzhou, China) using CRISPR-Cas9 technology. Lyz2-Cre mice were gifted from Dr. Xiaoyue Tan (Nankai University, China). Rfkfl/fl mice were mated with Lyz2-Cre mice to generate myeloid cell-specific RFK-knockout mice Rfkfl/flLyz2-Cre+ (termed RFK-KO-Mφ mice) and littermate Rfkfl/flLyz2-Cre- (termed RFK-WT-Mφ mice). All mice were on the C57BL/6 background. Mice were kept in individually ventilated cages within a temperature- and light-controlled room in an SPF facility and provided with unlimited food and water. All animal experiments and breeding conditions were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Tianjin Medical University.
2.2. Cell lines and primary cell culture
RAW264.7 cells (ATCC, TIB-71) and THP-1 Cell (ATCC, TIB-202) were cultured at 37 °C, 5 % CO2, in RPMI-1640 supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin-streptomycin (PS). HEK293T (ATCC, CRL-3216), Hepa 1–6 (ATCC, CRL-1830), and E0771 mouse breast cancer cells (ATCC, CRL-3461), were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10 % FBS and 1 % PS. For isolation of mouse peritoneal macrophages, each mouse was injected intraperitoneally with 1 ml of 3 % Brewer thioglycollate medium. On the 4th day, the mice were sacrificed and 8 ml PBS, pre-cooled to 4 °C and supplemented with 3 % FBS, was injected into the abdominal cavity. The peritoneal fluid was slowly extracted and centrifuged. Erythrocytes were lysed with red blood cell lysate (Solarbio) and resuspended in RPMI-1640 containing 10 % FBS. Bone marrow-derived macrophages (BMDMs) were isolated from mouse tibia and femur and cultured in RPMI-1640 containing 10 % FBS and 30 % L929 supernatant at 37 °C and 5 % CO2 for 7 days. For the macrophage polarization assay, macrophages were treated with 100 ng/ml IFN-γ (50709-MNAH, Sino Biological), 100 ng/mL IFN-γ + 100 ng/mL LPS (L4516, Sigma), 100 ng/mL IFN-γ + 100 ng/mL TNF-α (50349-MNAE, Sino Biological), or with 20 ng/mL IL-4 (404-ML, R&D Systems), 10 ng/mL IL-13 (210-13, PeproTech), 10 ng/mL IL-4 + 5 ng/mL IL-13, for the indicated times.
2.3. RNA extraction and RT-PCR analysis
RNA was extracted using TRIZOL reagent (#P118-05, Genstar), followed by reverse transcription using a reverse transcription system (#A236-04, Genstar). Subsequently, qPCR analysis was carried out using the SYBR Green PCR Mix (#B21203, Bimake) with designed primers in the ABI 7500 Detection System.
The data were normalized to expression of the control gene encoding β-actin in each individual sample. The qPCR primers used were as follows: Homo sapiens (human): RFK forward, 5ʹ-CACCTGCCTTACTTCTGCCG-3ʹ and reverse, 5ʹ-CCAACACTGGCCCAACCATAG-3ʹ; CXCL9 forward, 5ʹ-CAGTAGTGAGAAAGGGTCG-3ʹ and reverse, 5ʹ-CATCTGCTGAATCTGGGT-3ʹ; IL-6 forward, 5ʹ-ACTCACCTCTTCAGAACGAATTG-3ʹ and reverse, 5ʹ-CCATCTTTGGAAGGTTCAGGTTG-3ʹ; ARG1 forward, 5ʹ-GTGGAAACTTGCATGGACAAC-3ʹ and reverse, 5ʹ-AATCCTGGCACATCGGGAATC-3ʹ; RETNLB forward, 5ʹ-CCGTCCTCTTGCCTCCTTC-3ʹ and reverse, 5ʹ-CTTTTGACACTAGCACACGAGA-3ʹ. Mus musculus (Mus): Rfk forward, 5ʹ-CCCCACAGCCAATTTTCCTGA-3ʹ and reverse, 5ʹ-TGCTCACCACCATTTTATGGAC-3ʹ; Cxcl9 forward, 5ʹ-TCCTTTTGGGCATCATCTTCC-3ʹ and reverse, 5ʹ-TTTGTAGTGGATCGTGCCTCG-3ʹ; Il-6 forward, 5ʹ-TTGCCTTCTTGGGACTGAT-3ʹ and reverse, 5ʹ-TTGCCATTGCACAACTCTT-3ʹ; Il-1β forward, 5ʹ-GAAATGCCACCTTTTGACAGTG-3ʹ and reverse, 5ʹ-TGGATGCTCTCATCAGGACAG-3ʹ; Tnf-a forward, 5ʹ-CCTCTTCTCATTCCTGCTTG-3ʹ and reverse, 5ʹ-GTCACCCCGTCCACATCTT-3ʹ; Arg1 forward, 5ʹ-CCACAGTCTGGCAGTTGGAAG-3ʹ and reverse, 5ʹ-GGTTGTCAGGGGAGTGTTGATG-3ʹ; Mgl1 forward, 5ʹ-CAGAATCGCTTAGCCAATGTGG-3ʹ and reverse, 5ʹ-TCCCAGTCCGTGTCCGAAC-3ʹ; Mgl2 forward, 5ʹ-TTCAAGAATTGGAGGCCACT-3ʹ and reverse, 5ʹ-CAGACATCGTCATTCCAACG-3ʹ; Retnla forward, 5ʹ-CCAATCCAGCTAACTATCCCTCC-3ʹ and reverse, 5ʹ-CACTTGGTGGTTTGCTACG-3ʹ; Hk2 forward, 5ʹ-TGATCGCCTGCTTATTCACGG-3ʹ and reverse, 5ʹ-AACCGCCTAGAAATCTCCAGA-3ʹ; Pkm2 forward, 5ʹ-GCCGCCTGGACATTGACTC-3ʹ and reverse, 5ʹ-CCATGAGAGAAATTCAGCCGAG-3ʹ; Glut1 forward, 5ʹ-CAGTTCGGCTATAACACTGGTG-3ʹ and reverse, 5ʹ-GCCCCCGACAGAGAAGATG-3ʹ.
2.4. Protein isolation and immunoblot analysis
Cells were lysed using radioimmunoprecipitation assay lysis buffer supplemented with protease inhibitor cocktail. Equal amounts of protein were separated by SDS-PAGE for Western blotting. Target protein levels were analyzed with specific antibodies. Visualization was performed using an ECL chemiluminescence detection kit (Millipore). The antibodies used were as follows: RFK (1:1000; #15813-1-AP, Proteintech); iNOS (1:1000; #13120S, CST); ARG1 (1:1000; #93668S, CST); β-actin (1:1000; #sc-47778, Santa Cruz); Tubulin (1:1000; #ab7792, Abcam); FLAG (1:1000; #20543-1-AP, Proteintech); IL-1β (1:1000; #ab9722, Abcam); GST (1:1000; #10000-0-AP, Proteintech); His (1:1000; #AH367, Beyotime); HA (1:1000; #3724, CST); p-JAK1 (1:1000; #74129S, CST); JAK1 (1:1000, #3344S, CST); p-JAK2 (1:1000; #8082S, CST); JAK2 (1:1000, #3230S, CST).
2.5. Flow cytometry
Tumor tissue or Peritoneal cells were dissociated into a single cell suspension, and stained with specific surface antibodies in staining buffer containing 2 % BSA: Fixable Dye (#423105, Biolegend); anti-CD45-PE (#103105, Biolegend); anti-CD170-FITC (#155504, Biolegend); anti-Ly6C-Alexa fluor 488 (#127623, Biolegend); anti-Ly6G-APC-Cy7 (#127623, Biolegend); anti-F4/80-PE-Cy7 (#157307, Biolegend); anti-F4/80-PE-Cy5 (#123112, Biolegend); anti-CD11b-APC (#101211, Biolegend); and flow cytometric analysis or sorting was then performed using a digital BD Caliber flow cytometer (BD Biosciences). The data were analyzed by FlowJo (Tree Star) software.
