ABSTRACT
Background and Aims
Butalbital is an acute headache medication commonly prescribed for tension‐type headache (TTH), although discouraged by guidelines due to a risk of medication overuse headache (MOH). Considering spinal manipulative therapy (SMT) may reduce TTH frequency and intensity, we hypothesized adults with TTH receiving chiropractic SMT would be less likely to receive a butalbital prescription over 2 years versus matched controls not receiving SMT. We secondarily compared likelihood of MOH between cohorts.
Methods
We searched a United States medical records database of patients attending academic medical centers for adults with TTH, from 2013 to 2024, excluding those diagnosed with other headaches and seen in inpatient/emergency settings. We divided patients into two cohorts: (1) SMT and (2) non‐SMT, using propensity matching to control for demographics and other variables associated with likelihood of butalbital prescription and MOH.
Results
Three thousand one hundred and sixteen patients remained per cohort after matching. The incidence of butalbital prescription was lower in the SMT cohort compared to the non‐SMT cohort (SMT: 1.7%; non‐SMT: 3.8%), yielding an RR (95% CI) of 0.46 (0.33–0.63; p < 0.001). The incidence of MOH was lower in the SMT cohort versus non‐SMT cohort (SMT: 0.5%; non‐SMT: 1.2%), yielding an RR (95% CI) of 0.44 (0.25–0.80; p < 0.001).
Conclusion
Adults receiving chiropractic SMT had a significantly lower likelihood of butalbital prescription and, tentatively, MOH compared to matched controls not receiving SMT. These findings support current guideline recommendations favoring SMT in TTH care, though future studies should replicate and compare these findings with other nonpharmacologic clinicians and interventions.
Keywords: barbiturates, chiropractic, headache, spinal manipulation
1. Background
Tension‐type headache (TTH) is the most common type of headache, with a global prevalence of 26% [1, 2]. TTH is typically characterized by bilateral pressing, tightening head pain, and co‐occurs with neck pain in nearly 90% of patients [3]. It is occasionally treated with butalbital or butalbital‐containing medications for acute/abortive purposes [4, 5, 6]. However, this has been discouraged by practice guidelines due to poor efficacy and potential complications such as withdrawal, overuse, and medication overuse headache (MOH) [2, 7, 8, 9]. Patients also seek spinal manipulative therapy (SMT) for TTH relief [10, 11, 12]. While evidence supports the efficacy of SMT for TTH [13, 14], its association with butalbital prescription and MOH remains unknown [15].
First‐line medications for TTH include acetaminophen and aspirin [3, 7, 8, 16]. Butalbital, a barbiturate and sedative/hypnotic medication, is a second‐line therapy but carries the risk of cognitive impairment, dependence/addiction, and headache chronification [7, 9]. In addition, patients may develop MOH, described as a worsening of the primary headache after overuse or discontinuation of acute headache medication, and occurring at least 15 days per month [17]. Common butalbital‐containing medications used for headaches often include caffeine and either acetaminophen or aspirin. Butalbital‐containing medications have not demonstrated efficacy for headaches in comparison to other treatments or placebo [18].
Several studies suggest that butalbital is commonly prescribed for TTH. An examination of a US data set of ambulatory visits for nonserious, non‐migraine headaches found that either opioids or barbiturates were prescribed in approximately 15% of cases [4]. According to data from a single US academic headache center, one in five patients was currently using either opioids or barbiturates, while more than half of patients had been prescribed an opioid or barbiturate [5]. Finally, another study highlighted that butalbital prescription was more common than opioid prescription for TTH [6].
Chiropractors are non‐pharmacologic clinicians who frequently use SMT to manage musculoskeletal disorders [11]. Systematic reviews have found evidence that SMT may reduce TTH intensity and frequency compared to sham interventions or no treatment [13, 14]. In addition, SMT is recommended for TTH by the US Centers for Disease Control [19]. Considering SMT may benefit TTH, it remains plausible that patients receiving SMT may be less inclined to seek medications for acute TTH relief such as butalbital. As butalbital is not a first‐line medication for TTH, examination of its prescription could reflect acute exacerbations of recalcitrant headaches and/or medication guideline non‐adherence.
This study addresses gaps in the TTH literature by examining the association between SMT and butalbital prescription and MOH. We hypothesized that adults receiving chiropractic SMT for TTH would have a reduced likelihood of receiving a butalbital prescription over a 1‐year follow‐up compared to matched controls not receiving SMT, and secondarily compared the likelihood of MOH between cohorts.
2. Materials and Methods
2.1. Study Design
This study implemented a retrospective cohort design with a data range spanning 11 years to present coinciding with US headache recommendations discouraging butalbital as a first‐line intervention [9]. We adhered to a registered protocol [20]. Inclusion of patients ended 2 years before the query date (April 15, 2024), allowing for ascertainment of the outcomes. Study reporting conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline [21]. This study used deidentified, anonymized data from TriNetX (TriNetX Inc. Cambridge, MA, US) obtained via the University Hospitals Clinical Research Center Honest Broker. The University Hospitals Institutional Review Board (IRB; Cleveland, OH, US) considered the present study “not human subjects research,” therefore not requiring review board approval or patient consent (IRB number: STUDY20241256). In addition, TriNetX has received an exemption from Western IRB, which waives the need for patient consent.
The US TriNetX network includes over 124 million individuals from 89 academic medical centers and their affiliated community and ambulatory offices [22, 23], and approximately 145,000 unique patients receiving chiropractic SMT at the time of our query. Data are routinely collected and can be examined to conduct longitudinal and retrospective research [22, 23]. This data resource may be searched using standardized terminology such as the International Classification of Diseases, 10th Edition (ICD‐10) diagnosis codes, which are converted automatically to 9th Edition whenever necessary [22, 23]. Data span demographics, diagnoses, procedures, laboratory values, and medications [23]. TriNetX monitors standard levels of data quality with respect to conformance, consistency, and completeness [22, 23] and the data have demonstrable medication completeness meeting at least 87% [24]. Data are deidentified via contractual and technical safeguards [22, 23].
