Abstract
We report the use of a yeast one-hybrid system to isolate a transcriptional regulator of the sea urchin embryo hatching enzyme gene, SpHE. This gene is asymmetrically expressed along the animal-vegetal axis of sea urchin embryos under the cell-autonomous control of maternal regulatory activities and therefore provides an excellent entry point for understanding the mechanism that establishes animal-vegetal developmental polarity. To search for transcriptional regulators, we used a fragment of the SpHE promoter containing several individual elements instead of the conventional bait that contains a multimerized cis element. This screen yielded a number of positive clones that encode a new member of the Ets family, named SpEts4. This protein contains transcriptional activation activity, since expression of reporter genes in yeast does not depend on the presence of the yeast GAL4 activation domain. Sequences in the N-terminal region of SpEts4 mediate the activation activity, as shown by deletion or domain-swapping experiments. The newly identified DNA binding protein binds with a high degree of specificity to a SpHE promoter Ets element and forms a complex with a mobility identical to that obtained with 9-h sea urchin embryo nuclear extracts. SpEts4 positively regulates SpHE transcription, since mutation of the SpEts4 site in SpHE promoter transgenes reduces promoter activity in vivo while SpEts4 mRNA coinjection increases its output. As expected for a positive SpHE transcriptional regulator, the timing of SpEts4 gene expression precedes the transient expression of SpHE in the very early sea urchin blastula.
The Strongylocentrotus purpuratus hatching enzyme gene, SpHE, is transcribed transiently only in nonvegetal blastomeres during the cleavage and very early blastula stages of sea urchin development (20). The activation of SpHE is early and cell autonomous and therefore is very likely to be regulated by localized maternal transcription-regulatory activities partitioned asymmetrically along the animal-vegetal (AV) axis. Consequently, the SpHE gene represents an excellent entry point for determining how the AV axis is established, through the identification of trans-acting factors that regulate its transcription.
Previously, we reported that a relatively compact region of the SpHE promoter, consisting of 300 bp upstream of the transcription initiation site, is sufficient to sponsor both high-level transcriptional activity and correct nonvegetal spatial expression (26, 27). Although this regulatory region is small, nine cis-acting elements have been defined within it, which can form complexes with at least six different proteins, as shown by in vitro DNase I footprinting and electrophoretic mobility shift assays (EMSAs) (26). Most of these cis elements were found to be occupied when the gene is active in vivo but to be unoccupied and in a nucleosome-like configuration when the gene is inactive (28). Extensive mutational dissection of the SpHE regulatory region supports a model in which all of the DNA binding proteins confer positive function in nonvegetal blastomeres (26, 27). This suggests that an important mechanism establishing AV polarity of developmental potential in the early sea urchin embryo is partitioning of positive maternal regulatory activities to nonvegetal blastomeres.
To test this model, we have begun to investigate the SpHE transcriptional regulators. Here we report the cloning of an S. purpuratus egg cDNA encoding a member of the Ets family, using a nontraditional yeast one-hybrid genetic screening approach in which a large portion of the SpHE promoter sequence instead of individual multimerized cis elements was used as bait. Using this method, we isolated 13 strong positive clones containing overlapping segments of the same sequence, which encodes a member of the Ets transcription factor family. Because this protein contains a conserved Ets domain that is much more similar to that of Drosophila Ets4 (6) than to any other Ets domain, we have named it SpEts4. We demonstrate that the SpEts4 gene sequence encodes a protein that binds with the same specificity, and forms a complex of similar mobility in EMSA, as does the native protein found in nuclear protein extracts from very early blastulae. Using SpHE promoter transgenes, we show that the site to which SpEts4 binds confers positive regulatory activity and that exogeneously supplied recombinant SpEts4 augments promoter activity. Consistent with a positive function for SpEts4 in regulating SpHE transcription, SpEts4 transcripts accumulate in the egg and early embryo transiently and just prior to the burst of SpHE mRNA expression.
