Coronary artery calcification is common in elderly, diabetic, and dialysis-dependent patients, poses a great challenge to interventional therapy and significantly increases the risk of complications and poor prognosis.[1] Traditional rotational atherectomy and excimer laser coronary atherectomy are both special treatment techniques for severely calcified lesions, but they are difficult to use in clinical practice due to complicated operations. Intravascular lithotripsy (IVL) is a new way of treating calcified lesion. The principle of IVL is similar to that of urinary lithotripsy. The pulse sound pressure waves selectively interact with the high-density calcium to generate significant shearing force to rupture the calcification ring on the intimal side of the blood vesselsd and can produce impact oscillation to fragmentation on deep calcifications.[2-4] This case demonstrated the effect and safety of IVL in the treatment of elderly people with severe coronary calcification inside and outside the stent. To some extent, the comparative effect of IVL and excimer laser coronary atherectomy was demonstrated.
An 84-year-old man with episodic chest pain for 25 years was admitted to our hospital with recurrence for 1 month. In 1997, the patient sought treatment in another hospital due to sudden chest pain. The patient was considered to have acute myocardial infarction. Emergency coronary angiography was performed, and the anterior descending artery was completely occluded. Three stents were implanted in the anterior descending artery. In 2005, this patient complained of chest pain after mobilization. Follow-up coronary angiography revealed in-stent restenosis of the anterior descending artery, and two stents were again implanted in the stent. Thereafter, the secondary prevention of coronary heart disease was regularly used. One month prior, the patient developed chest tightness and shortness of breath after walking 400–500 meters and was admitted to our hospital with “unstable angina pectoris”. The patient had a history of hypertension for over 40 years and took 5 mg amlodipine besylate tablets QD and 47.5 mg metoprolol succinate sustained-release tablets QD, for a long time, and his blood pressure was well controlled. In 2018, left common carotid artery stent implantation was performed. No history of smoking. The main laboratory examination results after admission were as follows: TnT-hs of 0.0202 ng/mL (< 0.014 ng/mL); TC of 5.86 mmol/L; LDL-C of 3.97 mmol/L; euthyroid function; NT-pro BNP of 505 pg/mL; HbA1c of 5.8%; and eGFR of 60.744 mL/min·per 1.73 m2. Cardiac ultrasound showed that the basal segment of the ventricular septum was thickened by approximately 12 mm, there was a small amount of mitral valve regurgitation, the global systolic function of the left ventricle was normal (ejection fraction: 64%), and no obvious abnormality was found in diastolic function. ECG after admission is presented in Figure 1.
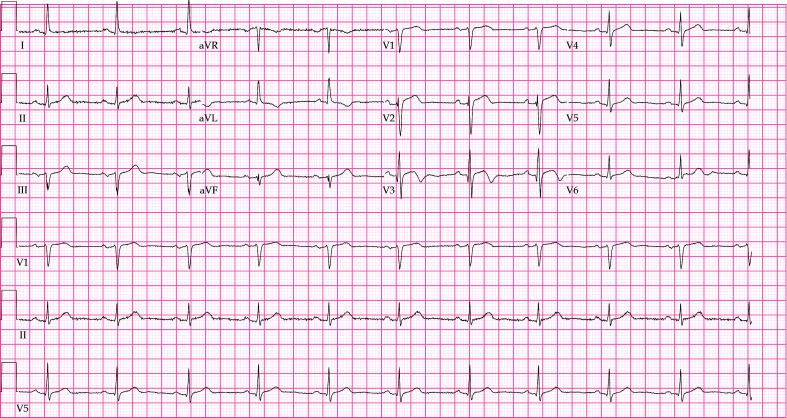
Figure 1.
ECG of the patient at admission: V2–V3 T waves were bidirectional or low-flat.
ECG: electrocardiogram.
