ABSTRACT
Background
The use of commercially extracted phytogenic compounds to maintain poultry health and productivity in the absence of in‐feed antibiotics is prohibitively costly in developing countries.
Objectives
The goal of the study is to determine the effect of dietary supplementation with Thymus schimperi leaf meal (TLM) on production performance, egg quality and haemato‐biochemical parameters of Bovan brown layers.
Methods
A total of 96 laying hens at 25 weeks of age were randomly assigned to 4 treatments with 6 replications each. The treatments include the control (standard commercial laying diet), TLM1.5 (control + 1.5% TLM), TLM2.5 (control + 2.5% TLM) and TLM3.5 (control + 3.5% TLM). Egg production, feed intake and feed conversion ratio were recorded for each replicate. Two eggs per replication were used to measure internal and external egg quality traits on a monthly basis. At the end of the trial, blood samples were collected from 2 birds/replicate for the determination of albumin, uric acid, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, luteinizing hormone, prolactin and progesterone.
Results
All blood parameters were within the normal ranges of the breed. Egg production, feed conversion ratio, internal egg quality traits and external egg quality traits of hens fed diets containing 2.5% TLM were significantly higher than the control. Furthermore, diets containing 2.5% TLM led to a significantly reduced feed conversion ratio compared to all other dietary treatments.
Conclusions
In conclusion, 2.5% TML is recommended to improve egg production and egg quality without adverse effect on hen health.
Keywords: antibiotics, egg albumen, feed conversion ratio, progesterone, thyme, yolk colour
Thyme leaf meal supplementation (2.5% of the diet) improved egg production and quality laying hen. Thyme leaf meal supplementation did not adversely affect the hens’ health. Thus, thyme leaf at a level of 2.5% would be an affordable source of essential oils to improve egg production and the quality of lying hens.
1. Introduction
Antibiotics are widely utilized in the poultry and animal husbandry industries for both preventative measures and to boost growth and productivity (Diaz‐Sanchez et al. 2015). However, due to antibiotic resistance and health issues related with antibiotic residuals in poultry products, the use of antibiotics as a feed supplement has been widely banned (Castanon 2007; El‐Sabrout, Khalifah, and Mishra 2023).
Many feed additives, such as prebiotics, probiotics, organic acids, phytogenic compounds and aromatic plant extracts, are now used as alternative feed additives in poultry production to improve health and performance (Palamidi, Paraskeuas, and Mountzouris 2023; El‐Sabrout, Khalifah, and Mishra 2023; El‐Sabrout, Dantas, and Souza‐Junior 2023).
Aromatic plants as feed additives in replacement of antibiotics have been widely researched. Phytogenic additives may positively affect feed intake and feed utilization of livestock (Gu et al. 2013; Marume et al. 2020; Su et al. 2021). They have antimicrobial, antioxidative and immunomodulatory effects when they are included in livestock diets (Horky et al. 2019; Saleh, Ahmed, and Ebeid 2019; Yalçin et al. 2020). Their essential oil content is the most important factor behind their positive effect in animal nutrition.
Thymus genus belongs to the Lamiaceae family. It has a high level of essential oils (1%–2.5%), flavonoids, tannins, phenolic acids, carbohydrates and triterpenes. The essential oil content and composition of thyme vary widely due to biotic and abiotic factors (Damtie et al. 2019). The primary bioactive phenolic components in thyme are thymol (5‐methyl‐2‐isopropylphenol) and carvacrol (5‐isopropyl‐2‐methylphenol) (Escobar et al. 2020).
Respiratory disease, microbial infections and pain have all been traditionally treated using thyme (Demir et al. 2008). The positive effect of thyme inclusion in broiler diets on bird health was reported by several studies. Thyme and its essential oils have been reported to suppress the growth of Salmonella typhimurium (Ibrahim et al. 2021) and Escherichia coli (Veloso et al. 2019). Inclusion of 0.1% and 0.5% thyme leaf meal in laying hen diets improved egg production, decreased feed conversion ratio and reduced E. coli in litter (Canan and Erhan 2007).
Previous studies on thyme leaf meal found positive effects on egg production and quality (0.5% [Zarei and Torki 2010], 1% [Cimrin 2019], 1.5% [Hammershøj and Steenfeldt 2015] and 2% [Yalçin et al. 2020]). However, higher inclusion level of thyme meal in laying hens diet may result in further improvement in egg production and quality. Although essential oils have strong antimicrobial functions, they may decrease production performance when fed in high levels (Horky et al. 2019).
Accordingly, the goal of the current study is to determine the effect of different levels of thyme meal supplementation on egg production, egg quality and health of laying hens.