MATERIALS AND METHODS
S. purpuratus egg cDNA library.
A total of 1 mg of total RNA from S. purpuratus eggs was used to extract poly(A)+ RNA by using the FastTrack 2.0 kit (Invitrogen). The cDNA library was made by using a cDNA library construction kit supplied by Clontech (Matchmaker Two-Hybrid System). Briefly, 8-μg aliquots of poly(A)+ RNA were reverse transcribed separately by using either random or oligo(dT) primers, and the cDNA products were combined and inserted into a shuttle vector, pGAD10, containing the GAL4 activation domain. Transformation by electroporation of Escherichia coli DH5α cells yielded >6 × 105 independent colonies. The library was amplified on 20- by 150-mm plates containing ∼30,000 colonies/plate.
Yeast one-hybrid screening of the sea urchin egg cDNA library.
The SpHE promoter sequence from bp −324 to −143 was used as the bait to select DNA binding domains encoded in the sea urchin egg cDNA library. Within this promoter region are recognition motifs for Otx, Rel, and Ets as well as three additional binding sites defined by EMSAs and DNase I footprinting (26). The bait was inserted into reporter plasmids pHISi and pLacZi, and the recombinant plasmids were introduced sequentially into the genome of the yeast strain Y4271. These plasmids and control plasmids, containing either p53 cis elements, p53blue, or DNA binding sequences (pGAD53m), were supplied in the Clontech Matchmaker One-Hybrid System kit. The transformants were tested for growth on medium lacking His (His− medium) in the presence of increasing concentrations of 3-aminotriazol (3-AT). Cells whose growth was inhibited by 5 mM 3-AT were selected as the host for the library screen. Transformation with the library was carried out by using LiCl-polyethylene glycol, and transformants grown on His− and Leu− selective medium were tested for β-galactosidase activity. Plasmids from putative positive clones were isolated from the yeast after homogenization with glass beads and then individually transferred into DH5α cells for amplification. To eliminate false positives, these plasmids were separately introduced into yeast cells containing either the SpHE bait or the p53 binding site, and the transformants were tested for β-galactosidase activity. The plasmids that conferred expression only in the SpHE host were chosen for further analysis. Inserts were sequenced by a combination of manual dideoxy sequencing and automated sequencing. DNA sequences were used to query the GenBank database, and sequence comparisons were made by using Clustal software (21).
Transcription activation domain mapping.
The SpEts4 activation domain was identified by using the yeast one-hybrid system. The GAL4 transcription activation domain in plasmids pGAD10 (for SpEts4) and pGAD424 (for p53) was removed by internal deletion of sequences from restriction site KpnI to EcoRI, and SpEts4 sequences were inserted. To test for activation activity in SpEts4, constructs encoding proteins with deletions in three separate regions (EtsΔ36–123, EtsΔ126–184 [pointed domain], and EtsΔ184–274) were transformed into SpHE bait-containing yeast cells. To test whether SpEts4 sequences could mediate activation when linked to a heterologous (p53) DNA binding domain, four fusion protein constructs, p53E1–275, p53EΔ126–184, p53E1–123, and p53E184–275 were prepared and used to transform p53 bait-containing yeast. SpEts4 transformants were tested for growth ability on His− plates with 5 mM 3-AT and for β-galactosidase activity. p53 transformants were tested only for β-galactosidase activity by filter lift assay as described in the Clontech Yeast Protocols Handbook.
In vivo transcription assays.
Measurements of promoter activity in vivo were made by using chimeric constructs carrying wild-type or mutated SpHE promoters linked to a bacterial chloramphenicol acetyltransferase (CAT) reporter gene. The mutation changed CGGAAC at bp −250 in the SpHE promoter to an EcoRI site, GAATTC. These constructs were microinjected into fertilized S. purpuratus eggs and assayed exactly as described previously (26).
For transactivation assays, capped, polyadenylated SpEts4 mRNA was synthesized by using the Message Machine kit from Ambion and purified according to the manufacturer’s instructions, and ∼0.2 pg was coinjected with 2,500 copies of the SpHE promoter transgene.
Developmental RNase protection assays.
RNA was prepared from eggs and embryos at selected developmental stages by using the TRIzol protocol (GIBCO-BRL). The quantity of RNA was determined by spectrophotometry, and its quality was verified by gel electrophoresis on denaturing formaldehyde-containing agarose gels. Probes complementary to SpEts4 mRNA (from bp −102 to +99 relative to the translation initiation site) and SpHE sequences (from bp +316 to +543 relative to the transcription start site and containing exon 1 and intron 1 sequences) were labeled with [α-32P]UTP to specific activities of 6 × 108 and 0.75 × 108 cpm/μg, respectively. The SpEts4 and SpHE probes protect 202- and 110-nucleotide fragments, respectively. One-half nanogram of probe was hybridized with 1.5 μg of total RNA to kinetic termination at 50°C for 18 h. RNase protection and electrophoresis of the protected products were conducted as described previously (30).
