Abstract
Myocardial infarction (MI) remains the leading cause of death related to cardiovascular diseases globally, presenting a significant clinical challenge due to the specificity of the lesion site and the limited proliferative capacity of cardiomyocytes (CMs) for repairing the infarcted myocardium. Extensive studies reported so far has focused on the utilization of hydrogel-based cardiac patches for MI treatment, highlighting their promising mechanical properties, conductivity, and ability to remodel the microenvironment post-repair. However, the majority of developed cardiac patches have been limited to the myocardial tissue surface via suturing or adhesive administration. Suturing inevitably leads to additional damage to the fragile myocardium, while uneven application of adhesives may result in patch displacement and compromised drug release. Based on these critical issues, we systematically summarize the advantages and drawbacks of using hydrogel patches for MI treatment with emphasis on elucidating various design strategies. Specifically, we first describe the changes in the pathological microenvironment following MI. Next, we discuss the biomimetic types of hydrogel patches, their functional design, and corresponding strategies for microenvironment adaptation, emphasizing adhesion mechanisms, wet adhesion design strategies, and fabrication techniques for hydrogel patches. Finally, we address the potential challenges and prospects of hydrogels as patches for MI therapy. The review is believed to provide theoretical guidance for the development of new therapeutic strategies for effectively MI treatment.
Keywords: Hydrogel, Patch, Adhesion mechanisms, Pathological microenvironment, Myocardial infarction
Graphical abstract
1. Introduction
Cardiovascular disease (CVD) is a leading cause of death worldwide, with myocardial infarction (MI) and subsequent heart failure presenting the highest morbidity and mortality rates among all CVDs. Although the incidence of MI has decreased over the past 50 years, the risk profile associated with MI has remained largely unchanged [1,2]. The treatment of MI typically involves highly invasive procedures that carry significant risk, and not all patients retain the capacity to remain conscious post-procedure. Recently, two less invasive surgical techniques have gained prominence: percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) [3,4]. PCI involves inserting a guidewire through an artery in the leg or arm to reach the coronary artery, followed by reperfusion through balloon dilation and stenting. CABG, often selected when PCI proves ineffective or in cases of multi-vessel disease, achieves reperfusion by grafting a vessel to bypass the occluded vessel, directly blood from the aorta to the infarcted area. However, both PCI and CABG have limitations [5]. The risk of restenosis post-stenting challenges PCI's efficacy, and although drug-eluting stents have mitigated this issue, they have not significantly lowered morbidity and mortality rates [6]. CABG is an expensive procedure, and patients continue to experience serious complications after the procedure, such as atrial fibrillation, thromboembolism, infections, and kidney damage [[7], [8], [9], [10]]. Current pharmacological and surgical interventions primarily focus on halting disease progression rather than effectively promoting cell proliferation or tissue regeneration in areas affected by MI. This limitation stems from several factors: the limited proliferative capacity of adult cardiomyocytes (CMs), the intricate ischemia/hypoxia characterizing the infarcted microenvironment, the demanding nature of clinical surgical procedures, and challenges in drug delivery due to the blind-end effect of vascular occlusion [11,12]. After a MI, a series of complex processes ensue, including myocardial cell necrosis, an intercellular inflammatory response, myocardial fibrosis, and tissue remodeling [13,14]. Consequently, MI treatment necessitates interventions not only to restore blood supply but also strategies aimed at encouraging cell proliferation and tissue regeneration, addressing both the superficial issues and the underlying pathology and microenvironmental changes. Therefore, there is an urgent need for therapeutic strategies tailored to the complex post-MI microenvironmental alterations to enhance treatment outcomes.
Tissue engineering and regenerative medicine show promise in aiding myocardial repair post MI [[15], [16], [17]]. Hydrogel materials have gained considerable interest in tissue engineering due to their impressive mechanical properties, biocompatibility and cell delivery ability [18,19]. The special properties of hydrogels also provide an extracellular matrix (ECM)-like microenvironment to retain CM proliferation and migration and provide mechanical support for the damaged ventricular wall [20,21]. As a result, hydrogels have received significant attention in MI therapy. In cardiac tissue engineering, hydrogels primarily exist in two forms: hydrogel patches and injectable hydrogels. As injectable hydrogels need to be injected directly into the myocardium, come with potential drawbacks such as extended tissue damage, susceptibility to inflammatory reactions, hydrogel degradation, and the requirement for myocardium-like properties [22,23]. In contrast, hydrogel patches offer notable advantages for MI therapy. These patches can exploit the properties of polymeric materials to undergo a sol-gel transition or utilize memory-smart responsive materials capable of restoring folded patches to their original shape under specific conditions. This allows for minimally invasive delivery of a hydrogel patch loaded with an active substance or cell to the infarcted area. The patch can then adhere to the surface of the infarcted myocardium in response to specific environmental stimuli, providing in-situ specific release and reducing the invasiveness of the procedure [24,25]. Furthermore, specific structural modifications can be incorporated into the hydrogel network construction to confer certain biological properties of the myocardium, such as stretchability, self-healing capability, and electrical conductivity [26].
After MI, the infarcted area leads to several adverse outcomes, including cardiomyocyte death, fibrotic scar formation, and loss of electrical conductivity, which can ultimately result in heart failure [27,28]. Consequently, hydrogel cardiac patches must be designed to replace damaged cardiomyocytes or myocardial tissue, thereby supporting normal cardiac function. These patches should possess suitable mechanical properties for structural support, electrical conductivity comparable to normal myocardium to restore electrical conduction, and therapeutic strategies tailored to the MI microenvironment. However, the design requirements, strategies, adhesion mechanisms, therapeutic mechanisms, and potential applications of hydrogel patches in the cardiac field still need further elucidation.
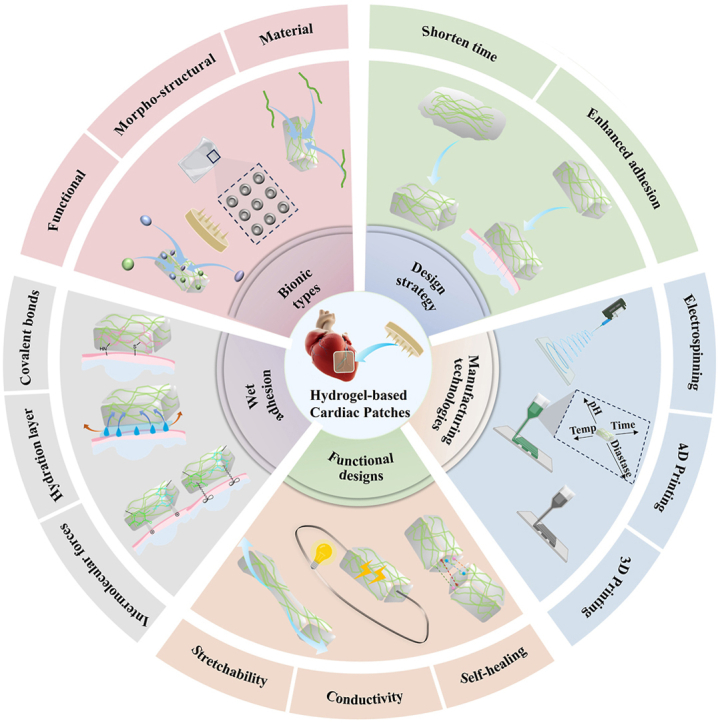
Compared to existing studies, this review distinguishes itself by providing a systematic summary of effective strategies for MI treatment utilizing hydrogel cardiac patches, as well as explores the various adhesion mechanisms of these patches to cardiac tissues and the design strategies employed in creating them. Specifically, this review discusses the evolving pathological stages after MI and the key challenges that must be addressed at each stage at the beginning followed by detailed description on the design of bioinspired hydrogel patches and their adhesion mechanisms. This review not only summarizes the existing information on various adhesion methods employed to enable adhesion of hydrogel patches to myocardial tissues for promoted functional recovery of the myocardium, but also outlines diverse fabrication methods for hydrogel patches tailored for cardiac repair. Finally, an outlook on the current challenges and future prospects of hydrogel patches for MI treatment is made (Scheme 1). In conclusion, the focus of this review is on the pathological changes after MI in relation to the rational design of hydrogel patches, with the aim of exploring new therapeutic methods for restoring damaged myocardial function and promoting myocardial regeneration.
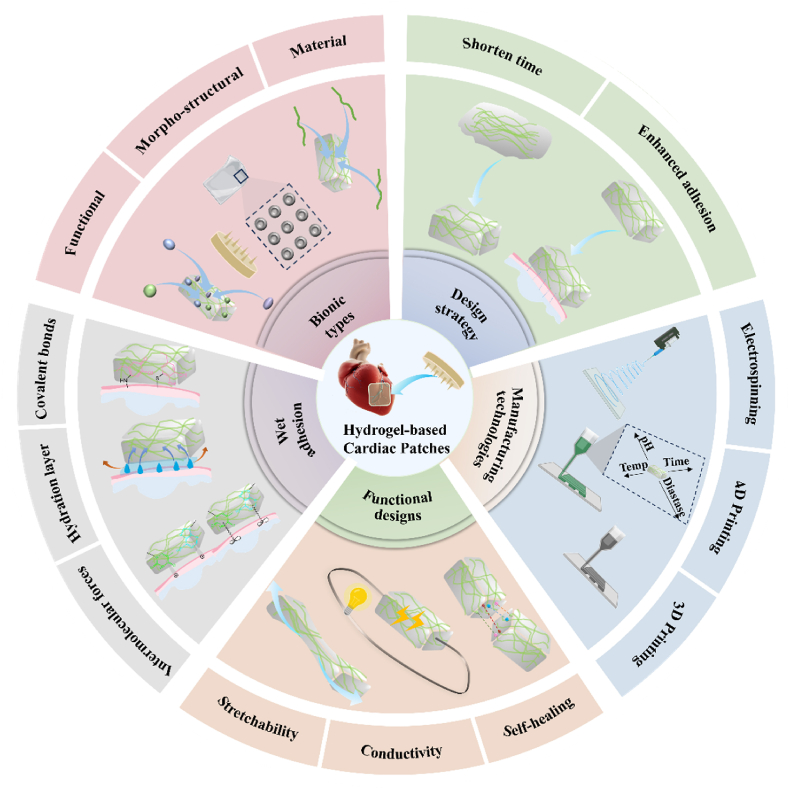
Scheme 1.
Diagram depicting the bionic design and manufacturing methodologies employed in the creation of hydrogel-based cardiac patches.
2. Pathological microenvironment after MI and corresponding therapeutic strategies
MI is characterized by the necrosis of myocardial tissue due to myocardial hypoxia and ischemia. This condition arises from obstruction in the coronary arteries, which can be attributed to factors such as endothelial damage, rupture of atheromatous plaques, or sustained coronary artery spasms [29,30]. The heart primarily relies on oxidative metabolism for energy production and is highly sensitive to changes in intracellular oxygen levels [31,32]. Decreased oxygen levels in the heart due to coronary artery blockage result in an increase in anaerobic ATP synthesis, leading to energy depletion in cardiac myocytes and mitochondrial dysfunction [33,34]. Notably, the proliferative capacity of postnatal CM progressively and consistently declines with age, not exceeding 0.5 % in a 60-year-old human heart [[35], [36], [37]]. CMs are the most mitochondria-rich organelles of all cells, and mitochondrial injury leads to increased cell membrane permeability, decreased ATPase activity, deficient energy synthesis, and diminished inhibition of the calcium pump. This situation results in elevated cytoplasmic calcium levels, compromised integrity of myocardial cell membrane, and uncontrolled release of cellular contents into the ECM. Consequently, chemokines attract inflammatory cells to the infarct zone, promoting ECM degradation and hastening the progression of MI to heart failure.
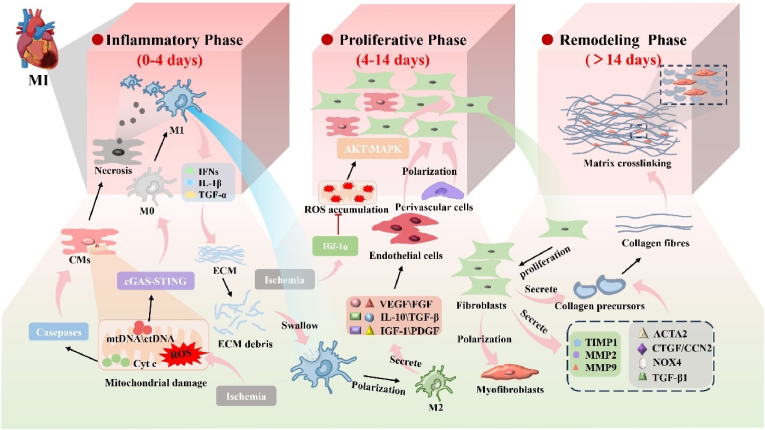
The post-MI pathological microenvironmental undergoes a complex and dynamic process, which can be divided into three distinct but partially overlapping phases: the inflammatory phase, the proliferative phase, and the remodeling phase [38,39]. Early inflammation is initiated by hypoxia and ischemic myocardial injury [40]. Mitochondria play a crucial role in regulating cardiomyocyte viability. Myocardial ischemia and hypoxia cause persistent mitochondrial damage, resulting in the intracellular accumulation of excessive fatty acids and an increase in reactive oxygen species (ROS). These alterations directly impair the membrane structure and internal components of mitochondria, leading to mitochondrial dysfunction and a subsequent shortage of ATP [41,42]. Following mitochondrial damage, Cytochrome C (Cyt c) and Mitochondrial DNA (mtDNA) are released into the cytosol [43]. Cyt c activation triggers the caspase pathway, promoting extensive and irreversible CM death [[44], [45], [46]]. Deceased cells release contents that recruit a large number of neutrophils and immune cells to infiltrate the infarcted area. Notably, the release of cytoplasmic DNA from CMs activate the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway in M0 macrophages, promoting their polarization into M1 macrophages, followed by the release of large amounts of pro-inflammatory factors and type Ⅰ interferons (IFN-1), which exacerbate the inter-cellular inflammatory cascade and worsen infarcted myocardium development [47,48]. Macrophage play a critical role in myocardial repair post-MI, initially phagocytosing necrotic cells and removing debris degraded by ECM [49]. With the stimulation of factors such as mtDNA and cell-free circulating DNA (cfDNA), M0 macrophages polarize to M1 macrophages and secrete inflammatory factors to promote inflammation. Pro-inflammatory genes exhibit robust intercellular communication with inflammatory cells, including macrophages [50,51]. In the later stages of myocardial repair, some M1 macrophages transition into M2 macrophages, known for their anti-inflammatory properties. These M2 macrophages are crucial in producing growth factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) to promote angiogenesis [52].
There is a partial overlap between the proliferative and remodeling phases in the post-MI period. During this phase, cardiac fibroblasts (CFs), a heterogeneous stromal cell population, play a prominent role in maintaining cardiac biomechanical integrity by regulating ECM deposition and turnover. Fibroblast differentiation is crucial for myocardial scar formation but also contributes to detrimental myocardial remodeling and sclerosis [53,54]. The primary factor governing CFs proliferation regulation is hypoxia-inducible factor-1α (HIF-1α), which acts as a key regulator of CFs hyperactivation and proliferation after ischemia by controlling metabolism, ROS accumulation, and intracellular signaling. Dysfunction of HIF-1α in CFs promotes their proliferation after MI, which is also associated with increased ROS levels [55,56]. CFs transform into myofibroblasts under the stimulation of growth factors, leading to significantly enhanced ECM synthesis in response to increased myocardial wall pressure, resulting in excessive scarring and contractile dysfunction [[57], [58], [59]]. By day 14 post MI, there is a significant increase in the fibrotic response. This is characterized by a notable rise in the expression of genes that encode ECM components, as well as remodeling proteases like tissue inhibitors of metalloproteinases (TIMPs) and matrix metalloproteinases. Moreover, there is an upregulation of fibrotic mediators such as ACTA2, CTGF/CCN2, TGF-β1, and NOX4 [56]. Various cytokines in pathways such as Wnt signaling, mitogen-activated protein kinases (MAPKs), and protein kinase B/phosphatidylinositol-3-kinase (PKB/PI3K) play a role in myocardial fibrosis formation [[60], [61], [62], [63]]. Additionally, a variety of immune cells, including neutrophils, monocytes/macrophages, and dendritic cells, along with their respective molecular signals, actively participate in balancing pro-fibrotic and anti-fibrotic responses [[64], [65], [66]].
During the fourth week post-MI, there is significant remodeling occurring at the infarct site. This process involves the secretion of TIMPs and MMPs, which collectively modify the ECM [67]. Collagen fibers are formed in the infarcted area during this stage, replacing the necrotic CMs and maturing the scar at the infarct site [68,69]. While the scar helps to maintain the structural integrity of the heart, it lacks the elasticity and contractile function of normal myocardial tissue [[70], [71], [72]]. CMs, as highly differentiated terminal cells, have limited regenerative capacity and are unable to repopulate the scar at a sufficient rate [73]. Consequently, the scar has a long-term impact on cardiac function. Furthermore, the newly formed scar matrix exhibits inconsistent electrical signals compared to the surrounding tissues, potentially leading to arrhythmias that may progress to heart failure. Therefore, using conductive biomaterials is crucial to enhance the physiological relevance of in vitro bioengineered cardiac tissue [72,74]. (Fig. 1).
Fig. 1.
Pathological microenvironmental changes in MI.
Based on the aforementioned process of MI development, it is evident that the pathological changes of MI occur gradually. Therefore, therapeutic strategies need to be designed according to the different pathological features at various stages to effectively achieve optimal repair. During the early stage of inflammation, therapeutic modalities should focus on promoting cardiomyocyte activity, inhibiting ROS, blocking cardiomyocyte apoptosis, and preventing ECM degradation. Additionally, due to the persistence of inflammation, it is necessary to develop strategies that modulate the inflammatory response to mitigate acute inflammation. The proliferative phase is primarily characterized by endothelial cell and fibroblast proliferation. Strategies aimed at treating this phase should focus on controlling macrophage phenotype and function, regulating matrix remodeling, modulating fibroblast activity, and promoting angiogenesis. In the final stage of remodeling, the scar formed within the infarcted area disrupts normal myocardial contraction, diastole, and electrical signal transmission. The primary strategy available at this stage appears involves replacing cardiomyocytes or myocardial tissue within the infarcted region with exogenous regenerative cells or artificial biomaterials designed to mimic myocardial tissue [75,76]. In summary, a rational design of drug- or cell-based delivery systems based on the pathological microenvironmental changes in MI may be an effective strategy to improve the therapeutic efficacy of MI (Fig. 1).
3. Bionic hydrogel patches
Bionic hydrogel patches are innovative biomaterials that mimic the form, function, and chemical structure of living organisms. They have garnered significant attention in the field of myocardial tissue engineering [77,78]. Researchers have developed bionic hydrogel patches that replicate the physical and biological properties of native myocardium, offering enhanced support for the regeneration and repair of infarcted myocardium [[79], [80], [81]]. To promote myocardial repair, these patches should possess excellent wet adhesion, elasticity, and contractility. Furthermore, they should exhibit similar electrical conductivity to cardiac tissues, enabling them to adapt to changes in shape during cardiac systole and diastole without detachment [82]. Apart from these mechanical properties, hydrogel patches utilized for myocardial repair must demonstrate outstanding biocompatibility, biodegradability, and wet adhesion [83,84]. However, it is important to note that different types of biomimetic hydrogel patches vary in composition, function, and mechanism of action, which will be discussed in the subsequent sections.