TriNetX complies with the Health Insurance Portability and Accountability Act (HIPAA), the US federal law that protects healthcare data privacy and security. TriNetX is certified to the International Organization for Standardization 27001:2013 standard and maintains an Information Security Management System to ensure the protection of the healthcare data it has access to and to meet the requirements of the HIPAA Security Rule. Data are deidentified per the de‐identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process of de‐identifying data is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. The TriNetX network contains data provided by participating healthcare organizations, each of which represents and warrants that it has all necessary rights, consents, approvals, and authority to provide the data to TriNetX under a Business Associate Agreement, so long as their name remains anonymous as and their data are utilized for research purposes. The data are attenuated to ensure that they do not include sufficient information to identify the healthcare organization that contributed specific patient information.
We used the natural language processing software available within TriNetX (Averbis, Freiburg im Breisgau, DE) [23], which uses algorithms to extract information from unstructured clinical data such as chart notes and test results [25, 26]. This software has demonstrated adequate reliability and accuracy compared to manual chart review, with a Kappa value of 0.79 (good) [26, 27]. Our use of natural language processing aimed to enhance the application of selection criteria and identification of propensity‐matched variables.
2.2. Participants
We included adults aged at least 18 years with a diagnosis of TTH (ICD‐10: G44.2). To standardize and improve data completeness and healthcare utilization between cohorts, we required a healthcare visit within 1 month and 2 years preceding, and within 1 day and 1 year following the index date of inclusion.
We divided patients into two cohorts beginning at an index date defined as (1) SMT; the first co‐occurrence of any of the Current Procedural Terminology (CPT) codes for chiropractic SMT (98940, 98941, 98942) and TTH diagnosis code, and (2) non‐SMT; the first co‐occurrence of an ambulatory office evaluation (CPT: 1013626) with a TTH diagnosis code.
We excluded patients at risk for serious secondary or other primary headache types [28]: those with brain tumors, cerebral infarction, complicated headache syndromes, MOH, migraine, posttraumatic headache or intracranial injury, temporal arteritis, transient ischemic attack, trigeminal autonomic cephalgia, trigeminal neuralgia, and vascular headache. To more thoroughly exclude migraineurs, we also excluded those prescribed antimigraine medications. To help exclude individuals with serious secondary headache etiologies, we excluded patients in emergency, inpatient, or critical care settings on the index date. Exclusions are summarized in Supporting Information S1: Table 1.
Table 1.
Baseline characteristics.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Variable (n (%) or mean (SD)) | SMT | Non‐SMT | SMD | SMT | Non‐SMT | SMD |
| N | 3118 | 141,039 | NA | 3116 | 3116 | NA |
| Demographics | ||||||
| Age at Index | 47.8 (16.9) | 47.6 (16.2) | 0.013 | 47.9 (16.9) | 47.8 (16.4) | 0.004 |
| Female | 2375 (76%) | 101207 (72%) | 0.101 | 2373 (76%) | 2385 (77%) | 0.009 |
| Male | 743 (24%) | 33568 (24%) | 0.001 | 743 (24%) | 729 (23%) | 0.011 |
| Hispanic or Latino | 65 (2%) | 18609 (13%) | 0.428 | 65 (2%) | 67 (2%) | 0.004 |
| Not Hispanic or Latino | 2708 (87%) | 93309 (66%) | 0.503 | 2706 (87%) | 2712 (87%) | 0.006 |
| American Indian or Alaska Native | 16 (1%) | 406 (< 1%) | 0.036 | 16 (1%) | 12 (< 1%) | 0.019 |
| Asian | 26 (1%) | 5795 (4%) | 0.212 | 26 (1%) | 27 (1%) | 0.003 |
| Black or African American | 125 (4%) | 25736 (18%) | 0.465 | 125 (4%) | 119 (4%) | 0.010 |
| Native Hawaiian or Other Pacific Islander | 10 (< 1%) | 882 (1%) | 0.044 | 10 (< 1%) | 10 (< 1%) | 0.000 |
| White | 2548 (82%) | 83227 (59%) | 0.513 | 2546 (82%) | 2525 (81%) | 0.017 |
| Other Race | 37 (1%) | 6272 (4%) | 0.198 | 37 (1%) | 48 (2%) | 0.030 |
| Diagnoses | ||||||
| Adverse socioeconomic circumstances | 62 (2%) | 6997 (5%) | 0.163 | 62 (2%) | 65 (2%) | 0.007 |
| Anxiety‐related disorders | 982 (31%) | 43225 (31%) | 0.018 | 981 (31%) | 988 (32%) | 0.005 |
| Cervicalgia | 1580 (51%) | 21068 (15%) | 0.823 | 1578 (51%) | 1609 (52%) | 0.020 |
| Chronic pain, not elsewhere classified | 615 (20%) | 21101 (15%) | 0.126 | 615 (20%) | 629 (20%) | 0.011 |
| Diseases of the digestive system | 1237 (40%) | 57994 (41%) | 0.029 | 1237 (40%) | 1231 (40%) | 0.004 |
| Mood disorders | 750 (24%) | 34154 (24%) | 0.004 | 749 (24%) | 724 (23%) | 0.019 |
| Nicotine dependence | 181 (6%) | 13978 (10%) | 0.153 | 181 (6%) | 179 (6%) | 0.003 |
| Overweight and obesity | 526 (17%) | 25437 (18%) | 0.031 | 526 (17%) | 501 (16%) | 0.022 |
| Psychoactive substance use | 265 (8%) | 19634 (14%) | 0.172 | 265 (9%) | 261 (8%) | 0.005 |
| Pregnancy, childbirth, and the puerperium | 215 (7%) | 8765 (6%) | 0.028 | 214 (7%) | 216 (7%) | 0.003 |
| Sleep disorders | 604 (19%) | 24306 (17%) | 0.055 | 604 (19%) | 588 (19%) | 0.013 |
| Tobacco use | 37 (1%) | 7922 (6%) | 0.246 | 37 (1%) | 41 (1%) | 0.012 |
| Prior treatments | ||||||
| Medication(s) (any) | 2881 (92%) | 129229 (92%) | 0.028 | 2879 (92%) | 2846 (91%) | 0.039 |
| Acetaminophen | 992 (32%) | 49214 (35%) | 0.065 | 991 (32%) | 937 (30%) | 0.037 |