2. Materials and Methods
2.1. Collection and Preparation of Thyme Leaves
Fresh thyme (Thymus schimperi) plants were collected from natural pasture in Serbo district, Jimma Zone, Oromia, Ethiopia in November 2021. Thyme leaves were picked manually and air‐dried in the shade. The air‐dried leaves were milled to pass a 1 mm screen and stored before diet formulation.
2.2. Description of the Study Area
The study was conducted at Dr. Tilahun Poultry farm, situated 350 km southwest of Addis Ababa (7° 39′ N, 36° 49′ E, 1.850 m.a.s.l).
2.3. Animals, Treatments and Management
A total of 96 Bovan Brown laying hens at 25 weeks of age were used in this study. The birds were allocated randomly to four experimental groups with each group having six replicate cages (4 hens/cage) in a barn having similar environmental conditions for all chickens. The experiment spanned a duration of 12 weeks, conducted between February and April 2022. The barn microclimate depended on the outside environment, with a relative humidity of 77% and an average temperature of 23°C. Digital electric ventilation was used to regulate the barn microenvironment, ensuring it matched the birds thermoneutral zone. All experimental cages had a nipple drinker and a separate feeder. The birds had free access to mash feed and clean drinking water during the study. The dietary treatments were the control (Commercial layer diets, T. schimperi leaf meal 1.5 [TLM1.5] [control + 1.5% TLM], TLM2.5 [control + 2.5% TLM] and TLM3.5 [control + 3.5% TLM]). The ingredient composition and nutritional analysis of the commercial concentrate used in the study are presented in Table 1.
TABLE 1.
Chemical composition of experimental feeds.
| Commercial concentrate | Thymus schimperi leaf meal | |
|---|---|---|
| Ingredient composition (%) | ||
| Toasted soybean meal | 20 | |
| Maize grain | 49 | |
| Noug a seed cake | 14 | |
| Wheat bran | 8 | |
| Limestone flour | 4.5 | |
| Meat and bone meal | 2 | |
| Vitamin and mineral premix b | 2 | |
| Salt | 0.5 | |
| Nutrient composition | ||
| Dry matter (%) | 90 | 91.3 |
| Crude protein (%) | 18 | 8.04 |
| Crude fibre (%) | 8 | 15.4 |
| Ether extract (%) | 8 | 3.59 |
| Total ash (%) | 7.5 | 9.51 |
| Calcium (%) | 3.23 | |
| Total phosphorus (%) | 0.74 | |
| Nitrogen free extract (%) | 48.5 | 27.8 |
| Metabolisable energy (MJ/kg DM) c | 12.6 | 6.43 |
| Total essential oil (g/kg) | 15.1 | |
| Thymol (g/kg) | 0.936 | |
| Carvacrol (g/kg) | 9.56 |
Guizotia abyssinica.
Vitamin premix per kg of diet: vitamin A—2.7 mg; vitamin D3—0.05 mg; vitamin E—18 mg; vitamin K3—2 mg; thiamine—1.8 mg; riboflavin—6.6 mg; pantothenic acid—10 mg; pyridoxine—3 mg; cyanocobalamin—0.015 mg; niacin—30 mg; biotin—0.1 mg; folic acid—1 mg; choline chloride—250 mg; antioxidant—100 mg; Fe—50 mg; Mn—100 mg; Zn—100 mg; Cu—10 mg; I—1 mg; Se—0.2 mg.
Calculated according to Wiseman (1987).
The birds were vaccinated against New Castle, fowl typhoid, fowl pox infectious bursal disease and Marek's disease upon arrival to the experimental barn. The birds were adapted to their respective treatment diet for 2 weeks before the 12‐week data collection period.
All birds were housed in an environmentally controlled barn with the temperature maintained at approximately 24°C. The barn had controlled ventilation and lighting (16L:8D).
2.4. Feed Intake and Body Weight Changes
Feed intake was recorded weekly. Feed refusal was subtracted from the feed offered to calculate feed intake. The hens were weighed at the start of the experiment and weekly thereafter.
2.5. Egg Production and Egg Quality
The number of eggs produced by each cage was recorded daily, including eggs that were broken. Hen‐day egg production, egg mass and FCR (feed consumption per kg of egg mass) were calculated as follows:
Two eggs/cages were collected monthly and individually marked before egg quality measurement. Egg length and width of each egg were measured using a vernier calliper, then used to calculate egg shape index. Eggshell was weighed after the shell membrane is removed using an analytical scale. Eggshell thickness was recorded as an average of 3 points (blunt, middle and sharp) using a micrometre screw gauge. Yolk weight, albumen weight, albumen height, Haugh unit and yolk colour were recorded for each egg. Haugh unit was calculated according to the following equation:
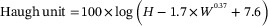 |
where H is the albumen height (mm), and W is egg weight (g). Egg shape index was calculated according to the following equation:
2.6. Blood Analysis
At the end of the egg production trial, blood samples were collected from the jugular vein of 2 hens/replicate into heparinized tubes. The tubes were centrifuged (Spectrafuge centrifuge, 22,668 rpm for 15 min), and then, the plasma was stored at −20°C. Total protein, albumin, uric acid, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase of serum were measured spectrophotometrically according to Sanghavin and Jivani (1979).