3.1. Types of bionic hydrogel patches
3.1.1. Morpho-structural bionic hydrogel patches
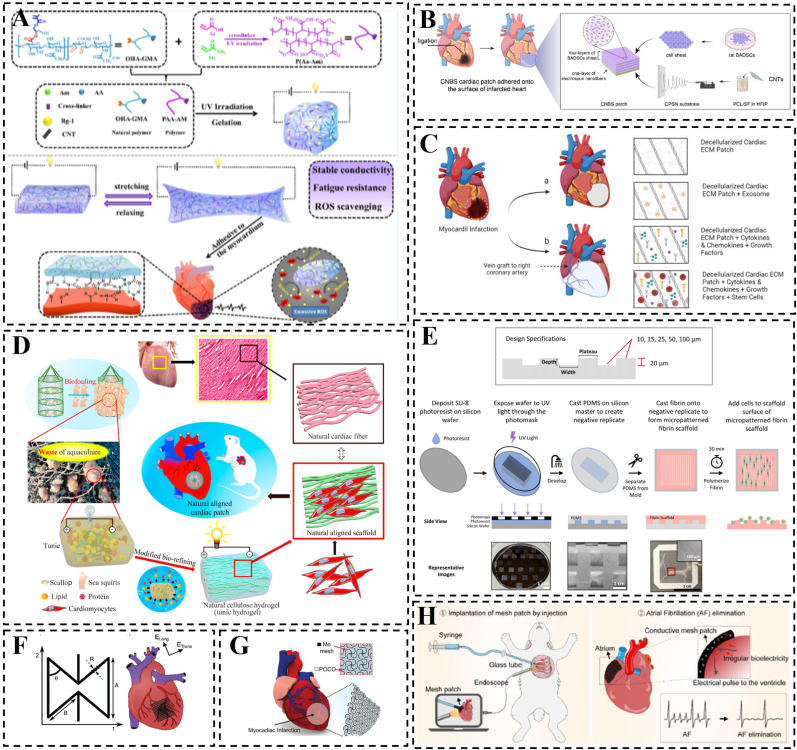
Morpho-structural biomimicry aims to modify the preparation conditions and processing techniques of hydrogels to replicate the physical form and microchemical structure of organisms, thereby achieving organism-like functions. In fact, this hydrogel is designed to improve its “own” properties, such as adhesion and mechanical properties, etc. In other words, it needs to acquire adhesion and appropriate mechanical properties because it needs to use them to promote cell and tissue repair in certain ways. For instance, this approach involves emulating the adhesive properties exhibited by organic groups found in mussels and the microsuckers found on octopuses tentacles to enhance the adhesion of hydrogel patches to substrates [85] (Fig. 2C). In the natural world, octopuses exhibit reversible physical adhesion by regulating the motion of their suckers, whereas snails achieve chemical adhesion by dispersing and intertwining sticky macromolecules present in their mucus [86,87]. Li et al. proposed a multifunctional bionic hydrogel patch inspired by the adhesive properties of snail mucus and octopus suction cups. The patch, made of tannin-grafted gelatin, silver-tannin nanoparticles, polyacrylamide (PAAm), and poly(N-isopropylacrylamide) (PNIPAm), combines microsorbent actuators with a tensile backing layer [88]. The silver-tannin nanoparticles provide excellent electrical conductivity, while the thermo-responsive PNIPAm acts like a ‘suction cup,’ enabling the patch to adhere to biological tissues and release VEGF in a controlled manner (Fig. 2A).
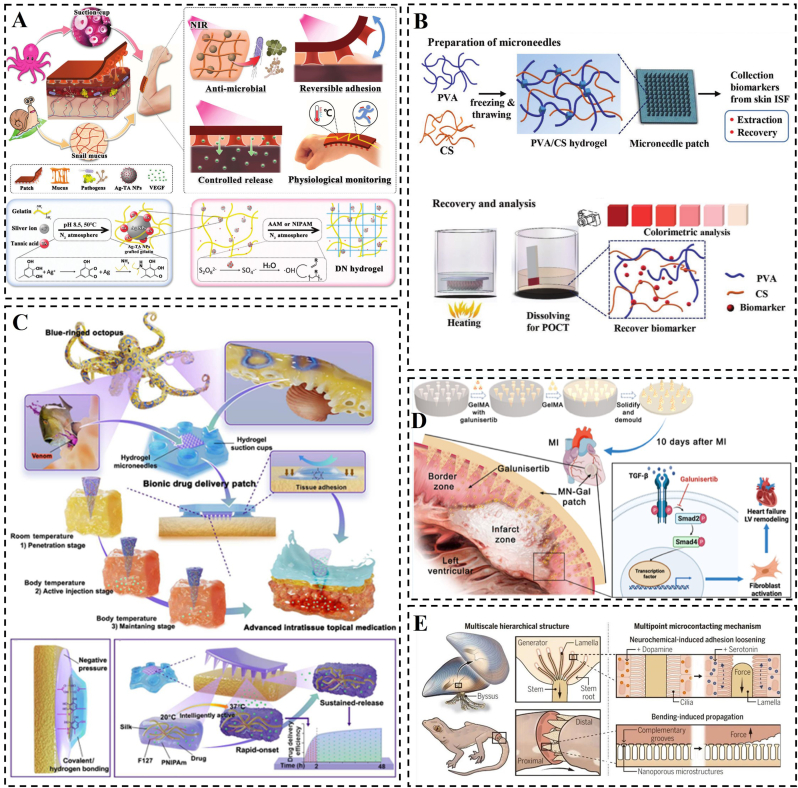
Fig. 2.
Construction or schematic representation of morpho-structural bionic hydrogels. A) Schematic representation of a multi-biologically inspired functional hydrogel patch. Reproduced with permission [88]. Copyright 2023, Wiley. B) Schematic depiction of the hydrogel microneedle patch designed for point-of-care testing of skin interstitial fluid, along with characterization details of the patch. Reproduced with permission [90]. Copyright 2020, Wiley. C) Blue-ringed octopus-inspired drug delivery patch. Reproduced with permission [85]. Copyright 2023, American Association for the Advancement of Science. D) Construction of galunisertib-loaded gelatin methacryloyl hydrogel microneedles and schematic diagram of their action. Reproduced with permission [91]. Copyright 2022, American Chemical Society. E) Multiscale hierarchical structure and Multipoint microcontacting mechanism. Reproduced with permission [89]. Copyright 2023, American Association for the Advancement of Science.
To achieve excellent compatibility between medical materials and tissues by designing biomimetic adhesive materials, Pan et al. explored the dynamic adhesion mechanism of the marine mussel. By analyzing the revealed multiscale hierarchical structure between pedicle filaments (a non-living proteinaceous material secreted by mussels) and self-tissues, they emphasized that the key to the controlled connection between mussel-pedicle dynamic biological interfaces lies in the multipoint microcontacting mechanism, rather than relying solely on surface-specific chemical interactions [89]. This multipoint microcontacting mechanism, observed in naturally dynamic multiscale stepwise biointerfaces, is not exclusive to mussels and is expected to provide valuable insights for the development of dynamic and histocompatible biomaterials. A similar multiscale step structure is also present at the interface between the two segments of the tail of reptilian lizards, such as geckos, ensuring a stable connection between the tail and the body. When threatened, localized disruption of micro-contact points triggered by tail oscillation can lead to the fracture of the entire interface (Fig. 2E). The design of hydrogel microneedle patches serves as an exemplification of the multipoint microcontact mechanism, He et al. developed skin interstitial fluid (ISF)-based microneedle patches for immediate detection, utilizing hydrogel microneedles composed of polyvinyl alcohol (PVA) and chitosan (CS) [90]. (Fig. 2B). Meanwhile, in recent years, there have been corresponding reports of hydrogel microneedle patches applied in the treatment of MI, Chen et al. designed a biocompatible hydrogel microneedle (MN) patch using gelatin methacryloyl for the treatment of excessive cardiac fibrosis after MI (Fig. 2D) [91].
When designing biomimetic adhesive hydrogel patches for myocardial repair, it is worth considering the replication of the unique chemical interactions observed in the adhesion mechanism of organisms such as mussels. This approach, combined with the physical construction of adhesive hydrogel patches utilizing the multipoint microcontacting mechanism, may lead to enhanced adhesion effects, thereby facilitating myocardial repair. Furthermore, the dynamic bio-surface interfaces found in mussels and other organisms offer a potential solution for improving the tissue compatibility of current biomaterials. The biomimetic construction of these structures holds promise for applications in interdisciplinary fields such as removable implantable materials (e.g., biosensors and medical implants) and dynamic brain-computer interfaces.
3.1.2. Material bionic hydrogel patches
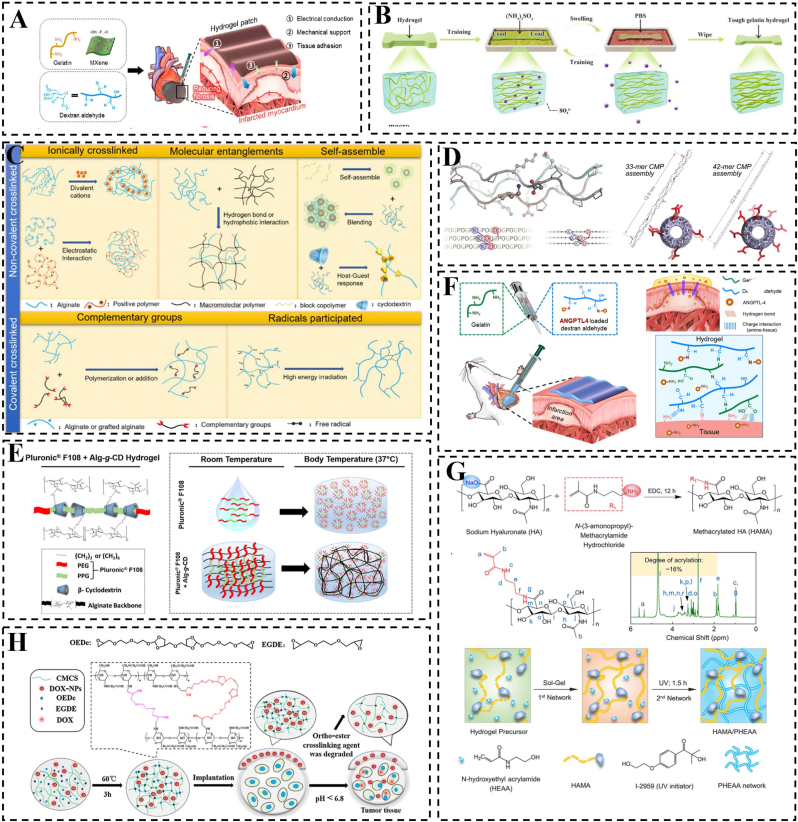
Material biomimicry aims to mimic the chemical composition of living organisms by altering the materials used in the construction of hydrogel patches and altering the cross-linking of hydrogels. Generally, these hydrogels are constructed using natural bioactive molecules such as alginate, cellulose, chitosan, collagen, cyclodextrin, gelatin, and hyaluronic acid [[92], [93], [94], [95], [96], [97], [98], [99], [100], [101]], which enhance biocompatibility and promote cell attachment and proliferation on biological tissues (Fig. 3B–G) [102]. This approach of bionic bonding materials overcomes the limitations of existing tissue adhesives by replicating the bonding mechanism of natural adhesives and employing biocompatible materials for their preparation [103]. Peng et al. chemically cross-linked hyaluronic acid methacryloyl (HAMA) and poly(N-hydroxyethyl acrylamide) (PHEAA) to create a biomimetic hyaluronic acid methacryloyl hydrogel with tough and adhesive properties (Fig. 3H) [104]. The mechanical properties of the bulk HEMA/PHEAA hydrogel exhibited a significantly improvement, reaching 0.45 MPa compared to the less than 0.1 MPa of normal hyaluronic acid hydrogel. The abundance of hydrogen bonding endowed the resulting hydrogels with exceptionally high adhesion to various non-porous matrices, making them suitable for a wide range of biological tissues, including the heart, with an interfacial toughness of approximately 1432 J/m2. However, cardiac patches lacking electrical conductivity may lead to arrhythmias and often fail to achieve optimal therapeutic outcomes. Addressing this concern, Lee et al. developed a conductive adhesive hydrogel cardiac patch (CAHCP) by combining two-dimensional titanium carbide (Ti3C2Tx) MXene with gelatin and dextran aldehyde (Fig. 3A) [26]. The CAHCP demonstrated material properties suitable for cardiac patch applications, such as an electrical conductivity of 18.3 mS/cm, similar to cardiac tissue, an elasticity of 30.4 kPa resembling that of cardiac tissue, strong tissue adhesion at 6.8 kPa, and resistance to various mechanical stresses. The CAHCP showed cytocompatibility and enhanced cardiomyocyte maturation in vitro. Moreover, it could be effectively applied to cardiac tissue, maintaining stable adherence to the beating epicardium.
Fig. 3.
Construction of natural polymer material bionic hydrogel. A) Schematic construction of CAHCP. Reproduced with permission [26]. Copyright 2023, American Chemical Society. B) Schematic representation of highly biocompatible and tough gelatin hydrogel preparation. Reproduced with permission [97]. Copyright 2023, Wiley. C) Different cross-linking methods for sodium alginate hydrogels. Reproduced with permission [98]. Copyright 2020, Elsevier. D) Design of sticky-ended synthetic collagen self-assemblies; comparison of molecular models depicting the symmetric self-assembly of 33-mer and 42-mer CMPs into nanofibers. Reproduced with permission [95]. Copyright 2023, Wiley. E) Diagram illustrating the physical cross-linking mechanism between Alg-g-CD macromolecules and Pluronic F108, as well as the impact of Pluronic F108 on the thermo-responsive behavior of the hydrogel network. Reproduced with permission [99]. Copyright 2015, American Chemical Society. F) Hydrogel cardiac patches based on gelatin and dextran aldehyde. Reproduced with permission [101]. Copyright 2024, Elsevier. G) Preparation and drug release of hydrogels based on carboxymethyl chitosan. Reproduced with permission [100]. Copyright 2019, Elsevier. H) Design strategies for bioactive dual network hydrogels. Reproduced with permission [104]. Copyright 2023, Frontiers Media S.A.