2.6. Enzyme-linked immunosorbent assay (ELISA)
Supernatants of cells were collected and measured using the indicated ELISA kits. IL-6 (#EK0411, Boster); IL-1β (#EK0394, Boster); TNF-α (#EK0527, Boster); CCL17 (#EK0682, Boster); CCL22 (#EK0447, Boster); the assays were performed in accordance with the reagent manufacturer's protocol.
2.7. Plasmid transfection, RNA interference, and reagents
Expression plasmids for RFK and iNOS were constructed using standard molecular biology techniques and cloned into the pLenti-3 × Flag/HA vector. Point mutations (RFK/iNOS) were generated by site-directed mutagenesis; a plasmid encoding wild-type RFK or iNOS protein was used as template. All RFK and iNOS mutant constructs were generated using Phusion Hot Start II DNA Polymerase (Thermo Fisher Scientific). The plasmids with different structure domains of iNOS (1–500/1–970/501–1144/971–1144) were generated based on pLenti-iNOS-HA. pGEX-4T-1-GST-RFK was generated by cloning RFK cDNA into the pGEX-4T-1-GST vector, which was linearized with EcoRI and Xho1 (Thermo Fisher Scientific). pET28a-His-iNOS was cloned by PCR of an iNOS cDNA fragment into the pET28a-His vector linearized with EcoRI (Thermo Fisher Scientific). All the plasmids and mutations that were generated were confirmed by sequencing. RAW264.7 cells, HEK293T cells, and primary macrophages were transfected with the appropriate plasmids and siRNA using Lipofectamine 2000/RNAiMAX (Invitrogen). The siRNA sequences were as follows: Mus-siRfk: 5ʹ-CCAGGCACTTAAACATAATTT-3ʹ; Hum-siRfk: 5ʹ-CCCATATTACAAGAATACGAA-3ʹ. The reagents used were as follows: Phorbol 12-myristate 13-acetate (PMA, #HY-18739, MCE); FAD (#HY-B1654, MCE); DETA NONOate (#HY-136278, MCE); Aminoguanidine hydrochloride (#HY-B1041, MCE); NAC (#HY-B0215, MCE); GSH (#G1404, Sigma-Aldrich); Ruxolitinib (#HY-50856, MCE).
2.8. Chitin administration
Chitin (#C9752, Sigma-Aldrich) was washed twice with PBS and then sonicated on ice. The suspension was filtered through a 100 μm filter and diluted to 30 mL with PBS. Each mouse was injected intraperitoneally with approximately 3 μg of chitin. Two days after the injection, peritoneal cells were harvested for flow cytometry and gene expression analysis.
2.9. CCl4-induced liver fibrosis model
Liver fibrosis was induced by intraperitoneal injection of CCl4 (1.0 ml/kg: #C27710, Acmec Biochemical), diluted 1:3 in corn oil, 3 times a week for 4 weeks (total 12 injections). Serum alanine aminotransferase (ALT) (#C009-2-1) and aspartate aminotransferase (AST) (#C010-2-1) were measured using a commercial kit (Nanjing Jiancheng Bioengineering Institute). For histological analysis, livers were dissected and fixed in 4 % paraformaldehyde solution overnight before staining with hematoxylin–eosin for routine examination or with Sirius red staining (#G1471, Solarbio) for visualization of fibrotic deposition. Fibrotic areas were quantitated by ImageJ software (NIH). Liver sections embedded in OCT were stained for immunofluorescence. Liver RNA was extracted for qPCR detection of fibrosis-related cytokine gene expression.
2.10. Tumor model and bone marrow adoptive transfer model
For the peritoneal tumor model, 1 × 105 B16–F10 melanoma cells were intraperitoneally injected into male and female C57BL/6 mice aged 6 weeks (n = 5 mice for each group). F4/80+ macrophages were sorted from peritoneal lavages collected on day 0, 3, and 6, respectively by flow cytometry and further analyzed by qPCR.
RFK-KO-Mφ and RFK-WT-Mφ mice aged 6–12 weeks were subcutaneously injected in the flank region with 1 × 106 E0771 or hepa1-6 tumor cells (n = 6–10 in each group). Tumor volume was measured every 2 days using the formula (tumor volume = ½ (L × W2)). Mice were sacrificed 24–25 days after subcutaneous injection of E0771 or hepa1-6 cells for tumor harvesting.
To establish a mouse model for bone marrow adoptive transfer with high levels of riboflavin, we followed two procedures. First, C57BL/6 mice were fed with a high riboflavin diet (#M21063001, BioPike) or normal diet for 2 weeks, and their bone marrow was collected. Second, C57BL/6 mice were subcutaneously injected with 1 × 106 E0771 cells per mouse (n = 6). Tumor volume was measured every 2 days from the 5th day after E0771 cell inoculation. After 7 days, bone marrow cells (5 × 106) were intraperitoneally injected into E0771 cell-inoculated mice (once every 5 days, 5 times [[23], [24], [25], [26]]. After 31 days, the mice were sacrificed.
2.11. FAD and nitric oxide concentration measurements
Mouse serum and intracellular levels of FAD were assessed using the FAD Assay Kit (#ab204710, Abcam) which implements a colorimetric assay in accordance with the manufacturer's instructions. Protein concentrations were then normalized using the Micro BCA Protein Assay Kit (Thermo Scientific, 23235).
NO in the cell supernatant were assessed using the NO Assay Kit (#A013-2-1, Nanjing Jiancheng Bioengineering Institute), which implements a colorimetric assay in accordance with the manufacturer's instructions. Protein concentrations were then normalized using the Micro BCA Protein Assay Kit (Thermo Scientific, 23235). Intracellular levels of NO were quantified by flow cytometry using a DAF-FM DA fluorescent probe (Beyotime, S0019).
2.12. Measurements of iNOS, PDH, IDH2, and ACO2 activity
Cellular iNOS activity was measured with the iNOS Activity Assay Kit (#Ab211083, Abcam), while PDH (#BC0385, Solarbio), IDH2 (#BC2165, Solarbio), and ACO2 (#BC4480, Solarbio) activity was evaluated using the corresponding Activity Assay Kit. The assays were conducted following the manufacturers’ protocols.
2.13. Immunofluorescence staining
Liver tissue or tumors were embedded in O.C.T. The entire cryomold was frozen in a dry ice bath and prepared for cryosection. Immunofluorescence staining was performed with primary antibodies against F4/80 (#ab16911, Abcam), CD11b (#ab133357, Abcam), ARG1, and IL-1β, on 5-μm-thick frozen sections. Sections were imaged using Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies and a fluorescence microscope, and images were analyzed using ImageJ software.
2.14. Immunohistochemistry
Tumors were collected and fixed in 4 % paraformaldehyde for 24–48 h, followed by dehydration in an ethanol gradient and embedding in paraffin. Subsequently, the tumors were sliced into 5-μm sections for immunostaining using primary and secondary antibodies. The nucleus was stained with DAPI, and fluorescence microscopy was employed for imaging analysis. The antibodies used were as follows: CD11b (1:200; #ab133357, Abcam), CD4 (1:200; #14-9766-82, Invitrogen), CD8a (1:200; #14-0808-82, Invitrogen), Foxp3 (1:200; # 14-5773-80, Invitrogen), F4/80 (1:200; #ab16911, Abcam), IL-1β (1:200; #ab9722, Abcam), ARG1 (1:1000; #93668S, CST).
2.15. Mitochondrial and glycolysis stress test assay
Peritoneal macrophages were seeded into 24-well Seahorse XFe-24 assay plates (Agilent) and then subjected to the indicated stimulations, after which they were washed and cultured in XF RPMI Medium (pH 7.4) for 1 h. The Seahorse XFe-24 software (Agilent) automatically calculated and recorded the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) through mitochondrial or glycolysis stress testing, respectively. To determine ATP production for OCR, 2.0 μM oligomycin (#HY-16589, MCE) was used. The maximum respiration rate was measured using 2.0 μM FCCP (#HY-100410, MCE). Next, 1.0 μM antimycin A (#MS0070-10MG, MKBio)/rotenone (#HY-B1756, MCE) was administered to cells to specifically measure non-mitochondrial respiration. Measurements were taken after each mitochondrial inhibitor was added and before introducing the subsequent inhibitor. To evaluate ECAR, we determined the fundamental glycolytic potential using 10 μM glucose. The maximum glycolytic capacity was measured with 1.5 μM oligomycin. The cells were treated with 50 μM of 2-deoxy-d-glucose (2-DG) (#ST1024, Beyotime) to ensure that the ECAR obtained in the experiment came from the glycolytic pathway. Measurements were captured after incorporating each glycolysis inhibitor and prior to the subsequent inhibitor being administered.