In vitro translation of SpEts4 protein.
The SpEts4 cDNA was transferred to pGEM Easy plasmid (Promega). The protein was synthesized by using 1 μg of linearized plasmid and the TNT coupled reticulocyte lysate kit (Promega) in the presence of [35S]methionine (40 pmol; 1,000 Ci/mmol). The labeled products (1 μl of the 50-μl reaction mixture) were assayed for size by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Control luciferase protein encoded from a plasmid provided by the manufacturer was synthesized and assayed in the same way.
EMSA.
EMSA was conducted by using in vitro-translated proteins and 9-h nuclear extracts as described previously (26). Probes (bp −274 or −261 to −241) containing only the Ets motif were end labeled with [γ-32P]ATP to a specific activity of ∼2.0 × 106 cpm/pmol. For each reaction, ∼4.5 fmol of probe was incubated on ice for 10 min with either an aliquot of the in vitro translation reaction mixture (see above) or nuclear extract protein; the latter was prepared as described previously (5). To test for specificity of binding, various competitor DNAs, each containing a different variant Ets binding site sequence, were added in a 200-fold molar excess. The reaction mixtures were then fractionated by electrophoresis through nondenaturing 5% acrylamide gels.
RESULTS
Isolation of trans-acting factors that interact with the SpHE promoter sequence by using a yeast one-hybrid screen of an egg cDNA library.
In order to identify the trans-acting factors involved in SpHE promoter activation, we used a nontraditional version of the yeast one-hybrid method. In general, this assay selects among cDNA sequences, fused to a vector-encoded transcriptional activation domain, for those whose protein products can bind target DNA and activate expression of a reporter gene in yeast cells (23). According to presently used methods, the one-hybrid approach is said to require a bait consisting of a multimerized cis element in order to increase the probability of protein-DNA interaction. However, because the SpHE promoter contains a large number of functional cis elements, and because the exact boundaries of some of these are difficult to define precisely, we used a single copy of the native promoter sequence from bp −324 to −143. This region includes all identified cis elements except two CCAAT sites that were excluded to avoid possible background resulting from known CCAAT transcription factors in yeast. Another reason for using this approach is that among the many cis elements regulating the SpHE promoter, none is detectably more important than the others for mediating either quantitative or spatial regulation (26–28). Finally, we wanted to increase the probability of selecting for strong protein-DNA interactions, which are likely to be favored by using the natural promoter sequence instead of multimerized elements. Because the SpHE gene is very likely activated by maternal transcription factors, we used RNA from eggs to build a cDNA library of fusions to the GAL4 activation domain in plasmid pGAD10.
The screening strategy consisted of two tests: (i) growth on His− medium in the presence of 3-AT and expression of β-galactosidase and (ii) elimination of false positives by double screening of selected transformants with an unrelated promoter bait and with the bait containing SpHE promoter sequences. First, a test cell line that contained integrated copies of SpHE promoter bait linked to either the histidine gene or the β-galactosidase gene was selected for growth on His− medium that was completely inhibited by 5 mM 3-AT. After transformation with the egg cDNA library, of 2 × 106 egg cDNA transformants of this cell, more than 100 colonies grew under 3-AT selection, and they all were positive for β-galactosidase activity, although at variable levels. The plasmid from each transformant was extracted and individually reintroduced into yeast cells containing either SpHE promoter bait or a p53 bait. Of these, 14 clones were SpHE specific and conferred the highest levels of β-galactosidase activity. Sequence analysis revealed that 13 clones shared a 1.8-kb sequence (Fig. 1). Of these 13 clones, 8 contained inserts of about 1.8 kb, and the remaining inserts ranged from 2.3 to 2.9 kb. Interestingly, many of these positive inserts were not linked in frame to a GAL4 activation domain, raising the possibility that the selected egg cDNA sequences encode a protein with its own transcription activation function as well as a DNA binding domain. Furthermore, several strong positive transformants contained plasmid inserts in opposite orientation with respect to the ADH1 promoter that drives transcription of fusion proteins. This observation suggests that sufficient transcription must have occurred from a downstream cryptic promoter.