3.1.3. Functional bionic hydrogel patches
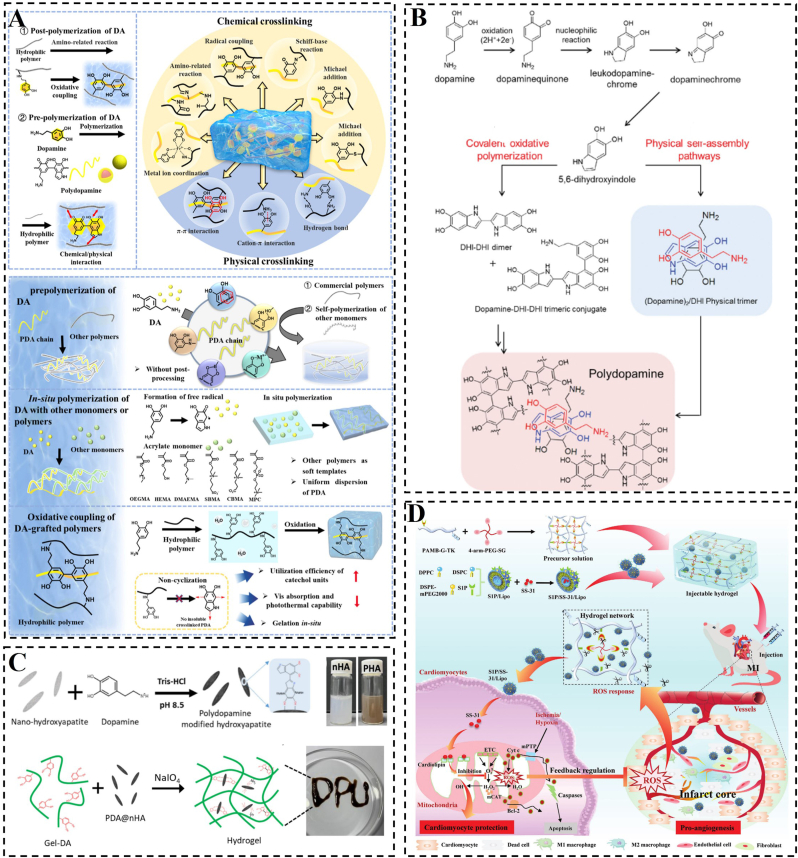
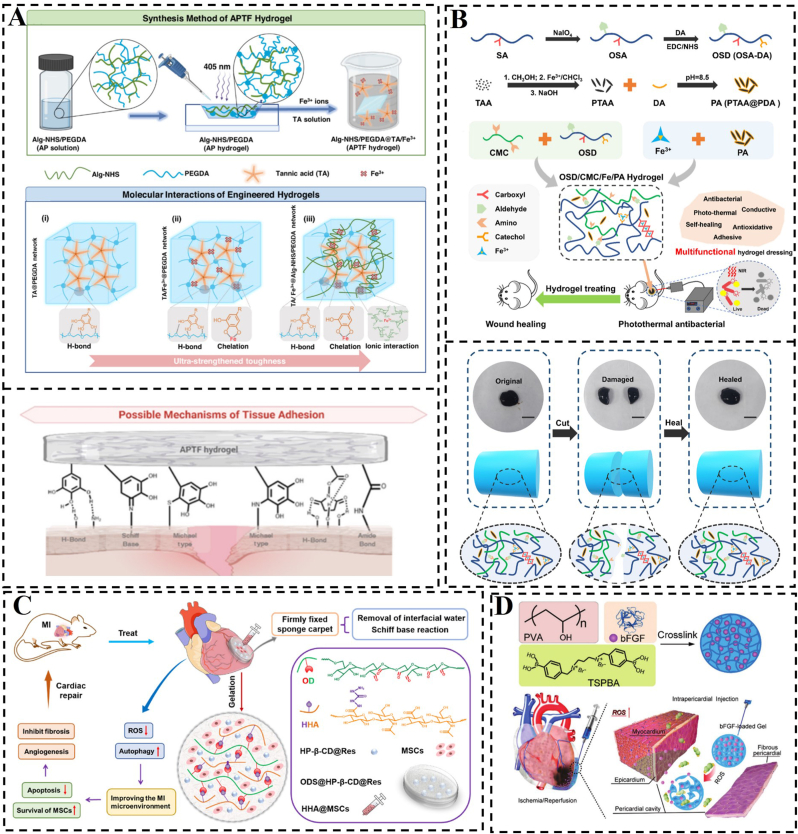
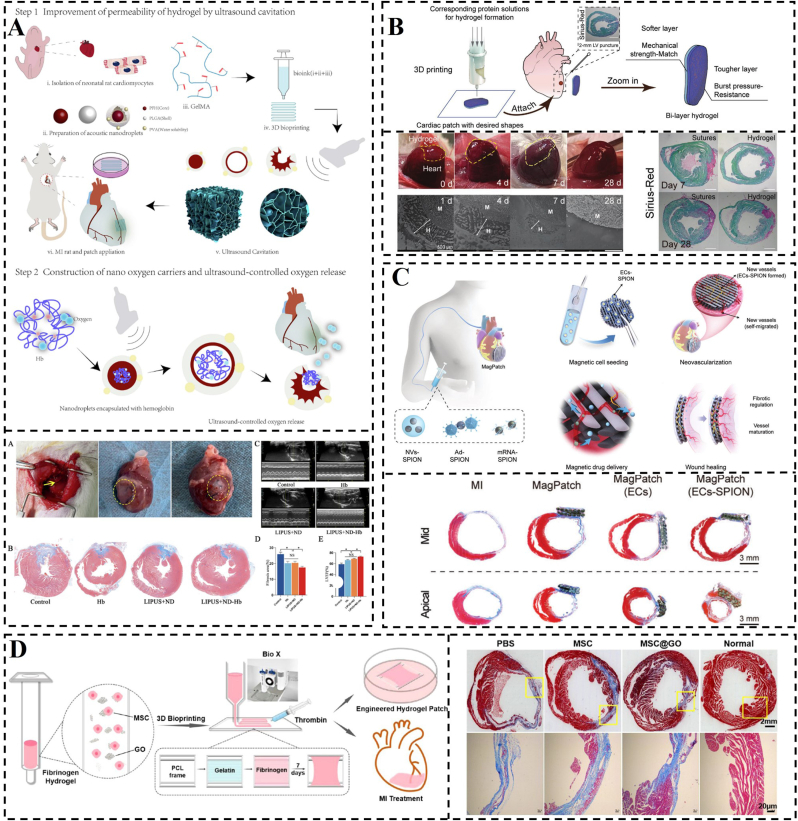
Functional biomimicry aims to incorporate drugs, cytokines, genes and other active ingredients into the construction of biomimetic hydrogels to replace or facilitate damaged cells and tissues to perform their normal functions, such as electrical conductivity, anti-inflammatory, antimicrobial, and facilitating wound healing. The starting point for the design of this hydrogel is not to improve its “own” properties, but to design it from the point of view of what functions are needed by damaged cells and tissues. However, it is also possible to use its “own” properties to better fulfil the corresponding functions. As we have reported in our previous work the application of functional bionic hydrogel loaded with SS-31 (elamipretide; D-Arg-Dmt-Lys-Phe-NH2) in MI (Fig. 4D) [43]. This hydrogel was designed to specifically target damaged mitochondria for controlled drug release, leading to the reduction of ROS and enhancement of CM activity. Another example of a high-quality nanoparticle used in constructing functional materials is polydopamine (PDA). PDA contains abundant functional groups that enable both covalent and non-covalent interactions with polymers. Additionally, PDA can anchor transition metal ions to form hydrogels (Fig. 4A and B) [[105], [106], [107]]. The inclusion of PDA confers various functions to hydrogels, such as adhesion, photothermal effect, UV protection, and antioxidant and antibacterial properties [[108], [109], [110], [111]]. In a study by Ma et al., functionalized nanohydroxyapatite (nHA) was combined with PDA to enhance the crosslink density between nHA and dopamine-modified gelatin (Gel-DA) [112]. The incorporation of PDA-functionalized nHA (PHA) led to a significant increase of approximately 200 kPa in the compressive strength of Gel-DA hydrogels, while maintaining a similar microstructure to nHA alone. The phenolic hydroxyl groups present in PHA promoted cell adhesion and proliferation within Gel-DA hydrogels (Fig. 4C). Indeed, many hydrogels with catechol polymers suffer from uncontrolled dopa oxidation, leading to a mix of products that compromise structural stability, drug release control, and therapeutic applications. The effective measures that can prevent the degradation or oxidation of dopamine-based hydrogels include, (i) incorporation of antioxidants, such as vitamin E or ascorbic acid, during hydrogel preparation for inhibiting oxidation reactions and enhancing material stability [113], (ii) minimizing exposure to oxygen during the preparation and storage of the hydrogel via working in an inert gas environment (such as nitrogen or helium) or utilizing sealed containers, (iii) maintaining the pH of the hydrogel in a neutral to slight acidic range (e.g. pH 4–6) for a significantly compromised oxidation process of dopamine that is more susceptible to oxidation at elevated pH levels [114], (iv) introduction of novel crosslinking networks and adhesive groups for hydrogel synthesis to mitigate the loss of functionality due to oxidized dopamine. For example, incorporating polymers capable of undergoing secondary crosslinking with dopamine-derived dopaquinone enables gradual reinforcement of the hydrogel for maintained hydrogel structural stability [115].
Fig. 4.
Schematic construction of functional biomimetic hydrogel. A) Crosslinking mechanisms and preparation process of PDA hydrogels. Reproduced with permission [105]. Copyright 2023, Elsevier. B) PDA synthesis occurs via two pathways. Reproduced with permission [107]. Copyright 2012, Wiley. C) PHA preparation and nanocomposite hydrogel formation schemes. Reproduced with permission [112]. Copyright 2023, Elsevier. D) Diagram depicting the formation and operational mechanism of an S1P/SS-31/Lipo-encapsulated ROS-responsive composite hydrogel, designed for effective MI treatment. Reproduced with permission [43]. Copyright 2022, Wiley.
To mimic cellular functions in living organisms, cells are often encapsulated in materials, Wang et al. developed hydrogel sheets loaded with human skin fibroblasts (HSF) and hydrogel sheets loaded with human umbilical vein endothelial cells (HUVEC) by integrating cell sheets with abundant ECM, high cell density, and strong cellular connectivity with biomimetic hydrogels [116]. This one-step process involved rapidly assembling the cell sheets through layer-by-layer stacking within the hydrogels, resulting in the formation of hydrogels containing cell sheets (CSH). The encapsulated cells exhibited excellent viability, proliferation, and migration ability within the hydrogel environment. Keykhaee et al. introduced a multifunctional hydrogel by combining alginate and gum Arabic [117]. They immobilized nerve growth factor in custom-designed mesoporous silica nanoparticles (MSNs) and incorporated these MSNs into hydrogels along with carnosine to enhance their therapeutic properties. The hydrogel reduces inflammation while promoting epithelial re-formation, angiogenesis, and collagen deposition. This method of designing cell membranes or encapsulating active factors and functional components associated with myocardial repair in nanoparticles is a promising approach for myocardial repair.
In summary, the design of biomimetic hydrogel patches doesn't rely on only one biomimetic strategy to achieve effective tissue repair. Instead, they combine multiple biomimetic solutions to achieve both biological and physicochemical excellence, so that the desired and significant regenerative effect is achieved.
3.2. Wet adhesion mechanism of bionic hydrogel patches
Recently, extensive research has been conducted on bionic hydrogel patches that exhibit high robustness, excellent deformability, and multiple functions. These patches hold great promise in various applications, particularly in the field of hydrogel adhesion to substrates, which is a current area of intense research [85,[118], [119], [120], [121]]. Due to their water-containing nature, hydrogel patches find primary applications in humid environments, such as artificial organs, tissue engineering, and human biomedical devices (Fig. 5D and E) [122,123]. In all these areas, including myocardial repair, the attachment of hydrogel patches to other surfaces is crucial for achieving a significant effect [124]. However, hydrogel patches exhibit poor adhesion to other surfaces when fully swollen [125]. Recent advancements have been made in enhancing the adhesion of hydrogels to biological tissue surfaces. In myocardial applications, adhesion is primarily achieved through the mutual binding of hydrogel patches with surface amino groups, sulfhydryl groups, and other reactive groups on the myocardium, forming covalent bonds or hydrogen bonds [126,127]. Cohesion denotes the contribution of all the covalent and non-covalent forces for hydrogel formation. However, the presence of an interfacial hydration layer and the failure of patch cohesion hinder the adhesion to the myocardial surface [128]. Furthermore, hydrogel patches primarily rely on covalent bonds and intermolecular forces to maintain cohesion [129]. Ensuring strong adhesion of the patch to the myocardium is dependent on eliminating the hydration layer and promoting the formation of covalent bonds or intermolecular forces. Despite the potential for hydrogel patches to adhere well to myocardial tissue, there is a risk of adherence to surrounding tissues, which could lead to inflammation. To enhance comprehension of the adhesion mechanism of hydrogel patches, we will segment the explanation into distinct paragraphs and offer thorough clarification.
Fig. 5.
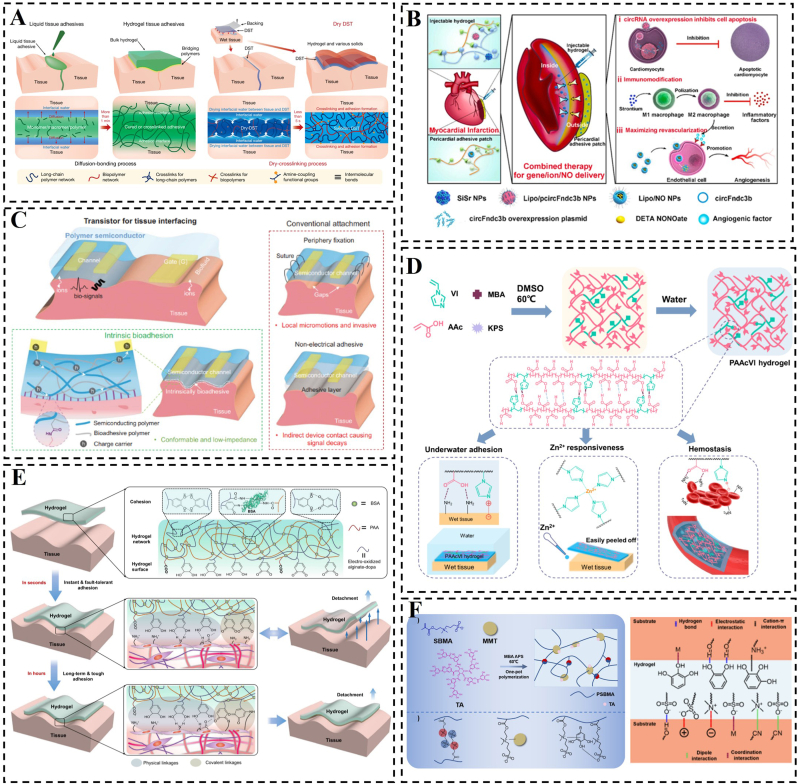
Schematic representation of the preparation of hydrogels and their adhesion mechanism. A) The dry double-sided tape and its mechanism of dry-crosslinking for adhering wet tissues and devices. Reproduced with permission [146]. Copyright 2019, Springer Nature. B) Schematic illustration of combined administration of injectable hydrogel with immune activity and a wet adhesive hydrogel cardiac patch designed for intramyocardial gene/ion delivery and epicardial NO delivery, aimed at restoring cardiac function post MI. Reproduced with permission [143]. Copyright 2023, Elsevier. C) Bioadhesive polymer semiconductors for electrochemical transistor-based tissue interfaces. Reproduced with permission [144]. Copyright 2023, American Association for the Advancement of Science. D) A hydrogel with tough underwater adhesive properties. Reproduced with permission [123]. Copyright 2022, American Chemical Society. E) Schematic illustration of the adhesion and fault-tolerant mechanism of a hydrogel tape. Reproduced with permission [122]. Copyright 2021, Springer Nature. F) Fabrication mechanism of the mussel-inspired adhesive hydrogels; possible adhesion mechanisms of the hydrogel. Reproduced with permission [134]. Copyright 2023, The Royal Society of Chemistry.
3.2.1. Hydration layer
Hydrogel patches, like many other materials, develop a hydrated layer on their surface when exposed to a liquid environment [130]. In the context of wet adhesion, this hydration layer acts as a watery film between the hydrogel and the substrate, significantly hindering adhesion. Furthermore, traditional polymer adhesives are prone to water erosion and may swell, ultimately resulting in failure [131]. In contrast, hydrogel, being a novel material with a three-dimensional network structure, can rapidly swell in water without dissolving. This characteristic lays the foundation for its application in wet adhesion [128]. Despite significant progress in wet adhesion of hydrogels, constructing a stable and long-lasting wet-adhesive hydrogel remains a challenging task [132,133]. When hydrogel patches adhere to the myocardium or other substrate surfaces, the presence of a hydrated layer restricts the adhesion between the hydrogel and the biological tissue (Fig. 5F) [134]. Furthermore, water droplets can accumulate in the interstitial space, reducing the macroscopic contact area between the hydrogel and the substrate. Moreover, prolonged exposure of the adhesive portion of the hydrogel patch to the liquid environment leads to the penetration of water molecules into the hydrogel. This can induce plasticization, swelling, erosion, degradation, or hydrolysis, ultimately leading to cohesive failure of the hydrogel [[135], [136], [137]]. That is why the removal of the hydration layer is a key prerequisite for the design of adhesive hydrogel patches.
In the presence of a hydrated layer, wet adhesion of hydrogels is usually achieved by means of repelling the hydration layer or absorbing the hydration layer. One method of achieving repulsion is to use hydrophobic groups and utilize their hydrophobic effect to eliminate the hydration layer at the hydrogel-tissue contact interface [[138], [139], [140]]. For example, spraying a hydrophobic solvent onto the hydrogel surface can create a hydrophobic thin layer that repels the hydration layer [139]. Similarly, monomers with long carbon chains or aromatic ring structures can facilitate hydrophobic interactions. Upon contact with water, monomers with long carbon chains tend to cluster together quickly, forming cohesive structures that repel water molecules from the surface of the target object. This allows the hydrogel and substrate to adhere through other intermolecular forces, bypassing any hindrance from water [141,142]. The aromatic ring structure's versatility enables the modification of various functional groups to regulate the ability to repel the hydrated layer [128]. In addition, water-absorbing materials can also be used to remove the water at the interface, enabling the bonding of the hydrogel patch to the substrate and achieving the desired effect. To this end, Zhang et al. used a pre-anchored sponge with a removable hydration layer to bond an Nitric oxide (NO)-laden liposomal pericardial adhesion hydrogel patch to the myocardial surface, facilitating the release of NO nanomedicines in the epicardium and promoting angiogenesis (Fig. 5B) [143]. Furthermore, Li et al. aimed to achieve multifunctional properties by creating a dual network film through the blending of a semiconducting polymer with a bioadhesive brush polymer [144]. The polymer backbone was modified with long side chains that were functionalized with carboxylic acids and N-hydroxysuccinimide esters (NHS). These functional groups enabled electrostatic and covalent interactions between the material and the tissue, leading to the acceleration of the elimination of the hydration layer and ultimately resulting in rapid adhesion.
The absence of the hydration layer poses challenges for hydrogel design, while the presence of hygroscopic substances or hydrophilic groups within the hydrogel facilitates absorption of the hydration layer. This absorption leads to strong adhesion of the hydrogel to the substrate [128]. Wei et al. developed multifunctional composite hydrogels using polyglutamic acid, polylysine, casein as a blowing agent, and calcium as a solidifying agent, resulting in hydrophilic properties, humectant capabilities, and adhesive qualities due to the hydrophilic and positively charged amino groups in the composite hydrogels [145]. Additionally, Yuk et al. introduced a novel tissue adhesive called dry double-sided tape (DST), which combines natural polymers with crosslinked poly(acrylic acid) grafted with N-hydrosuccinimide ester [146]. Dry DST quickly absorbs the hydration layer from the tissue surface upon contact, transitioning to a hydrogel state and establishing temporary physical adhesion with the tissue surface through hydrogen bonding and electrostatic interaction forces (Fig. 5A).
3.2.2. Covalent bonds
A covalent bond is formed when two or more atoms share their outer electrons, ideally reaching a saturated electronic state and resulting in a chemically stable structure. Covalent bonds are stronger than physical interactions and can substantially improve the cohesion of hydrogel patches and their adhesion to biological tissues. Hydrogels that contain organic ligands can typically react with functional groups on the tissue surface, forming covalent bonds such as amides, imines, siloxanes, and carbon-nitrogen bonds, thus achieving wet adhesion [[147], [148], [149]]. Myocardial tissue possesses reactive functional groups such as sulfhydryl and hydroxyl groups on its surface. Hydrogel patches that are rich in organic ligands can establish covalent bonds with these groups, thereby improving the adhesion between the hydrogel and myocardial tissue. Wu et al. established a wet-adhesive hydrogel cardiac patch that can be quickly adhered to cardiac tissues by removing interfacial water from the tissue surface by capillary action, and then stabilized and adhered to the surface of the myocardium by using the aldehyde group contained in the hydrogel, which forms a Schiff base with the amino group on the surface of the myocardium (Fig. 6C) [124]. Taking inspiration from the adhesive properties of mussels, various wet-adhesive hydrogels functionalized with catechol groups have been developed. Tannic acid (TA), which are polymeric compounds composed of multiple catechol units and phenolic carboxylic acid units bound by ester bonds, can form covalent bonds with biological tissues, achieving wet adhesion. Zheng et al. designed a hydrogel network using NHS-fixed alginate (Alg-NHS), poly(ethylene glycol) diacrylate (PEGDA), TA, and Fe3+ ions to introduce different modes of covalent and non-covalent interactions [126]. The synergistic interplay between these components significantly enhanced the adhesive strength of the hydrogel patches (Fig. 6A). Additionally, for hydrogel patches that are chemically crosslinked via non-dynamic covalent bonds, breaking these bonds results in a loss of cohesion. Once these bonds are disrupted, the hydrogel cannot be re-bonded and will no longer maintain its bulk form, leading to a loss of the patch's fundamental functionality and therapeutic efficacy.
Fig. 6.
Hydrogels built using covalent bonds. A) Schematic construction of the hydrogel and its adhesion mechanism. Reproduced with permission [126]. Copyright 2023, Elsevier. B) Scheme for the preparation of the hydrogel and its self-repairing properties. Reproduced with permission [150]. Copyright 2023, Elsevier. C) Construction of a hydrogel cardiac patch and its mechanism map. Reproduced with permission [124]. Copyright 2022, Elsevier. D) Schematic diagram of a hydrogel patch introducing borate bonds. Reproduced with permission [155]. Copyright 2021, Wiley.