| Antidepressants | 1218 (39%) | 44168 (31%) | 0.163 | 1216 (39%) | 1166 (37%) | 0.033 |
| Aspirin | 455 (15%) | 15822 (11%) | 0.101 | 453 (15%) | 435 (14%) | 0.017 |
| Benzodiazepines | 638 (20%) | 32124 (23%) | 0.056 | 638 (20%) | 652 (21%) | 0.011 |
| Butalbital | 45 (1%) | 11090 (8%) | 0.308 | 45 (1%) | 58 (2%) | 0.033 |
| Caffeine | 100 (3%) | 14042 (10%) | 0.275 | 100 (3%) | 115 (4%) | 0.026 |
| Ibuprofen | 672 (22%) | 27463 (19%) | 0.052 | 670 (22%) | 648 (21%) | 0.017 |
| Opioid analgesics | 902 (29%) | 48602 (34%) | 0.119 | 902 (29%) | 871 (28%) | 0.022 |
| Sedatives/hypnotics | 230 (7%) | 9026 (6%) | 0.039 | 230 (7%) | 233 (7%) | 0.004 |
| Skeletal muscle relaxants | 698 (22%) | 32402 (23%) | 0.014 | 698 (22%) | 672 (22%) | 0.020 |
| Chiropractic care* (spinal or extraspinal manipulation) | 2668 (86%) | 93 (0%) | 3.432 | 2666 (86%) | 57 (2%) | 3.148 |
Note: Reported for descriptive purposes only and not matched (*). Counts of 10 should be interpreted with caution as TriNetX rounds count 1–9 up to 10 for de‐identification purposes.
Abbreviation: SMD, Standardized mean difference.
2.3. Variables
We used propensity matching to reduce bias [29], balancing key variables present within 1 year preceding the index date (Supporting Information S1: Table 2). We matched medications that are often used for TTH which may influence MOH likelihood: acetaminophen, antidepressants, aspirin, butalbital, caffeine, ibuprofen, and skeletal muscle relaxants [8, 17, 30]. We matched additional variables associated with MOH including age, sex, chronic pain, anxiety, depression, eating disorders, obesity, gastrointestinal disorders, neck pain, sedatives, sleep disorders, substance use disorders, a measure of adverse socioeconomic and psychosocial circumstances, and tobacco use [17, 31, 32, 33]. We matched pregnancy, which would reduce the likelihood of butalbital prescription [34]. We also matched race and ethnicity, which may generally influence prescribing behaviors for headaches [35]. Finally, we matched any prescription medication, to control for potential differences in pharmacological care preferences between cohorts [36].
Table 2.
Primary outcome of butalbital prescription.
| Before matching | After matching | |||
|---|---|---|---|---|
| SMT | Non‐SMT | SMT | Non‐SMT | |
| Number of patients | 3118 | 141,039 | 3116 | 3116 |
| Butalbital, n | 54 | 8,232 | 54 | 118 |
| Butalbital, % (95% CI) | 1.7 (1.3–2.2) | 5.8 (5.7–6.0) | 1.7 (1.3–2.2) | 3.8 (3.1–4.5) |
| RR (95% CI) | 0.30 (0.23–0.39; p < 0.001) | (Reference) | 0.46 (0.33–0.63; p < 0.001) | (Reference) |
Abbreviations: 95% CI, 95% confidence intervals; RR, risk ratio; SMT, spinal manipulative therapy.
For our primary outcome, we identified butalbital prescriptions (RxNorm: 19860), rather than the broader category of barbiturates. Barbiturates may be used to treat other conditions such as seizures or for anesthesia, whereas butalbital is primarily used for headaches [34].
We used an outcome assessment window of 2 years to give greater insights into the long‐term management of TTH and the slow development of MOH (ICD‐10: G44.4) [31]. The outcome assessment window began the day following the index date, to further exclude patients already diagnosed with MOH, and because our focus is on long‐term, longitudinal outcomes rather than immediate care.
2.4. Required Study Size
We calculated a total required sample size of 5076 using data from a prior study regarding MOH [17]. Considering this outcome is less common than butalbital prescription, it allowed us to adequately power the study for both our primary and secondary outcomes. We used G*Power (Kiel University, DE), and Z‐tests to determine a difference between two independent proportions (0.015 vs. 0.030), two‐tailed α error of 0.05, a power of 0.95, and an allocation ratio of 1.
2.5. Statistical Methods
We used built‐in features of the TriNetX software for statistical analysis. Propensity scores were derived using logistic regression to estimate the log odds of non‐SMT cohort assignment, ranging from 0 (lowest likelihood) to 1 (highest likelihood). Matching will implement a greedy nearest‐neighbor algorithm, a 1:1 matching ratio, using a calliper of 0.01 pooled standard deviations. Baseline characteristics will be compared using Pearson χ2 and independent‐sample t‐tests. Standardized mean difference (SMD) was used to assess between‐cohort balance, with a threshold of < 0.1 [37]. We did not make imputations for missing data. We used R (version 4.2.2, Vienna, AT) [38]. To calculate 95% confidence intervals (CIs) and the ggplot2 package [39] to plot propensity score density and cumulative incidence of butalbital prescription as a sensitivity analysis. The risk ratios (RR) for butalbital prescription were calculated by dividing the incidence proportion in the SMT cohort by the non‐SMT cohort. As a secondary outcome, we calculated the RR for MOH. We calculated p‐values for RRs using the chi‐square test and evaluated significance using a two‐tailed alpha level of p ≤ 0.05.
For additional secondary outcomes, we assessed the adequacy of propensity matching by calculating RRs for negative control outcomes that we expected to remain uninfluenced by SMT [40], including antibiotics and proton pump inhibitors. We aimed for point estimates for each outcome suggestive of between cohort balance (i.e., 0.73 ≥ RR ≤ 1.38) [41]. We also examined the median, mean, and standard deviation of follow‐up SMT visits.