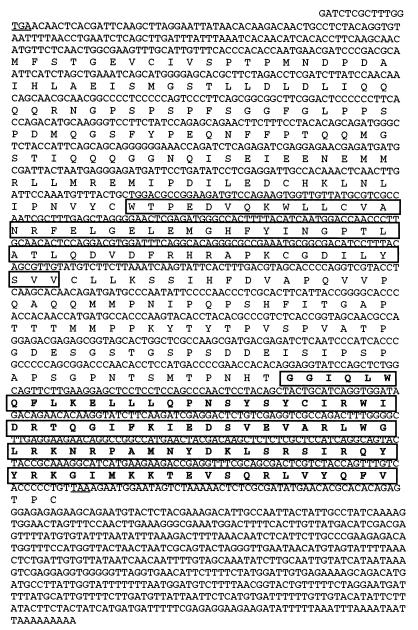
FIG. 1.
Sequence of the SpEts4 cDNA and its predicted translation product. Stop codons upstream of the translation initiation site and at the end of the open reading frame are underlined. The region in the C-terminal quarter of the protein that is similar to the conserved DNA binding domains of Ets factors is shown boxed and in boldface, and a region in the middle of the protein with similarity to the pointed domain and thought to be involved in protein-protein interactions is also boxed. Transcriptional activation is mediated by sequences upstream of amino acid 123 (see Fig. 3).
The selected cDNAs encode a novel sea urchin Ets family transcription factor.
The longest open reading frame obtained from the 13 selected egg cDNAs predicts a protein of 363 amino acids (Fig. 1). The translation start site is defined by the codon for the first methionine residue downstream from an in-frame stop codon, whose local context conforms to the translation consensus sequence (17). Comparison of the following open reading frame to sequences in the GenBank database shows that the C-terminal amino acid region comprises a conserved domain of about 85 amino acids that is related to the DNA binding domain of the Ets family of transcription factors (Fig. 1). The consensus core sequence recognized by Ets factors is C/AGGAA/T, which appears in the SpHE −300 promoter at bp −250 and −107. Only the −250 site was included in the bait sequence, suggesting that this Ets site can bind the Ets family member selected in our screen.
Comparison of Ets (DNA binding) domain sequences in the database to the recovered sea urchin Ets sequence indicates that the closest relative is Drosophila Ets4 (6). In the Ets domain, the amino acid residues are 82% identical, while the identity to all other Ets domain sequences is much lower (35 to 54%) (Fig. 2). The newly cloned sea urchin Ets protein is distinct from the Ets2 factor previously reported for these embryos (52% identical in the DNA binding domain) (7, 19). Ets transcription factors contain a second conserved sequence in the N-terminal region, called the pointed domain. Corresponding sequence is not available for Drosophila Ets4. Of the available sequences, the pointed domain of the vertebrate Ets protein, Tel (11), is most closely related to a region of SpEts4 (amino acids 127 to 183) (Fig. 1), having 40% identity and 57% similarity; this is comparable to the level of identity between the Ets domains of SpEts4 and Tel, which is only 44%. The function of the pointed domain is not well understood, but it is thought to mediate dimerization of Tel proteins. This is because Tel-ABL tyrosine kinase fusion proteins, which are created as a result of a chromosomal translocation, dimerize, causing constitutive activation of the kinase and an oncogenic transformation (11). From these comparisons and the currently available data, we conclude that Drosophila Ets4 is the closest known relative of the newly identified sea urchin Ets factor, and we accordingly have named the sea urchin factor SpEts4.
FIG. 2.
Sequence comparisons of the DNA binding domains of selected members of the Ets family. The percent identity between the Ets domain of each Ets factor and that of SpEts4 is given at the right. Asterisks indicate residues that are identical in all seven proteins. Dots represent conserved similar amino acids.
SpEts4 contains a transcription activation domain.