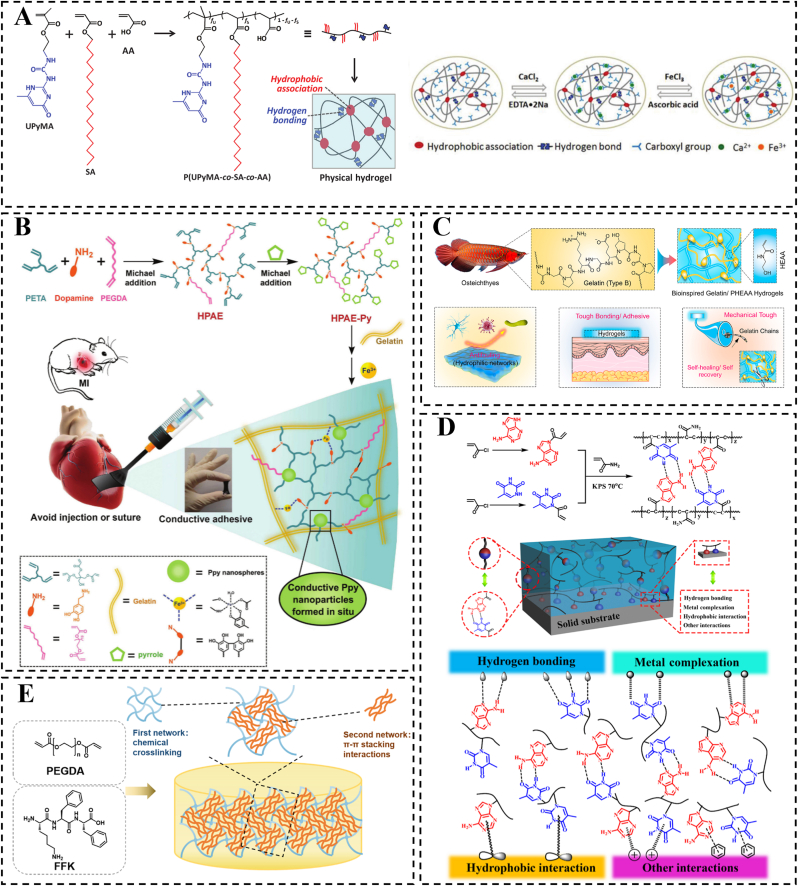
The coordination bond is a unique type of covalent bond that exhibits pH dependence and reversible properties. This distinctive characteristic not only maintains the stability typical of covalent bonds but also imparts reversible wet adhesion properties to the hydrogel. In a study by Qiao et al., a series of hydrogels were created using sodium oxidized alginate grafted dopamine/carboxymethyl chitosan/Fe3+ (OSD/CMC/Fe3+ hydrogels) with polydopamine encapsulated poly(thiophene-3-acetic acid) [150]. These hydrogels feature dynamic Schiff base and Fe3+ coordination bonds, allowing for autonomous repair in case of disruption. On a larger scale, the hydrogel demonstrates self-healing properties, enabling it to adapt to complex wound surfaces (Fig. 6B). Chemical cross-linking of hydrogels using irreversible covalent bonds prevents hydrogel patches from re-cohesive after bond breakage, significantly impacting tissue adhesion and repair ability. Therefore, in addition to ligand bonds, various dynamic covalent bonds are commonly utilized in the construction of hydrogels to meet specific functional requirements. There is a growing interest in developing hydrogel patches with dynamic covalent bonds to enable self-healing and promote tissue repair. For instance, dynamic borate, imine, and acylhydrazone bonds have been incorporated to create hydrogel networks [[151], [152], [153]]. Liu et al. developed a new hydrogel by combining two different kinetically dynamic covalent bonds, namely the boronic acid ester bond and the acylhydrazone bond [154]. These hydrogels were synthesized through dynamic interactions between the phenylboronic acid moiety and PVA, along with the ketone segment reacting with adipic dihydrazide (ADH), all achieved without the need for a catalyst. The active boronic acid ester bonds enable rapid gelation kinetics and provide the hydrogels with self-repairing capabilities, while the strong acylhydrazone bonds enhance the mechanical strength of the hydrogel network. This is especially true in myocardial repair applications, where the phenylborate bond is not only a dynamic bond but also a ROS-responsive bond. Li et al. introduced the phenylborate bond to synthesize a ROS-responsive hydrogel to deliver bFGF for myocardial repair. After injecting the therapeutic gel into the pericardial cavity, the hydrogel formed an epicardial patch in situ on the heart surface [155]. The patch scavenges ROS, significantly inhibits cardiomyocyte apoptosis, and promotes cardiomyocyte proliferation.
3.2.3. Intermolecular forces
Intermolecular forces, encompassing non-covalent interactions like electrostatic forces, hydrogen bonding, hydrophilic-hydrophobic interactions, van der Waals interactions, π-π stacking, and π-cation interactions, are frequently employed in the design of hydrogels and for adhering to biological tissues (Fig. 7D and E) [[156], [157], [158], [159], [160]]. In comparison to covalent bonding, these non-covalent interactions are relatively weak and generally reversible these non-covalent interactions are relatively weak and therefore reversible [161]. As a result, these interactions provide hydrogels with intriguing features such as fatigue resistance, self-repair, thermal and/or pH responsiveness, self-recovery capabilities, shape memory functionality, and remodeling properties. These discoveries have stimulated further exploration of promising applications in diverse fields [162]. Drawing inspiration from these non-covalent interaction mechanisms, bioelectronic engineering explores novel biomimetic technologies that seek to reversible and robust interfacial bonding between hydrogels and biological tissues. For example, hydrogen bonding serves as an excellent non-covalent mechanism for crafting versatile materials, and its strong binding capacity and reversibility enhance the cohesive ability of hydrogel patches as well as their adhesion to biological tissues [123]. Wang et al. proposed a novel biomimetic approach by integrating gelatin extracted from bony fish into a hydrophilic polymer network to form a unique tough, adhesive, and self-repairing composite hydrogel [163]. The results showed that the gelatin/poly(N-hydroxyethyl acrylamide) (PHEAA) hydrogel possessed high mechanical strength of ∼1.0 MPa and high interfacial toughness exceeding 1000 J/m2. In addition, due to the abundant hydrogen bonding in the composite crosslinked network, the gelatin/PHEAA hydrogel maintained high and reversible adhesion even under highly dynamic conditions (Fig. 7C). However, it is worth noting that hydrogel patches can be designed with multiple non-covalent interactions, multiple covalent bonds, or a combination of covalent and non-covalent interactions, which may offer enhanced adhesion to biological tissues and superior repair capabilities.
Fig. 7.
Wet adhesive hydrogels constructed with partially noncovalent forces. A) Schematic depictions of the synthesis of (UPyMA-co-SA-co-AA) and the metal ion-induced triple shape memory effect mechanism. Reproduced with permission [127]. Copyright 2018, Wiley. B) Schematic depiction of a conductive and adhesive hydrogel formation, applied by directly painting onto the surface of MI hearts in SD rats. Reproduced with permission [166]. Copyright 2018, Wiley. C) Illustration of composite crosslinked Gelatin/PHEAA hydrogels comprising type B gelatin extracted from Osteichthyes skin and super-hydrophilic PHEAA. Reproduced with permission [163]. Copyright 2022, Elsevier. D) Schematic depiction of the adhesive hydrogel and potential adhesion mechanisms, encompassing hydrogen bonding, metal complexation, hydrophobic association, and other interactions such as cation-π and/or π-π stacking. Reproduced with permission [160]. Copyright 2017, American Chemical Society. E) Schematic diagram of the π−π stacked hydrogel synthesis. Reproduced with permission [157]. Copyright 2023, American Chemical Society.
To enhance the mechanical and stimulus response properties of hydrogels, researchers have explored the construction of dual or multiple non-covalent cross-linking bonds. Chang et al. demonstrated the production of tough hydrogel by solution casting and subsequent solubilization of a film consisting of ureido-pyrimidinone methyl acrylate-co-staryl acrylate-co-PAA [127]. These gels utilize hydrophobic interactions among crystallizable alkyl chains and four hydrogen bonds facilitated by ureido-pyrimidinone (UPy) motifs, effectively acting as double cross-linking mechanisms within the hydrogel structure. The synergistic interplay between hydrophobic interactions and hydrogen bonds resulted in remarkable mechanical properties, with tensile rupture stresses reaching 4.6 MPa and strains at break up to 680 %. The UPy motifs facilitated the crystallization of the alkyl chains, while the hydrophobic alkyl chains stabilized the UPy-UPy hydrogen bonds. Additionally, these hydrogels exhibited responsiveness to various external stimuli, such as temperature, pH, and ions. The presence of hydrogen bonds and hydrophobic interactions also contributed to the enhancement of the hydrogels' wet adhesion properties (Fig. 7A).
In the context of MI treatment, the design of hydrogel patches necessitates robust wet adhesion to myocardial tissue. To enhance this adhesion strength, it is beneficial to combine and synergize multiple adhesion mechanisms. Furthermore, the adhesion properties of cardiac patches are intricately linked to the distances between the chemical groups involved in various types of non-covalent interactions. These interactions include hydrogen bonds, electrostatic interactions, van der Waals forces, and hydrophobic interactions. The strength of these interactions typically varies with the distance between the chemical groups. Specifically, when two chemical groups are in close proximity, the intensity of non-covalent interactions tends to increase, thereby enhancing adhesion. For instance, hydrogen bonds can form and function effectively only at appropriate distances and orientations. The optimal distance between a hydrogen atom and an electronegative atom (such as -COOH, -NH2, or -OH) is approximately 1.5–2.5 Å for effective hydrogen bonding formation [164]. Within this range, hydrogen bonds can significantly improve the adhesion of the patch to biological tissues. Similarly, electrostatic interactions rely on the distance between molecules; when the distance is too great, the interaction force diminishes considerably. When the distance between cations (such as -NH4+) and anions (such as -COO-) falls within a few to tens of angstroms, the electrostatic interaction is notably strengthened. Therefore, ensuring that charged groups are in close proximity during the design of cardiac patches can enhance their adhesion to cardiac tissue. The strength of van der Waals forces is inversely related to the distance between molecules, typically being most significant at distances of 3–5 Å [165]. Hydrophobic groups tend to cluster together to avoid water, and the closer they are to one another, the stronger the hydrophobic interactions. In cardiac patches, increasing the number of hydrophobic groups and optimizing their arrangement can further enhance adhesion to cardiac tissue. Thus, when designing cardiac patches, it is crucial to consider the various types of non-covalent interactions and the distances between their chemical groups to optimize adhesion. By carefully selecting and arranging these chemical groups, the performance of the patch in biological environments can be effectively improved, thereby enhancing its therapeutic efficacy. In addition to excellent adhesion properties, the conductivity of the adhesive patch is also critical for MI treatment and may facilitate the clinical translation of such designs (Fig. 7B) [166].
3.3. Design strategies for wet adhesion hydrogels
Hydrogel patches utilized after MI must attain stable adhesion to the myocardium for therapeutic efficacy. However, the liquid environment surrounding the heart significantly influences patch adhesion, rendering the design of wet-adhesive hydrogel patches a persistent and formidable challenge [167]. Wet adhesion hydrogels can be broadly classified into two categories based on their structures and properties: gel-type hydrogels and sol-type hydrogels. Gel-type hydrogels are polymers that form a three-dimensional network structure in water. They absorb a substantial amount of water, transitioning into a gel state that allows for direct adhesion to wet surfaces through molecular interactions. However, the adhesion interface of gel-type hydrogels is often influenced by liquid interference, affecting the overall adhesion effectiveness. On the other hand, sol-type hydrogels are colloidal solutions that transform into a gel state when stimulated to a certain degree. Sol-type hydrogels exhibit excellent fluidity, enabling close contact with the interface and achieving superior adhesion. However, traditional sol-type hydrogels have extended curing times. As a result, current research on sol-type hydrogels focuses on reducing curing times, while gel-type hydrogels aim to enhance contact area by eliminating the hydration layer [168]. To achieve stable wet adhesion of hydrogels, it is essential to overcome their drawbacks and explore complementary or even synergistic design strategies for hydrogel patches suitable for myocardial repair or other tissue repair. In the following sections, we provide a detailed overview of these two types of hydrogels and their respective design approaches in the context of wet adhesion.
3.3.1. Gel-type hydrogels
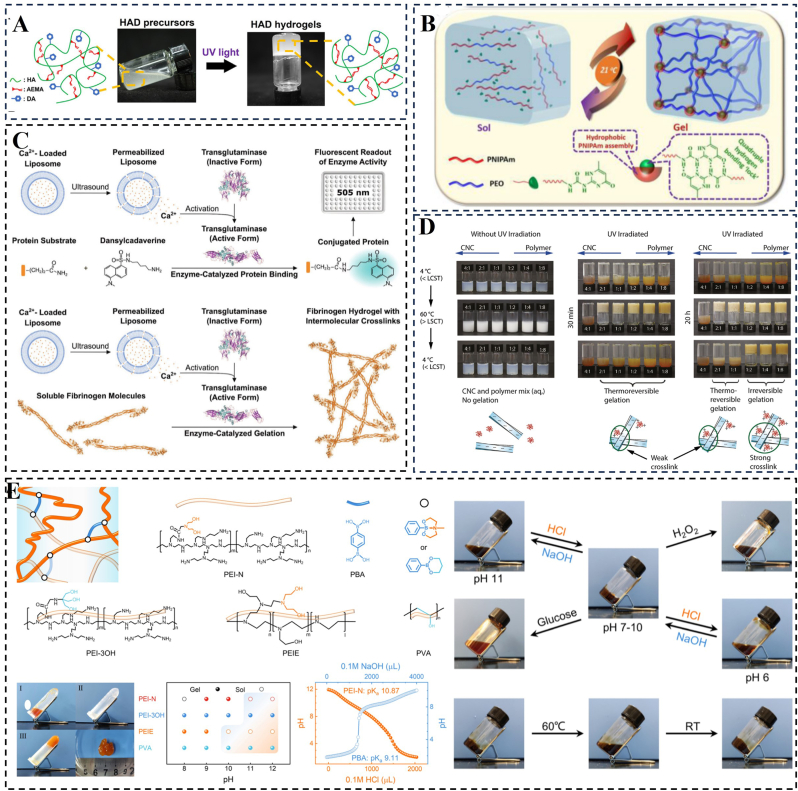
Gel-type hydrogels, commonly used in medical applications like fillers, drug release carriers, and tissue engineering, exhibit a soft texture and excellent elasticity akin to human soft tissue [169]. Hydrogel formation can be induced through various methods such as light irradiation, pH adjustment, heating, cooling, and ultrasound-triggered enzymatic gelation (Fig. 8) [[170], [171], [172], [173], [174]]. However, in applications like myocardial repair and tissue repair, the presence of a hydrated layer on the hydrogel's surface can hinder adhesion. Therefore, the current focus in the field of wet adhesion for gel-type hydrogels is on addressing this issue caused by the hydrated layer [137,175]. In previous sections, we have provided a detailed description of methods to eliminate the hydration layer. Additionally, the hydrophilicity or hydrophobicity of the gel surface can be adjusted by altering the chemical structure of the polymer or incorporating surfactants. For example, modifying monomers to introduce long alkyl chains and aromatic ring structures or utilizing absorbent materials for pre-water absorption can achieve the desired adjustment.
Fig. 8.
Hydrogels can be formed using a variety of triggering agents. A) Hydrogel formation after photocrosslinking. Reproduced with permission [171]. Copyright 2023, Ivyspring International. B) Schematic representation of reversible sol-gel transition of hydrogels prepared under temperature switching. Reproduced with permission [174]. Copyright 2017, American Chemical Society. C) Schematic representation of ultrasound-triggered enzyme catalysis and hydrogelation. Reproduced with permission [170]. Copyright 2020, Wiley. D) Sol-gel transitions illustrated by the vial inversion tests. Reproduced with permission [172]. Copyright 2020, American Chemical Society. E) Schematic representation of a traditional borate hydrogel; gel photographs; pH-dependent sol-gel phase diagram; multiresponsive properties of hydrogels. Reproduced with permission [173]. Copyright 2023, American Chemical Society.
In addition, Chen et al. created a sandcastle worm-inspired powder containing TA-functionalized cellulose nanofibers (TA-CNF), PAA, and polyethyleneimine (PEI) [125]. The powder exhibits rapid absorption of a wide range of body fluids and undergoes a transformation into a hydrogel. The resulting hydrogel demonstrates rapid, self-reinforcing, and reproducible wet adhesion to adipose tissue. Its dense physically cross-linked network allows for excellent 14-fold ductility and the ability to self-heal even after immersion in water. In the design of wet adhesion gels, it is often necessary to incorporate functional groups into the polymer structure that can interact with the target surface, promoting mechanisms such as hydrogen bonding, van der Waals forces and other molecular interactions. Ma et al. developed a wet-adhesive porous hydrogel (PVA/PAAc-N+) using wet-induced phase separation-solvent exchange and in-situ light curing methods. The PAAc-N+ layer contains carboxylic acid and NHS ester groups, facilitating non-covalent and covalent adhesion to tissue surfaces [176]. The PVA layer, with high crystallinity and abundant hydrogen bonding, allows for ionic cross-linking with carboxylate ions in the PAAc-N+ layer. The resulting PVA/PAAc-N+ hydrogel had a swelling ratio of 0.29 and an adhesive strength of 63.1 kPa. This dual-layer design offers an outer protective layer that resists postoperative tissue adhesion, reducing the risk of inflammation.
In summary, gel-type hydrogels generally exhibit weaker adhesion to tissues compared to sol-type hydrogels due to the presence of a hydration layer. The removal of this hydration layer poses a significant challenge for gel-type hydrogels with weak adhesion. Therefore, employing synergistic effects inspired by bionic microstructures found in organisms such as sandcastle worms and mussels, in combination with dynamically adhering tough hydrogels, may offer more efficient drainage solutions. Additionally, the design of bi- or multilayer hydrogel patches with an outer protective layer could be a promising direction for myocardial repair and other tissue repair applications. The inner layer would adhere to the infarcted area via the synergistic effect of the adhesion mechanisms, while the outer layer, made of a non-adhesive material, would cover the adhesive hydrogel. This design approach can help prevent tissue adhesion with surrounding tissues, reducing the likelihood of inflammation.
3.3.2. Sol-type hydrogels
Sol-type hydrogels are polymers that exist in a dispersed state, forming a homogeneous solution in a solvent. The rheological properties of a sol play a crucial role in coating and adhesion, with smaller particles being more likely to form a uniform cover layer, increasing the contact area with the substrate and enhancing adhesion. Optimal viscosity and rheological properties contribute to improved coating uniformity and adhesion. However, the development of sol-gel hydrogels has primarily focused on reducing curing time [128]. For example, Shen et al. modified β-cyclodextrin (β-CD) with acryloyl chloride and copolymerized it with NIPAM and AA to create a temperature-sensitive hydrogel [169]. The critical phase transition temperature of this sol-gel hydrogel was controlled at 19 °C, achieving a rapid sol-gel phase transition within 2–10 s. Sol-type hydrogels can undergo a gel shift in response to stimuli such as temperature, pH, or ionic concentration. By designing temperature-responsive sols, sol-type hydrogels can achieve adhesion to myocardial tissue under body temperature conditions. Barbier et al. developed a temperature-responsive copolymer by grafting poly(N-isopropylacrylamide-Stat-N-tert-butylacrylamide) onto hyaluronic acid [25]. The transition temperature of this solution could be accurately adjusted between 10 and 35 °C by modifying the composition of the low critical solvation temperature side chains. By investigating the characteristics of temperature-sensitive materials and optimizing them, it is anticipated that viscous gels can be formed at body temperature.
In summary, sol-type hydrogels typically have high adhesive strength due to full contact and strong mechanical interlocking at the molecular level, but typically fail by cohesion. To overcome this limitation, rapid reaction of group polymerization or cross-linking can significantly enhance cohesion and establish strong interfacial adhesion. By invoking different chemical bonds and regulating the interaction of the hydrogel with the surface groups of the substrate, sol-gels can be cured within seconds or minutes, offering a solution for achieving reversible adhesion.
