Highlights
-
•
Symptoms of malaria can be vague and non-specific.
-
•
Consider malaria in anyone with fever within 1 year of travel to an endemic area.
-
•
Examination of thick and thin blood films remains the gold standard for malaria diagnosis.
-
•
Severe malaria is a medical emergency. Primary and secondary care clinicians must be aware of the signs of severe malaria.
-
•
Climate change, drug resistance and vaccines are changing the profile of malaria.
Keywords: Malaria, Fever in returning traveller, Tropical medicine
Abstract
Malaria remains a major global health problem. Transmission occurs in 84 countries across five continents, with almost 250 million cases and over 600,000 deaths each year. Primary and secondary care clinicians in the UK need to be alert to the prospect of malaria presenting in returning travellers. They must be aware of the signs of severe malaria, the need for prompt diagnosis and treatment, and the importance of seeking specialist advice. With emerging resistance, climate change and the roll-out of the first malaria vaccines, the landscape of malaria is changing. Here we discuss the past, present and future of malaria.
Past
Malaria, the mosquito-borne disease caused by the protozoa Plasmodium, has been present in the human population for thousands of years; fevers most likely caused by malaria were described in China in 2700 BC and in Greece in 800 BC.1 The close relationship between humans and malaria has shaped the human genome, driving mutations responsible for conditions such as sickle cell disease, thalassaemia and glucose-6-phosphate dehydrogenase (G6PD) deficiency.1 Although now considered a traveller’s disease in the UK, malaria is believed to have historically been endemic, causing ‘marsh fever’ or ‘ague’ in certain low-lying areas of England.2
Present
Epidemiology
Concerted global health efforts to increase the distribution of vector control methods, diagnostics and treatments have averted 2.1 billion malaria cases and 11.7 million deaths since 2000.3 However, progress has stalled, and even reversed, since 2015. According to the World Health Organization (WHO) World Malaria Report 2023, an estimated 249 million cases of malaria occurred in 2022, leading to 608,000 deaths. Most deaths (75%) occur in children under 5 years in sub-Saharan Africa.3
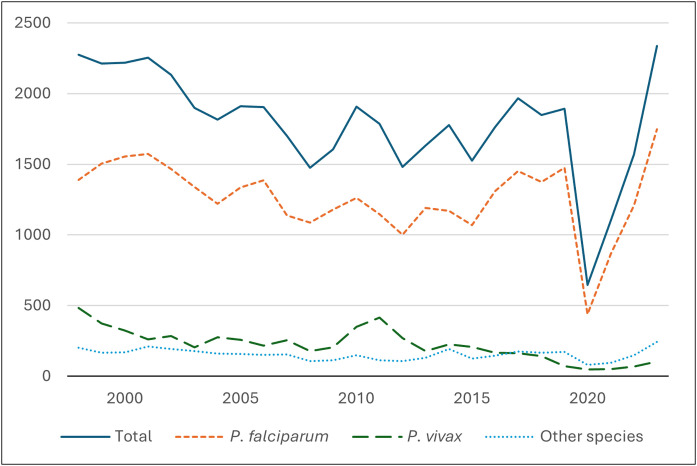
An average of 1,600 imported malaria cases are recorded in the UK each year.4 After a dip caused by the COVID-19 pandemic, 2,093 cases were reported by the UK Health Security Agency in 2023, the highest number in the past 25 years (Fig. 1).4
Fig. 1.
Annual number of UK imported malaria cases (1998–2023). Adapted from UK Health Security Agency data.4
Aetiology
Malaria is introduced by the bite of an infected female Anopheles mosquito. Multiple Plasmodium species cause malaria in humans, including P. falciparum, P. vivax, P. ovale (wallikeri and curtisi), P. malariae and P. knowlesi.5
P. falciparum accounts for 97% of global malaria cases, mainly in sub-Saharan Africa. It is the most likely species to cause severe illness due to its propensity to ‘sequester’ infected erythrocytes in end-organ microvasculature.5 P. vivax is geographically the most widespread species; 3.3 billion people live in endemic areas, primarily in South America and south and south-east Asia.3,6 P. vivax and P. ovale spp. cause relapsing disease due to the development of hyponozoites, which reside in the liver and can reactivate to produce blood-stage infections (clinically indistinguishable from acute primary infection) weeks, months or even years after the initial inoculation. Relapses account for approximately 60–80% of all P. vivax clinical cases in endemic areas.5
Clinical features
The symptoms and signs of malaria are variable and non-specific. It is impossible to diagnose or exclude malaria on clinical features alone. The most important first step is to consider the possibility of malaria (Table 1).7
Table 1.