SpEts4 appears to have transcription activation activity, since, as discussed above, linkage of the GAL4 activation domain to SpEts4 sequence was not required for strong expression of reporter genes in the yeast one-hybrid screen. To eliminate possible activation via GAL4 peptides binding in trans to SpEts4, positive clones lacking GAL4 sequences were tested for β-galactosidase expression (not shown) and growth in 3-AT-containing His− medium (Fig. 3C; compare sector 2 [+GAL4] with sector 3 [−GAL4]). Both assays show that transcription activity was retained after deletion of GAL4 sequence. In the case shown in Fig. 3C, GAL4 sequence was in frame with SpEts4 in the parent clone; after GAL4 deletion, a slight but detectable reduction in activity was observed, consistent with both GAL4 and SpEts4 sequences’ providing activation activity. In cases in which GAL4 and SpEts4 sequences were not linked in frame, removal of GAL4 had no effect (data not shown).
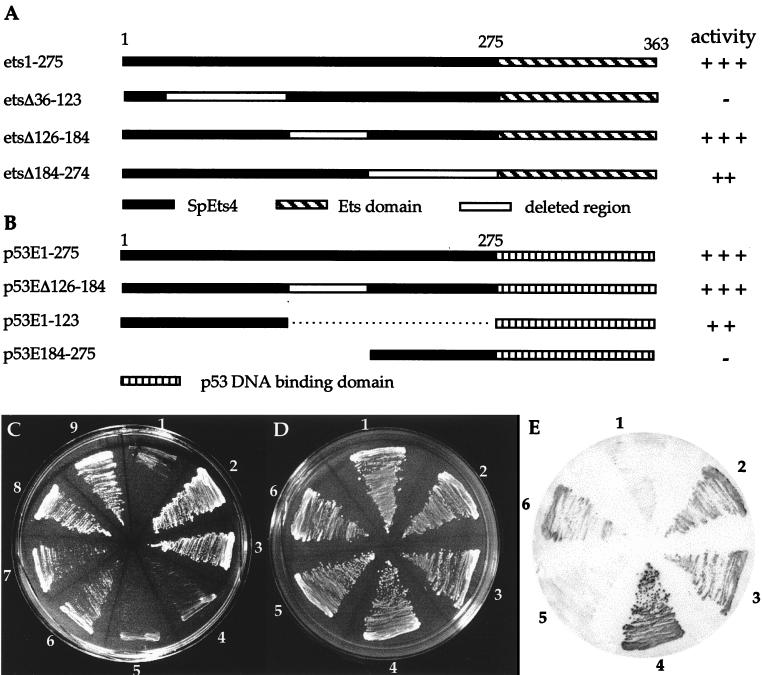
FIG. 3.
Mapping of the SpEts4 transcription activation domain by the yeast one-hybrid assay. (A) Constructs encoding SpEts4 mutated by internal deletions. Each of these constructs lacks the GAL4 activation domain. (B) Constructs encoding fusion proteins of SpEts4 sequences linked to the p53 DNA binding domain. Numbers refer to SpEts4 amino acid sequences. Relative activities of reporter gene expression as measured by growth under His− selection in 5 mM 3-AT or β-galactosidase expression are shown at the right. (C) Growth of yeast cells under His− selection transformed with plasmids expressing wild-type or mutant SpEts4 proteins. 1, negative control transformed with nonrecombinant plasmid only; 2, positive control with wild-type SpEts4 in pGAD10, containing the GAL4 activation domain linked in frame; 3, wild-type SpEts4, lacking GAL4 sequences; 4 and 5, EtsΔ36–123 in forward and reverse orientations, respectively; 6 and 7, EtsΔ184–274 in forward and reverse orientations, respectively; 8 and 9, EtsΔ126–184 in forward and reverse orientations, respectively. (D and E) Transcriptional activation provided by p53-SpEts4 fusion proteins. (D) Growth of yeast containing an integrated copy of the p53 bait and transformed with plasmids encoding the p53-SpEts4 fusion proteins diagrammed in panel B. (E) Yeast colonies from panel D were analyzed for β-galactosidase expression. 1, negative control (untransformed cells); 2, p53E1–275; 3, p53Δ126–184; 4, positive control, containing the GAL4 domain; 5, p53E184–275; 6, p53E1–123.