3. Results
3.1. Participants
Before propensity matching, there were 3118 patients in the SMT cohort and 141,039 in the non‐SMT cohort. After matching, 3116 patients remained per cohort. Before matching, patients in the SMT cohort were more often female and White, less often Hispanic or Latino, Black or African American or other race and a greater proportion of patients were diagnosed with cervicalgia and chronic pain (SMD > 0.1; Table 1). Prior prescriptions also varied, with the SMT cohort having a greater proportion of those prescribed aspirin or antidepressants, yet a lower proportion of those prescribed butalbital or caffeine (SMD > 0.1). After matching, all key variables were ideally matched (SMD < 0.1). A greater proportion of patients in the SMT cohort had received chiropractic care for a non‐TTH disorder before the index date of inclusion, yet previous chiropractic care was not matched and only reported for descriptive purposes.
3.2. Descriptive Data
There was an adequate mean number of data points per patient per cohort (SMT: 4180; non‐SMT: 3629). After matching, the proportion of unknown demographic variables was similar between cohorts: unknown ethnicity (both cohorts: 11%; SMD = 0.008), unknown sex (SMT: 0%; non‐SMT: < 1%; SMD = 0.80), and unknown age (both cohorts: 0%, SMD = 0). Plotted post‐matching propensity score densities overlapped, suggesting that covariates were adequately balanced (Figure 1). Together, these findings suggest that there were minimal between‐cohort differences with respect to data density, completeness, and covariate balance.
Figure 1.
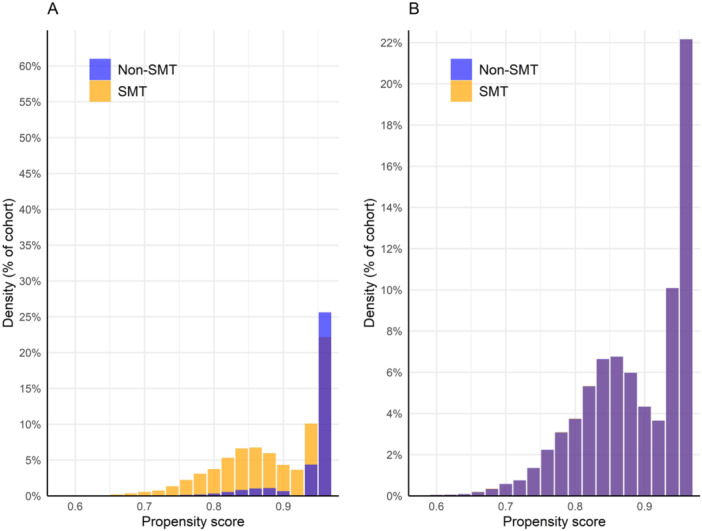
Propensity score density graph. Propensity scores before (A) and after (B) matching. The orange bars represent the spinal manipulative therapy (SMT) cohort while the blue bars represent the non‐SMT cohort. After matching, propensity score densities overlap closely, suggesting adequate covariate balance.
3.3. Primary Outcome
The incidence of butalbital prescription through 2 years' follow‐up from the index date of inclusion was lower in the SMT cohort compared to the non‐SMT cohort (Table 2). After propensity matching, 1.7% of the SMT cohort had received a butalbital prescription, compared to 3.8% of the non‐SMT cohort, translating to an RR (95% CI) of 0.46 (0.33–0.63; p < 0.001). A plot highlighted a curvilinear increase in cumulative incidence of butalbital prescription in the non‐SMT cohort compared to a more linear pattern in the non‐SMT cohort (Figure 2).
Figure 2.
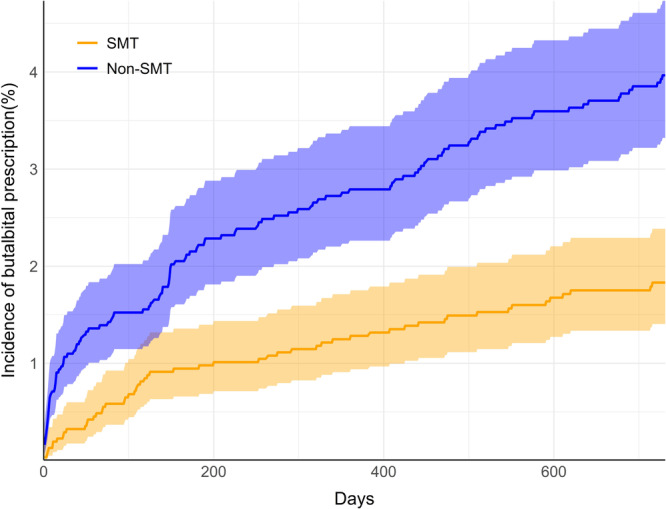
Cumulative incidence of butalbital prescription. Incidence curves in the spinal manipulative therapy cohort (SMT; orange) and non‐SMT (blue) are shown through two years' follow‐up (730 days). Shaded regions highlight 95% confidence intervals.
3.4. Secondary Outcomes
The incidence of MOH through 2 years' follow‐up from the index date of inclusion was lower in the SMT cohort compared to the non‐SMT cohort (Table 3). After propensity matching, 0.5% of the SMT cohort had been diagnosed with MOH, compared to 1.2% of the non‐SMT cohort, translating to an RR (95% CI) of 0.44 (0.25–0.80; p < 0.001). A plot highlighted an earlier increase in the cumulative incidence of MOH compared to the non‐SMT cohort (Figure 3).
Table 3.
Secondary outcome of medication overuse headache.
| Before matching | After matching | |||
|---|---|---|---|---|
| SMT | Non‐SMT | SMT | Non‐SMT | |
| Number of patients | 3118 | 141,039 | 3116 | 3116 |
| MOH, n | 16 | 1068 | 16 | 36 |
| MOH, % (95% CI) | 0.5 (0.3–0.8) | 0.8 (0.7–0.8) | 0.5 (0.3–0.8) | 1.2 (0.8–1.5) |
| RR (95% CI) | 0.68 (0.41–1.11; p < 0.1186) | (Reference) | 0.44 (0.25‐0.80; p < 0.001) | (Reference) |
Abbreviations: 95% CI, 95% confidence intervals; MOH, medication overuse headache; RR, risk ratio; SMT, spinal manipulative therapy.