3.4. Design requirements for hydrogel cardiac patch
When designing a hydrogel cardiac patch, it is crucial to first ensure that the patch achieves strong adhesion to the myocardial surface. Effective adhesion is essential for the patch to function properly and deliver therapeutic benefits. Additionally, the performance of the hydrogel patch after adhesion plays a critical role in its overall effectiveness (Table 1). In the following paragraphs, we will outline the key properties that are important for hydrogel cardiac patches.
Table 1.
Design requirements of hydrogels-based cardiac patch.
| Design requirements | Presentation | Relevant parameters or requirements | Ref |
|---|---|---|---|
| Conductivity | Cardiac patches must possess electrical conductivity to facilitate electrical signaling and coordinate the heart's electrical activity. |
|
[177] |
| |||
| Mechanical properties |
|
|
[181] |
| Biocompatibility | The patch material must exhibit low immunogenicity to minimize the risk of bodily rejection. | Synthesize hydrogels using materials derived from natural components or those with low immunogenicity. | [196] |
| Adhesion | Ensure that patches remain securely attached during cardiac contractions and in wet conditions. | One objective is to enhance interfacial drainage capacity, while the other is to increase the concentration of surface adhesion groups. | [124] |
3.4.1. Conductivity
The spontaneous activity of the sinus node generates action potentials that are transmitted to cardiomyocytes in both the left and right atria, initiating the rhythmic contractions of myocardial tissue and the cardiac systolic-diastolic cycle, which pumps blood throughout the body. After MI, the partial loss of electrical conduction and the progressive collapse of the myocardial wall exacerbate the transition from MI to heart failure. The conductive hydrogel patch addresses these defects by acting as a “bridge” that facilitates electrical signaling between infarcted fiber islands and healthy myocardium. Its internal conductive network efficiently transmits electrical signals from healthy regions to damaged areas, minimizing signal loss and distortion. By preventing premature myocardial failure during the systolic-diastolic cycle, the patch helps maintain normal cardiac function. However, excessive conductivity of the patch may result in increased current, leading to overstimulation of heart tissue. This can induce arrhythmias, disrupt normal electrical activity, or even precipitate cardiac arrest. High conductivity may also cause localized heating, interfering with normal cardiac function and potentially resulting in tissue damage or inflammatory responses. In the context of cardiac electrical signaling, excessive conductivity can distort or interfere with signal transmission. Conversely, insufficient conductivity may hinder effective electrical signaling, failing to provide the necessary stimulation for cardiac tissue repair or reconstruction. Ideally, the conductivity of the patch should approximate that of normal human myocardial tissue, which exhibits a transverse conductivity of approximately 5 × 10⁻⁵ S/cm and a vertical conductivity of around 1.6 × 10⁻³ S/cm [177].
To restore myocardial electrical integrity post-infarction, designing conductive hydrogels to re-establish synchronized systole and diastole at the infarction site has become a promising approach. Recently, conductive components such as metal nanoparticles, carbon nanomaterials, and conductive polymers have been incorporated into hydrogel matrices to create conductive hydrogels with electrical properties comparable to natural myocardial tissues. This enhancement aims to improve their effectiveness in applications like bioelectronics and cardiac tissue repair [178]. Liang et al. developed a novel approach to create a paintable and quickly bondable conductive hydrogel cardiac patch. This was achieved through the Fe3+-triggered simultaneous polymerization of covalently linked pyrrole and dopamine within a hyperbranched chain structure. The in situ formation of conductive polypyrrole serves a dual purpose: it enhances electrical conductivity and provides a unique method for crosslinking the hydrogel network. This innovation offers a suture-free strategy for cardiac tissue engineering, providing potential advancements in repairing myocardial tissue post-infarction [166]. Lee et al. pioneered the development of a highly conductive and adhesive hydrogel utilizing MXene nanosheets, aimed at engineering cardiac patches to enhance the function of MI regions. This innovative hydrogel serves as a paintable solution for repairing infarcted cardiac tissue, emphasizing the critical roles of mechanical support, electrical conduction, and tissue adhesion in cardiac tissue engineering [26]. While hydrogel patches incorporating conductive materials hold promise for a range of biomedical applications, including cardiac repair, important considerations remain. The cytocompatibility, biosafety, and degradability of conductive nanomaterials necessitate thorough evaluation. Addressing these factors is crucial for advancing the clinical viability of electroactive tissue engineering technologies in practical medical settings.
3.4.2. Mechanical properties
The mechanical properties are critical parameters in the design of cardiac patches. As MI progresses, the infarcted area ultimately develops into a hardened fibrotic scar that lacks elasticity, plasticity, and electrical conductivity, thereby impairing normal cardiac function. Hydrogel patches can provide mechanical support due to their elasticity and strength. The primary reasons include: (ⅰ) Cardiac patches are typically constructed from materials with high mechanical strength and suitable stiffness, such as polymers, natural biomaterials, or composites. These materials undergo stretching, bending, and compression during the heart's contraction and relaxation cycles, which helps maintain the structural integrity of the heart, supports normal heartbeat, and compensates for the mechanical deficiencies in the infarcted area [91,179], (ⅱ) After MI, the myocardium in the affected region becomes weakened and dilated. The mechanical support provided by the patch can prevent the worsening of these pathological changes and assist in maintaining the heart's normal structure and function, (ⅲ) The heart experiences complex stresses and strains during contraction and relaxation. Cardiac patches efficiently dissipate and transmit these stresses through their elastic and plastic properties, (ⅳ) The design of the patches can optimize interactions with the surrounding myocardial tissue, reducing localized stress concentrations and alleviating the mechanical burden on the damaged area. This uniform stress distribution helps lessen the overall load on the heart and enhances its pumping efficiency. Notably, some cardiac patches feature adjustable elastic moduli, allowing them to adapt to the heart's movements during contraction and relaxation. This adaptability enables the patches to provide appropriate support under varying physiological conditions, reducing additional stress caused by asynchrony with cardiac motion.
Regardless of the administration forms of hydrogels either embedded within the myocardium or attached to myocardium surface for MI treatment, the mechanical properties of incorporated hydrogels should closely align with the mechanical modulus of healthy myocardial tissue and exhibit fatigue resistance and self-healing properties to enhance mechanical stability. Injectable hydrogels should offer comparable mechanical modulus, fatigue resistance, and self-healing capabilities to ensure uniform distribution and stability within the myocardium. Conversely, hydrogel patches need to closely align with the myocardial surface's mechanical properties, providing strong adhesion, resistance to fatigue, and durability under dynamic cardiac motion. Both approaches must exhibit biocompatibility with the myocardial environment to prevent adverse reactions. Overall, the structural equivalence in both cases involves matching the mechanical and functional characteristics of healthy myocardial tissue, although each method has unique considerations based on its mode of application. The stiffness of normal rat myocardial tissue ranges from 0.1 to 140 kPa, while that of normal human myocardial tissue varies between 20 kPa at end-diastole and 500 kPa at end-systole [180]. Furthermore, the tensile strength of human myocardium ranges from 3 to 15 kPa, with stiffness values of 10–20 kPa at early diastole and 200–500 kPa at end-diastole [181]. Consequently, by adjusting mechanical properties such as elastic modulus and strength, the hydrogel patches can be customized to meet patients' cardiac needs. This customization ensures that the patches provide optimal support for the damaged heart, thereby enhancing their effectiveness in promoting repair and recovery. In summary, hydrogel patches can improve the mechanical properties of damaged cardiac tissue, increase local strength and elasticity, maintain the heart's shape and function, and facilitate tissue recovery by simulating or enhancing the heart's natural mechanics.
Hydrogels can generally be engineered with adjustable mechanical properties by varying component concentrations, utilizing multiple forces, and incorporating highly mechanical materials, which will be discussed below. (i) Varying the concentration of components. Varying the concentration of components is one of the most common methods to enhance the mechanical properties of hydrogels. Efraim et al. developed hydrogels with tailored mechanical properties using decellularized porcine cardiac ECM combined with varying amounts of chitosan. Their study demonstrated that these mechanically robust hydrogels preserved normal cardiac function and mitigated damage from MI [182]. (ii) Utilizing multiple forces: Yu et al. developed an electromechanically coupled hydrogel patch featuring dynamic covalent and non-covalent cross-linking, combined with cellular therapy. This patch improved electrical connectivity between healthy and infarcted regions, with its conductivity and sensitivity tailored to match myocardial tissues. The electrically conductive patch, in conjunction with cellular therapy, effectively prevented myocardial fibrosis and promoted neovascularization [183]. (iii) Incorporation of highly mechanical components: Graphene oxide (GO) is a highly mechanically conductive material that, when integrated into hydrogels, increases the network complexity and enhances the hydrogel's mechanical strength. For instance, Shin et al. investigated reduced graphene oxide (rGO)-encapsulated GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. The incorporation of rGO into the GelMA matrix significantly improved both the electrical conductivity and mechanical properties of the material [184]. In addition, in a study by Mei et al., a three-dimensionally printed conductive hydrogel patch containing MSCs @GO was proposed for efficient repair of MI [185]. Incorporating GO flakes has been shown to enhance both the mechanical properties and electrical conductivity of hydrogel patches, making them excellent candidates for cardiac repair applications. Therefore, adjustable mechanical hydrogel patches hold great promise for future clinical translation.
3.4.3. Rheology
Rheology, the study of the flow and deformation of materials, is critical for optimizing mechanical properties, enhancing biocompatibility, improving durability, and facilitating implantation. To effectively adapt to cardiac motion, hydrogels must mimic the elasticity and flexibility of cardiac tissue. A thorough understanding and optimization of these rheological properties can enhance the adaptability, stability, and functionality of patches. Key aspects include improving the ease of surgical application, developing functionalized hydrogels with features such as self-healing and drug release, and replicating the biomechanical properties of cardiac tissue to provide more natural support [186]. Consequently, specific rheological parameters must be considered in the design of hydrogel heart patches to ensure optimal performance. These parameters include: (i) Storage modulus (G′): This measures the elastic behavior of the hydrogel. For heart patches, G′ should be sufficiently high to provide adequate support and maintain the structural integrity of the patch under the mechanical stresses exerted by the heart. Typically, a storage modulus in the range of 1–10 kPa is desirable to match the elasticity of heart tissue. (ii) Loss modulus (G″): This reflects the viscous behavior of the hydrogel. The loss modulus must be optimized to ensure that the patch can effectively absorb and dissipate energy from cardiac motion. A loss modulus that is not excessively high will prevent excessive damping, which could impair the patch's ability to absorb impacts. Ideally, a G″ value that is roughly in the same range as G′ will provide favorable viscoelastic properties. (iii) G′ and G″ crossover point (Gelation point): The gelation point, where G′ and G″ intersect, indicates the transition from a liquid-like to a gel-like state. For heart patches, this point should be optimized to ensure that the hydrogel transitions to a gel state at physiological temperatures, typically around 37 °C, ensuring that the patch remains stable and effective once implanted. (iv) Viscosity: The viscosity of the hydrogel significantly influences its handling and application. A moderate viscosity is necessary for the proper placement and spreading of the hydrogel over heart defects. A viscosity range of 1–10 Pa s is typically suitable, facilitating easy application without excessive flow. (v) Shear thinning behavior: The hydrogel should exhibit shear-thinning properties, which means that its viscosity decreases under shear stress. This behavior enhances the ease of application and allows the patch to conform to the shape of the cardiac tissue. The degree of shear thinning should be sufficient to facilitate smooth application while maintaining stability during and after implantation [187]. (vi) Self-healing properties: Rheological tests should also evaluate the hydrogel's ability to self-heal after being subjected to mechanical stress. This capability is crucial for ensuring the long-term functionality and durability of the patch. A hydrogel exhibiting robust self-healing characteristics will return to its original rheological state after deformation. (vii) Elastic recovery: The hydrogel should demonstrate high elastic recovery, allowing it to revert to its original shape after deformation. This property ensures that the patch maintains its functional integrity and conforms effectively to the cardiac tissue over time. Thus, the hydrogel rheology of hydrogels are essential for advancing cardiac patch technology.
3.4.4. Effects of hydrogel properties on cells
Hydrogels can provide ECM-like structures that mimic the cellular environment, thereby supporting the activity of the encapsulated cells. However, the influence of various hydrogel properties on cell behavior is complex. A comprehensive understanding of how these properties impact cell behavior is essential for designing more effective hydrogel patches for MI [188]. Recent research has increasingly shown that the physical properties of hydrogels—such as pore size, degradability, and stiffness—can modulate cell behavior, including proliferation, migration, and differentiation. These effects are primarily mediated through alterations in mechanotransduction signals [189]. Variations in pore sizes within the hydrogel network create differing degrees of spatial confinement for cells, influencing both individual cell behavior and multicellular organization. McAndrew et al. demonstrated that stem cells cultured in gelatin-based scaffolds with smaller pore sizes were more likely to differentiate into bone lineages [190]. Fu et al. designed hydrogels with three distinct pore sizes: large (287.99 ± 74.58 μm), medium (205.22 ± 55.58 μm), and small (146.45 ± 3.15 μm). They assessed the impact of these pore sizes on the vascularization of endothelial progenitor cells both in vitro and in vivo. Their experiments revealed that a medium pore size was optimal for hydrogel vascularization in vitro, while a large pore size was more effective for in vivo hydrogel vascularization. Among the various physical properties of hydrogels, matrix stiffness exerts the most significant influence on cell behavior as a critical variable parameter. Hydrogel stiffness transmits essential mechanical signals that affect cellular phenotypes, including morphology, polarity, migration, differentiation, and maturation. These matrices can mimic the microenvironment of different tissues—such as neurons, adipose tissue, cartilage, cancellous bone, and blood vessels—thereby influencing the phenotype and functionality of the final cell types [191]. Matrix stiffness plays a vital role in converting mechanical forces into biochemical signals, thereby affecting cell fate through established mechanical signaling pathways. These pathways include integrin-dependent focal adhesion kinase (FAK), Rho/Rho-associated protein kinase (ROCK), Yorkie homologue Yes-associated protein (YAP)/TAZ, and Wnt/β-catenin signaling pathways. By closely matching the stiffness of the hydrogel to the natural rigidity of myocardial ECM, cell-specific differentiation can be promoted. This alignment facilitates the differentiation of cells into myocardial tissue cells by providing a stiffness environment similar to that of the native tissue. Wang et al. prepared six types of hydrogels with a wide stiffness range of 1.2–28.9 kPa through dynamic covalent cross-linking of gelatin derivatives and oxidized hyaluronic acid. This hydrogel, featuring gradient stiffness, served as a modeling platform to guide the adhesion, migration, and differentiation of rat bone marrow stem cells (rBMSCs) [192]. Nattasit et al. developed a polyacrylamide hydrogel with adjustable stiffness and demonstrated that induced pluripotent stem cell-derived embryoid bodies cultured in a hydrogel with a stiffness of 28.1 ± 2.3 kPa displayed exhibited enhanced CM differentiation and structural maturation of myogenic fibers [193].
Furthermore, the composition of cardiac patches and the incorporation of cytokines play a significant role in influencing cell survival, proliferation, and migration. For example, these patches are composed of ECM components, including collagen, fibronectin, and glycosaminoglycans, which provide essential growth factors and reduce the risk of immune rejection. Adequate blood supply is critical for cell survival and proliferation, as it facilitates the delivery of oxygen and nutrients. Consequently, patch design should incorporate strategies to promote angiogenesis, thereby ensuring that cells receive sufficient nourishment. The addition of appropriate growth factors, such as VEGF and FGF, can further enhance cell proliferation and migration [194,195].
Therefore, the rational design of hydrogel scaffolds with physiologically matched stiffness and pore size, as alternatives to natural ECM, is highly beneficial for cardiac engineering research. Additionally, electrospinning and bioprinting technologies have garnered significant attention in tissue engineering applications. Electrospinning excels in creating scaffolds with high specific surface area and well-defined pore structures, which facilitate cell attachment and growth. Bioprinting technologies enable the fabrication of intricate 3D structures and precise microscopic features on demand. The growing interest in cardiac patch fabrication highlights the relevance of these technologies, which we will explore further in the following section.
4. Cardiac patches
Tissue-engineered cardiac patches show great promise as a therapeutic option for individuals with MI. These patches aim to integrate superior mechanical properties and biological functionalities to effectively repair damaged myocardial tissue (Fig. 9C and D) [183,197,198]. The ideal cardiac patches should closely mimic the structural characteristics and biological functions of myocardial tissue. They should also have the capability to be loaded with drugs that promote myocardial repair. By replacing infarcted myocardial tissue and enhancing contractile function, these patches have the potential to significantly improve outcomes for individuals with MI. Cardiac patches typically closely resemble the structure of the natural ECM and possess similar electrical conductivity properties as the heart. Conductive cardiac patches bypass abnormal areas in damaged hearts where electrical signals fail to conduct or conduct slowly. By restoring normal conduction pathways in the heart, these patches help prevent reperfusion arrhythmias and promote proper cardiac function (Fig. 9G and H) [199,200]. The arrangement of CMs in cardiac patches is essential to maintain the microstructure and biological function of native tissues. Therefore, numerous studies have focused on patterning cardiac patches to align CMs and create functional cardiac tissue (Fig. 9E and F) [201,202]. In the application of hydrogel patches for myocardial repair, it is important for the patches to be porous, allowing rapid transfer of nutrients and oxygen to cells in the infarct microenvironment, which facilitates crosstalk, cell attachment, expansion and maturation (Fig. 9A) [21,203]. In summary, the preparation of cardiac patches requires physiologically accurate scaffold microstructures, mechanical structures to support heart beat kinetics, appropriate biocompatibility and degradation rates, and electrical conductivity similar to that of cardiac tissue. To achieve effective myocardial repair with cardiac patches, a combination of cells, bionic scaffolds that mimic the structure and function of natural tissues, and signals is necessary. Exciting results have been achieved with cardiac patches made using electrostatic spinning or three-dimensional printing techniques, effectively promoting myocardial repair. However, many of these patches face challenges with removal or degradation. By integrating hydrogel materials with these fabrication techniques, the benefits of both can be synergized: novel fabrication methods allow for precise personalization of cardiac patch structures, while hydrogels create an ECM-like environment that enhances cell adhesion, proliferation, and myocardial repair. In the following paragraphs, we provide a detailed summary of applications in cardiac patch fabrication using electrostatic spinning technology, 3D printing technology, and smart response materials.
Fig. 9.
Schematic design of various types of cardiac patches. A) Synthesis of conductive hydrogel patches with high elasticity and fatigue properties and schematic representation of their effects. Reproduced with permission [21]. Copyright 2022, American Chemical Society. B) Schematic illustration of the fabrication of CNBS cardiac patches. Reproduced with permission [210]. Copyright 2023, KeAi Communications Co. C) The interplay between decellularized cECM patch, growth factors, and further seeding of stem cells. The Reproduced with permission [198]. Copyright 2023, MDPI. D) The cardiac patch derived from sea squirts, a byproduct of marine culture waste, showed promise for treating MI. By treating sea squirts waste with acid or similar methods, researchers could create a conductive and well-aligned hydrogel, which notably improved the cardiac function of MI rats. Reproduced with permission [197]. Copyright 2020, Elsevier. E) Schematic illustrations and representative images of micropatterned scaffold fabrication. Reproduced with permission [202]. Copyright 2023, Wiley. F) Illustration depicting the bow-tie dimensions and alignment of the auxetic cardiac patch on the heart. Reproduced with permission [201]. Copyright 2018, Wiley. G) Schematic illustration of a cardiac patch mounted on the epicardial surface. Reproduced with permission [199]. Copyright 2023, Wiley. H) Illustration showing an injectable mesh cardiac patch designed for eliminating atrial fibrillation. Reproduced with permission [200]. Copyright 2023, Wiley.