To map the general location of transcription activation activity in the SpEts4 protein, constructs encoding proteins with deletions of three separate regions upstream of the Ets domain were transfected into yeast cells containing the SpHE promoter bait. These constructs, diagrammed in Fig. 3A, constitute a series of internal deletions that either flank or include the pointed domain. Deletion of the pointed domain (EtsΔ126–184) did not reduce activity (Fig. 3C, sectors 8 and 9), while EtsΔ36–123 was completely inactive when tested for growth under His− selection (Fig. 3C, sectors 4 and 5) or for β-galactosidase activity. These results suggest that the upstream region, but not the pointed domain, supplies a transactivating function. The activity of the Δ184–274 protein was lower than that of either the full-length protein or pointed-domain deletion mutant (Fig. 3C, sectors 6 and 7). However, it is unlikely that this reflects the presence of a second independent activation region, because the Δ36–123 protein, which contains it, is entirely inactive. Instead, sequences between amino acids 184 and 274 may be required for optimal activity of the upstream domain by providing either a coactivation or structural function.
Since deletion of amino acids 36 to 123 resulted in loss of transcriptional activation, it was important to determine whether this sequence was sufficient to provide positive activity when linked to a heterologous DNA binding domain, p53. Plasmids encoding this fusion protein as well as those encoding other SpEts4 sequences (diagrammed in Fig. 3B) were transformed into yeast containing the p53 promoter bait linked to the reporter β-galactosidase and grown to approximately equal densities (Fig. 3D). Transcription activation mediated by SpEts4 sequence is reflected by both the time of appearance and intensity of the blue reaction product (Fig. 3E). By each of these criteria, p53E1–275 (sector 2) and p53EΔ126–184 (sector 3) are strongly positive and comparable to the positive control plasmid, pGAD53m, containing the GAL4 activation domain (sector 4). Further, sequences between amino acids 1 and 123 (p53E1–123; sector 6) also confer promoter activity at a somewhat reduced level compared to the wild type. A requirement for downstream sequences (amino acids 184 to 275) for full activity was also observed in the deletion analysis (cf. growth in sectors 6 and 7 with that in either sectors 8 and 9 or sectors 2 and 3 in Fig. 3C). In contrast, the activity of the fusion containing only amino acids 184 to 275 (p53E184–275; sector 5) is similar to background levels obtained for untransformed p53 bait cells (sector 1). These results confirm the deletion analysis and lead to the conclusion that transcriptional activation is mediated by sequences upstream of the pointed domain in SpEts4.
SpEts4 protein translated in vitro binds specifically to the −250 Ets site in the SpHE promoter.
To test whether SpEts4 protein can bind specifically to the SpHE Ets site, a plasmid containing the SpEts4 open reading frame was transcribed in vitro, and the RNA was translated in the presence of [35S]methionine by using a rabbit reticulocyte lysate. As shown in Fig. 4A, several labeled products were obtained; the predominant one of these was about 47 kDa, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. This value is reasonably close to the size predicted from the inferred amino acid sequence, which is 41 kDa. These proteins formed a complex with a SpHE probe (bp −274 to −241) containing the Ets CGGAA core and flanking sequences, as shown by the EMSA results in Fig. 4B. In contrast, no complex was formed when a template encoding luciferase was used in parallel reactions.
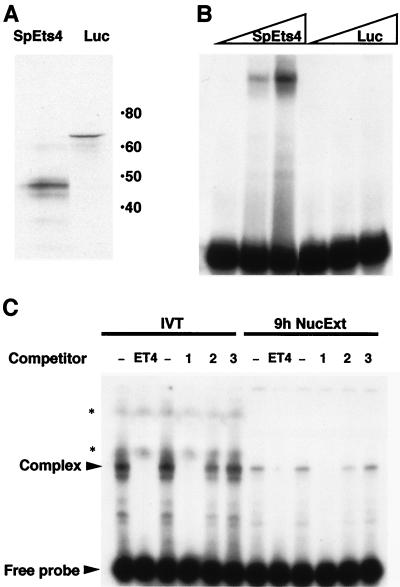
FIG. 4.