2.7. Feed and Essential Oil Component Analysis
Feed samples were ground to pass a 1 mm screen, then analysed for dry matter, ash, ether extract and crude fibre according to AOAC (2006) (Methods 930.15, 942.05, 2003.05 and 962.09, respectively). The micro‐Kjeldahl method was used to analyse nitrogen content of the feed and crude protein was calculated as nitrogen ×6.25. Ether extract, crude fibre and crude protein were subtracted from organic matter to obtain nitrogen‐free extract. Metabolizable energy of the feeds was calculated according to Pauzenga (1985) equations:
where CP is crude protein, EE is ether extract and NFE is a nitrogen‐free extract. The shade‐dried and powdered aerial parts of the thyme plant were subjected to hydro‐distillation for 4 h using a Clevenger‐type apparatus to obtain the essential oil. The oil was then dried using anhydrous sodium sulphate and stored at +4°C before being used for further analysis. The essential oil composition of dried thyme leaves was analysed with GC–MS (Agilent‐6890, MS:5973, NJ, USA) using an HP‐5 MS 30 m column.
2.8. Statistical Analysis
One‐way analysis of variance was used to determine the effect of the dietary treatment on production performance, egg quality and blood biochemistry of laying hens. Fisher's least significant difference was used to compare means at p = 0.05. Statistical Analysis System was used to carry out the statistical analyses (SAS 2012).
3. Results
3.1. Production Performance
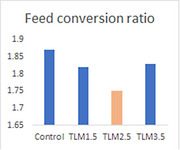
Nutritional and ingredient composition of the experimental feed is presented in Table 1. The effect of the dietary treatment on egg production is presented in Table 2. Final weight of TLM1.5 birds was significantly higher than the control. The dietary treatment significantly decreased feed intake of the birds. Egg mass and daily egg production were significantly improved by the treatment. Egg weights of TLM2.5 and TLM3.5 were significantly higher than those of the control. The dietary treatment significantly decreased FCR with TLM2.5 showing the lowest value.
TABLE 2.
The effects of dietary Thymus schimperi leaf meal on the production performance and egg quality of laying hens.
| Treatment a | ||||||
|---|---|---|---|---|---|---|
| Parameter | Control | TLM1.5 | TLM2.5 | TLM3.5 | SEM b | p c |
| Production performance | ||||||
| Initial bird weight (kg) | 1.7 | 1.69 | 1.7 | 1.7 | 0.003 | 0.991 |
| Final bird weight (kg) | 1.82b | 1.92a | 1.82b | 1.81b | 0.011 | <0.001 |
| Feed intake (g/day/bird) | 104a | 101b | 99.4b | 97.3c | 0.744 | <0.001 |
| Bird weight gain (kg) | 0.11b | 0.22a | 0.11a | 0.117b | 0.027 | <0.001 |
| Egg mass (g/bird/day) | 42.3c | 49a | 44.8b | 44.6b | 0.706 | <0.001 |
| Daily egg production (%) | 80.4c | 87a | 82.8b | 82.6b | 0.649 | <0.001 |
| Egg weight (g/day) | 57.9c | 58.8c | 60.3b | 65.3a | 0.567 | <0.001 |
| Feed conversion ratio | 1.87a | 1.82b | 1.75c | 1.83b | 0.042 | <0.001 |
| External traits | ||||||
| Shell thickness (mm) | 0.31c | 0.50a | 0.42b | 0.38b | 0.019 | <0.001 |
| Shell weight (g) | 6.95c | 7.42ab | 7.74a | 7.11bc | 0.146 | <0.001 |
| Shell weight (% of egg weight) | 11.9b | 12.8a | 12.7a | 11.5b | 0.205 | <0.001 |
| Shell breaking strength (N) | 35b | 35.5b | 39.6a | 34.4b | 0.428 | <0.001 |
| Egg width (mm) | 4.19 | 4.26 | 4.31 | 4.32 | 0.052 | 0.254 |
| Egg length (mm) | 5.12b | 5.45a | 5.46a | 5.61a | 0.064 | <0.001 |
| Egg shape index (%) | 74.7c | 78.1bc | 79.6b | 84.3a | 1.32 | <0.001 |
| Internal traits | ||||||
| Yolk weight (g) | 14.2c | 14.6c | 15.1b | 15.8a | 0.178 | <0.001 |
| Yolk weight (% of egg weight) | 24.6 | 24.8 | 25.1 | 25.3 | 0.356 | 0.718 |
| Albumen weight (g) | 36.7b | 36.6b | 37.5b | 39.4a | 0.595 | <0.001 |
| Albumen weight (% of egg weight) | 63.4 | 62.3 | 62.3 | 63.2 | 0.404 | 0.218 |
| Albumen height (mm) | 5.88 | 6.27 | 6.44 | 5.54 | 0.329 | 0.335 |
| Haugh unit | 88.2c | 88.4c | 88.9b | 89.4a | 0.157 | <0.001 |
| Yolk colour | 6.5c | 7.33b | 8.66a | 7.66b | 0.25 | <0.001 |
Note: Means in a row with different superscripts are significantly different (p ≤ 0.05).