2.16. Mitochondrial respiratory complex (MRC) activity
Mitochondria were isolated from PEMs. A Micro BCA Protein Assay Kit (Thermo Scientific, 23235) was used to quantify mitochondrial samples. The activity of MRC complexes I–V was assessed using commercial kits (Abbkine KTB1850/KTB1860/KTB1870/KTB1880/KTB1890) according to the manufacturer's instructions.
2.17. Recombinant protein purification
Purified iNOS was produced from BL21 (DE3) competent E. coli cells that were transformed with the pET28a-His-iNOS plasmid. The cultures were grown at 37 °C until they reached an OD of 0.6 at 600 nm, and were then induced with 0.1 mM IPTG for 18 h at 18 °C. The resulting cell pellets were collected and subjected to sonication in a lysis buffer containing 20 mM Tris (pH 7.5), 0.1 % Triton X-100, 1 mg/mL lysozyme, and 1 μg/mL DNase. The proteins were then enriched using TALON Metal Affinity Resin (Takara, 635503), eluted using 100 mM imidazole, and finally concentrated into a buffer solution of 25 mM Tris (pH 8.0), 0.4 M NaCl.
GST-RFK was produced from BL21(DE3) competent E. coli cells transformed with pGEX-4T-1-GST-RFK and induced with 0.1 mM IPTG for 18 h at 18 °C. Cell pellets were lysed as described above. Proteins were enriched using GST-Sefinose resin (Sangon, C600031). GST-fusion proteins were eluted with 10 mM reduced glutathione in 50 mM Tris (pH 8.0). GST protein was purified from pGEX-4T-1-GST by the same procedure as for GST-RFK. Eluted proteins were dialyzed overnight against PBS before use in pulldown.
2.18. GST/His pulldown
The cell pellets of E. coli that were transformed with pGEX-4T-1-GST-RFK or pET28a-His-iNOS were collected and lysed as described above. After centrifugation at 10,000 g for 15 min and incubation with GST-Sephinose resin for 4 h, proteins were eluted and subjected to SDS-PAGE.
2.19. Biotin–streptavidin pulldown assay
Biotin-RFK-peptide (aa1-30/aa60-92/aa101-130) or biotin-peptide-scramble (Synbio Technologies) was incubated with streptavidin beads with rotation at 4 °C for 2 h, followed by incubation with purified iNOS or HEK293T lysates overexpressing HA-iNOS at 4 °C overnight. The beads were then washed, subjected to immunoblotting, and assessed for the expression of iNOS.
2.20. Immunoprecipitation and mass spectrometry analysis
RFK-Flag was overexpressed in 293T cells, the immunoprecipitation used an anti-Flag antibody, followed by the elution using an 3 × Flag peptide. The Flag-immunoprecipitated material was Coomassie-stained. Portions of the Coomassie-stained gel were excised and analyzed by mass spectrometry.
2.21. Statistical analyses
The figure legends indicate the sample size and level of significance. Statistical significance was determined using a two-tailed Student's t-test. One-way analysis of variance (ANOVA) was used to analyze the difference between more than two groups. Fluorescence imaging was conducted in a blinded manner. The mean values ± SEM of all quantitative data are presented from a minimum of three independent experiments. Data were analyzed using GraphPad Prism software (GraphPad Software). A significance level of less than 0.05 was used to determine statistical significance (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Results with a p-value of 0.05 or greater were considered not significant (NS).
3. Results
3.1. RFK promotes M(IFN-γ) but inhibits M(IL-4) polarization through its enzyme activity
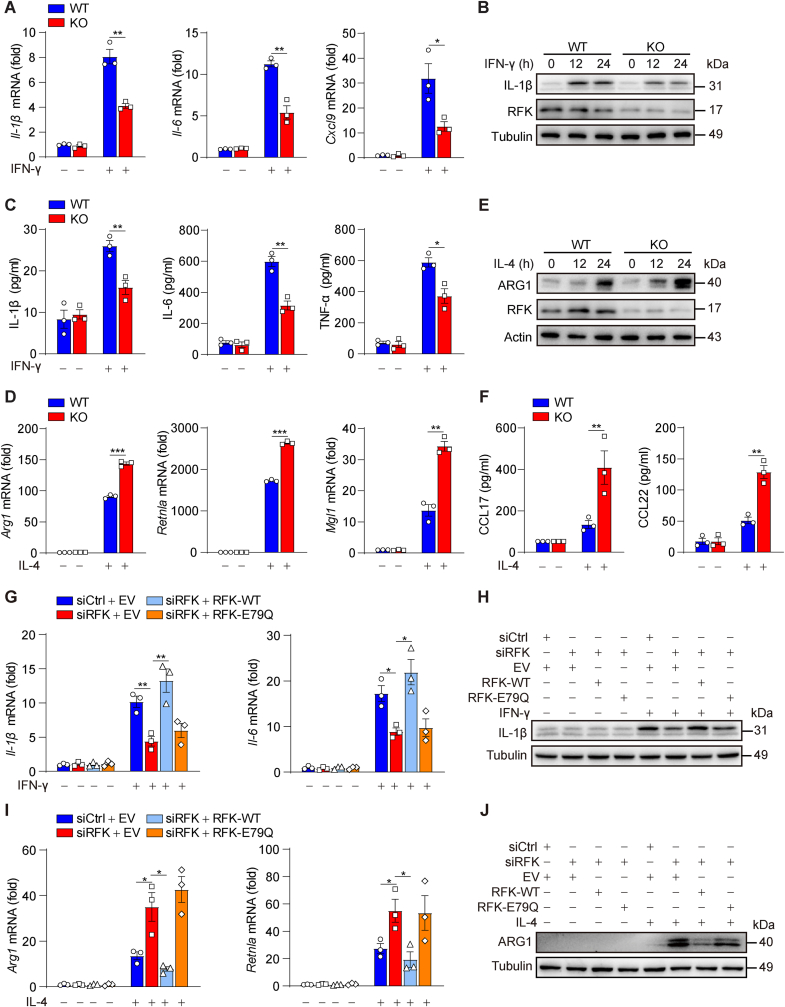
We first investigated whether the expression and function of RFK, the key enzyme in riboflavin metabolism, are related to macrophage polarization. Primary peritoneal elicited macrophages (PEMs) (Figs. S1A and S1B) and bone marrow-derived macrophages (BMDMs) (Figs. S1C and S1D) were stimulated with various known M1-activating agents, including IFN-γ alone, IFN-γ plus LPS, and IFN-γ plus TNF-α, or with M2-activating cytokines, including IL-4, IL-13, and IL-4 plus IL-13. Consistent with previous reports [16,17], RFK is constitutively expressed in macrophages (Figs. S1A–F). Moreover, the expression of RFK was reduced under M1 polarization models and elevated under M2 polarization models (Figs. S1A–F). An in vivo decrease of RFK was also observed in sorted peritoneal macrophages of peritoneal B16–F10 tumor-bearing mice (Fig. S1G), which suggested that RFK is an important regulator in macrophage polarization. To investigate the effect of RFK on macrophage polarization, we developed Rfkfl/flLyz2-Cre+ (RFK-KO-Mφ) mice, which have a myeloid cell-specific Rfk deletion compared with their littermate Rfkfl/flLyz2-Cre- (RFK-WT-Mφ) mice (Figs. S1H–K). The results showed that there was no difference in the differentiation or number of primary peritoneal macrophages between RFK-KO-Mφ mice and RFK-WT-Mφ mice, indicating that myeloid RFK knockout had no significant effect on bone marrow development in mice (Fig. S1L–N). Next, we found that the expression of M1 marker genes including IL-1β, IL-6, Cxcl9, and TNF-α was suppressed in Rfk-ablated BMDMs (Fig. 1A–C). Conversely, RFK deletion upregulated the expression of M2-specific marker genes including ARG1, Retnla, MGL1, and CC chemokine ligands 17 (CCL17) and 22 (CCL22) (Fig. 1D–F). Consistent with these findings, similar suppression of M1 markers and upregulation of M2 markers were observed in RAW264.7 cells and THP-1–differentiated macrophages in which RFK expression was inhibited with a specific interfering RNA (siRNA) (Figs. S2A–F).