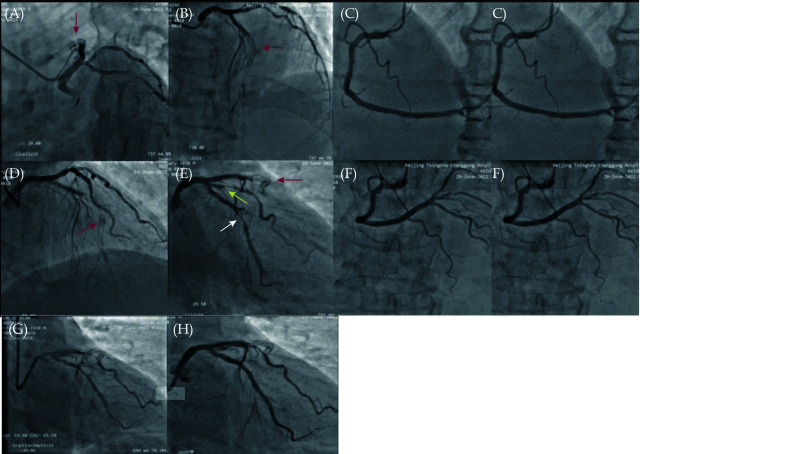
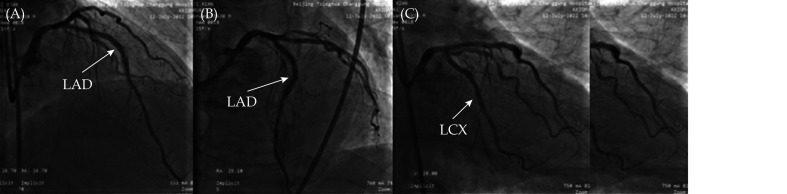
Coronary angiography in our hospital (Figure 2A-2F) showed complete occlusion of the anterior descending artery in the stent (red arrow), and the anterior blood flow was TIMI grade 0. The proximal end of the circumflex branch had 80%–90% stenosis, and the proximal end of the obtuse margin branch had 80% stenosis. The anterior blood flow was TIMI grade 3. No obvious stenosis was found in the right coronary artery. First, interventional treatment of the circumflex branch was performed, and a Resolute 2.5 × 26 mm stent was implanted, which was released at 12 atm. An NC TREK 2.75 × 12 mm balloon was postdilated at a pressure of 16 atm. Stent surgery is shown in Figure 2G & 2H.
Figure 2.
Coronary angiography of the anterior descending artery showed the stent shadow, the stent was completely occluded (A, B, D, E with red arrow), and the anterior blood flow was TIMI grade 0. The distal circumflex branch showed 80%–90% stenosis (E with white arrow), and the anterior blood flow was TIMI grade 3. The proximal end of the first obtuse margin showed 80% stenosis (E with yellow arrow). No significant stenosis was found in the right coronary artery (C & F). No obvious collateral circulation was found. Rotary branch stent surgery: stent positioning (G) and postoperative stent (H).