Control, TLM1.5, TLM2.5 and LM3.5 = commercial laying hen concentrate, control + 1.5% Thymus schimperi leaf meal, control + 2.5% Thymus schimperi leaf meal and control + 3.5% Thymus schimperi leaf meal, respectively.
SEM: standard error mean.
* = p ≤ 0.05. ns = p > 0.05.
3.2. Internal and External Egg Quality
Shell thickness and egg length were significantly improved by the treatment. Shell weight, shape index, yolk weight and Haugh unit of TLM2.5 and TLM3.5 eggs were significantly higher than the control.
Albumen weight of TLM3.5 eggs was significantly higher than that of the control. Yolk colour was significantly improved by the dietary treatment.
3.3. Blood Biochemistry
Blood analysis of the experimental birds is presented in Table 3. Aspartate amino transferase and alanine amino transferase of TLM2.5 were significantly lower than those of the control. Albumin and alkaline phosphatase levels in the supplemented birds were significantly higher compared to the control birds. Luteinizing hormone and progesterone of TLM2.5 were significantly higher than the control. Uric acid and prolactin were not significantly affected by the dietary treatment.
TABLE 3.
Blood biochemical parameters of laying Bovan brown layers supplemented with different levels of dried thyme leaf meal.
| Treatment a | ||||||
|---|---|---|---|---|---|---|
| Parameter | Control | TLM1.5 | TLM2.5 | TLM3.5 | SEM b | p c |
| Aspartate amino transferase (IU/L) | 175c | 156b | 150b | 136a | 4.03 | <0.001 |
| Alanine amino transferase (IU/L) | 3.97c | 2.9ab | 3.2b | 2.4a | 0.214 | <0.001 |
| Albumin (mmol/L) | 1.43c | 1.67ab | 1.72b | 1.57a | 0.034 | <0.001 |
| Alkaline phosphatase (IU/L) | 348d | 464c | 805b | 904a | 18 | <0.001 |
| Uric acid (mmol/L) | 2.77 | 2.2 | 3.37 | 2.29 | 0.419 | 0.218 |
| Luteinizing hormone (ng/mL) | 0.396ab | 0.341b | 0.437a | 0.378ab | 0.026 | <0.001 |
| Prolactin (ng/mL) | 0.047 | 0.112 | 0.132 | 0.12 | 0.058 | 0.244 |
| Progesterone (ng/mL) | 0.32b | 0.396b | 1.12a | 0.614b | 0.165 | <0.001 |
Note: Means in a row with different superscripts are significantly different (p ≤ 0.05).
Control, TLM1.5, TLM2.5 and LM3.5 = commercial laying hen concentrate, control + 1.5% Thymus schimperi leaf meal, control + 2.5% Thymus schimperi leaf meal and control + 3.5% Thymus schimperi leaf meal, respectively.
SEM: standard error mean.
* = p ≤ 0.05. ns = p > 0.05.
4. Discussion
4.1. Production Performance
The objective of this study was to determine the viability of using TLM supplementation as an affordable source of dietary essential oils to improve laying hen production performance.
Our results showed a clear effect of TLM on feed intake and weight gain, which agrees with studies summarized by Akbari, Torki, and Kaviani (2016) and Yalçin et al. (2020).
Thyme essential oil was found to improve digestive enzyme secretion in the poultry gut (Wade et al. 2018; Basmacioğlu et al. 2010), resulting in an improvement in nutrient digestibility, which could explain the improvement in growth and egg production (Abd El‐Hack et al. 2022). Supplementing chicken diet with essential oils improved the morphology of the intestinal villi (Xiao et al. 2022), which might improve the absorption of nutrients in the small intestine, thereby increasing egg production. However, the intestinal morphology was not assessed in the current study.
As the study showed that TLM supplementation increased serum progesterone and luteinizing hormone levels, this may be one of the mechanisms behind the improvement in egg production in the supplemented hens. The improvement in egg production in response to supplementation with thyme leaf in the current study is consistent with Saleh, Ahmed, and Ebeid (2019) who reported that adding phytoestrogen to laying hens diet increases steroidogenesis and laying rate (Shang et al. 2018).