Fig. 1.
RFK promotes M(IFN-γ) but inhibits M(IL-4) polarization through its enzyme activity. A–C Bone marrow-derived macrophages (BMDMs) of Rfkfl/flLyz2-Cre- (WT) and Rfkfl/flLyz2-Cre+ (KO) mice were stimulated with IFN-γ for 12 h or the indicated times, followed by detection of the intracellular or secreted levels of M1 marker expression by qPCR (A) or Western blot (B) or ELISA analysis (C). D–F RFK-WT and RFK-KO BMDMs were stimulated with IL-4 for 24 h or the indicated times, followed by detection of the intracellular or secreted levels of M2 marker expression by qPCR (D) or Western blot (E) or ELISA analysis (F). G–J RAW264.7 cells were transfected with siControl or siRFK, together with an RNA interference (RNAi)-resistant FLAG-tagged WT-RFK ectopic expression plasmid or an RFK enzyme activity mutation plasmid (E79Q), and then stimulated either with IFN-γ for 12 h to detect M1 marker expression, or with IL-4 for 24 h to detect M2 marker expression, by qPCR (G, I) or Western blot (H, J). Data are presented as mean ± SEM. n = 3 per group (A, C, D, F, G, and I). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Conversely, M1 markers were upregulated and M2 markers were suppressed in RFK-overexpressing RAW264.7 cells (Figs. S2G–I). Furthermore, RFK overexpression significantly induced suppression of M1 markers in siRNA-mediated RFK-silenced RAW264.7 cells. However, the levels of M1 markers in cells that overexpressed RFK-E79Q (an enzyme-inactivating mutation) was similar to those in RFK-deficient RAW264.7 cells, indicating that the enzyme activity of RFK is essential for its promotion of macrophage M(IFN-γ) and M(LPS + IFN-γ) polarization (Fig. 1G, H and S2J, K). Moreover, RFK enzyme activity is critical for the suppression of macrophage M(IL-4) polarization (Fig. 1I and J). These findings together indicate that RFK regulates macrophage polarization through its enzyme activity.
3.2. RFK regulates macrophage polarization in vivo
One of the important functions of M(IL-4) polarized macrophages is to recruit eosinophils through ARG1, which can be modeled by chitin administration [27]. Two days after intraperitoneal injection of chitin to RFK-WT-Mφ and RFK-KO-Mφ mice, eosinophil populations in the peritoneal cavity were detected by flow cytometry. Myeloid RFK deficiency increased peritoneal eosinophil recruitment and increased Arg1 expression in peritoneal macrophages (Figs. S3A–D), indicating that RFK plays a negative regulatory role in the M(IL-4) phenotype. Another important function of M(IL-4) macrophages in vivo is to promote the regression of fibrosis by enhancing expression of matrix metalloproteinases, inhibiting CD4+ T cell responses via ARG1, phagocytosing damaged hepatocytes, and inducing senescence and apoptosis of activated hepatic stellate cells [[27], [28], [29], [30], [31], [32]]. We further investigated the role of myeloid RFK in regulating the M(IL-4) phenotype in the CCl4-induced hepatic fibrosis model, and found that the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were lower in RFK-KO-Mφ mice than in RFK-WT-Mφ mice (Fig. S3E). Furthermore, myeloid RFK-deficient mice showed alleviated hepatic injury and fibrosis (Figs. S3F and G). In addition, we found that myeloid RFK deficiency significantly enhanced the proportion of ARG1+ macrophages in the liver (Fig. S3H), which further suggested that RFK plays an important role in inhibiting the function of M(IL-4) macrophages in vivo.
To further explore the effect of myeloid RFK on the polarization of tumor-associated macrophages (TAMs) in vivo, RFK-WT-Mφ and RFK-KO-Mφ mice were challenged by inoculation of murine hepatocellular carcinoma cells (Hepa 1–6) and Mouse Breast Cancer Cells (E0771). RFK-KO-Mφ mice showed increased tumor growth (Fig. 2A, B and S4A, B). Immunohistochemical analysis revealed fewer CD11b+ myeloid cells, CD4+, and CD8a+ T cells, alongside more FoxP3+ regulatory T cells in RFK-KO-Mφ tumors compared to RFK-WT-Mφ tumors (Fig. 2C and S4C). Fluorescence-activated cell sorting (FACS) analysis revealed reductions in CD11b+Ly6G−Ly6Chigh monocytes and F4/80+ macrophages, while CD11b+Ly6G+Ly6Clow neutrophils remained unchanged in RFK-KO- Mφ tumors (Fig. 2D and E). Furthermore, qPCR analysis of isolated F4/80+ macrophages exhibited decreased Il-1β and increased Arg1 expression (Fig. 2F), as confirmed by immunohistochemical analysis (Figs. S4D and E) and immunofluorescence staining (Figs. S4F and G). These findings suggest that myeloid RFK deficiency leads to a shift from M1 to M2 polarization in TAMs.
Fig. 2.
RFK regulates macrophage polarization in vivo. A–F RFK-WT-Mφ and RFK-KO-Mφ mice were subcutaneously injected with 2 × 106 Hepa1-6 cells per mouse. Tumor volume was calculated every 2 days beginning 5 days after cell inoculation (A). Excised tumor and tumor weights on the 25th day are shown (B). Immunohistochemical staining were performed on tumor sections of RFK-WT-Mφ and RFK-KO-Mφ mice with the indicated antibodies; representative images are shown. Scale bars, 50 μm. Histological semiquantification was performed (C). The proportions of monocytes (P1, CD11b+Ly6G−Ly6Chigh) and neutrophils (P2, CD11b+Ly6G+Ly6Clow) in tumor tissues of RFK-WT-Mφ and RFK-KO-Mφ mice were analyzed by flow cytometry (D). Representative fluorescence-activated cell sorting (FACS) and quantification of macrophages (P1, F4/80+CD11b+) sorted from tumor tissues of RFK-WT-Mφ and RFK-KO-Mφ mice (E), and the expression of Il-1β and Arg1 was detected by qPCR (F). Data are presented as mean ± SEM. n = 6 per group (D–F); n = 9 per group (A and B); n = 10 per group (C). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.3. RFK modulates macrophage polarization through FAD
As a key enzyme in riboflavin metabolism, RFK is essential in providing FAD [15]. We then explored whether FAD might be responsible for modulating macrophage polarization. Interestingly, IFN-γ stimulation enhanced intracellular FAD at 15 and 30 min, while IL-4 stimulation did not change the level FAD at either time points in PEMs (Fig. S5A). Interestingly, pretreating cells with the JAK inhibitor ruxolitinib significantly suppressed the IFN-γ-induced increase in FAD, suggesting that activation of the IFN-γ-JAK signaling pathway may be crucial for enhancing RFK activity (Fig. S5B). Addition of exogenous FAD supported M(IFN-γ) but suppressed M(IL-4) polarization (Fig. 3A–D). Moreover, FAD treatment restored the macrophage polarization phenotype of RFK-KO BMDMs to the level seen in RFK-WT BMDMs (Fig. 3A–D). Consistently, supplementation of FAD increased the level of FAD in RFK-WT BMDMs and the difference in FAD level between RFK-WT and RFK-KO BMDMs decreased (Fig. S5C).
Fig. 3.
RFK modulates macrophage polarization through FAD. A–D RFK-WT and RFK-KO BMDMs were pretreated with FAD for 6 h and then stimulated with IFN-γ for 12 h or IL-4 for 24 h, to detect M1 or M2 marker expression by qPCR (A, C) or Western blot (B, D). Data are presented as mean ± SEM. n = 3 per group (A, C). NS, not significant (p ≥ 0.05), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
We also found a consistent phenomenon in RFK-deficient RAW264.7 cells and PEMs. RFK deletion inhibited M(IFN-γ) and M(LPS + IFN-γ) polarization while promoting M(IL-4) polarization; however, this effect was rescued upon replenishing FAD (Figs. S5D–I). These results indicate that RFK regulates macrophage polarization through its downstream metabolite FAD.