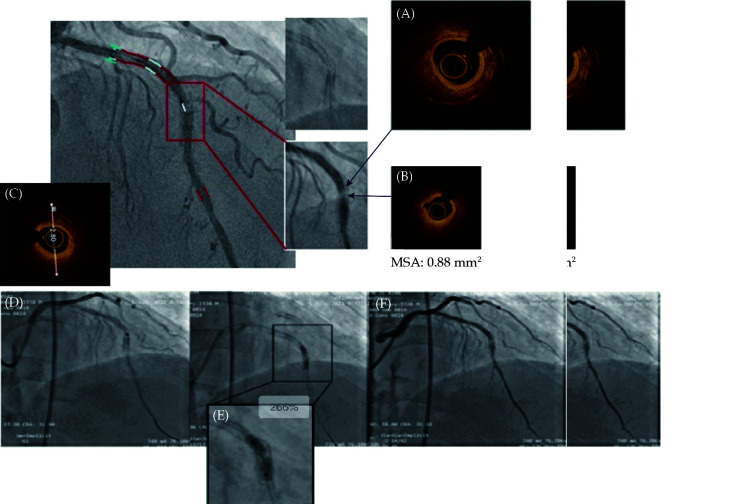
Then, interventional treatment of the LAD was performed. The Fielder XT guidewire could not pass through the lesion with the support of the Fincross microcatheter. The Gaia 2 guidewire was replaced, and the occluded segment could be passed through. Optical coherence tomography (OCT) examination was performed after predilation of the Sprinter 1.5 × 15 mm and Sprinter 2.0 × 20 mm balloons at 12 atm. Diffuse hyperplasia and local circular calcification were found in the stent. The MSA was 0.88 mm2. This was a double-layer metal stent. There was calcified plaque outside the stent (Figure 3A-3C). Excimer laser ablation (LAD) was performed. A 0.9 mm laser catheter was chosen. The energy density was 60 mJ/mm2, and the pulse frequency was 45 Hz. Contrast media were used as ablation media. A total of 6 ablation procedures were performed, but the laser catheter could not pass through the calcified lesion. A 1.4 mm laser catheter was replaced with an energy density of 45–60 mJ/mm2 and a pulse frequency of 45 Hz. The catheter could still not pass the lesion due to poor trackability. Then, a NC Sprinter 3.0 × 15 mm balloon was used for high-pressure dilation. The pressure was 26 atm. The balloon could still not be fully expanded, and the mid-stent showed severe residual stenosis (Figure 3D-3F). The operation was terminated.
Figure 3.
(A–C) OCT examination of the LAD after predilation: the MSA of the mid-section of the stent was 0.88 mm2. This is a double-layer metal stent, and the hyperplasia in the stent turned into ring calcification. The diameter of the inner stent was 2.8 mm. (D): excimer laser plaque ablation; the laser catheter could not be passed through the calcified lesion; (E): the 3.0 × 15 mm NC Sprinter balloon could not be fully inflated at 26 atm; and (F): the final angiographic result of excimer laser ablation, showing severe residual stenosis in the mid-segment of the stent.
OCT: optical coherence tomography.
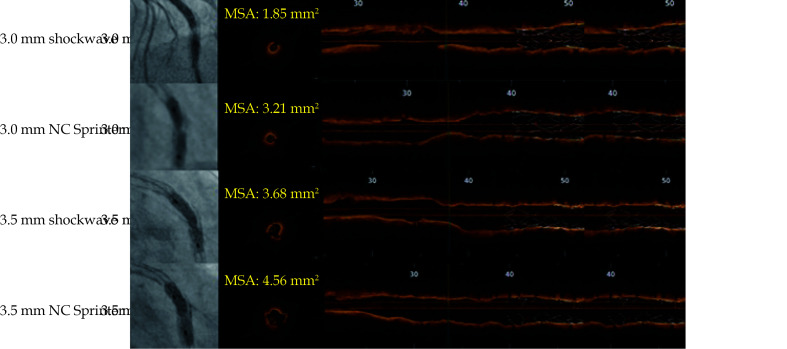
The patient was treated again after 7 days. The OCT showed the original stent diameter was 2.8 mm. So a 3.0 × 12 mm shockwave balloon was selected and successfully passed through the residual calcified LAD lesion. After dilation at 4 atm, 8 shockwave therapy sessions were performed, with each session maintained for 10 s. Then, the pressure was increased to 6 atm. The review OCT showed that the MSA was 1.85 mm2. The SMA was 3.21 mm2 after dilation of a 3.0 × 15 mm NC Sprinter balloon at 26 atm. The original stent diameter was observed to increase to 3.4 mm. Hence a 3.5 × 12 mm shockwave balloon was replaced, and another 8 cycles of shockwave therapy were performed and then with MSA of 3.68 mm2. The SMA was 4.56 mm2 after dilation of a 3.5 × 15 mm NC Sprinter balloon at 26 atm (Figure 4). The final result is shown in Figure 5A-5C. The stent was patent, the residual stenosis was < 20%, the anterior blood flow was TIMI grade 3, and the surgery was ended. He was given 100 mg aspirin QD, 75 mg clopidogrel bisulfate QD, dual antiplatelet therapy and secondary prevention of coronary heart disease. The patient’s symptoms were significantly relieved.