Figure 3.
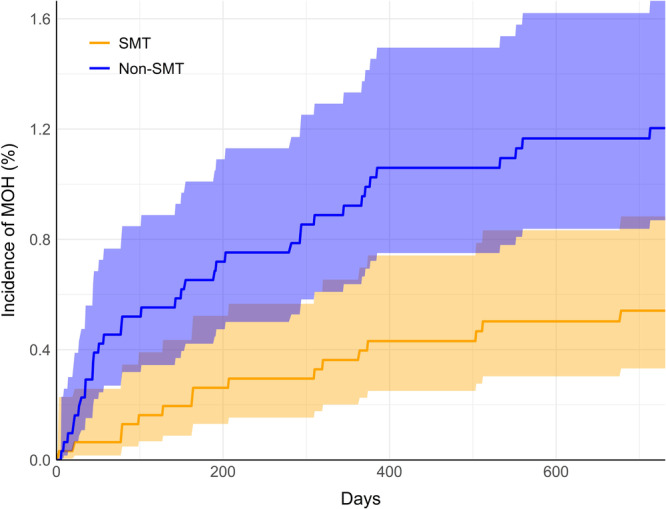
Cumulative incidence of medication overuse headache (MOH). Incidence curves in the spinal manipulative therapy cohort (SMT; orange) and non‐SMT (blue) are shown through two years' follow‐up (730 days). Shaded regions highlight 95% confidence intervals.
Follow‐up SMT visits were common in the SMT cohort, with 73% of patients having more than one follow‐up visit, at a mean of 15 visits [SD = 17] and median of 9.
Prescription of negative outcome control medications was similar when comparing the SMT to the non‐SMT cohort, with antibiotics prescribed in 25.1% versus 23.6% (RR = 1.07; 95% CI: 0.98,1.16; p = 0.1481) and proton pump inhibitors prescribed in 29.9% versus 28.8% (RR = 1.04; 95% CI: 0.96,1.12; p = 0.3302). These findings provide further evidence that cohorts were adequately balanced through propensity matching.
4. Discussion
The present study findings support our hypothesis that adults receiving initial SMT for TTH have a significantly reduced likelihood of butalbital prescription compared to matched controls not receiving SMT through 2 years' follow‐up, as well as a reduced likelihood of MOH. Analysis of cumulative incidence suggests that between‐cohort differences in outcomes of butalbital prescription and MOH may stem from the initial care‐receiving period and persist thereafter. These findings suggest there is potential usefulness of SMT in managing TTH extending beyond improvement in headache frequency or intensity, and reinforce guidelines recommending SMT for TTH patients [19, 42].
The present findings could be explained as a function of care pathways. Chiropractors are nonpharmacologic providers and do not prescribe butalbital, therefore they are not faced with pressure to prescribe medications for TTH. A previous scoping review found evidence to suggest that prioritizing a nonmedical provider at the first point of care for low back pain was associated with a reduction in opioid prescriptions [43]. It is plausible but unclear whether this mechanism would also apply to butalbital prescription and MOH in the context of TTH. Regardless, the present study findings are likely not explained by chiropractic patients' desire to avoid prescription medications. As we propensity matched on past medications, over 90% of patients in both cohorts were already prescribed medications at baseline.
The observed reduction in butalbital prescriptions may be explained by the clinical benefits of SMT for TTH. Evidence from recent systematic reviews suggests that SMT may reduce the pain intensity of TTH [14, 44, 45, 46]. Accordingly, this may reduce patients' likelihood of subsequent specialist visits or encounters where they may be prescribed butalbital. Secondarily, considering butalbital is a risk factor for development of MOH [17], any reduction in butalbital prescription could correspond with a reduction in MOH likelihood.
The present findings could have important implications. While both outcomes reflected a statistically significant decrease in RR with SMT, the absolute risk differences were relatively small. Despite being a smaller magnitude between cohorts, differences in the likelihood of MOH (0.7%) may be more clinically relevant than differences in butalbital prescription (2.1%). MOH is a chronic, debilitating headache that adversely affects quality of life, work, and income, and is more impactful than TTH itself [17]. MOH is difficult to treat and requires a multidisciplinary approach [17]. Accordingly, a small but significant decrease in the likelihood of MOH may be clinically relevant.
Future studies could build on the present findings by examining the likelihood of butalbital prescription across a range of clinician types, including chiropractors and other nonpharmacologic practitioners (e.g., acupuncturists, physical therapists) as well as prescribing clinicians (e.g., neurologists, primary care physicians). This would help determine if our observed association is explained by a general effect of nonpharmacologic care or is more related to the SMT intervention. Second, this study could be repeated while also considering the costs of care attributed to MOH. Considering the low incidence of MOH, a prospective randomized controlled trial may be challenging unless multicenter recruitment is possible.
4.1. Strengths and Limitations
The present study was strengthened by adherence to a registered protocol [20], by having a multidisciplinary team, use of a large national data set, and propensity matching strategy. Furthermore, there are several markers of validity of our study, such as the demographics including a majority of females, among whom TTH is most prevalent, a moderately high comorbid neck pain prevalence, and past use of several medications used to treat TTH among both cohorts [3]. While specific estimates within TTH populations are limited, our incidence of butalbital prescription and MOH appear in agreement with the reported literature [4, 5, 6, 31].