Stability of chicken macrofloral ecosystem improves disease resistance, nutrient absorption and immune functions (Pan and Yu 2013; Shang et al. 2018). Therefore, the improvement in production in the current study might be ascribed to the improvement in the stability of the gut microbiome due to thyme leaf meal (Hong et al. 2012; Pan and Yu 2013; Fernandez et al. 2019; Sigolo et al. 2021; Su et al. 2021). On the gut microbiota level, essential oils in TLM might improve the abundance of Firmicutes and Megamonas, microorganisms in caecum ecosystem that are efficient in cellulose digestion, providing propionate as a glycogenic source to citric acid for egg production (Sun et al. 2016).
The negative effect of TLM on feed intake, although small, could be related to the unpleasant smell and taste of thymol and carvacrol to poultry (Zhai et al. 2018).
The improvement in weight gain without concurrent increase in feed intake may explain the decrease in FCR as a result of TLM supplementation.
4.2. Internal and External Egg Quality
The improvement in shell weight and thickness in TLM compared to the control pinpoints that eggs produced by the hens supplemented with TLM have stronger shells, which would reduce egg loss during handling and transport. The increase in serum concentration of calcium as a result of phytoestrogen supplementation was reported by Lu et al. (2018), which is most likely responsible for the improvement in eggshell characteristics observed in this study. Our study also showed an improvement in yolk weight due to TLM supplementation, which may result from the apparent improvement in nutrient digestion and absorption indicated by the improved FCR associated with TLM supplementation (Vlaicu et al. 2022).
The inclusion of the TLM positively influenced some internal quality parameters. Haugh unit, an indication to measure albumen quality (Eisen, Bohren, and McKean 1962), was improved by TLM supplementation, indicating an improvement in egg freshness. This is in agreement with Cheng et al. (2022), who reported on a positive effect of essential oils on egg quality. Supplementation of TLM increased the intensity of yolk colour, which could be linked to xanthophylls found naturally in thyme and thyme‐like plants. In line with the current study, positive effects of supplementation of fennel or hot red pepper (Abou‐Elkhair, Selim, and Hussein 2018) and encapsulated essential oils and organic acids (Wiseman 1987) on egg yolk colouration have been reported.
4.3. Blood Biochemistry
Previous studies on thyme leaf meal assessed different levels of inclusion in laying hen diet on egg production and quality with levels of 0.5% (Torki, Ghasemi, and Zarei 2010), 1% (Cimrin 2019), 1.5% (Hammershøj and Steenfeldt 2015) and 2% (Yalçin et al. 2020). Although essential oils have strong antimicrobial functions, they might negatively impact monogastric animal health (kidneys and liver function) and reduce production when fed in high level (Horky et al. 2019). High level of essential oils (beyond 2 g/kg feed) in laying hen diet is related with a reduction in feed intake and egg production performance (Marume et al. 2020). Aspartate amino transferase, alanine amino transferase, albumin and alkaline phosphatase are widely used to evaluate liver health (Cruz et al. 2018). Our study shows that, although aspartate amino transferase, alanine amino transferase, albumin and alkaline phosphatase were significantly affected by the treatment, they remained within the normal range reported for broiler chickens (Meluzzi et al. 1992). Accordingly, the increasing level of TLM supplementation did not negatively affect the liver function of laying hens. High uric acid level in blood serum reflects kidney malfunction (Srivastava et al. 2018). Once again, uric acid level in all birds was in the normal range of laying hens, although it significantly varied among treatments. Overall, supplementation of the experimental hen diets with TLM up to 3.5% did not interfere with liver and kidney function.
Wide variation in essential oil level and composition of thyme leaf due to biotic and abiotic factors was reported (Tohidi, Rahimmalek, and Trindade 2019). This variability poses a key challenge to the use of thyme leaf meal as a feed supplement. Accordingly, determination of this variability would help in recommending the level of TLM, which is associated with the best egg production performance and quality.
5. Conclusion
It is concluded that TML2.5 improved egg production and external and internal egg traits compared to the control. Furthermore, it has the lowest feed conversion ratio among all the experimental treatments. Thus, thyme at a level of 2.5% could be used as an affordable source of essential oils to improve egg production and the quality of laying hens.
Author Contributions
Abdulwahid Yasin: investigation, writing–original Draft. Metekia Tamiru: conceptualization, investigation, writing–original, writing–review, funding acquisition, supervision. Ashraf Alkhtib: conceptualization, writing–original draft, writing–review and editing, supervision. Abdo Mohammed: conceptualization, investigation, writing–review and editing, funding acquisition, supervision. Tagesse Tadesse: writing–original draft. Jane Wamatu: writing–review and editing. Emily Burton: writing–review and editing.
Ethics Statement
Prior to the start of experiment, compliance of the experiment to the guidelines of animal experimentation was checked and approved by Department of animal science, College of Agriculture and Veterinary Medicine, Jimma University with Ref. No.: AnSc/18/m/2021.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.70146.