Figure 4.
IVL treatment process: MSA of 3.0 × 12 mm shockwave balloon treatment was 1.85 mm2, then dilated by 3.0 × 15 mm NC Sprinter balloon at 26 atm, MSA was 3.21 mm2, MSA of 3.5 × 12 mm shockwave balloon treatment was 3.68 mm2, and MSA was 4.56 mm2 after dilation of 3.5 × 15 mm NC Sprinter balloon at 26 atm.
IVL: intravascular lithotripsy.
Figure 5.
Final angiographic results.
(A): Right anterior oblique + head position; (B): head position directly; and (C): right anterior oblique + foot position. LAD: left anterior descending; LCX: left circumflex.
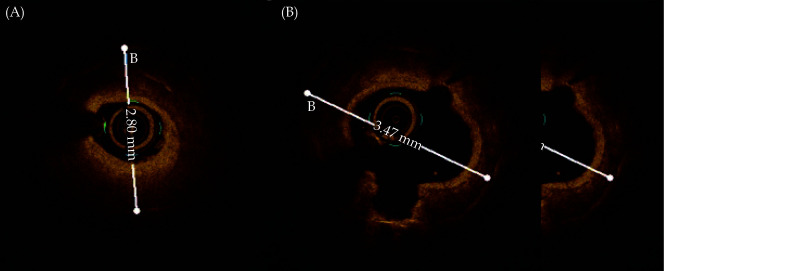
Severe calcified lesions pose enormous challenges to coronary intervention. These lesions obstruct the passage of the device, affect the selection of stent length, interfere with the expansion of the stent and the integrity of the lesion coverage, and the long-term prognosis is poor.[5,6] In this patient, the anterior descending artery was completely occluded during coronary angiography. OCT revealed that circular calcification localized in the mid-section of the stent. At the same time, we found that there were at least two to three layers of metal overlapping in the annular calcification in the middle section of the stent. There was also calcification outside the stent which led to poor expansion of original stent. And it was speculated that this was the site of the recurrent in-stent restenosis. Rotational atherectomy is considered an effective way to treat severely calcified lesions, but the ROTAXUS study (Rotational atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease) did not show clinical benefits brought by rotational atherectomy.[7] Because excimer laser plaque ablation can abate calcified lesions outside the stent, it can effectively correct the poor expansion of the stent caused by calcification outside the stent. Rotational atherectomy can address calcification within the stent, but cannot address calcification outside the stent. Therefore, we preferred excimer laser plaque ablation to address this lesion. However, the 0.9 mm and 1.4 mm laser catheters both used contrast agent as the ablation medium, and the energy density of 60 mJ/mm2 and the pulse frequency of 45 Hz could not effectively ablate the calcified lesions there. IVL is a conditioning technique for calcified lesion that is newly used in clinical practice. IVL is a type of balloon catheter that can emit pressure pulses in a circular manner and can produce impact oscillation to fragment on both superficial and deep calcifications.