However, several limitations should be noted. As an observational study, we cannot conclude that SMT was causative of reductions in butalbital prescription or MOH. There may have been unmeasured confounding related to the severity of the TTH, headache days per month, socioeconomic markers, clinician type initially seen (i.e., neurologist, primary care, pediatrician, pain management), over‐the‐counter medications not documented in the medical record, drug interactions, off‐label prescription of butalbital, and lifestyle factors, including stress, physical activity levels, and sleep quality [5, 17, 31, 32]. In general, patient characteristics that may influence health opportunities and outcomes such as occupation, education, and religion are poorly represented in the data set. SMT is a broad term referring to the use of manual therapies directed to the joints of the spine [47, 48, 49, 50]. Considering our study focused solely on chiropractic SMT, our findings may not be generalizable to all SMT techniques, which may differ in real‐world administration, effectiveness, safety, and utilization for TTH from SMT used by chiropractors in the US [47, 48]. Patients' clinical chart information could be incorrect, leading to documentation bias with selection criteria and propensity matching. Examination of the likelihood of specialty‐specific encounters over follow‐up was not possible given the constraints of the data set.
We were unable to validate the query given our use of deidentified multicenter data. Study findings may only generalize to academic settings in the United States, considering the use of SMT and/or butalbital may vary in other countries.
5. Conclusion
Our findings reveal a significant reduction in likelihood of both butalbital prescription and, tentatively, MOH, through 2‐years' follow‐up among adults with TTH receiving SMT compared to matched controls. These findings reinforce clinical practice guidelines already recommending SMT for TTH. However, additional research is needed to corroborate our results and examine the association between a broader variety of nonpharmacologic interventions and butalbital prescription and MOH.
Author Contributions
Robert J. Trager: conceptualization, methodology, software, formal analysis, investigation, resources, data curation, writing–original draft, writing–review and editing, visualization, supervision, project administration, funding acquisition. Timothy J. Williamson: conceptualization, methodology, investigation, writing–review and editing. Pratheek S. Makineni: conceptualization, methodology, investigation, writing–review and editing. Lindsay H. Morris: conceptualization, methodology, investigation, writing–review and editing.
Disclosure
The lead author Robert J. Trager affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Ethics Statement
This study used de‐identified, deidentified, anonymized data from TriNetX (TriNetX Inc., Cambridge, MA, US) obtained via the University Hospitals Clinical Research Center Honest Broker. The University Hospitals Institutional Review Board (Cleveland, OH, US) considered the present study “not human subjects research,” therefore not requiring review board approval or patient consent (IRB number: STUDY20241256).
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting information.
Acknowledgements
This publication was made possible through the support of the Clinical Research Center of University Hospitals Cleveland Medical Center (UHCMC) and the Case Western Reserve University Clinical and Translational Science Collaborative (CTSC) 4UL1TR000439. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of UHCMC or National Institutes of Health. The work conducted by RT received support from the Elisabeth Severance Prentiss Foundation (Cleveland, OH) through general funding. This project is also supported by the Clinical and Translational Science Collaborative of Northern Ohio, which is funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Science Award grant, UM1TR004528. The study did not receive a specific grant and the funders were not involved in developing the study protocol or any key decisions.
Data Availability Statement
The minimal, de‐identified, deidentified, aggregated data for baseline characteristics and plots of propensity score density and cumulative incidence of butalbital prescription and MOH are available in Figshare (https://doi.org/10.6084/m9.figshare.25757271) [51].
References
- 1. Stovner L. J., Hagen K., Linde M., and Steiner T. J., “The Global Prevalence of Headache: An Update, With Analysis of the Influences of Methodological Factors on Prevalence Estimates,” Journal of Headache and Pain 23 (2022): 34, 10.1186/s10194-022-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steiner T. J. and Martelletti P., “Aids for Management of Common Headache Disorders in Primary Care,” Journal of Headache and Pain 8, no. Suppl 1 (2007): S2. [PubMed] [Google Scholar]
- 3. Ashina S., Mitsikostas D. D., Lee M. J., et al., “Tension‐Type Headache,” Nature Reviews Disease Primers 7 (2021): 24. [DOI] [PubMed] [Google Scholar]
- 4. Mafi J. N., Edwards S. T., Pedersen N. P., Davis R. B., McCarthy E. P., and Landon B. E., “Trends in the Ambulatory Management of Headache: Analysis of NAMCS and NHAMCS Data 1999–2010,” Journal of General Internal Medicine 30 (2015): 548–555, 10.1007/s11606-014-3107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minen M. T., Lindberg K., Wells R. E., et al., “Survey of Opioid and Barbiturate Prescriptions in Patients Attending a Tertiary Care Headache Center,” Headache: Journal of Head and Face Pain 55 (2015): 1183–1191, 10.1111/head.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pershing M., Hirekhan O., Syed A., Elliott J. O., and Toot J., “Documentation of International Classification of Headache Disorders Criteria in Patient Medical Records: A Retrospective Cohort Analysis,” Cureus 16, no. 1 (2024): e52209, 10.7759/cureus.52209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bendtsen L., Evers S., Linde M., Mitsikostas D. D., Sandrini G., and Schoenen J., “EFNS Guideline on the Treatment of Tension‐Type Headache – Report of an EFNS Task Force,” European Journal of Neurology 17 (2010): 1318–1325, 10.1111/j.1468-1331.2010.03070.x. [DOI] [PubMed] [Google Scholar]