Acknowledgements
The authors would give sincerely gratitude to Dr. Tilahun for allowing this experiment to be conducted in his commercial layer farm. Universal Clinic Chemical Pathology Diagnostic Laboratory (Jimma, Ethiopia) and Jimma University specialized hospital (Jimma, Ethiopia) are acknowledged for blood analysis. Jimma University, Animal Nutrition and Post‐harvest and Food Science Technology Laboratory is appreciated for their support in feed analysis.
Abdulwahid Yasin, Metekia Tamiru and Ashraf Alkhtib contributed equally to the manuscript.
Funding: The authors received no specific funding for this work.
Data Availability Statement
Data are available under reasonable request.
References
- Abd El‐Hack, M. E. , El‐Saadony M., Saad A., et al. 2022. “Essential Oils and Their Nanoemulsions as Green Alternatives to Antibiotics in Poultry Nutrition: A Comprehensive Review.” Poultry Science 101, no. 2: 101584. 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou‐Elkhair, R. , Selim S., and Hussein E.. 2018. “Effect of Supplementing Layer Hen Diet With Phytogenic Feed Additives on Laying Performance, Egg Quality, Egg Lipid Peroxidation and Blood Biochemical Constituents.” Animal Nutrition 4: 394–400. 10.1016/j.aninu.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari, M. , Torki M., and Kaviani K.. 2016. “Single and Combined Effects of Peppermint and Thyme Essential Oils on Productive Performance, Egg Quality Traits, and Blood Parameters of Laying Hens Reared Under Cold Stress Condition (6.8±3°C).” International Journal of Biometeorology 60: 447–454. 10.1007/s00484-015-1042-6. [DOI] [PubMed] [Google Scholar]
- AOAC . 2006. Official Methods of Analysis. Gaithersburg, MD, USA: AOAC. [Google Scholar]
- Basmacioğlu, H. , Baysal S., Misirlioğlu Z., Polat M., Yilmaz H., and Turan N.. 2010. “Effects of Oregano Essential Oil With or Without Feed Enzymes on Growth Performance, Digestive Enzyme, Nutrient Digestibility, Lipid Metabolism and Immune Response of Broilers Fed on Wheat–Soybean Meal Diets.” British Poultry Science 51: 67–80. [DOI] [PubMed] [Google Scholar]
- Canan, Ş. , and Erhan M.. 2007. “Effect of Dietary Thyme on Laying Hen's Performance and E. coli Concentration in Feces.” International Journal of Natural and Engineering Sciences 1: 55–58. [Google Scholar]
- Castanon, J. I. R. 2007. “History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds.” Poultry Science 60: 2466–2471. [DOI] [PubMed] [Google Scholar]
- Cheng, H. , Chen J., Tang S., Guo S., He C., and Qu X.. 2022. “Effects of Essential Oil/Palygorskite Composite on Performance, Egg Quality, Plasma Biochemistry, Oxidation Status, Immune Response and Intestinal Morphology of Laying Hens.” Poultry Science 101: 101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimrin, T. 2019. “Thyme (Thymbra spicata L.), Rosemary (Rosmarinus officinalis L.) and Vitamin E Supplementation of Laying Hens.” South African Journal of Animal Sciences 49: 914–921. 10.4314/SAJAS.V49I5.15. [DOI] [Google Scholar]
- Cruz, C. , Freitas E., Braz N., Salles R., and da Silva I.. 2018. “Blood Parameters and Enzymatic and Oxidative Activity in the Liver of Chickens Fed with Calcium Anacardate.” Revista Ciencia Agronomica 49: 343–352. 10.5935/1806-6690.20180039. [DOI] [Google Scholar]
- Damtie, D. , Braunberger C., Conrad J., Mekonnen Y., and Beifuss U.. 2019. “Composition and Hepatoprotective Activity of Essential Oils from Ethiopian Thyme Species (Thymus serrulatus and Thymus schimperi).” Journal of Essential Oil Research 31: 120–128. 10.1080/10412905.2018.1512907. [DOI] [Google Scholar]
- Demir, E. , Kilinc K., Onyedi B., and Üniversitesi E.. 2008. “Comparative Effects of Mint, Sage, Thyme and Flavomycin in Wheat‐Based Broiler Diets the Possibilities of Using Low‐Protein Diets in the Feeding of Japanese Quails.” Archiva Zootechnica 11: 54–63. [Google Scholar]
- Diaz‐Sanchez, S. , D'Souza D., Biswas D., and Hanning I.. 2015. “Botanical Alternatives to Antibiotics for Use in Organic Poultry Production.” Poultry Science 94: 1419–1430. 10.3382/ps/pev014. [DOI] [PubMed] [Google Scholar]