4.1. Fabrication of cardiac patches using electrospinning
Various tissues in the human body, such as the extracellular matrix, muscle, and heart, are characterized by fibrous structures that play essential roles in providing mechanical strength and carrying out important biological functions. Traditional hydrogels often lack these fibrous structures and have weak mechanical properties, which limits their potential applications [204]. Electrospinning has become a popular method in tissue engineering for creating cardiac patches due to its ability to produce high aspect ratio fibers resembling the extracellular matrix, offering excellent physical strength, and providing the capability to incorporate drugs [205,206]. Electrostatic spinning enables the preparation of biocompatible polymeric materials like gelatin, poly(lactic acid) (PLA), and polycaprolactone (PCL) for the preparation of cardiac patches. This choice of materials helps reduce foreign body reactions and rejection [[207], [208], [209]]. In addition, functional substances such as drugs and growth factors can be directly embedded or compounded into the nanofibres to achieve the drug-releasing function of cardiac patches, which can help to promote cardiac tissue regeneration and repair.
To this end, Wei et al. developed a novel cardiac patch by layering sheets of brown adipose-derived stem cells (BADSCs) and combining them with electrospun polycaprolactone/silk-protein nanofibers (CPSNs) containing multi-walled carbon nanotubes (CNTs) [210]. The CPSN-BADSCs cardiac patch has shown the ability to influence macrophage polarization and improve gap junction remodeling, ultimately contributing to the restoration of cardiac function. Hybrid cardiac patches, composed of electrospun nanofibers and cell sheets, offer a new approach to cardiac remodeling post ischemic MI (Fig. 9B). While traditional cardiac patches require grafting and suturing onto the infarcted heart for reparative effects, adhesive hydrogel patches may be more advantageous for cardiac patch applications. However, monolayer adhesive patches require grafting and suturing onto the infarcted heart surface to achieve reparative capability, adhesive hydrogel patches may be more favorable for cardiac patch applications. However, mono-layer adhesive patches used for tissue repair often form tissue adhesions with the surrounding tissues, leading to inflammation. To address this issue, Zhang et al. developed a two-layer Janus patch with an inner tissue-facing multifunctional electrospun hydrogel patch (MEHP) [211]. The MEHP is prepared by co-electrostatic spinning of gelatin methacrylamide (GelMA) and zinc oxide nanoparticles, followed by treatment with TA. The inner MEHP possesses excellent mechanical properties, adhesion strength, and remarkable antioxidant, anti-inflammatory, and antimicrobial properties, allowing it to adhere to the injury site. The outer layer consists of electrospun poly-L-lactic acid, serving as a physical barrier to prevent the invasion of foreign cells and tissues into the defect site and reduce the formation of tissue adhesions. This bilayer strategy for the hydrogel patch can be applied in myocardial repair to mitigate the unfavorable effects of tissue adhesion and subsequent inflammation between the cardiac patch and the surrounding tissues.
Electrostatic spinning technology enables the preparation of nanoscale fibre structures that closely resemble the natural tissue structure of the human body. These fibre structures promote cell attachment, proliferation, differentiation, and contribute to tissue regeneration. Furthermore, the diameter, arrangement, and structure of the fibres can be precisely controlled by adjusting electric field parameters and the polymer solution's characteristics. Regulating cardiac patch properties to meet diverse clinical needs is crucial. However, electrostatic spinning technology still faces challenges and limitations, with fiber alignment induction being a key issue [168]. Various factors can impact fiber alignment, resulting in uneven or crossed misalignment that can affect the structure and properties of cardiac patches. The morphological and shape anisotropy play a significant role in influencing mechanical strength and various cellular functions like adhesion, proliferation, and alignment. Additionally, conventional electrostatic spinning methods often result in two-dimensional (2D) structures with densely stacked nanofiber layers and limited porosity, limiting their application in tissue engineering where cell infiltration is necessary for forming three-dimensional (3D) tissue structures. Therefore, there is a growing trend to develop new technologies capable of producing scaffolds with robust 3D nanofiber structures.
4.2. Fabrication of cardiac patches using 3D printing
3D printing technology is employed in the manufacturing of cardiac patches by utilizing specialized equipment, materials, and computer-aided design (CAD) software. This process involves converting three-dimensional structural data of the infarcted area of the heart into a printed model, which is then constructed layer by layer to form an actual cardiac patch. This technique has garnered significant attention in the medical and biomedical fields [[212], [213], [214]]. Bioinks utilized in bioprinting are injectable liquids containing cells or biomaterials. The most common type of bioink is a cell suspension, consisting of a mixture of cells and growth factors derived from human tissue, cultured stem cells, fibroblasts, among others (Fig. 10D) [185]. Aside from cell suspensions, various non-cell-based materials can serve as components of bioinks (Fig. 10C) [215]. For example, the commonly used biodegradable material, polylactic acid/hydroxyacetic acid copolymer, allows for the printing of scaffold structures that gradually degrade post-implantation [216,217]. Additionally, the ECM, a complex biomaterial essential for cell growth and development, can also be extracted to prepare specific types of bioinks that provide a cell growth environment mimicking the natural surroundings.
Fig. 10.
Cardiac patches made by bioprinting technology. A) Schematic of the workflow of a 3D printing cardiac patch for MI; photographs of in vivo experiments with cardiac patches and related data. Reproduced with permission [223]. Copyright 2023, Elsevier. B) Hydrogel bilayer heart patch fabricated by 3D printing technology. Reproduced with permission [203]. Copyright 2022, Wiley. C) A bilayer intrinsically magnetic epicardial patch prepared by 3D printing. Reproduced with permission [215]. Copyright 2023, Wiley. D) Schematic Illustration of the Effects of 3D Bioprinting of Conductive Hydrogel Patch on Cardiac Repair for the Treatment of MI; Four weeks after treatment, fibrosis was assessed according to histological analysis of Masson's trichromatic staining. Reproduced with permission [185]. Copyright 2024, American Chemical Society.
In recent years, hydrogels have gained significant attention as highly promising biomaterial candidates due to their resemblance to the natural ECM and their ability to provide a hydrated environment conducive to cell proliferation [218,219]. Commonly used biogel materials include gelatin, alginate, and gelatin-alginate composite gels [[220], [221], [222]]. These gel materials offer the necessary support and protection for cell attachment and growth. Wang et al. developed nanodroplets that respond to ultrasound-triggered phase transition and integrated them into GelMA hydrogel as a bioink for 3D bioprinting [223]. The introduction of nanodroplets and ultrasonic irradiation created pores within the hydrogel, enhancing its permeability. Hemoglobin was encapsulated into these nanodroplets (ND-Hb) to produce oxygen carriers. Studies demonstrated that ND-Hb patches, when exposed to low-intensity pulsed ultrasound, led to the highest cell survival rate. This non-invasive and efficient method effectively increased the hydrogel's permeability, enabling better substance exchange within the cardiac patch. Additionally, ultrasound-controlled oxygen release enhanced graft cell viability and supported the repair of infarcted tissue (Fig. 10A). In addition, Jiang et al., used 3D printing to design a protein-based bilayer hydrogel cardiac patch, with the first layer having properties such as soft mechanical strength similar to cardiac tissue, and strong interfacial adhesion to cardiac tissue. The second layer serves as a robust physical barrier against rupture pressure caused by cardiac bleeding, and can effectively prolong the storage and release of therapeutic molecules [203] (Fig. 10B).
Although 3D bioprinting improves the reproducibility and accuracy of stent fabrication and holds significant potential in cardiac patch manufacturing, there are still limitations associated with current bioinks and printing processes. Many bioinks derived from natural sources lack structural stability and deform immediately after extrusion from the nozzle, complicating the printing process. The fabrication of cardiac scaffolds composed of micro/nanofibers is notably constrained by the low throughput of conventional 3D printing techniques. In contrast, Focused Rotational Jet Spinning (FRJS) has emerged as a promising additive manufacturing technology for the creation of cardiac patches and other cardiac structures. Proposed by Chang et al., FRJS enables the production of micro/nanofiber scaffolds with programmable 3D arrangements. This innovative method facilitates the rapid generation of long fibers that can be easily shaped into heart valves and other implantable devices. Experimental studies have demonstrated that aligning fibers in the ventricular direction is crucial for optimizing cardiac performance [224]. Additionally, Motta et al. utilized FRJS technology to fabricate valves using poly(L-propylene glycol-ε-caprolactone) fiber scaffolds, which promote rapid cellular infiltration and exhibit mechanical properties akin to those of natural heart valves. This manufacturing process shows considerable potential for advancing the development of synthetic heart valves and other cardiac patches [225]. Moreover, simultaneously replicating both the external geometry and internal structures, such as blood vessels, of a given organ presents a significant challenge in MI treatment. To address this issue, Fang et al. developed a technique called Sequential Printing in Reversible Ink Templates (SPIRIT). This method employs a microgel-based biphasic bioink that acts as both an effective bioink and a suspension medium for embedded 3D printing, owing to its shear-thinning and self-repairing properties. When encapsulating human induced pluripotent stem cells (hiPSCs), the microgel-based biphasic bioinks were utilized to 3D print cardiac tissues and organoids, facilitating extensive stem cell proliferation and differentiation into cardiac cells. By incorporating these microgel-based biphasic bioinks, the SPIRIT strategy successfully generated ventricular models with perfused vascular networks, a capability that existing 3D printing methods have yet to achieve [226].
Moreover, the geometry of 3D-printed patches merely corresponds to the initial infarcted area at the time of fabrication. However, the heart's curved surface changes shape in response to its dynamic beating, and static 3D structures are unable to adapt to such dynamic conditions. This lack of adaptability may result in the loss of loaded components. Therefore, developing cardiac patches with high electrical conductivity and flexibility and achieving gapless attachment to conform to the geometry of the infarcted myocardial region remains a challenge. In recent years, 4D printing of dynamically structured cardiac patches has begun to be extensively investigated and may be able to alleviate the limitations of 3D printing by allowing for adaptive responses and thus gapless attachment to the infarcted region under dynamic conditions of the heart.
4.3. Fabrication of cardiac patches using 4D printing and smart response technology
4D Printing is an innovative manufacturing technique based on 3D printing technology that utilizes smart responsive materials combined with 3D bioprinting to create dynamic structures that can be transformed into desired shapes according to the needs of the environment [227]. In the case of cardiac patches, 4D printing can be used to produce adaptive patches with adjustable properties that can be seamlessly integrated into the target tissue after implantation [228]. The materials made by 4D printing technology exhibit reversible behavior in response to environmental stimuli, which is actually achieved through the incorporation of smart response materials in the printing process. Smart response materials have the ability to respond to external stimuli such as changes in temperature, pH, ionic concentration or other physiological conditions [229,230]. For instance, shape memory polymers (SMPs), widely used in 4D printing, can transform from a rigid polymer to an elastic polymer and back to a rigid polymer again, recovering their original shape after stimulation [231]. Consequently, smart-responsive materials can adapt their shapes in response to different stimuli, ensuring a better fit with the target tissue environment. In a study by Hann et al., thermo-responsive SMPs were employed to fabricate a spherical 4D smart minimally invasive cardiac cellular carrier capable of transforming into an in-situ cardiac patch within the infarcted region of myocardial tissue [24]. The results showed that the 4D carrier has a good deformation ability from a spherical carrier to a fully stretched transformation into a patch at human body temperature, and the fabricated 4D spherical carrier will fold before injection and restore its initial shape in the targeted region after delivery to transform into a patch adhering to the infarcted region without cell loss for repairing the damaged myocardial tissue.
In summary, 4D printing technology may lead to a novel therapeutic strategy using tunable smart-responsive materials bound or loaded with desired drugs or bioactive molecules, allowing cardiac patches to adapt to individual differences in dynamic cardiac structure of MI patients and release bound or loaded components in response to specific conditioned stimuli, thus improving myocardial repair. In addition, the invasiveness of the procedure can be reduced when implanting cardiac patches through the use of 4D printing technologies, such as the widespread development of collapsible smart cardiovascular implants, which are cardiac patches that are accessed with minimal invasiveness through two small incisions. Currently, there are few reports on hydrogel-based cardiac patches created using 4D printing technology. However, the combination of 4D printing and hydrogel materials has seen extensive application in tissue repair fields. For instance, Lu et al. utilized 4D printing to create a multifunctional hydrogel dressing with a thermo-responsive backbone (i.e., PNIPAM) that facilitates wound contraction when activated by body temperature. The resulting hydrogel exhibits several notable properties: strong tissue adhesion, temperature-responsive contraction, effective hemostasis, biocompatibility, biodegradability, and inflammation modulation [232]. Its design allows it to conform perfectly to wounds of various complex shapes and depths, thereby promoting wound closure and enhancing tissue regeneration. In the future, integrating 4D bioprinting with hydrogel materials presents a promising strategy for advancing cardiac repair and therapy. Considering the intricate changes in the microenvironment following MI, the future integration of 4D bioprinting technology with hydrogel materials, which harness the microenvironment's target responsiveness, promises to be a highly effective strategy for advancing cardiac repair and therapy.
Definitely, hydrogel-based technologies present promising solutions to some of the posed challenges. However, each of the novel manufacturing technologies, such as electrospinning, 3D printing, and 4D printing—has distinct advantages and limitations (Table 2). For example, hydrogels can significantly improve the biocompatibility of electrospinning technology. Without this enhanced biocompatibility, the technology would be limited by its inherent characteristics. Electrospinning is particularly advantageous for producing high surface area and nanofiber structures, which are especially beneficial for integration with hydrogel matrices. The combination of hydrogels and bioprinting enables the creation of complex and well-structured cardiac patches tailored for personalized medicine applications. Electrospinning is especially effective in fabricating nanofibrous structures that closely mimic the native tissue environment, thereby promoting better patch integration and functional recovery. Additionally, 3D printing allows precise control over the architecture and customization of the patch, ensuring a seamless fit and effective function within the targeted area. 4D printing enhances this capability by enabling the patch to dynamically respond to environmental stimuli after implantation, thus providing adaptability that can improve long-term functionality and integration.
Table 2.
Advantages and as limitations of manufacturing technologies.
| Manufacturing Methods | Advantages | Limitations | Ref. |
|---|---|---|---|
| Pure hydrogel-based patches | Highly biocompatible, ECM-like environment with straightforward preparation. | It cannot determine the microgeometry, making it challenging to achieve a specific spatial structure. | [233,234] |
| Electrospinning | Diverse chemical compositions, large specific surface area, and high porosity. | Complex manufacturing process and low mechanical strength. | [235,236] |
| 3D printing | It provides a three-dimensional environment that supports cell growth based on varying requirements. | Higher cost and less mechanical. | [237,238] |
| 4D printing | It utilizes 3D printing technology, allowing for the modification of the material's shape or properties in response to intelligent stimuli. | Higher costs. | [239,240] |
Compared to traditional water gel patches, these advanced manufacturing technologies offer significant improvements, including enhanced mechanical strength, better tissue integration for improved interaction with surrounding myocardial tissue, and the ability to create customized geometries tailored to specific patient needs. However, it is important to acknowledge that these advanced technologies also introduce increased complexity in manufacturing processes and potentially higher costs. Despite these challenges, the advantages of improved patch performance and patient-specific customization render these technologies valuable advancements in cardiac tissue engineering.
5. Prospects and challenges
MI remains one of the leading causes of death worldwide, prompting extensive research into both pharmacological and biomaterial interventions for its treatment. Recently, hydrogels have attracted significant research attention due to their exceptional properties. Despite challenges such as the potential for re-induced myocardial damage from hydrogel injection and uncertainties surrounding optimal dosage, hydrogel patches represent a promising approach for MI management. However, hydrogel-based cardiac patches also present both opportunities and risks.
From a design perspective, the porous structure of hydrogels makes them ideal carriers for various drugs and active ingredients. Their excellent biocompatibility further enhances their potential in medical applications, positioning hydrogels as highly promising materials in medicine [[241], [242], [243]]. The advantageous properties of hydrogel cardiac patches have stimulated extensive research in recent years, indicating that these patches could provide a novel therapeutic strategy at various stages of MI progression. Early application of hydrogel patches at the onset of MI symptoms may slow or halt disease progression, thereby minimizing the size of the infarcted myocardium. During the inflammatory phase, hydrogel patches infused with anti-inflammatory agents could mitigate the inflammatory response and subsequent fibrosis, thereby alleviating cardiac stress. In the proliferative and remodeling phases, hydrogel patches containing bioactive agents, such as stem cells and growth factors, could support myocardial regeneration and repair, promoting the recovery of damaged myocardium [26,71,198]. In addition to their biocompatibility and electrical conductivity that help maintain normal cardiac structure and function, certain hydrogel patches possess monitoring and tracking capabilities. These features enable real-time assessments of cardiac status, facilitating timely adjustments to treatment plans [[244], [245], [246]]. However, the design of hydrogels presents several challenges. Careful consideration must be given to the properties of conductive materials, including their degradability and toxicity. In addition, both excessive and insufficient mechanical strength may compromise the efficacy of myocardial repair. There is an increasing need to further investigate how factors such as hardness, pore size, and other properties influence cell behavior and to determine the specific requirements for their application in MI treatment.
At the manufacturing level, traditional hydrogel patches often lack precisely customized structures. However, advancements such as electrospinning for creating performance-tuned nanofibers and printing technologies for custom designs offer promising solutions. Despite this progress, the integration of hydrogel with these manufacturing technologies remains limited, and it is unclear whether professional barriers exist. Expanding the combination of hydrogels with advanced manufacturing technologies could enable the development of biomimetic hydrogels, yielding more accurate patches tailored to meet patients' individual needs. Furthermore, these advanced technologies should be progressively optimized to reduce costs and facilitate subsequent clinical translation. We believe that, in the future, the integrating of hydrogels with innovative technologies will enhance their advantages and address existing limitations, potentially leading to new and more effective therapeutic strategies for MI treatment.