- 8. Steiner T. J., Jensen R., Katsarava Z., et al., “Aids to Management of Headache Disorders in Primary Care (2nd Edition): On Behalf of The European Headache Federation and Lifting the Burden: The Global Campaign Against Headache,” Journal of Headache and Pain 20 (2019): 57, 10.1186/s10194-018-0899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loder E., Weizenbaum E., Frishberg B., and Silberstein S., “Choosing Wisely in Headache Medicine: The American Headache Society's List of Five Things Physicians and Patients Should Question,” Headache: Journal of Head and Face Pain 53 (2013): 1651–1659, 10.1111/head.12233. [DOI] [PubMed] [Google Scholar]
- 10. Moore C., Leaver A., Sibbritt D., and Adams J., “Prevalence and Factors Associated With the Use of Primary Headache Diagnostic Criteria by Chiropractors,” Chiropractic & Manual Therapies 27 (2019): 33, 10.1186/s12998-019-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beliveau P. J. H., Wong J. J., Sutton D. A., et al., “The Chiropractic Profession: A Scoping Review of Utilization Rates, Reasons for Seeking Care, Patient Profiles, and Care Provided,” Chiropractic & Manual Therapies 25 (2017): 35, 10.1186/s12998-017-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y., Dennis J. A., Leach M. J., et al., “Complementary and Alternative Medicine Use Among Us Adults With Headache or Migraine: Results From the 2012 National Health Interview Survey,” Headache: Journal of Head and Face Pain 57 (2017): 1228–1242, 10.1111/head.13148. [DOI] [PubMed] [Google Scholar]
- 13. Demont A., Lafrance S., Gaska C., Kechichian A., Bourmaud A., and Desmeules F., “Efficacy of Physiotherapy Interventions for the Management of Adults With Cervicogenic Headache: A Systematic Review and Meta‐Analyses,” PM & R: Journal of Injury, Function, and Rehabilitation 15 (2023): 613–628, 10.1002/pmrj.12856. [DOI] [PubMed] [Google Scholar]
- 14. Repiso‐Guardeño A., Moreno‐Morales N., Armenta‐Pendón M. A., Rodríguez‐Martínez M. C., Pino‐Lozano R., and Armenta‐Peinado J. A., “Physical Therapy in Tension‐Type Headache: A Systematic Review of Randomized Controlled Trials,” International Journal of Environmental Research and Public Health 20 (2023): 4466, 10.3390/ijerph20054466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore C., Leaver A., Sibbritt D., and Adams J., “The Features and Burden of Headaches Within a Chiropractic Clinical Population: A Cross‐Sectional Analysis,” Complementary Therapies in Medicine 48 (2020): 102276, 10.1016/j.ctim.2019.102276. [DOI] [PubMed] [Google Scholar]
- 16. Becker W. J., Findlay T., Moga C., Scott N. A., Harstall C., and Taenzer P., “Guideline for Primary Care Management of Headache in Adults,” Canadian Family Physician Medecin de famille canadien 61 (2015): 670–679. [PMC free article] [PubMed] [Google Scholar]
- 17. Ljubisavljevic S., Ljubisavljevic M., Damjanovic R., and Kalinic S., “A Descriptive Review of Medication‐Overuse Headache: From Pathophysiology to the Comorbidities,” Brain Sciences 13 (2023): 1408, 10.3390/brainsci13101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tfelt‐Hansen P. C. and Diener H.‐C., “Why Should American Headache and Migraine Patients Still be Treated With Butalbital‐Containing Medicine?,” Headache: The Journal of Head and Face Pain 52 (2012): 672–674, 10.1111/j.1526-4610.2012.02115.x. [DOI] [PubMed] [Google Scholar]
- 19. Dowell D., Ragan K. R., Jones C. M., Baldwin G. T., and Chou R., “CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022,” MMWR: Recommendations and Reports 71 (2022): 1–95, 10.15585/mmwr.rr7103a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trager R. J., Williamson T. J., Makineni P. S., and Morris L. H., “Association Between Chiropractic Spinal Manipulation, Butalbital Prescription, and Medication Overuse Headache in Adults With Tension‐Type Headache: Protocol for a Retrospective Cohort Study,” Published Online First: 1 April 2024, 10.17605/OSF.IO/7UZ68. [DOI] [Google Scholar]
- 21. Von Elm E., Altman D. G., Egger M., Pocock S. J., Gøtzsche P. C., and Vandenbroucke J. P., “The Strengthening the Reporting of Observational Studies in Epidemiology (Strobe) Statement: Guidelines for Reporting Observational Studies,” Annals of Internal Medicine 147 (2007): 573–577, 10.2471/blt.07.045120. [DOI] [PubMed] [Google Scholar]
- 22. Topaloglu U. and Palchuk M. B., “Using a Federated Network of Real‐World Data to Optimize Clinical Trials Operations,” JCO Clinical Cancer Informatics 2 (2018): 1–10, 10.1200/CCI.17.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palchuk M. B., London J. W., Perez‐Rey D., et al., “A Global Federated Real‐World Data and Analytics Platform for Research,” JAMIA Open 6 (2023): ooad035, 10.1093/jamiaopen/ooad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evans L., London J. W., and Palchuk M. B., “Assessing Real‐World Medication Data Completeness,” Journal of Biomedical Informatics 119 (2021): 103847, 10.1016/j.jbi.2021.103847. [DOI] [PubMed] [Google Scholar]
- 25. Daumke P., Heitmann K. U., Heckmann S., Martínez‐Costa C., and Schulz S., “Clinical Text Mining on FHIR,” Studies in Health Technology and Informatics 264 (2019): 83–87, 10.3233/SHTI190188. [DOI] [PubMed] [Google Scholar]
- 26. Pokora R. M., Le Cornet L., Daumke P., Mildenberger P., Zeeb H., and Blettner M., “Validation of Semantic Analyses of Unstructured Medical Data for Research Purposes,” Das Gesundheitswesen 82 (2020): S158–S164, 10.1055/a-1007-8540. [DOI] [PubMed] [Google Scholar]
- 27. Legnar M., Daumke P., Hesser J., et al., “Natural Language Processing in Diagnostic Texts From Nephropathology,” Diagnostics 12 (2022): 1726, 10.3390/diagnostics12071726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Do T. P., Remmers A., Schytz H. W., et al., “Red and Orange Flags for Secondary Headaches in Clinical Practice: SNNOOP10 List,” Neurology 92 (2019): 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gokhale M., Stürmer T., and Buse J. B., “Real‐World Evidence: The Devil Is in the Detail,” Diabetologia 63 (2020): 1694–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scher A., Lipton R., Stewart W., and Bigal M., “Patterns of Medication Use by Chronic and Episodic Headache Sufferers in the General Population: Results From the Frequent Headache Epidemiology Study,” Cephalalgia 30 (2010): 321–328, 10.1111/j.1468-2982.2009.01913.x. [DOI] [PubMed] [Google Scholar]