- Eisen, E. , Bohren B., and McKean H.. 1962. “The Haugh Unit as a Measure of Egg Albumen Quality.” Poultry Science 41: 1461–1468. 10.3382/ps.0411461. [DOI] [Google Scholar]
- El‐Sabrout, K. , Dantas M., and Souza‐Junior J.. 2023. “Herbal and Bee Products as Nutraceuticals for Improving Poultry Health and Production.” World's Poultry Science Journal 79: 223–242. [Google Scholar]
- El‐Sabrout, K. , Khalifah A., and Mishra B.. 2023. “Application of Botanical Products as Nutraceutical Feed Additives for Improving Poultry Health and Production.” Veterinary World 16: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar, A. , Pérez M., Romanelli G., and Blustein G.. 2020. “Thymol Bioactivity: A Review Focusing on Practical Applications.” Arabian Journal of Chemistry 13: 9243–9269. 10.1016/j.arabjc.2020.11.009. [DOI] [Google Scholar]
- Fernandez, M. , Kembro J., Ballesteros M., Caliva J. M., Marin R. H., and Labaque M. C.. 2019. “Dynamics of Thymol Dietary Supplementation in Quail (Coturnix japonica): Dataset on Thymol Bioavailability, Egg Yolk Fatty Acids Profile and Performance Traits.” Data in Brief 24: 103884. 10.1016/j.dib.2019.103884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, H. , Shi S., Chang L., Tong H., Wang Z., and Zou J.. 2013. “Safety Evaluation of Daidzein in Laying Hens: Part II. Effects on Calcium‐Related Metabolism.” Food and Chemical Toxicology 55: 689–692. 10.1016/j.fct.2012.12.064. [DOI] [PubMed] [Google Scholar]
- Hammershøj, M. , and Steenfeldt S.. 2015. “Organic Egg Production. II: The Quality of Organic Eggs Is Influenced by Hen Genotype, Diet and Forage Material Analyzed by Physical Parameters, Functional Properties and Sensory Evaluation.” Animal Feed Science and Technology 208: 182–197. 10.1016/j.anifeedsci.2015.07.012. [DOI] [Google Scholar]
- Hong, J. , Steiner T., Aufy A., and Lien T.. 2012. “Effects of Supplemental Essential Oil on Growth Performance, Lipid Metabolites and Immunity, Intestinal Characteristics, Microbiota and Carcass Traits in Broilers.” Livestock Science 144: 253–262. 10.1016/j.livsci.2011.12.008. [DOI] [Google Scholar]
- Horky, P. , Skalickova S., Smerkova K., and Skladanka J.. 2019. “Essential Oils as a Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals.” Animals 9: 1–15. 10.3390/ani9060352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, D. , Abdelfattah‐Hassan A., Badawi M., et al. 2021. “Thymol Nanoemulsion Promoted Broiler Chicken's Growth, Gastrointestinal Barrier and Bacterial Community and Conferred Protection against Salmonella typhimurium .” Scientific Reports 11: 1–20. 10.1038/s41598-021-86990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, L. , Chen N., Nayeem F., et al. 2018. “Novel Effects of Phytoestrogenic Soy Isoflavones on Serum Calcium and Chloride in Premenopausal Women: A 2‐Year Double‐Blind, Randomized, Placebo‐Controlled Study.” Clinical Nutrition 37: 1862–1870. 10.1016/j.clnu.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marume, U. , Mokagane J., Shole C., and Hugo A.. 2020. “ Citrullus lanatus Essential Oils Inclusion in Diets Elicit Nutraceutical Effects on Egg Production, Egg Quality, and Physiological Characteristics in Layer Hens.” Poultry Science 99: 3038–3046. 10.1016/j.psj.2020.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meluzzi, A. , Primiceri G., Giordani R., and Fabris G.. 1992. “Determination of Blood Constituents Reference Values in Broilers.” Poultry Science 71: 337–345. 10.3382/ps.0710337. [DOI] [PubMed] [Google Scholar]
- Palamidi, I. , Paraskeuas V., and Mountzouris K.. 2023. “Dietary and Phytogenic Inclusion Effects on the Broiler Chicken Cecal Ecosystem.” Frontiers in Animal Science 3: 1–15. 10.3389/fanim.2022.1094314. [DOI] [Google Scholar]
- Pan, D. , and Yu Z.. 2013. “Intestinal Microbiome of Poultry and Its Interaction with Host and Diet.” Gut Microbes 5: 108–119. 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauzenga, U. 1985. “Feeding Parent Stock.” Zootecnica International 17: 22–24. https://cir.nii.ac.jp/crid/1571698600845198080. [Google Scholar]
- Saleh, A. , Ahmed E., and Ebeid T. A.. 2019. “The Impact of Phytoestrogen Source Supplementation on Reproductive Performance, Plasma Profile, Yolk Fatty Acids and Antioxidative Status in Aged Laying Hens.” Reproduction in Domestic Animals 54, no. 6: 846–854. 10.1111/rda.13432. [DOI] [PubMed] [Google Scholar]