[4] In contrast to rotational atherectomy, IVL does not cause mechanical tissue damage to the lesions, nor does it reduce plaque volume. However, larger and thicker calcified plaques can be shaken to create cracks, and the plaques can be released by dilation of the balloon to a pressure of 4 to 6 atm. Several studies have evaluated the safety of IVL.[8] Disrupt CAD III[9] was a multicenter prospective single-arm study to evaluate the safety and efficacy of IVL in the treatment of coronary artery calcification with stenosis. The primary safety endpoint was the 30-day MACE-free rate. The primary efficacy endpoint was freedom from MACEs during hospitalization. A total of 431 patients from 4 countries were enrolled. The primary safety endpoint, the 30-day MACE-free rate, was 92.2%; the primary efficacy endpoint, the success rate of interventional surgery, was 92.4%; the mean calcification length was 18.8 mm, the calcification curvature was 76.5 degrees, and the calcification thickness was 0.25 mm. OCT showed that multiplane longitudinal calcification cracks could be seen in 67.4% of the lesions after IVL treatment. DISRUPT CAD[10] (Shockwave Coronary Rx Lithoplasty Study) is another multicenter prospective single-arm study to evaluate the conditioning effect of IVL in coronary calcified lesions before stent implantation. The primary safety endpoint was 30-day MACE. The primary study endpoint was clinical success (residual stenosis < 50% after stent implantation and no MACE during hospitalization). Thirty-day MACE events accounted for 5% and 95% of the patients who achieved clinical success. We applied IVL to this patient whose excimer laser treatment had failed. The diameter of the shock balloon was selected based on the OCT lumen diameter (1:1), as shown in Figure 6A & 6B. After two shock wave balloon treatments of 3.0 mm and 3.5 mm, multiple cracks appeared in the circular calcification, and the lumen gradually enlarged (Figure 7). In the end, the calcified lesion was successfully released, and the poorly expanded stent was modified, which may reduce the incidence of in-stent restenosis in the future. This patient demonstrated the safety of IVL in the interventional treatment of elderly patients with severe calcification in the stent. When the diameter of the lesion is suitable for the passage of a shock wave balloon, it can also be used as a preferred treatment, saving surgical time and cost.
Figure 6.
OCT before shockwave balloon treatment showed that the diameter of the inner stent was 2.8 mm (A) and the diameter of the inner stent after 3.0 mm shockwave and 3.0 mm NC Sprinter balloon dilation was 3.47 mm (B).
OCT: optical coherence tomography.
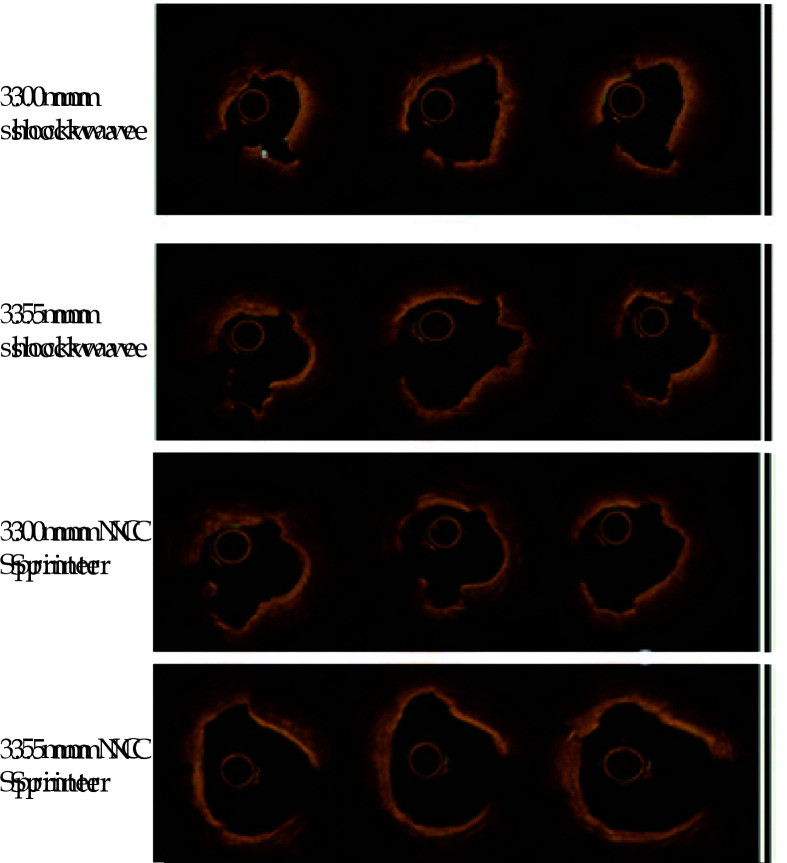
Figure 7.