Common pitfalls in the management of malaria.7
|
Common symptoms include fever, chills, myalgia and headache. Gastrointestinal symptoms (diarrhoea is common in children), jaundice or respiratory symptoms may occur and can contribute to misdiagnosis. Most patients do not have a specific fever pattern.7
Individuals who experience repeated malaria infections (eg in high transmission areas) develop a degree of naturally acquired immunity. They may experience fewer symptoms or have asymptomatic parasitaemia. However, immunity is lost quickly and does not guarantee protection from future illness.8
Malaria is classified as either uncomplicated or severe. Features of severe disease are outlined in Table 2. A parasitaemia of >10% is an indicator of severe malaria. In the UK, a lower threshold (>2%) is adopted due to a largely non-immune population.
Table 2.
One or more of the following criteria without an identified alternative cause:
|
In a UK setting, consider managing any patient with a parasitaemia of >2% as severe malaria.
Malaria should be considered in any patient presenting with a febrile illness within 1 year of travel to an endemic area.7 P. falciparum has a minimum incubation period of 6 days, and most cases occur within 3 months of exposure. Antimalarial prophylaxis may delay the onset of symptoms. Relapse infections due to P. vivax and P. ovale spp. may present later than 6 months.
Diagnosis
Travellers returning from malaria-endemic areas with a history of fever must undergo urgent investigation. This usually requires referral to secondary care. Examination of thick and thin blood smears by microscopy remains the gold-standard investigation.9 If the initial blood film is negative, and clinical suspicion is high, it should be repeated after 12–24 h and again after a further 24 h. Rapid diagnostic tests (RDTs), based upon detection of parasite antigens, may be performed, but do not offer an equivalent alternative to blood films due to lower sensitivity, histidine-rich protein 2 mutations preventing detection of P. falciparum, and an inability to fully speciate (eg if mixed infections) or quantify parasitaemia.9 Molecular tests (including quantitative real-time PCR) are becoming increasingly available.9 Malaria is a notifiable disease.10
Treatment
Falciparum malaria
The first-line treatment for non-severe falciparum malaria is an oral artemisinin combination therapy (ACT) such as artemether-lumefantrine (Riamet).7 If an ACT is not immediately available (or contraindicated), an alternative antimalarial should be started instead (Table 3). If a patient has taken malaria prophylaxis, the same drug should not be used for treatment.11 If a patient is vomiting or cannot tolerate oral therapy, parenteral therapy should be used as for severe malaria.11
Table 3.
| Uncomplicated P. falciparum malaria | |
|---|---|
| Artemether-lumefantrine (Riamet) | Recommended first-line ACT Four tablets at 0, 8, 24, 34, 48 and 60 h (>35 kg) Take with milk or fatty food |
| Dihydroartemisinin-piperaquine | Second-line ACT Three tablets daily for 3 days (36–60 kg) or four tablets daily for 3 days (>60 kg) |
| Atovaquone-proguanil (Malarone) | Four tablets daily for 3 days Not to be used if patient took Malarone prophylaxis Take with milk or fatty food |
| Quinine PLUS doxycycline or clindamycin | Quinine 600 mg 8 hourly for 5–7 days PLUS doxycycline 200 mg daily or clindamycin 450 mg 8 hourly for 7 days |
| Severe or complicated malaria | |
| IV Artesunate | First-line treatment 2.4 mg/kg at 0, 12 and 24 h, then once daily If not available, do not delay treatment, give IV quinine as alternative Can cause QTc prolongation – check ECG |
| IV Quinine | Alternative if IV artesunate not available 20 mg/kg loading dose over 4 h, followed by 10 mg/kg three times a day |
| Complete full course of oral treatment when patient can tolerate oral therapy | |
| Non-falciparum malaria | |
| Artemether-lumefantrine (Riamet) | Recommended first-line ACT Four tablets at 0, 8, 24, 34, 48 and 60 h (>35 kg) Take with milk or fatty food |
| Chloroquine | 620 mg initially, 310 mg at 6, 24 and 48 h (NB Avoid if infection acquired in areas with known chloroquine resistance) |
| Severe/complicated malaria – treat as per severe/complicated P. falciparum malaria | |
| Prevention of relapse (P. vivax / P. ovale spp.) | |
| Primaquine | 30 mg daily for 14 days (P. vivax) or 15 mg daily for 14 days (P. ovale spp.) Check for G6PD deficiency prior to treatment Reduced dosing may be used in mild-moderate G6PD deficiency – consult a specialist |
| Pregnancy | |
| Artemether-lumefantrine (Riamet) | First-line treatment in second/third trimester Seek specialist advice regarding use in first trimester |
| Quinine plus clindamycin | Safe in all trimesters |
| Severe malaria | IV artesunate IV quinine can be given as an alternative until IV artesunate available |
| Relapse prevention | Do not give primaquine during pregnancy Treat with chloroquine 310 mg weekly until pregnancy complete Do not use primaquine while breast feeding until infant G6PD status is known |
Severe/complicated malaria
This is a medical emergency. Patients should be managed on a high dependency unit and specialist advice should be sought.7,11 The first-line treatment is IV artesunate (Table 3). If this is not available, IV quinine should be started without delay.7 Once patients can take oral medication, a full course of ACT or alternative should be completed.7 Response to treatment should be assessed by serial blood films to ensure clearance of parasitaemia.