- Sanghavin, N. , and Jivani N.. 1979. “A Colorimetric Method for the Determination of Nitrazepam.” Talanta 26: 63–64. 10.1016/0039-9140(79)80158-7. [DOI] [PubMed] [Google Scholar]
- SAS . 2012. SAS/STAT 12.1 User's Guide Survey Data Analysis. Cary, NC: : SAS Institute Inc. [Google Scholar]
- Shang, Y. , Kumar S., Oakley B., and Kim W. K.. 2018. “Chicken Gut Microbiota: Importance and Detection Technology.” Frontiers in Veterinary Science 5: 254. 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigolo, S. , Milis C., Dousti M., et al. 2021. “Effects of Different Plant Extracts at Various Dietary Levels on Growth Performance, Carcass Traits, Blood Serum Parameters, Immune Response and Ileal Microflora of Ross Broiler Chickens.” Italian Journal of Animal Science 20: 359–371. 10.1080/1828051X.2021.1883485. [DOI] [Google Scholar]
- Srivastava, A. , Kaze A., McMullan C., Isakova T., and Waikar S.. 2018. “Uric Acid and the Risks of Kidney Failure and Death in Individuals With CKD.” American Journal of Kidney Diseases 71: 362–370. 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, G. , Wang L., Zhou X., et al. 2021. “Effects of Essential Oil on Growth Performance, Digestibility, Immunity, and Intestinal Health in Broilers.” Poultry Science 100: 101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, B. , Wang X., Bernstein S., et al. 2016. “Marked Variation Between Winter and Spring Gut Microbiota in Free‐Ranging Tibetan Macaques (Macaca thibetana).” Scientific Reports 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidi, B. , Rahimmalek M., and Trindade H.. 2019. “Review on Essential Oil, Extracts Composition, Molecular and Phytochemical Properties of Thymus Species in Iran.” Industrial Crops and Products 134: 89–99. 10.1016/j.indcrop.2019.02.038. [DOI] [Google Scholar]
- Torki, M. , Ghasemi R., and Zarei M.. 2010. “Adding Medicinal Herbs Including Garlic (Allium sativum) and Thyme (Thymus vulgaris) to Diet of Laying Hens and Evaluating Productive Performance and Egg Quality Characteristics.” American Journal of Animal and Veterinary Sciences 5: 151–154. http://jn.nutrition.org/cgi/content/abstract/131/3/955S. [Google Scholar]
- Veloso, R. , Fronza N., Júnior A., Carvalho V., Fujinawa M., and da Silveira S.. 2019. “Potential of Thyme Essential Oil on Arugula Sanitization.” Ciencia e Agrotecnologia 43: 1–10. 10.1590/1413-7054201943006819. [DOI] [Google Scholar]
- Vlaicu, P. , Untea A., Turcu R., Saracila M., Panaite T., and Cornescu G.. 2022. “Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality.” Foods 11: 1105. 10.3390/foods11081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, M. , Manwar S., Kuralkar S., Waghmare S., Ingle V., and Hajare S.. 2018. “Effect of Thyme Essential Oil on Performance of Broiler Chicken.” Journal of Entomology and Zoology Studies 6: 25–28. [Google Scholar]
- Wiseman, J. 1987. Feeding of Non‐Ruminant Livestock. Nottingham: Butterworth and Co. Ltd. [Google Scholar]
- Xiao, G. , Zheng L., Yan X., et al. 2022. “Effects of Dietary Essential Oils Supplementation on Egg Quality, Biochemical Parameters, and Gut Microbiota of Late‐Laying Hens.” Animals 12, no. 19: 2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalçin, S. , Eser H., Onbaşilar İ., and Yalçin S.. 2020. “Effects of Dried Thyme (Thymus vulgaris L.) Leaves on Performance, Some Egg Quality Traits and Immunity in Laying Hens.” Ankara Universitesi Veteriner Fakultesi Dergisi 67: 303–311. 10.33988/auvfd.677150. [DOI] [Google Scholar]
- Zarei, M. , and Torki M.. 2010. “Adding Medicinal Herbs Including Garlic (Allium sativum) and Thyme (Thymus vulgaris) to Diet of Laying Hens and Evaluating Productive Performance and Egg Quality Characteristics.” American Journal of Animal and Veterinary Sciences 5: 151–154. [Google Scholar]
- Zhai, H. , Liu H., Wang S., Wu J., and Kluenter A. M.. 2018. “Potential of Essential Oils for Poultry and Pigs.” Animal Nutrition 4: 179–186. 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available under reasonable request.