3.4. RFK regulates macrophage polarization through NO
RFK has been shown to physically and functionally couple TNFR1 to NADPH oxidase, enhancing FAD incorporation into NADPH oxidase, which boosts NADPH oxidase activity and increases TNF-induced reactive oxygen species (ROS) production [17]. Since IFN-γ is known to induce ROS production via NADPH oxidase [[33], [34], [35]], we investigated whether RFK regulates M(IFN-γ) polarization through ROS. Both antioxidants NAC and glutathione (GSH) reduced the mRNA expression of Il-6 and Il-1β, suggesting that ROS plays a role in M(IFN-γ) polarization (Fig. S6A). However, neither NAC nor GSH could counteract the up-regulation of M1 marker expression caused by RFK overexpression (Fig. S6A). This indicates that RFK likely regulates macrophage polarization through mechanisms beyond ROS.
It has been reported that FMN and FAD are co-factors for iNOS and that RFK deficiency impairs NO production [[16], [17], [18]]. The level of NO was lower in RFK-KO than in RFK-WT BMDMs, whether stimulated by IFN-γ or LPS + IFN-γ or IL-4 (Fig. 4A, and S6B, C). We also found that the addition of the NO donor diethylamine (DETA) NONOate upregulated M1 markers and suppressed M2 markers, and rescued the macrophage polarization phenotype induced by RFK deficiency (Fig. 4B–E, and S6D, E). Conversely, treatment with the NO synthase inhibitor aminoguanidine hydrochloride (AG), which inhibits the activity of the major nitric oxide synthase iNOS in macrophages, reduces M1 marker expression, promotes M2 markers, and rescues the macrophage polarization phenotype induced by RFK knockout or overexpression (Fig. 4F–I and S6F). These findings together indicate that RFK regulates macrophage polarization through NO.
Fig. 4.
RFK regulates macrophage polarization through NO. A RFK-WT and RFK-KO BMDMs were stimulated with LPS + IFN-γ or IFN-γ for the indicated times and the concentration of NO in the cell culture supernatant was then determined. B–E RFK-WT and RFK-KO BMDMs were pretreated with NO donor for 6 h and then stimulated with IFN-γ for 12 h or IL-4 for 24 h, followed by detection of M1 or M2 marker expression by qPCR (B, D) or Western blot (C, E). F–I RFK-WT and RFK-KO BMDMs were pretreated with AG for 6 h and then stimulated with IFN-γ for 12 h or IL-4 for 24 h, followed by detection of M1 or M2 marker expression by qPCR (F, H) or Western blot (G, I). Data are presented as mean ± SEM. n = 3 per group (A, B, D, F, and H). NS, not significant (p ≥ 0.05), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.5. RFK deficiency modulates energy metabolism through NO-mediated remodeling of the TCA cycle and mitochondrial electron transport chain
Cellular energy metabolism is critical for macrophage polarization [7,11,36,37]. RFK deficiency upregulated expression of glycolytic genes including hexokinase 2 (HK2), glucose transporter type 1 (GLUT1), and pyruvate kinase M2 (PKM2) (Figs. S7A and B). Using a Seahorse extracellular flux analyzer to determine extracellular acidification rate (ECAR) or oxygen consumption rate (OCR), we found that RFK deficiency significantly decreased ECAR, and increased OCR in IFN-γ- or IL-4-treated PEMs (Fig. 5A–D and Figs. S7C–F). We further found that blocking mitochondrial ATP synthase activity with oligomycin (OM), and 2-deoxy-d-glucose (2-DG)-mediated blocking of glycolytic flux, abolished energy metabolism reprogramming (Figs. S8A–F) and macrophage polarization phenotypes (Figs. S8G and H) induced by RFK deficiency, indicating that RFK modulates macrophage polarization through glucose-fueled energy metabolism. Furthermore, we found that RFK deficiency increased the enzymatic activities of PDH (pyruvate dehydrogenase), IDH2 (isocitrate dehydrogenase 2), and ACO2 (aconitase 2) (Fig. 5E–G), decreased citrate concentration (Fig. 5H), and enhanced the activities of mitochondrial electron transport complexes (Fig. 5I and J).
Fig. 5.
RFK deficiency modulates energy metabolism through NO-mediated remodeling of the TCA cycle and mitochondrial electron transport chain. A–D Analysis of extracellular acidification rate (ECAR) or oxygen consumption rate (OCR) of RFK-WT and RFK-KO PEMs by Seahorse assay. WT and KO PEMs were pretreated with NO donor for 6 h and then stimulated with IFN-γ for 12 h or IL-4 for 24 h. ECAR was analyzed by addition of glucose, oligomycin, and 2-deoxy-d-glucose (A, B). OCR was analyzed by addition of oligomycin, FCCP, and antimycin A plus rotenone (C, D). E–J RFK-WT and RFK-KO PEMs were pretreated with NO donor for 6 h and then stimulated with IFN-γ for 12 h or with IL-4 for 24 h, followed by analysis of PDH, IDH2, and ACO2 activities (E–G), citrate concentration (H), or mitochondrial complex activities (I, J). Data are presented as mean ± SEM. n = 3 per group (E–J); n = 4 per group (B and D); n = 5 per group (A and C). NS, not significant (p ≥ 0.05), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
NO has been reported to cause glucose metabolism reprogramming and mitochondrial dysfunction by inhibiting the enzymatic activities of PDH, ACO2, IDH, and mitochondrial electron transport complexes [9,38,39]. We then explored whether NO plays a role in energy metabolism reprogramming induced by RFK deficiency. FAD supplementation fully restored the energy metabolism changes caused by RFK deficiency, while further addition of NO synthase inhibitor AG negated the effects of FAD supplementation in RFK-deficient cells (Figs. S7A–F). Additionally, we found that the addition of NO donor upregulated ECAR (Fig. 5A and B), suppressed OCR (Fig. 5C and D) and the enzyme activities of PDH, ACO2, and IDH2, and rescued the alterations in TCA cycle remodeling (Fig. 5E–H) and mitochondrial electron transport complexes induced by RFK deficiency (Fig. 5I and J). FAD modulates the protein stability of HIF-1α, which is critical for controlling immune cell metabolism and functions [40,41]. We found no obvious changes in HIF-1α expression in RFK-KO BMDMs treated with or without NO donor compared with that in RFK-WT BMDMs (Fig. S8I). These results suggest that RFK reprograms macrophage energy metabolism through NO.
3.6. RFK interacts with iNOS to boost iNOS activity and reprogram macrophage energy metabolism and polarization phenotypes
The riboflavin metabolites FMN and FAD are cofactors of iNOS [18]. Using ClusPro protein docking analysis based on the indicated crystal structures (Fig. S9A), we next asked whether RFK, the key enzyme in the riboflavin metabolism pathway, interacted with iNOS to boost iNOS activity and NO production. We conducted tandem-affinity purification of Flag-tagged RFK expressed in 293T cells. The immunopurified material was analyzed by mass spectrometry (MS) to identify RFK protein partners, including iNOS. Notably, many of the identified partners are involved in JAK-STAT-related signaling pathways, such as JAK, STAT, SRC, and RAC1 (Fig. S9B). Combined with the results in Fig. S5B, this further suggest that the JAK-STAT signaling pathway may regulate RFK activity. Immunoprecipitation assays confirmed that both ectopically expressed and endogenous RFK interacted with iNOS (Fig. 6A and B). Purified recombinant RFK and iNOS proteins could also interact with each other (Fig. 6C and D). We also analyzed the interaction at various time points after cytokine treatment. IFN-γ stimulation increased RFK-iNOS interaction at short time period (5–60 min) and long time period (6–12 h) (Figs. S9C and D), whereas IL-4 did not significantly change the interaction at either time period (Figs. S9E and F). We then further explored which domain of iNOS interacted with RFK, and found that iNOS aa (1–500) interacted with RFK (Fig. 6E and S9G-I).
Fig. 6.