At the surgical level, injectable hydrogels for myocardial repair are typically administered at five predetermined sites within the infarcted area. This method can lead to additional myocardial damage and may result in inaccurate dosing. In this context, patch-based therapeutic strategies present promising alternatives. Unlike injectable methods, patches can be precisely applied over the infarcted area, potentially minimizing further damage and enhancing the consistency of therapeutic delivery. These strategies may offer better control over hydrogel placement and distribution, leading to more effective myocardial repair and regeneration. However, the current procedure for implanting cardiac patches often requires open-heart surgery, which poses challenges for MI patients due to their compromised condition. This underscores the importance of developing minimally invasive approaches for delivering cardiac patches in future clinical applications. Furthermore, traditional cardiac patches typically rely on adhesion methods such as sutures, staples, or adhesives, which carry inherent risks, including bleeding, cytotoxicity, infection, and foreign body reactions [247]. In contrast, hydrogel patches provide various strategies for myocardial adhesion, including bionic structures, covalent bonding, molecular forces, and co-adhesion mechanisms. These patches can be delivered and implanted on the cardiac surface in a minimally invasive manner, significantly less invasive than traditional open-heart surgery. For instance, collapsible hydrogel carriers fabricated using 4D printing, as discussed in previous chapters, can be placed at the lesion site through two small incisions and then return to their original shape, adhering in response to in vivo environmental stimuli. This approach may offer greater convenience and safety compared to traditional surgical methods [23,24]. However, few hydrogel-based 4D-printed cardiac patches have been reported. 4D-printed hydrogel-based patches can be customized to accommodate individual patient differences, changing shape in response to the microenvironment to fit varying cardiac geometries. In the future, this customization has the potential to enhance efficacy and reduce side effects. Nonetheless, a concerted effort by researchers is still required to develop more precise and effective customized cardiac patches.
Several considerations must be taken into account when designing hydrogel cardiac patches. A key factor is ensuring strong adhesion to the myocardium; If the patch detaches, the entire restorative design and function will be compromised. Robust adhesion mechanisms are essential for maintaining the patch's effectiveness and stability. However, a monolayer hydrogel patch that adheres not only to the myocardium but also to surrounding tissues may potentially trigger undesirable inflammation. Consequently, future developments may focus on bi- or multi-layer cardiac patches with an outer protective layer. The bilayer design can provide anti-adhesive properties to the outer hydrogel, helping to prevent unwanted tissue adhesion. Additionally, multilayer hydrogel may enable the sequential delivery of therapeutic components tailored to the different stages of MI. However, the application of multilayer hydrogels in MI treatment remains challenging. The drug release from each layer must be synchronized with the corresponding stage of MI, meaning the degradation rate of each layer should match the timing of the relevant stage. Furthermore, the fabrication of multi-layer hydrogel cardiac patches necessitates advanced technology for producing and stacking hydrogel sheets. It also remains uncertain whether the ultra-thin structure of the hydrogel would compromise adhesion and drug delivery capabilities [[248], [249], [250]]. Additionally, powders inspired by organisms such as the sandcastle worm exhibit rapid absorption of a wide range of body fluids, converting into a hydrogel with swift, self-reinforcing, and reproducible wet adhesion. These powders hold significant promise as tissue binders and repair materials, representing a strategy that combines the advantages of both powders and hydrogels. However, further scientific investigation is required to ascertain their applicability for myocardial repair.
In summary, focused research is essential to advance the biomedical applications of supramolecular hydrogel patches for the treatment of myocardial ischemia. Key areas for improvement in the development of hydrogel cardiac patches include: (i) in-depth clarification on the wet adhesion mechanisms of these patches. Specifically, further investigation is needed to understand how chemical groups and their spatial distances influence the magnitude of the forces between them, as this will impact the overall adhesion of the patch. (ii) In vivo applications of bi- or multi-layer hydrogels for tissue repair to significantly improve the fabrication of hydrogel-based cardiac patches. This approach has the potential to broaden the applicability of hydrogels in biomedical and tissue engineering fields, offering enhanced functionality and adaptability for diverse therapeutic needs. (iii) continued exploration of hydrogel patches with improved or tunable mechanical properties is critical for tissue engineering, particularly in myocardial repair. Enhanced functionalization and intelligence of these hydrogels, such as electrical conductivity and multi-stimulus responsiveness, will further improve their functionality. The specific performance range required for hydrogel-based patches for MI must be more clearly defined. (iv) Prioritizing the biomimetic nature of hydrogels is essential to enhance biocompatibility, provide an optimal environment for cell growth, and minimize adverse effects on tissues. (v) Integration of minimally invasive techniques with advanced manufacturing technologies to enhance the design of hydrogel-based cardiac patches and improve their effectiveness in treating patients with mild to moderate heart failure after MI. (vi) More attention on material safety, feasibility and simplicity within the translational clinical research system to ensure reproducibility and stability of results, as well as suitability for large-scale industrial production early in the development process.
6. Conclusion
Remarkable preclinical progress has been achieved in the utilization of hydrogel patches for MI treatment. Recently, diverse biomimetic hydrogel patch types have been developed to attain biological functionality, with the field trending towards the utilization of multilayer hydrogel patches. This paper provides a review of recent advancements in hydrogel patches for MI and innovative fabrication techniques aimed at expediting their application in myocardial repair. However, it is important to note that the field is still in its early stages, particularly considering the lack of additional insights resulting from the combination of MI pathogenesis and hydrogel patch fabrication techniques. Consequently, in the preceding sections, our focus was on MI pathogenesis, the wet adhesion mechanism, and design strategies of hydrogel cardiac patches. Additionally, the final section delved into various innovative manufacturing techniques for cardiac patches and the rational design of hydrogel myocardial patches. In conclusion, recent years have witnessed remarkable advancements in utilizing hydrogels as patches for myocardial repair. Therefore, the development of novel hydrogel patches capable of serving as carriers for therapeutic molecules and substitutes for damaged tissues to restore represents a crucial area for future research in the field of hydrogels and tissue repair.
CRediT authorship contribution statement
Zhenqiu Liu: Writing – original draft, Formal analysis, Data curation. Zhi Zheng: Writing – original draft, Supervision, Project administration, Investigation. Jiahao Xie: Formal analysis, Data curation. Hua Wei: Writing – review & editing, Supervision, Project administration, Funding acquisition. Cui-Yun Yu: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Hunan Science and Technology Innovation Leading Talent Project (2022RC3080), National Natural Science Foundation of China (81471777, 82304429, 82373826), Key R & D Program of Hunan Province (2021SK2036, 2023SK2043), Post-doctoral funding from the University of South China (220XQD106), and Hunan Provincial Natural Science Foundation of China (2023JJ40570, 2023JJ50138, 2022JJ40381).
Contributor Information
Zhi Zheng, Email: zhengzhi@usc.edu.cn.
Hua Wei, Email: weih@usc.edu.cn.
Cui-Yun Yu, Email: yucuiyunusc@hotmail.com.
Data availability
No data was used for the research described in the article.
References
- 1.Gaidai O., et al. Curr. Probl. Cardiol. 2023;48(5) doi: 10.1016/j.cpcardiol.2023.101622. [DOI] [PubMed] [Google Scholar]
- 2.Sakalaki M., et al. Clin. Res. Cardiol. 2023 [Google Scholar]
- 3.Ueki Y., et al. J. Am. Coll. Cardiol. 2022;79(6):513. doi: 10.1016/j.jacc.2021.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Park S., et al. Korean Circ. J. 2023;53(3):113. doi: 10.4070/kcj.2022.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L.Y., et al. JAMA Cardiol. 2020;5(6):631. doi: 10.1001/jamacardio.2020.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou L., et al. Risk Manag. Healthc. Pol. 2024;17:1779. doi: 10.2147/RMHP.S468235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Tanneur C., et al. J. Cardiothorac. Vasc. Anesth. 2015;29(6):1624. doi: 10.1053/j.jvca.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Rydén L., et al. Circulation. 2014;130(23):2005. doi: 10.1161/CIRCULATIONAHA.114.010622. [DOI] [PubMed] [Google Scholar]
- 9.Elbadawi A., et al. J. Thorac. Cardiovasc. Surg. 2023;165(2):672. doi: 10.1016/j.jtcvs.2021.03.081. [DOI] [PubMed] [Google Scholar]
- 10.Cao J., et al. Front. Cardiovasc. Med. 2024;vol. 11 doi: 10.3389/fcvm.2024.1398700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Z., et al. ACS Appl. Mater. Interfaces. 2021;13(34) doi: 10.1021/acsami.1c09658. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.-J., et al. J. Clin. Invest. 2018;128(11):5018. doi: 10.1172/JCI99659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seung H., et al. Basic Res. Cardiol. 2022;117(1):16. doi: 10.1007/s00395-022-00927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y., et al. Bioact. Mater. 2021;6(2):520. doi: 10.1016/j.bioactmat.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto K., et al. Cell Stem Cell. 2018;22(1):91. doi: 10.1016/j.stem.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Wu W.-q., et al. Drug Deliv. Transl. Res. 2019;9:920. doi: 10.1007/s13346-019-00627-0. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q., et al. Mater. Today Bio. 2024;25 doi: 10.1016/j.mtbio.2024.100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feig V.R., et al. Nat. Commun. 2018;9(1):2740. doi: 10.1038/s41467-018-05222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu H., et al. J. Mater. Chem. B. 2021;9(48) doi: 10.1039/d1tb01914g. [DOI] [PubMed] [Google Scholar]
- 20.Shen Z., et al. ACS Biomater. Sci. Eng. 2020;6(7):3957. doi: 10.1021/acsbiomaterials.0c00340. [DOI] [PubMed] [Google Scholar]
- 21.Shi T., et al. ACS Appl. Mater. Interfaces. 2023;15(11) doi: 10.1021/acsami.2c23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu W., et al. Gels. 2022;8(7):423. [Google Scholar]
- 23.He S., et al. Adv. Healthcare Mater. 2023;12(19) [Google Scholar]
- 24.Hann S.Y., et al. Int. J. Nanomed. 2023;18:1809. doi: 10.2147/IJN.S402855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbier L., et al. Carbohydr. Polym. 2023;310 doi: 10.1016/j.carbpol.2023.120715. [DOI] [PubMed] [Google Scholar]
- 26.Lee M., et al. ACS Nano. 2023;17(13) doi: 10.1021/acsnano.3c00933. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura Y., et al. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119289. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F., et al. ACS Nano. 2024;18(14) doi: 10.1021/acsnano.4c00509. [DOI] [PubMed] [Google Scholar]
- 29.Niccoli G., et al. Eur. Heart J. 2016;37(13):1024. doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 30.Saleh M., Ambrose J.A. F1000Research. 2018;(7):1378. doi: 10.12688/f1000research.15096.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nabeebaccus A.A., et al. Cardiovasc. Res. 2022;118(17):3305. doi: 10.1093/cvr/cvac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barteková M., et al. Free Radic. Biol. Med. 2021;169:446. doi: 10.1016/j.freeradbiomed.2021.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z., et al. J. Pharmaceut. Anal. 2021;11(6):764. doi: 10.1016/j.jpha.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X., et al. Clin. Chim. Acta. 2024;552 [Google Scholar]
- 35.Abbas N., et al. Basic Res. Cardiol. 2020;115(5):52. doi: 10.1007/s00395-020-0816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derks W., Bergmann O. Circ. Res. 2020;126(4):552. doi: 10.1161/CIRCRESAHA.119.315408. [DOI] [PubMed] [Google Scholar]
- 37.van Berlo J.H., et al. Nature. 2014;509(7500):337. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Z., et al. J. Contr. Release. 2021;335:216. doi: 10.1016/j.jconrel.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Liu W., et al. Small Methods. 2024 [Google Scholar]
- 40.Bavia L., et al. Immunol. Lett. 2018;200:18. doi: 10.1016/j.imlet.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Xie L., et al. Cell. Signal. 2023;112 doi: 10.1016/j.cellsig.2023.110919. [DOI] [PubMed] [Google Scholar]
- 42.Ting Xin, Lu C. Aging-US. 2020;12(5):4474. doi: 10.18632/aging.102899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Z., et al. Adv. Healthcare Mater. 2022;11(19) doi: 10.1002/adhm.202200990. [DOI] [PubMed] [Google Scholar]
- 44.Liu C., et al. Analyst. 2018;143(1):208. doi: 10.1039/c7an01515a. [DOI] [PubMed] [Google Scholar]
- 45.Kalpage H.A., et al. Int. J. Biochem. Cell Biol. 2020;121 doi: 10.1016/j.biocel.2020.105704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geng L., et al. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3207. doi: 10.26355/eurrev_201805_15082. [DOI] [PubMed] [Google Scholar]
- 47.Rech L., et al. Life Sci. 2022;291 doi: 10.1016/j.lfs.2021.120263. [DOI] [PubMed] [Google Scholar]
- 48.Xue T., et al. Hellenic J. Cardiol. 2022;63:32. doi: 10.1016/j.hjc.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Lindsey M.L. Cardiovasc. Res. 2018;114(12):1571. doi: 10.1093/cvr/cvy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z., et al. Eur. J. Pharmacol. 2024;967 doi: 10.1016/j.ejphar.2024.176398. [DOI] [PubMed] [Google Scholar]
- 51.Abe Y., et al. Biochem. Biophys. Res. Commun. 2024;690 doi: 10.1016/j.bbrc.2023.149272. [DOI] [PubMed] [Google Scholar]
- 52.Cheng Y., Rong J. Curr. Drug Targets. 2018;19(6):651. doi: 10.2174/1389450118666171031115025. [DOI] [PubMed] [Google Scholar]
- 53.Hume R.D., et al. JACC (J. Am. Coll. Cardiol.): Basic Transl. Sci. 2023;8(6):658. doi: 10.1016/j.jacbts.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ke D., et al. J. Transl. Med. 2024;22(1):560. doi: 10.1186/s12967-024-05353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi J., et al. Front. Physiol. 2020;11:669. doi: 10.3389/fphys.2020.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janbandhu V., et al. Cell Stem Cell. 2022;29(2):281. doi: 10.1016/j.stem.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X., et al. Theranostics. 2022;12(13):5824. doi: 10.7150/thno.75200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eschenhagen T. J. Clin. Invest. 2018;128(5):1731. doi: 10.1172/JCI121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan J., et al. Eur. J. Pharmacol. 2024;963 doi: 10.1016/j.ejphar.2023.176277. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q., et al. Signal Transduct. Targeted Ther. 2022;7(1):78. doi: 10.1038/s41392-022-00925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao H., et al. Biomed. Pharmacother. 2019;110:685. doi: 10.1016/j.biopha.2018.11.098. [DOI] [PubMed] [Google Scholar]
- 62.Cinato M., et al. Theranostics. 2021;11(13):6491. doi: 10.7150/thno.55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vilahur G., et al. Cardiovasc. Res. 2018;114(14):1860. doi: 10.1093/cvr/cvy201. [DOI] [PubMed] [Google Scholar]
- 64.Dolling M., et al. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.726153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang X., et al. vol. 9. 2022. (Frontiers in Cardiovascular Medicine). [Google Scholar]
- 66.She G., et al. J. Am. Heart Assoc. 2019;8(1) doi: 10.1161/JAHA.118.010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng D.-Y., et al. Life Sci. 2016;151:61. doi: 10.1016/j.lfs.2016.02.097. [DOI] [PubMed] [Google Scholar]
- 68.Mendiola E.A., et al. Acta Biomater. 2024;173:109. doi: 10.1016/j.actbio.2023.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu X., et al. J. Clin. Invest. 2018;128(5):2127. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rog-Zielinska E.A., et al. Trends Mol. Med. 2016;22(2):99. doi: 10.1016/j.molmed.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu A., et al. Adv. Healthcare Mater. 2021;11(2) [Google Scholar]
- 72.Walker B.W., et al. Biomaterials. 2019;207:89. doi: 10.1016/j.biomaterials.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ali S.R., et al. Proc. Natl. Acad. Sci. USA. 2014;111(24):8850. doi: 10.1073/pnas.1408233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu C., et al. Biomaterials. 2023;296 [Google Scholar]
- 75.Zhang J., et al. J. Nanobiotechnol. 2023;21(1):298. doi: 10.1186/s12951-023-02063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y., et al. J. Mater. Chem. B. 2024;12(1):39. doi: 10.1039/d3tb02101g. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y., et al. Mater. Horiz. 2023;10(10):4033. doi: 10.1039/d3mh00326d. [DOI] [PubMed] [Google Scholar]
- 78.Luo L., et al. Chem. Commun. 2023;59(64):9675. doi: 10.1039/d3cc01274c. [DOI] [PubMed] [Google Scholar]
- 79.Gao F., et al. Small Methods. 2023;8(4) doi: 10.1002/smtd.202300753. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Y., et al. Sci. Bull. 2022;67(17):1776. doi: 10.1016/j.scib.2022.07.032. [DOI] [PubMed] [Google Scholar]
- 81.Chen W., et al. Small. 2022;18(27) doi: 10.1002/smll.202203033. [DOI] [PubMed] [Google Scholar]
- 82.Lee M., et al. Int. J. Nanomed. 2022;17:6181. doi: 10.2147/IJN.S386763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y., Qiu X. WIREs Nanomed. Nanobiotechnol. 2022;14(4):e1787. doi: 10.1002/wnan.1787. [DOI] [PubMed] [Google Scholar]