OCT images showing progressive treatment with 3.0 mm and 3.5 mm shockwave balloons.
Compared with 3.0 mm shockwave therapy, after 3.5 mm shockwave therapy, the original cracks were widened, and new cracks were added. After two rounds of shockwave therapy, 3.0 mm and 3.5 mm NC Sprinter postdilation balloons were used for high-pressure postdilation, both of which could further expand the luminal area. OCT: optical coherence tomography.
ACKNOWLEDGMENTS
This work was supported by Fengtai District Commission of Health (Fengtai District Health System Science and Research Project NO.2023-81). All authors had no conflicts of interest to disclose.
References
- 1.Kassimis G, Didagelos M, De Maria GL, et al Shockwave intravascular lithotripsy for the treatment of severe vascular calcification. Angiology. 2020;71:677–688. doi: 10.1177/0003319720932455. [DOI] [PubMed] [Google Scholar]
- 2.Ozdemir D, Karimi Galougahi K, Petrossian G, et al Calcific plaque modification by acoustic shockwaves: intravascular lithotripsy in cardiovascular interventions. Curr Cardiol Rep. 2022;24:519–528. doi: 10.1007/s11886-022-01674-9. [DOI] [PubMed] [Google Scholar]
- 3.Kereiakes DJ, Virmani R, Hokama JY, et al Principles of intravascular lithotripsy for calcific plaque modification. JACC Cardiovasc Interv. 2021;14:1275–1292. doi: 10.1016/j.jcin.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 4.Barbato E, Gallinoro E, Abdel-Wahab M, et al Management strategies for heavily calcified coronary stenoses: an EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group. Eur Heart J. 2023;44:4340–4356. doi: 10.1093/eurheartj/ehad342. [DOI] [PubMed] [Google Scholar]
- 5.Bourantas CV, Zhang YJ, Garg S, et al Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158–1164. doi: 10.1136/heartjnl-2013-305180. [DOI] [PubMed] [Google Scholar]
- 6.Sotomi Y, Onuma Y, Dijkstra J, et al Impact of implantation technique and plaque morphology on strut embedment and scaffold expansion of polylactide bioresorbable scaffold: insights from ABSORB Japan trial. Circ J. 2016;80:2317–2326. doi: 10.1253/circj.CJ-16-0818. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Y, Tateishi H, Cavalcante R, et al Serial assessment of tissue precursors and progression of coronary calcification analyzed by fusion of IVUS and OCT: 5-year follow-up of scaffolded and nonscaffolded arteries. JACC Cardiovasc Imaging. 2017;10:1151–1161. doi: 10.1016/j.jcmg.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Tian F, Zhou SS, Liu J, et al Treatment of severely calcified coronary artery disease by intravascular lithotripsy primary outcomes and 180-day follow-up from the Chinese SOLSTICE Trial. J Geriatr Cardiol. 2023;20:32–39. doi: 10.26599/1671-5411.2023.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kereiakes DJ, Hill JM, Ben-Yehuda O, et al Evaluation of safety and efficacy of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: design and rationale for the Disrupt CAD III trial. Am Heart J. 2020;225:10–18. doi: 10.1016/j.ahj.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Kereiakes DJ, Hill JM, Shlofmitz RA, et al Intravascular lithotripsy for treatment of severely calcified coronary arteries: 2-year results-disrupt CAD III study. JACC Cardiovasc Interv. 2023;16:2472–2474. doi: 10.1016/j.jcin.2023.07.010. [DOI] [PubMed] [Google Scholar]