Supportive management should include:
-
•
careful management of fluid balance to ensure perfusion but prevent pulmonary oedema
-
•
close monitoring of blood glucose and correction of hypoglycaemia
-
•
monitoring for signs of secondary bacterial infection or shock, taking blood cultures and treating with broad spectrum antibiotics if present
-
•
renal replacement therapy if required for correction of acidosis or fluid/electrolyte imbalance
-
•
control of seizures.
-
•
exchange transfusion is no longer recommended. Blood transfusion may be indicated for symptomatic anaemia.7
Non-falciparum malaria
Patients with non-falciparum malaria can often be managed as outpatients. However, admission should be considered as severe, even fatal, cases can occur.11 First-line treatment is artemether-lumefantrine. Chloroquine is an alternative if unavailable (Table 3).11,12
In P. vivax and P. ovale spp. malaria, additional treatment is needed to prevent relapse. Primaquine is the treatment of choice in the UK (Table 3).7 G6PD screening should be performed before initiating treatment, as life-threatening haemolysis may occur in patients with G6PD deficiency.7
Pregnancy
Malaria in pregnancy is associated with a higher risk of severe disease in the mother and an increased risk of miscarriage or stillbirth.7 Patients should be managed promptly by infectious diseases and obstetric specialists. Congenital malaria can occur, and neonatal testing may be indicated. Artemether-lumefantrine is the treatment of choice in the second and third trimester (Table 3).7 Recent updates to the WHO guidelines also recommend the use of artemether-lumefantrine in the first trimester.12 Quinine plus clindamycin may be used at any stage of pregnancy. IV artesunate is advised for severe malaria at all stages of pregnancy due to the high risks of undertreated malaria.7 Primaquine is contraindicated in pregnancy. Relapse prevention should be managed as outlined in Table 3.
Follow-up
A repeat full blood count and blood film are recommended approximately 14 days after treatment due to a risk of post-treatment haemolysis, particularly following severe malaria or artemisinin therapy.7
Future
The WHO Global Technology Strategy calls for a reduction in malaria cases and deaths of 90% by 2030 from 2015.3 However, climate change, conflict, and drug and insecticide resistance are impeding progress towards this target.3
Malaria vaccines have the potential to close some of this gap. Two vaccines, RTS,S/AS01 and R21/Matrix-M, have been recommended by the WHO for the prevention of P. falciparum malaria in children living in endemic areas from 5 months of age.3 They target the initial pre-erythrocytic stage of infection and have an efficacy of approximately 75% after three doses over one malaria season when given with seasonal malaria chemoprophylaxis.13, 14, 15 A fourth dose is required at 12 months. RTS,S/AS01 reduced all-cause mortality in early childhood by 13% in a pilot malaria vaccine programme in Ghana, Kenya and Malawi, illustrating the remarkable potential of malaria vaccines when combined with current control methods.3 However, efficacy is partial and wanes over time, highlighting a need for more efficacious and durable vaccines. One strategy to achieve this is to target multiple stages of the parasite’s life cycle, including the blood-stage of infection.16,17 Vaccines are not currently recommended for travellers.
Clinicians treating returning travellers in the UK need to be alert to emerging resistance patterns. Artemisinin resistance is increasing, particularly in south-east Asia,3 and is of great concern. Treatment options continue to evolve, such as the licensing of tafenoquine in the USA and Australia in 2018 as a single dose anti-hypnozoite drug for P. vivax.18
Climate change is also impacting mosquito-borne diseases. The geographical distribution of vector populations is increasing, and floods are causing disease outbreaks.3 The UK currently has six mosquito species capable of transmitting malaria. Modelling suggests favourable conditions for P. vivax transmission could occur in south-east England by 2030.19 Although sustained indigenous transmission of malaria in the UK is unlikely, imported cases may occur more frequently, even from countries in Europe.20 Primary and secondary care clinicians in the UK must therefore remain vigilant to the prospect of malaria presenting in returning travellers.