- 84.Wang L., et al. Nat. Biomed. Eng. 2021;5(10):1157. doi: 10.1038/s41551-021-00796-9. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Z., et al. Sci. Adv. 2023;9:2213. [Google Scholar]
- 86.He Y., et al. Theranostics. 2018;8(18):5159. doi: 10.7150/thno.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma V., et al. Int. J. Biol. Macromol. 2022;222:3045. doi: 10.1016/j.ijbiomac.2022.10.079. [DOI] [PubMed] [Google Scholar]
- 88.Li W., et al. Adv. Sci. 2023;10(25) doi: 10.1002/advs.202301088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan G., Li B. Science. 2023;382(6672):763. doi: 10.1126/science.adl2002. [DOI] [PubMed] [Google Scholar]
- 90.He R., et al. Adv. Healthcare Mater. 2020;9(4) [Google Scholar]
- 91.Chen H., et al. ACS Appl. Mater. Interfaces. 2022;14(36) doi: 10.1021/acsami.2c12386. [DOI] [PubMed] [Google Scholar]
- 92.Burdick J.A., Prestwich G.D. Adv. Mater. 2011;23(12):H41. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao Y., et al. J. Mater. Chem. B. 2023;11(13):2801. doi: 10.1039/d2tb02808e. [DOI] [PubMed] [Google Scholar]
- 94.Liu H., et al. Int. J. Biol. Macromol. 2023;253 doi: 10.1016/j.ijbiomac.2023.126506. [DOI] [PubMed] [Google Scholar]
- 95.Tanrikulu I.C., et al. Adv. Sci. 2023;11(3) doi: 10.1002/advs.202303228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian B., Liu J. Int. J. Biol. Macromol. 2023;235 doi: 10.1016/j.ijbiomac.2023.123902. [DOI] [PubMed] [Google Scholar]
- 97.Yuan X., et al. Adv. Sci. 2023;10(24) doi: 10.1002/advs.202301665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang M., Zhao X. Int. J. Biol. Macromol. 2020;162:1414. doi: 10.1016/j.ijbiomac.2020.07.311. [DOI] [PubMed] [Google Scholar]
- 99.Miao T., et al. Biomacromolecules. 2015;16(12):3740. doi: 10.1021/acs.biomac.5b00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao J., et al. Carbohydr. Polym. 2019;225 doi: 10.1016/j.carbpol.2019.115237. [DOI] [PubMed] [Google Scholar]
- 101.Lee M., et al. Bioact. Mater. 2024;31:395. doi: 10.1016/j.bioactmat.2023.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hakim L.K., et al. Evid. base Compl. Alternative Med. 2021;2021:1. [Google Scholar]
- 103.Sergi R., et al. Materials. 2020;13(23):5560. doi: 10.3390/ma13235560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng Z., et al. Front. Bioeng. Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1159507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu Y., et al. Prog. Polym. Sci. 2023;146 [Google Scholar]
- 106.Fredi G., et al. ACS Omega. 2020;5(31) doi: 10.1021/acsomega.0c02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong S., et al. Adv. Funct. Mater. 2012;22(22):4711. [Google Scholar]
- 108.Fu Y., et al. Mater. Horiz. 2021;8(6):1618. doi: 10.1039/d0mh01985b. [DOI] [PubMed] [Google Scholar]
- 109.Batul R., et al. Biomater. Sci. 2017;5(7):1204. doi: 10.1039/c7bm00187h. [DOI] [PubMed] [Google Scholar]
- 110.Du L., et al. Colloids Surf. B Biointerfaces. 2022;214 doi: 10.1016/j.colsurfb.2022.112469. [DOI] [PubMed] [Google Scholar]
- 111.Michalicha A., et al. Carbohydr. Polym. 2021;256 doi: 10.1016/j.carbpol.2020.117524. [DOI] [PubMed] [Google Scholar]
- 112.Ma W., et al. Int. J. Biol. Macromol. 2023;240 doi: 10.1016/j.ijbiomac.2023.124287. [DOI] [PubMed] [Google Scholar]
- 113.Yang Y., et al. Spectrochim. Acta Mol. Biomol. Spectrosc. 2022;265 doi: 10.1016/j.saa.2021.120385. [DOI] [PubMed] [Google Scholar]
- 114.Umek N., et al. Front. Mol. Neurosci. 2018;11:467. doi: 10.3389/fnmol.2018.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng Z., et al. J. Contr. Release. 2024;371:570. doi: 10.1016/j.jconrel.2024.06.015. [DOI] [PubMed] [Google Scholar]
- 116.Wang H., et al. Bioact. Mater. 2023;28:420. doi: 10.1016/j.bioactmat.2023.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keykhaee M., et al. Carbohydr. Polym. 2023;321 doi: 10.1016/j.carbpol.2023.121179. [DOI] [PubMed] [Google Scholar]
- 118.Amaral K.R., et al. Adv. Healthcare Mater. 2023;12(28) doi: 10.1002/adhm.202301513. [DOI] [PubMed] [Google Scholar]
- 119.Araújo M., et al. Biomater. Sci. 2021;9(19):6510. doi: 10.1039/d1bm00118c. [DOI] [PubMed] [Google Scholar]
- 120.Khaliq T., et al. Int. J. Pharm. 2023;643 doi: 10.1016/j.ijpharm.2023.123244. [DOI] [PubMed] [Google Scholar]
- 121.Hu S., et al. Chem. Mater. 2024;36(3):1054. [Google Scholar]
- 122.Xue B., et al. Nat. Commun. 2021;12(1):7156. doi: 10.1038/s41467-021-27529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X., et al. ACS Appl. Mater. Interfaces. 2022;14(31) doi: 10.1021/acsami.2c08245. [DOI] [PubMed] [Google Scholar]
- 124.Wu T., et al. Bioact. Mater. 2023;21:20. doi: 10.1016/j.bioactmat.2022.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Y., et al. J. Colloid Interface Sci. 2023;646:472. doi: 10.1016/j.jcis.2023.05.009. [DOI] [PubMed] [Google Scholar]
- 126.Zheng Y., et al. Bioact. Mater. 2023;29:214. doi: 10.1016/j.bioactmat.2023.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chang X., et al. Macromol. Rapid Commun. 2018;39(14) doi: 10.1002/marc.201700806. [DOI] [PubMed] [Google Scholar]
- 128.Ma X., et al. J. Mater. Chem. A. 2022;10(22) [Google Scholar]
- 129.Wang X., et al. ACS Appl. Mater. Interfaces. 2023;15(39) doi: 10.1021/acsami.3c11117. [DOI] [PubMed] [Google Scholar]
- 130.Lee D.U., et al. Bioact. Mater. 2024;34:112. doi: 10.1016/j.bioactmat.2023.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fu C., et al. 2023. Advanced Materials. [Google Scholar]
- 132.Xue W., et al. Biomater. Sci. 2022;10(9):2417. doi: 10.1039/d2bm00183g. [DOI] [PubMed] [Google Scholar]
- 133.Peng X., et al. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ji Z., et al. Soft Matter. 2024;20(3):629. doi: 10.1039/d3sm01503c. [DOI] [PubMed] [Google Scholar]
- 135.Mubarok W., et al. Gels. 2022;8(6):387. doi: 10.3390/gels8060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou Y., Jin L. Soft Matter. 2020;16(24):5740. doi: 10.1039/d0sm00663g. [DOI] [PubMed] [Google Scholar]
- 137.Wang S., et al. Adv. Mater. Technol. 2022;8(6) [Google Scholar]
- 138.Fan X., et al. Mater. Horiz. 2021;8(3):997. doi: 10.1039/d0mh01231a. [DOI] [PubMed] [Google Scholar]
- 139.Liu J., et al. ACS Appl. Mater. Interfaces. 2020;13(1):1535. doi: 10.1021/acsami.0c18916. [DOI] [PubMed] [Google Scholar]
- 140.Wei J., et al. Mater. Horiz. 2021;8(10):2761. doi: 10.1039/d1mh00998b. [DOI] [PubMed] [Google Scholar]
- 141.Tsuchiya K., et al. Int. J. Mol. Sci. 2023;24(14) doi: 10.3390/ijms241411768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martín V.I., et al. J. Colloid Interface Sci. 2017;491:336. doi: 10.1016/j.jcis.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 143.Zhang X., et al. Nano Today. 2023;50 [Google Scholar]
- 144.Li N., et al. Science. 2023;381(6658):686. doi: 10.1126/science.adg8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wei R., et al. Macromol. Biosci. 2021;21(5) doi: 10.1002/mabi.202000367. [DOI] [PubMed] [Google Scholar]
- 146.Yuk H., et al. Nature. 2019;575(7781):169. doi: 10.1038/s41586-019-1710-5. [DOI] [PubMed] [Google Scholar]
- 147.Wang P., et al. Sci. China Mater. 2021;65(1):246. doi: 10.1007/s40843-021-1724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li J., et al. Science. 2017;357(6349):378. doi: 10.1126/science.aah6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang B., et al. ACS Appl. Mater. Interfaces. 2021;13(30) doi: 10.1021/acsami.1c10051. [DOI] [PubMed] [Google Scholar]
- 150.Qiao L., et al. Bioact. Mater. 2023;30:129. doi: 10.1016/j.bioactmat.2023.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guo R., et al. Biomacromolecules. 2017;18(4):1356. doi: 10.1021/acs.biomac.7b00089. [DOI] [PubMed] [Google Scholar]
- 152.Yu R., et al. Mater. Sci. Eng. C. 2021;127 doi: 10.1016/j.msec.2021.112210. [DOI] [PubMed] [Google Scholar]
- 153.Guo Z., et al. Soft Matter. 2017;13(40):7371. doi: 10.1039/c7sm00916j. [DOI] [PubMed] [Google Scholar]
- 154.Liu Y., et al. Polymers. 2020;12(2):487. [Google Scholar]
- 155.Li Z., et al. Adv. Funct. Mater. 2021;31(15) doi: 10.1002/adfm.202007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He N., et al. ACS Omega. 2019;4(25) doi: 10.1021/acsomega.9b02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu L., et al. ACS Biomater. Sci. Eng. 2023;9(8):4761. doi: 10.1021/acsbiomaterials.3c00579. [DOI] [PubMed] [Google Scholar]
- 158.Liu Y., et al. ACS Appl. Mater. Interfaces. 2023;15(17) doi: 10.1021/acsami.3c02176. [DOI] [PubMed] [Google Scholar]
- 159.Xiang L., et al. Acta Biomater. 2020;117:294. doi: 10.1016/j.actbio.2020.09.043. [DOI] [PubMed] [Google Scholar]
- 160.Liu X., et al. ACS Appl. Mater. Interfaces. 2017;9(20) doi: 10.1021/acsami.7b04832. [DOI] [PubMed] [Google Scholar]
- 161.Li S., et al. J. Mater. Chem. B. 2021;9(22):4423. doi: 10.1039/d1tb00523e. [DOI] [PubMed] [Google Scholar]
- 162.Wang W., et al. Prog. Polym. Sci. 2017;71:1. [Google Scholar]
- 163.Wang S., et al. Compos. B Eng. 2022;241 [Google Scholar]
- 164.Nakano S., et al. Inorg. Chem. 2021;60(5):3065. doi: 10.1021/acs.inorgchem.0c03345. [DOI] [PubMed] [Google Scholar]
- 165.Lim H.S., et al. J. Porous Mater. 2022;30(2):599. [Google Scholar]
- 166.Liang S., et al. Adv. Mater. 2018;30(23) doi: 10.1002/adma.201704235. [DOI] [PubMed] [Google Scholar]
- 167.Lu Y., et al. Acta Biomater. 2022;153:386. doi: 10.1016/j.actbio.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 168.Ghovvati M., et al. Adv. Healthcare Mater. 2022;11(13) doi: 10.1002/adhm.202200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen J., et al. Biomacromolecules. 2023;25(1):55. doi: 10.1021/acs.biomac.3c00708. [DOI] [PubMed] [Google Scholar]
- 170.Nele V., et al. Adv. Mater. 2020;32(7) doi: 10.1002/adma.201905914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu X., et al. Theranostics. 2023;13(15):5365. doi: 10.7150/thno.87639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hörenz C., et al. Biomacromolecules. 2020;21(2):830. doi: 10.1021/acs.biomac.9b01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kang H., et al. Chem. Mater. 2023;35(6):2408. [Google Scholar]
- 174.Zhang G., et al. ACS Macro Lett. 2017;6(7):641. doi: 10.1021/acsmacrolett.7b00275. [DOI] [PubMed] [Google Scholar]
- 175.Lee J.N., et al. ACS Appl. Mater. Interfaces. 2021;13(15) doi: 10.1021/acsami.1c02141. [DOI] [PubMed] [Google Scholar]
- 176.Ma P., et al. Adv. Mater. 2023;36(11) [Google Scholar]
- 177.Meyers K., et al. Gels. 2021;7(2):53. doi: 10.3390/gels7020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ye G., et al. Theranostics. 2020;10(5):2047. doi: 10.7150/thno.38876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee D.J., et al. J. Biol. Eng. 2019;13:6. doi: 10.1186/s13036-019-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Omens J.H. vol. 69. 1998. p. 559. (Progress in Biophysics & Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 181.Chen Q.Z., et al. Biomaterials. 2008;29(1):47. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 182.Efraim Y., et al. Acta Biomater. 2017;50:220. doi: 10.1016/j.actbio.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 183.Yu C., et al. ACS Nano. 2022;16(10) doi: 10.1021/acsnano.2c05168. [DOI] [PubMed] [Google Scholar]
- 184.Shin S.R., et al. Small. 2016;12(27):3677. doi: 10.1002/smll.201600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mei T., et al. ACS Biomater. Sci. Eng. 2024;10(4):1892. doi: 10.1021/acsbiomaterials.3c01837. [DOI] [PubMed] [Google Scholar]
- 186.von Lospichl B., et al. Colloids Surf. B Biointerfaces. 2017;159:477. doi: 10.1016/j.colsurfb.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 187.Shin M., et al. Polymer. 2021;229 [Google Scholar]
- 188.Tikhonova T.N., et al. Cells. 2022;11(24):4137. doi: 10.3390/cells11244137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martino F., et al. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McAndrews K.M., et al. Tissue Eng. 2014;20(23–24):3252. doi: 10.1089/ten.tea.2013.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Allen J.L., et al. Mol. Biol. Cell. 2012;23(18):3731. doi: 10.1091/mbc.E12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Q., et al. ACS Appl. Mater. Interfaces. 2022;14(42) doi: 10.1021/acsami.2c14924. [DOI] [PubMed] [Google Scholar]
- 193.Nattasit P., et al. Macromol. Biosci. 2023;23(7) doi: 10.1002/mabi.202300021. [DOI] [PubMed] [Google Scholar]
- 194.Yuan Y., et al. J. Future Foods. 2024;4(3):280. [Google Scholar]
- 195.Tang D., et al. Exp. Mol. Med. 2019;51(5):1. doi: 10.1038/s12276-019-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lin X., et al. Adv. Healthcare Mater. 2022;11(9) [Google Scholar]
- 197.He Y., et al. Bioact. Mater. 2021;6(7):2000. doi: 10.1016/j.bioactmat.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Akbarzadeh A., et al. Bioengineering. 2023;10(1):106. doi: 10.3390/bioengineering10010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ryu H., et al. Adv. Sci. 2023;10(27) doi: 10.1002/advs.202303429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dai J., et al. 2024. Advanced Healthcare Materials. [Google Scholar]
- 201.Kapnisi M., et al. Adv. Funct. Mater. 2018;28(21) doi: 10.1002/adfm.201800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.English E.J., et al. J. Biomed. Mater. Res. 2023;111(9):1309. doi: 10.1002/jbm.a.37530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jiang X., et al. Adv. Mater. 2022;34(19) doi: 10.1002/adma.202201411. [DOI] [PubMed] [Google Scholar]
- 204.Chen Y., et al. Biomater. Adv. 2022;136 doi: 10.1016/j.bioadv.2022.212803. [DOI] [PubMed] [Google Scholar]
- 205.AlAttar A., et al. J. Mech. Behav. Biomed. Mater. 2023;147 doi: 10.1016/j.jmbbm.2023.106157. [DOI] [PubMed] [Google Scholar]
- 206.Gkouti E., et al. Materials. 2023;16(22):7095. doi: 10.3390/ma16227095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Feng B., et al. Acta Biomater. 2019;83:211. doi: 10.1016/j.actbio.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 208.Ghorbani M., et al. Int. J. Biol. Macromol. 2020;165:1312. doi: 10.1016/j.ijbiomac.2020.09.225. [DOI] [PubMed] [Google Scholar]
- 209.Großhaus C., et al. Small. 2020;16(44) [Google Scholar]
- 210.Wei X., et al. Bioact. Mater. 2023;27:271. doi: 10.1016/j.bioactmat.2023.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang Q., et al. ACS Nano. 2023;17(17) doi: 10.1021/acsnano.3c01647. [DOI] [PubMed] [Google Scholar]
- 212.Das S., et al. APL Bioeng. 2021;5(3) doi: 10.1063/5.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rosellini E., et al. Front. Bioeng. Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1339120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Noor N., et al. Adv. Sci. 2019;6(11) doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Qian B., et al. Adv. Sci. 2023;10(36) doi: 10.1002/advs.202303033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bassand C., et al. J. Contr. Release. 2023;353:864. doi: 10.1016/j.jconrel.2022.11.052. [DOI] [PubMed] [Google Scholar]
- 217.Sun F., et al. Int. J. Mol. Sci. 2022;23(10):5831. doi: 10.3390/ijms23105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shin Y.J., et al. Acta Biomater. 2021;119:75. doi: 10.1016/j.actbio.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 219.Distler T., Boccaccini A.R. Acta Biomater. 2020;101:1. doi: 10.1016/j.actbio.2019.08.044. [DOI] [PubMed] [Google Scholar]
- 220.Asim S., et al. Adv. Healthcare Mater. 2023;12(17) doi: 10.1002/adhm.202203148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shang W., et al. Biofabrication. 2017;9(2) doi: 10.1088/1758-5090/aa6ed8. [DOI] [PubMed] [Google Scholar]
- 222.Mirek A., et al. J. Mater. Chem. B. 2022;10(43):8862. doi: 10.1039/d2tb01268e. [DOI] [PubMed] [Google Scholar]
- 223.Wang H., et al. Int. J. Biol. Macromol. 2023;244:125139. doi: 10.1016/j.ijbiomac.2023.125139. [DOI] [PubMed] [Google Scholar]
- 224.Chang H., et al. Bioeng. 2021;18:456852. [Google Scholar]
- 225.Motta S.E., et al. Matter. 2023;6:1860. [Google Scholar]
- 226.Fang Y., et al. Adv. Mater. 2023;35(22) doi: 10.1002/adma.202205082. [DOI] [PubMed] [Google Scholar]
- 227.Wang Y., et al. Adv. Mater. 2022;34(20) doi: 10.1002/adma.202201210. [DOI] [PubMed] [Google Scholar]
- 228.Kabirian F., et al. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.873453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Melocchi A., et al. Adv. Drug Deliv. Rev. 2021;173:216. doi: 10.1016/j.addr.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 230.Huang L., et al. Adv. Mater. 2016;29(7) [Google Scholar]
- 231.Yan S., et al. vol. 6. 2023. p. 234. (Research). [Google Scholar]
- 232.Lu Z., et al. Adv. Healthcare Mater. 2024;13(10) [Google Scholar]
- 233.Li K., et al. Materials. 2020;13(22):5277. [Google Scholar]
- 234.Nowak J.F., et al. J. Biomech. Eng. 2023;145(1) doi: 10.1115/1.4055038. [DOI] [PubMed] [Google Scholar]
- 235.Sun Y., et al. RSC Adv. 2019;9(44) doi: 10.1039/c9ra04966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pensa N.W., et al. Biomater. Res. 2019;23:22. doi: 10.1186/s40824-019-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sreepadmanabh M., et al. Biomed. Mater. 2023;18(4) doi: 10.1088/1748-605X/acd315. [DOI] [PubMed] [Google Scholar]
- 238.Iftekar S.F., et al. Polymers. 2023;15(11):2519. doi: 10.3390/polym15112519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sheikh A., et al. Drug Discov. Today. 2023;28(1) doi: 10.1016/j.drudis.2022.103391. [DOI] [PubMed] [Google Scholar]
- 240.Liu S., et al. Adv. Mater. Technol. 2023;8(12) doi: 10.1002/admt.202201716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kumar R., Parashar A. WIREs Comput. Mol. Sci. 2023;13(4) [Google Scholar]
- 242.Liu Y., et al. Biomater. Sci. 2024;12(4):837. doi: 10.1039/d3bm01645e. [DOI] [PubMed] [Google Scholar]
- 243.Shi W., et al. Regen. Ther. 2024;25:174. doi: 10.1016/j.reth.2023.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shen S., et al. Adv. Healthcare Mater. 2023;12(29) doi: 10.1002/adhm.202301990. [DOI] [PubMed] [Google Scholar]
- 245.Zhang J., et al. Adv. Mater. 2023;35(9) doi: 10.1002/adma.202210463. [DOI] [PubMed] [Google Scholar]
- 246.Li J., et al. Nano-Micro Lett. 2023;15(1):105. doi: 10.1007/s40820-023-01079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen S., et al. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.630745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jin L., et al. Gels. 2021;7(4):172. [Google Scholar]
- 249.Ouyang L., et al. ACS Appl. Mater. Interfaces. 2018;10(15) doi: 10.1021/acsami.7b19537. [DOI] [PubMed] [Google Scholar]
- 250.Liu G., et al. Front. Chem. 2018;6:439. doi: 10.3389/fchem.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.