RFK interacts with iNOS and boosts iNOS activity. A Immunoprecipitation of exogenous iNOS-hemagglutinin (HA) with an anti-HA antibody and Western blot analyses with the indicated antibodies were performed using HEK293T cell extracts. B Immunoprecipitation of endogenous RFK with an anti-iNOS antibody and Western blot analyses with the indicated antibodies were performed using RAW264.7 cell extracts. C, D A His pulldown assay (C) or a GST pulldown (D) assay was performed by mixing bacterially expressed His-iNOS and GST-RFK. E HEK293T cells were transfected with plasmids expressing RFK and iNOS-WT, iNOS (aa 1–500), or iNOS (aa 501–1144); immunoprecipitation was conducted with an anti-HA antibody and Western blot analyses with the indicated antibodies. F, G A streptavidin pulldown assay was performed. Biotin-tagged scrambled, RFK (aa 1–30), RFK (aa 60–92), or RFK (aa 100–130) peptides were incubated with lysates from iNOS-HA overexpressing HEK293T cells (F) or bacterially expressed His-iNOS (G). H iNOS and RFK mutants were expressed in HEK293T cells. Immunoprecipitation of exogenous RFK-FLAG mutants with an anti-FLAG antibody and Western blot analyses with the indicated antibodies were performed. I, J RFK-WT and RFK-KO PEMs were transfected with plasmids expressing RFK or the indicated RFK mutation, and then stimulated with IFN-γ for 12 h. Immunoprecipitation with an anti-iNOS antibody and Western blot analyses were performed with the indicated antibodies. iNOS activity was analyzed normalized to the amount of immunoprecipitated iNOS, determined by densitometry of western blots. K, L RFK-WT and RFK-KO PEMs were transfected with an RNA interference (RNAi)-resistant RFK ectopic expression plasmid, RFK-S64A or RFK-Y91A mutation plasmid, pretreated with FAD/FAD + AG/FAD + 2-DG for 6 h, and then stimulated with IFN-γ for 12 h or with IL-4 for 24 h, followed by detection of M1 (K) or M2 (L) marker expression by qPCR. Data are presented as mean ± SEM. n = 3 per group (the upper panels of J, and K, L); n = 4 per group (the lower panels of J). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
We next used the ConSurf algorithm (https://consurf.tau.ac.il) to evaluate the amino acid conservation and exposure in the structural model of RFK, and synthesized biotin-labeled peptides RFK (aa 1–30), RFK (aa 60–92), and RFK (aa 101–130) to identify the key functional domain of RFK for its interaction with iNOS (Fig. S9J). Using a streptavidin pulldown assay, we found that RFK (aa 60–92) but not the other two peptides could interact with cellular iNOS (Fig. 6F) and recombinant iNOS protein (Fig. 6G). We tried to pinpoint the interaction site at the amino acid level. According to the conservation and exposure of amino acids, we constructed RFK mutants, Immunoprecipitation assays showed that mutation at S64 and Y91 but not at G66, P69, Y70, E79, or F86 affects the affinity of RFK with iNOS (Fig. 6H and S9K). Furthermore, the activity of iNOS and the level of NO mediated by S64A and Y91A was much lower than that mediated by WT-RFK (Fig. 6I, J and S9L). These results indicate that S64 and Y91 of RFK is necessary for RFK binding with iNOS and thus for regulating the activity of RFK-bound iNOS.
Furthermore, overexpression of RFK restored the M1 and M2 markers of RFK-deficient macrophages to their levels in WT macrophages, while its S64A and Y91A mutant only partially rescued the phenotype, suggesting that the S64 and Y91 site is important for RFK regulation of macrophage polarization (Fig. 6K and L). Supplementation with FAD restored the macrophage polarization phenotype in S64A and Y91A mutant-transfected KO PEMs, and this restoration was blocked by the NOS inhibitor AG and the glucose metabolism inhibitor 2-DG (Fig. 6K and L). These results indicate that the RFK S64 and Y91 sites are critical for RFK's binding to iNOS, which promotes its nitric oxide synthase activity and thus regulates glucose metabolism reprogramming and macrophage polarization.
3.7. High riboflavin diet inhibits tumor progression
To further investigate the effects of RFK-mediated riboflavin metabolism on macrophage polarization and tumor progression in vivo, bone marrow cells (BMs) derived from mice fed with control or high riboflavin diet were adoptively transferred to mice, inoculated 7 days previously with E0771 cells, every 5 days for a total of 5 transfers (Fig. 7A). We found that BMs of mice fed with high riboflavin diet, which has a higher level of the downstream metabolite FAD, expressed higher levels of M1 markers but lower levels of M2 markers compared with BMs of the control group (Fig. 7B–E). Further subcutaneous tumor models demonstrated that mice receiving adoptively transferred BMs from a high riboflavin diet, had smaller tumor volumes and weights compared to the control group (Fig. 7F and G). Additionally, immunohistochemical analysis revealed that these tumors had a higher presence of F4/80+ macrophages with an elevated M1 phenotype and a reduced M2 phenotype compared to tumors from control mice (Fig. 7H). All these results indicate that riboflavin metabolism can reprogram macrophage polarization and inhibit tumor growth in vivo (Fig. 8).
Fig. 7.
High riboflavin diet inhibits tumor progression. A–H Schematic overview of bone marrow (BM) adoptive transfer, tumor induction, and analysis (A). C57BL/6 mice were fed normal or high riboflavin diet for 2 weeks then the FAD concentration in BMs was measured (B). BMs were then stimulated with either LPS + IFN-γ or IFN-γ for 12 h to detect M1 marker expression (C, D), or stimulated with IL-4 for 24 h to detect M2 marker expression (E) by qPCR. C57BL/6 mice were subcutaneously injected with 2 × 106 E0771 cells per mouse, tumor volume was calculated every 2 days beginning 5 days after cell inoculation. Seven days later, BMs of mice fed normal or high riboflavin diet for 2 weeks were intraperitoneally injected into mice inoculated with E0771 cells (once every 5 days, 5 times); excised tumor and tumor weights on the 31st day are shown (F, G). Immunohistochemical staining was performed with the indicated antibodies on tumor sections. Representative images are shown (H). Scale bars, 100 μm. Histological semiquantification was performed. Data are presented as mean ± SEM. n = 3 per group (C–E); n = 5 per group (B and G); n = 6 per group (F and H). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Fig. 8.
RFK interacts with and activates iNOS to orchestrate macrophage polarization. RFK deletion significantly inhibits M(IFN-γ) polarization, but enhances M(IL-4) polarization. Exogenous FAD or riboflavin supplementation could regulate macrophage polarization both in vitro and in vivo. RFK interacts with iNOS and enhances iNOS catalytic activity and NO production, which modulate macrophage polarization by reprogramming TCA cycle and mitochondrial electron transport chain.
4. Discussion
Our study shows that the expression of riboflavin kinase (RFK), a key enzyme in the metabolism of the essential mammalian nutrient riboflavin, is highly regulated during macrophage polarization. RFK expression was reduced under macrophage M1 polarization models and elevated under M2 polarization models. Genetic ablation of RFK significantly inhibits M(IFN-γ) polarization, but enhances M(IL-4) polarization through its enzyme activity. Furthermore, exogenous FAD reverses the polarization changes caused by RFK deficiency. Thus, RFK-mediated riboflavin metabolism represents a negative feedback mechanism by macrophages to avoid ‘overreaction’ during polarization.
In vivo, myeloid RFK-deficient mice showed increased tumor growth in subcutaneous tumor models by suppressing M(IFN-γ) but promoting M(IL-4) polarization. Due to the enhanced M(IL-4) polarization phenotype, RFK-deficient mice also showed less severe liver fibrosis in the CCl4-induced liver fibrosis model and enhanced recruitment of eosinophils in the chitin-induced M2 polarization model (Fig. S3). All these results indicate that RFK-mediated riboflavin metabolism can reprogram macrophage polarization to change the outcomes of macrophage-mediated immune responses.
Our results indicate that myeloid knockout of RFK does not significantly affect the proportion or quantity of peritoneal macrophages, suggesting that RFK does not influence bone marrow development. The reduced number of tumor-infiltrating macrophages in RFK-KO-Mφ mice may be attributed to various factors in the tumor microenvironment, such as cytokines, chemokines, nutrients, and interactions with neighboring cells, which could alter macrophage proliferation, apoptosis, chemotaxis, or differentiation. It would be interesting to conduct specific labeling experiments to track and quantify the proportions and survival of different macrophage subsets in tumors from RFK-KO-Mφ and RFK-WT-Mφ mice.