- 31. Hagen K., Linde M., Steiner T. J., Stovner L. J., and Zwart J.‐A., “Risk Factors for Medication‐Overuse Headache: An 11‐year Follow‐Up Study. The Nord‐Trøndelag Health Studies,” Pain 153 (2012): 56–61, 10.1016/j.pain.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 32. Westergaard M. L., Glümer C., Hansen E. H., and Jensen R. H., “Medication Overuse, Healthy Lifestyle Behaviour and Stress in Chronic Headache: Results From a Population‐Based Representative Survey,” Cephalalgia 36 (2016): 15–28, 10.1177/0333102415578430. [DOI] [PubMed] [Google Scholar]
- 33. Da Silva A. N. and Lake A. E., “Clinical Aspects of Medication Overuse Headaches,” Headache: Journal of Head and Face Pain 54 (2014): 211–217, 10.1111/head.12223. [DOI] [PubMed] [Google Scholar]
- 34. Skibiski J. and Abdijadid S., Barbiturates (StatPearls. StatPearls Publishing: Treasure Island (FL), 2023). [PubMed] [Google Scholar]
- 35. Devine J. W., Farley J. F., and Hadsall R. S., “Patterns and Predictors of Prescription Medication Use in the Management of Headache: Findings From the 2000 Medical Expenditure Panel Survey,” Headache: Journal of Head and Face Pain 45 (2005): 1171–1180, 10.1111/j.1526-4610.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 36. Sharma R., Haas M., and Stano M., “Patient Attitudes, Insurance, and Other Determinants of Self‐Referral to Medical and Chiropractic Physicians,” American Journal of Public Health 93 (2003): 2111–2117, 10.2105/ajph.93.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen J. W., Maldonado D. R., Kowalski B. L., et al., “Best Practice Guidelines for Propensity Score Methods in Medical Research: Consideration on Theory, Implementation, and Reporting. A Review,” Arthroscopy: Journal of Arthroscopic & Related Surgery 38 (2022): 632–642, 10.1016/j.arthro.2021.06.037. [DOI] [PubMed] [Google Scholar]
- 38. R Core Team , R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing, 2022). [Google Scholar]
- 39. Wickham H., ggplot2: Elegant Graphics for Data Analysis (Springer‐Verlag New York, 2016). [Google Scholar]
- 40. Lipsitch M., Tchetgen Tchetgen E., and Cohen T., “Negative Controls: A Tool for Detecting Confounding and Bias in Observational Studies,” Epidemiology 21 (2010): 383–388, 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nordahl‐Hansen A., Øien R. A., Volkmar F., Shic F., and Cicchetti D. V., “Enhancing the Understanding of Clinically Meaningful Results: A Clinical Research Perspective,” Psychiatry Research 270 (2018): 801–806, 10.1016/j.psychres.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 42. Hawk C., Whalen W., Farabaugh R. J., et al., “Best Practices for Chiropractic Management of Patients With Chronic Musculoskeletal Pain: A Clinical Practice Guideline,” The Journal of Alternative and Complementary Medicine 26 (2020): 884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zouch J., Comachio J., Bussières A., et al., “Influence of Initial Health Care Provider on Subsequent Health Care Utilization for Patients With a New Onset of Low Back Pain: A Scoping Review,” Physical Therapy 102 (2022): pzac150, 10.1093/ptj/pzac150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coelho M., Ela N., Garvin A., et al., “The Effectiveness of Manipulation and Mobilization on Pain and Disability in Individuals With Cervicogenic and Tension‐Type Headaches: A Systematic Review and Meta‐Analysis,” Physical Therapy Reviews 24 (2019): 29–43, 10.1080/10833196.2019.1572963. [DOI] [Google Scholar]
- 45. Jung A., Eschke R.‐C., Struss J., Taucher W., and Luedtke K., “Effectiveness of Physiotherapy Interventions on Headache Intensity, Frequency, Duration and Quality of Life of Patients With Tension‐Type Headache: A Systematic Review and Network Meta‐Analysis,” Cephalalgia 42 (2022): 944–965. [DOI] [PubMed] [Google Scholar]
- 46. Cumplido‐Trasmonte C., Fernández‐González P., Alguacil‐Diego I. M., and Molina‐Rueda F., “Manual Therapy in Adults With Tension‐Type Headache: A Systematic Review,” Neurología (English Edition) 36, no. 7 (2021): 537–547. 10.1016/j.nrleng.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 47. Farì G., Mariconda C., Dell'Anna L., et al., “How to Evaluate the Efficacy of Manipulations in Spine Disorders—A Comprehensive Review of New and Traditional Outcome Measures,” Clinics and Practice 14 (2024): 1478–1495, 10.3390/clinpract14040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bernetti A., La Russa R., de Sire A., et al., “Cervical Spine Manipulations: Role of Diagnostic Procedures, Effectiveness, and Safety From a Rehabilitation and Forensic Medicine Perspective: A Systematic Review,” Diagnostics 12 (2022): 1056, 10.3390/diagnostics12051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Degenhardt B., van Dun P. L. S., Jacobson E., et al., “Profession‐Based Manual Therapy Nomenclature: Exploring History, Limitations, and Opportunities,” Journal of Manual & Manipulative Therapy 32 (2024): 96–110, 10.1080/10669817.2023.2288495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hurwitz E. L., “Epidemiology: Spinal Manipulation Utilization,” Journal of Electromyography and Kinesiology 22 (2012): 648–654, 10.1016/j.jelekin.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 51.[dataset] Trager R: Chiropractic, tension‐type headache, and medication overuse headache data. 2024, 10.6084/m9.figshare.25757271. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- [dataset] Trager R: Chiropractic, tension‐type headache, and medication overuse headache data. 2024, 10.6084/m9.figshare.25757271. [DOI]
Supplementary Materials
Supporting information.
Data Availability Statement
The minimal, de‐identified, deidentified, aggregated data for baseline characteristics and plots of propensity score density and cumulative incidence of butalbital prescription and MOH are available in Figshare (https://doi.org/10.6084/m9.figshare.25757271) [51].