CRediT authorship contribution statement
Jo Salkeld: Writing – review & editing, Writing – original draft, Conceptualization, Visualization. Andrew Duncan: Writing – review & editing, Writing – original draft, Conceptualization, Visualization. Angela M. Minassian: Writing – review & editing, Conceptualization, Supervision.
Declaration of competing interest
Angela M Minassian has an immediate family member who is an inventor on patent applications relating to RH5 malaria vaccines. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This article has an accompanying continuing medical education (CME) activity. Completion of this CME activity enables RCP members to earn two external CPD credits. The CME questions are available at: https://cme.rcp.ac.uk/.
References
- 1.Nosten F, Richard-Lenoble D, Danis M. A brief history of malaria. Presse Medicale. 2022;51(3) doi: 10.1016/j.lpm.2022.104130. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD. Life & times the last British malaria outbreak. Br J Gen Pract. 2020;70(693):182–183. doi: 10.3399/bjgp20X709073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. World malaria report 2022 [Internet]. 2023 [cited 2024 Jun 25]. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023.
- 4.UK Health Security Agency. Imported malaria in the UK: statistics [Internet]. [cited 2024 Jun 25]. Available from: https://www.gov.uk/government/publications/imported-malaria-in-the-uk-statistics.
- 5.Poespoprodjo JR, Douglas NM, Ansong D, Kho S, Anstey NM. Malaria. Lancet. 2023 Dec 16;402(10419):2328–45. [DOI] [PubMed]
- 6.Battle KE, Lucas TCD, Nguyen M, et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet. 2019;394(10195):332–343. doi: 10.1016/S0140-6736(19)31096-7. Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lalloo DG, Shingadia D, Bell DJ, Beeching NJ, Whitty CJM, Chiodini PL. UK malaria treatment guidelines 2016. J Infect. 2016;72(6):635–649. doi: 10.1016/j.jinf.2016.02.001. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 9.Rogers CL, Bain BJ, Garg M, Fernandes S, Mooney C, Chiodini PL. British Society for Haematology guidelines for the laboratory diagnosis of malaria. Br J Haematol. 2022;197(3):271–282. doi: 10.1111/bjh.18092. May 1. [DOI] [PubMed] [Google Scholar]
- 10.UK Health Security Agency. Notifiable diseases and causative organisms: how to report [Internet]. [cited 2024 Jun 25]. Available from: https://www.gov.uk/guidance/notifiable-diseases-and-causative-organisms-how-to-report.
- 11.The Hospital for Tropical Diseases UCLH. Malaria diagnosis and treatment guideline. 2016 [cited 2024 Jul 5]; Available from: https://www.uclh.nhs.uk/application/files/2216/5762/6808/Malaria_Guideline_final_10_04_18.pdf.
- 12.World Health Organisation. WHO guidelines for malaria, 16 October 2023 [Internet]. 2023 [cited 2024 Jun 25]. Available from: https://www.who.int/publications/i/item/guidelines-for-malaria.
- 13.Datoo MS, Dicko A, Tinto H, et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet. 2024;403(10426):533–544. doi: 10.1016/S0140-6736(23)02511-4. Feb 10. [DOI] [PubMed] [Google Scholar]
- 14.Chandramohan D, Zongo I, Sagara I, et al. Seasonal Malaria Vaccination with or without Seasonal Malaria Chemoprevention. N Engl J Med. 2021;385(11):1005–1017. doi: 10.1056/NEJMoa2026330. Sep 9. [DOI] [PubMed] [Google Scholar]
- 15.Rts SCTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silk SE, Kalinga WF, Salkeld J, et al. Blood-stage malaria vaccine candidate RH5.1/Matrix-M in healthy Tanzanian adults and children; an open-label, non-randomised, first-in-human, single-centre, phase 1b trial. Lancet Infect Dis. 2024 doi: 10.1016/S1473-3099(24)00312-8. Jun. [DOI] [PubMed] [Google Scholar]
- 17.Dobaño C, Moncunill G, Bassat Q. Getting closer to an effective multi-stage malaria vaccine. Lancet Infect Dis. 2024 doi: 10.1016/S1473-3099(24)00356-6. Jun. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigo C, Rajapakse S, Fernando D. Vol. 2020. John Wiley and Sons Ltd; 2020. Tafenoquine for preventing relapse in people with Plasmodium vivax malaria. (Cochrane Database of Systematic Reviews). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medlock JM, Hansford KM, Vaux AGC, et al. Assessment of the public health threats posed by vector-borne disease in the United Kingdom (UK) Int J Environ Res Public Health. 2018;15(10) doi: 10.3390/ijerph15102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danis K, Baka A, Lenglet A, et al. Autochthonous Plasmodium vivax malaria in Greece. Euro Surveill. 2011;16(42) [PubMed] [Google Scholar]