We speculate that riboflavin content and RFK expression vary in resident macrophages, which may be exposed to a variety of extracellular environments. It will be interesting to study whether RFK- or riboflavin metabolism-controlled macrophage activation contributes to the distinct phenotypes of resident macrophage populations. Deficiency of riboflavin can lead to abnormal development, neurodegeneration, skin disorders, and cardiovascular disease [15,42]. Thus, it is likely that some of these effects are also mediated through disturbance of macrophage polarization. Further experiments will be needed to clarify whether and how macrophage RFK deficiency affects the phenotypes and functions of other immune cells and ultimately pathologies and outcomes in different animal models.
RFK couples tumor necrosis factor (TNF) receptor 1 to the NADPH oxidase subunit p22phox, so as to enhance the binding of FAD to the NADPH oxidase catalytic subunit Nox and its activity to produce ROS [17]. This RFK-dependent ROS production is critical for TNF-mediated cell death and defense against Listeria monocytogenes [17,19]. Although ROS promotes IFN-γ-induced M1 polarization (Fig. S6A), we found that NO but not ROS is critical for RFK-mediated regulation of M(IFN-γ) polarization. These differences may originate in different signaling pathways for TNF and IFN-γ. IFN-γ with or without LPS stimulates iNOS to mainly produce nitric oxide, and TNF stimulates NADPH oxidase to produce large amounts of ROS in macrophages [17].
Consistent with this, NO has also been shown to enhance M(LPS + IFN-γ) polarization [24,25,30]. Others have argued that NO can also affect cellular energy metabolism through mitochondrion-independent pathways such as stabilizing HIF-1α [38,43], while the present study reveals that neither exogenous nor endogenous NO can influence macrophage HIF-1α expression level. A recent report showed that NO derived from iNOS mediates the nitration of tyrosine residues in IRF5, leading to the suppression of M(LPS + IFN-γ) polarization with no significant effect on M(IL-4) polarization [44]. Another study showed that while exogenous NO promotes M(IL13+IL-4) polarization, endogenous NO has no effect on M(IL-4) polarization [43]. These differences may be attributable to the different types and incubation times of macrophage polarization stimuli, which needs further detailed investigation. Consistent with previous reports [[45], [46], [47]], we found that WT macrophages treated with IL-4 do express iNOS and have biologically relevant levels of nitrites. Additionally, we found that RFK deficiency decreased NO production both at baseline and upon IL-4 stimulation. This may facilitate RFK-deficient macrophages to undergo IL-4-induced polarization.
Our mechanistic studies have uncovered a physical interaction between RFK and iNOS, bringing FMN and FAD into close proximity to iNOS, facilitating the electron transfer from NADPH and activation of iNOS, and fitting with earlier findings that RFK deficiency decreases NO production [16,17]. Exogenous FAD rescued the reduced NO production and reprogrammed macrophage polarization induced by RFK deficiency. We speculate that the complex of RFK and iNOS contains FAD synthase. The FMN converted from RF, catalyzed by RFK, can be further transformed to FAD locally by FAD synthase, which meets the requirements of iNOS for both FMN and FAD. Since FAD can be hydrolyzed to FMN by FAD synthase, FAD pyrophosphatase, or other FAD-degrading enzymes [48,49], exogenous FAD can simultaneously provide enough FAD and FMN as cofactors to promote the activity of iNOS and rescue the polarization phenotype of macrophages caused by RFK deficiency.
Previous studies have demonstrated that macrophage polarization states are closely linked to their metabolic profiles [7,11,36,37]. M1 macrophages are characterized by high ECAR, reflecting their reliance on glycolysis for rapid energy production, whereas M2 macrophages exhibit increased OCR, primarily utilizing oxidative phosphorylation to support their long-term repair and anti-inflammatory functions [7,50]. Our results showed that the RFK–FAD–iNOS–NO signaling pathway promotes M(IFN-γ) while suppressing M(IL-4) polarization through a mitochondrion-dependent mechanism involving TCA cycle remodeling and dysfunction of mitochondrial electron transport complexes. This suggests that RFK deficiency reprograms macrophage energy metabolism, thereby influencing their polarization phenotypes.
In summary, our work reveals the important role of RFK-mediated riboflavin metabolism in regulating macrophage polarization. We not only identify RFK as a new target for regulating the activity of iNOS, but also expand the understanding of the post-translational regulation of iNOS activity.
CRediT authorship contribution statement
Xiao Shan: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Funding acquisition. Zemin Ji: Validation, Software, Resources. Baochen Wang: Investigation. Yanan Zhang: Methodology. Hongyuan Dong: Resources. Weijia Jing: Validation. Yanzhao Zhou: Validation. Penghui Hu: Validation, Supervision. Yan Cui: Resources. Zihan Li: Methodology. Sujun Yu: Resources. Jinxue Zhou: Supervision. Ting Wang: Writing – original draft, Funding acquisition. Long Shen: Methodology, Funding acquisition. Yuping Liu: Writing – original draft, Project administration, Data curation. Qiujing Yu: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. Xiaoyue Tan (Nankai University, China) for kindly providing Lyz2-Cre mice and several cell lines. This research was supported by grants from the Postdoctoral Fellowship Program of CPSF GZB20240116 (X.S.); the National Natural Science Foundation of China: 32471218 (Q.Y.), 82273088 (Q.Y.), 82073051 (T.W.), and 82273217 (L.S.); and the Tianjin Municipal Natural Science Foundation of China: 22JCQNJC00040 (L.S.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103413.
Contributor Information
Xiao Shan, Email: 18355157713@163.com.
Long Shen, Email: shenlong@ihcams.ac.cn.
Yuping Liu, Email: 18981838972@163.com.
Qiujing Yu, Email: yuqiujing@uestc.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Funes S.C., Rios M., Escobar-Vera J., Kalergis A.M. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–195. doi: 10.1111/imm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass C.K., Natoli G. Molecular control of activation and priming in macrophages. Nat. Immunol. 2016;17(1):26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung J., Zeng H., Horng T. Metabolism as a guiding force for immunity. Nat. Cell Biol. 2019;21(1):85–93. doi: 10.1038/s41556-018-0217-x. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill L.A., Pearce E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213(1):15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Bulnes P., Saiz M.L., Lopez-Larrea C., Rodriguez R.M. Crosstalk between hypoxia and er stress response: a key regulator of macrophage polarization. Front. Immunol. 2019;10:2951. doi: 10.3389/fimmu.2019.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theivendran S., Gu Z., Tang J., Yang Y., Song H., Yang Y., Zhang M., Cheng D., Yu C. Nanostructured organosilica nitric oxide donors intrinsically regulate macrophage polarization with antitumor effect. ACS Nano. 2022;16(7):10943–10957. doi: 10.1021/acsnano.2c03348. [DOI] [PubMed] [Google Scholar]
- 10.Rendra E., Riabov V., Mossel D.M., Sevastyanova T., Harmsen M.C., Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242–253. doi: 10.1016/j.imbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Van den Bossche J., O'Neill L.A., Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38(6):395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Tabas I., Bornfeldt K.E. Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circ. Res. 2020;126(9):1209–1227. doi: 10.1161/CIRCRESAHA.119.315939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson J.J., Suchindran C.M., Roggenkamp K.J. Micronutrient intakes in two US populations of older adults: lipid research clinics program prevalence study findings. J. Nutr. Health Aging. 2009;13(7):595–600. doi: 10.1007/s12603-009-0169-8. [DOI] [PubMed] [Google Scholar]
- 14.Preziosi P., Galan P., Deheeger M., Yacoub N., Drewnowski A., Hercberg S. Breakfast type, daily nutrient intakes and vitamin and mineral status of French children, adolescents, and adults. J. Am. Coll. Nutr. 1999;18(2):171–178. doi: 10.1080/07315724.1999.10718846. [DOI] [PubMed] [Google Scholar]
- 15.Powers H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003;77(6):1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 16.Schramm M., Wiegmann K., Schramm S., Gluschko A., Herb M., Utermohlen O., Kronke M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur. J. Immunol. 2014;44(3):728–741. doi: 10.1002/eji.201343940. [DOI] [PubMed] [Google Scholar]
- 17.Yazdanpanah B., Wiegmann K., Tchikov V., Krut O., Pongratz C., Schramm M., Kleinridders A., Wunderlich T., Kashkar H., Utermohlen O., Bruning J.C., Schutze S., Kronke M. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460(7259):1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 18.Stuehr D.J., Cho H.J., Kwon N.S., Weise M.F., Nathan C.F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc. Natl. Acad. Sci. U.S.A. 1991;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cinelli M.A., Do H.T., Miley G.P., Silverman R.B. Inducible nitric oxide synthase: regulation, structure, and inhibition. Med. Res. Rev. 2020;40(1):158–189. doi: 10.1002/med.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357(Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75(6):639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 23.Wang T., Dong Y., Yao L., Lu F., Wen C., Wan Z., Fan L., Li Z., Bu T., Wei M., Yang X., Zhang Y. Adoptive transfer of metabolically reprogrammed macrophages for atherosclerosis treatment in diabetic ApoE (-/-) mice. Bioact. Mater. 2022;16:82–94. doi: 10.1016/j.bioactmat.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Divangahi M., Chen M., Gan H., Desjardins D., Hickman T.T., Lee D.M., Fortune S., Behar S.M., Remold H.G. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat. Immunol. 2009;10(8):899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divangahi M., Desjardins D., Nunes-Alves C., Remold H.G., Behar S.M. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat. Immunol. 2010;11(8):751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coulombe F., Jaworska J., Verway M., Tzelepis F., Massoud A., Gillard J., Wong G., Kobinger G., Xing Z., Couture C., Joubert P., Fritz J.H., Powell W.S., Divangahi M. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40(4):554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Pesce J.T., Ramalingam T.R., Mentink-Kane M.M., Wilson M.S., El Kasmi K.C., Smith A.M., Thompson R.W., Cheever A.W., Murray P.J., Wynn T.A. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4) doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji L., Zhao X., Zhang B., Kang L., Song W., Zhao B., Xie W., Chen L., Hu X. Slc6a8-Mediated creatine uptake and accumulation reprogram macrophage polarization via regulating cytokine responses. Immunity. 2019;51(2):272–284 e7. doi: 10.1016/j.immuni.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Pellicoro A., Ramachandran P., Iredale J.P., Fallowfield J.A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014;14(3):181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 30.Feng M., Ding J., Wang M., Zhang J., Zhu X., Guan W. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int. J. Biol. Sci. 2018;14(9):1033–1040. doi: 10.7150/ijbs.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kisseleva T., Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021;18(3):151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 32.Yu B., Qin S.Y., Hu B.L., Qin Q.Y., Jiang H.X., Luo W. Resveratrol improves CCL4-induced liver fibrosis in mouse by upregulating endogenous IL-10 to reprogramme macrophages phenotype from M(LPS) to M(IL-4) Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109110. [DOI] [PubMed] [Google Scholar]
- 33.Hodny Z., Reinis M., Hubackova S., Vasicova P., Bartek J. Interferon gamma/NADPH oxidase defense system in immunity and cancer. OncoImmunology. 2016;5(2) doi: 10.1080/2162402X.2015.1080416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han C., Sheng Y., Wang J., Zhou X., Li W., Zhang C., Guo L., Yang Y. NOX4 promotes mucosal barrier injury in inflammatory bowel disease by mediating macrophages M1 polarization through ROS. Int. Immunopharm. 2022;104 doi: 10.1016/j.intimp.2021.108361. [DOI] [PubMed] [Google Scholar]
- 35.Bai A., Moss A., Rothweiler S., Serena Longhi M., Wu Y., Junger W.G., Robson S.C. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat. Commun. 2015;6:8819. doi: 10.1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natoli G., Pileri F., Gualdrini F., Ghisletti S. Integration of transcriptional and metabolic control in macrophage activation. EMBO Rep. 2021;22(9) doi: 10.15252/embr.202153251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langston P.K., Shibata M., Horng T. Metabolism supports macrophage activation. Front. Immunol. 2017;8:61. doi: 10.3389/fimmu.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey J.D., Diotallevi M., Nicol T., McNeill E., Shaw A., Chuaiphichai S., Hale A., Starr A., Nandi M., Stylianou E., McShane H., Davis S., Fischer R., Kessler B.M., McCullagh J., Channon K.M., Crabtree M.J. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep. 2019;28(1):218–230 e7. doi: 10.1016/j.celrep.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmieri E.M., Gonzalez-Cotto M., Baseler W.A., Davies L.C., Ghesquiere B., Maio N., Rice C.M., Rouault T.A., Cassel T., Higashi R.M., Lane A.N., Fan T.W., Wink D.A., McVicar D.W. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020;11(1):698. doi: 10.1038/s41467-020-14433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor C.T., Scholz C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022;18(9):573–587. doi: 10.1038/s41581-022-00587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang S.J., Park Y.S., Cho J.H., Moon B., An H.J., Lee J.Y., Xie Z., Wang Y., Pocalyko D., Lee D.C., Sohn H.A., Kang M., Kim J.Y., Kim E., Park K.C., Kim J.A., Yeom Y.I. Regulation of hypoxia responses by flavin adenine dinucleotide-dependent modulation of HIF-1alpha protein stability. EMBO J. 2017;36(8):1011–1028. doi: 10.15252/embj.201694408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thakur K., Tomar S.K., Singh A.K., Mandal S., Arora S. Riboflavin and health: a review of recent human research. Crit. Rev. Food Sci. Nutr. 2017;57(17):3650–3660. doi: 10.1080/10408398.2016.1145104. [DOI] [PubMed] [Google Scholar]
- 43.Drehmer D., Mesquita Luiz J.P., Hernandez C.A.S., Alves-Filho J.C., Hussell T., Townsend P.A., Moncada S. Nitric oxide favours tumour-promoting inflammation through mitochondria-dependent and -independent actions on macrophages. Redox Biol. 2022;54 doi: 10.1016/j.redox.2022.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu G., Zhang R., Geng S., Peng L., Jayaraman P., Chen C., Xu F., Yang J., Li Q., Zheng H., Shen K., Wang J., Liu X., Wang W., Zheng Z., Qi C.F., Si C., He J.C., Liu K., Lira S.A., Sikora A.G., Li L., Xiong H. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat. Commun. 2015;6:6676. doi: 10.1038/ncomms7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiroi M., Sakaeda Y., Yamaguchi H., Ohmori Y. Anti-inflammatory cytokine interleukin-4 inhibits inducible nitric oxide synthase gene expression in the mouse macrophage cell line RAW264.7 through the repression of octamer-dependent transcription. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/369693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi J.H., Liu L.N., Song D.D., Liu W.W., Ling C., Wu F.X., Wang T.T., Liu B., Cui N.P., Qin Y., Ni Z.Y. TRAF3/STAT6 axis regulates macrophage polarization and tumor progression. Cell Death Differ. 2023;30(8):2005–2016. doi: 10.1038/s41418-023-01194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang T., Wang R., Liu H., Wang L., Li J., Wu S., Chen X., Yang X., Zhao Y. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis. Life Sci. 2021;266 doi: 10.1016/j.lfs.2020.118903. [DOI] [PubMed] [Google Scholar]
- 48.Giancaspero T.A., Busco G., Panebianco C., Carmone C., Miccolis A., Liuzzi G.M., Colella M., Barile M. FAD synthesis and degradation in the nucleus create a local flavin cofactor pool. J. Biol. Chem. 2013;288(40):29069–29080. doi: 10.1074/jbc.M113.500066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giancaspero T.A., Galluccio M., Miccolis A., Leone P., Eberini I., Iametti S., Indiveri C., Barile M. Human FAD synthase is a bi-functional enzyme with a FAD hydrolase activity in the molybdopterin binding domain. Biochem. Biophys. Res. Commun. 2015;465(3):443–449. doi: 10.1016/j.bbrc.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 50.Mills E.L., O'Neill L.A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 2016;46(1):13–21. doi: 10.1002/eji.201445427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.