SpEts4 protein synthesized in vitro binds the SpHE −250 promoter element. (A) SpEts4 and luciferase (Luc) (control) proteins were synthesized in vitro in the presence of [35S]methionine by using a coupled transcription-translation system. The autoradiograph of products separated by polyacrylamide gel electrophoresis shows a major band at 47 kDa for SpEts4, which is similar to the size of the predicted translation product of SpEts4 cDNA. Numbers on the right are molecular masses in kilodaltons. (B) Aliquots of the translation reaction mixtures (0.04, 0.2, and 1 μl) were used in EMSAs with a 32P-labeled DNA probe containing the Ets cis element (bp −274 to −241) from the SpHE promoter. (C) EMSA was carried out (as described for panel B) with either 9-h sea urchin embryo nuclear extract (NucExt) (2 μg) or in vitro-translated (IVT) SpEts4 protein (0.5-μl translation reaction mixture). The probe represented SpHE promoter sequence from bp −261 to −241, and, where indicated, a 200-fold molar excess of unlabeled competitor DNA fragments was included. Lanes: −, no competitor; ET4, same as the probe sequence; 1, 2, and 3, E74, PU.1, and 59/60, respectively (three different Ets cis elements for which the factor in sea urchin embryo nuclear extracts has different affinity). Bands marked with asterisks represent signal from the IVT protein, labeled with [35S]methionine.
To help determine whether SpEts4 corresponds to the endogenous embryo protein that binds the −250 Ets cis element, complexes formed with in vitro-translated protein and nuclear extract protein were compared (Fig. 4C). Two characteristics of the complexes formed with factors from these two sources strongly suggest that they are the same protein. First, the complexes have the same mobility. Second, when each of these complexes was competed by a series of different Ets motif sequence variants, both were competed equally effectively by the SpHE Ets and E74 motifs, but neither was competed as well by the PU.1 or the sea urchin Ets2 site (59/60) (8). The sequences of these competitors are listed in Fig. 5. Although all of the sequences contain the same GGAA core motif, as expected, residues both 5′ and 3′ to the core in the −250 SpHE Ets site and E74 are more similar to each other than those in elements to which binding is weak or undetectable. We conclude that SpEts4 and a factor in 9-h nuclear extracts bind to different Ets elements with similar relative affinities.
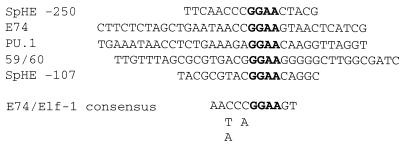
FIG. 5.
Sequences of SpHE Ets sites and Ets competitors.
We also tested the other putative Ets motif located at bp −107 in the SpHE promoter for binding to in vitro-translated SpEts4 and 9-h nuclear extracts. In neither case was a complex detected (data not shown), leading us to conclude that the upstream site is the only site in the SpHE promoter to which SpEts4 binds. As shown in Fig. 5, this result undoubtedly reflects the fact that the sequences surrounding the Ets core sequence at bp −107 differ significantly from those found at the −250 SpHE Ets4 or E74 sites to which SpEts4 binds.
SpEts4 positively activates the SpHE promoter-containing transgenes.
Our previous studies led to the conclusion that most, and probably all, cis elements in the SpHE promoter bind positively acting factors (27). Relevant to the present studies is the demonstration that an Ets site-containing partial promoter from bp −240 to −310 fused to the basal promoter region (bp −90 to +20) retained significant, spatially correct transcriptional activity (27). We tested whether SpEts4 behaves as a positive regulator of SpHE promoter-driven transgenes in vivo by using three independent assays: by mutation of the Ets cis element, by linking the Ets site to the basal promoter, and by in vivo transactivation via coinjected SpEts4 mRNA.
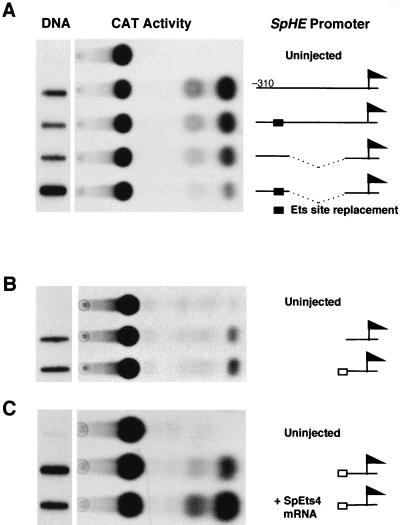
Because EMSAs showed that the −240 to −310 region tested previously contains binding sites for SpOtx and an unidentified factor as well as SpEts4 (26), we prepared constructs that specifically test the contribution of the SpEts4 element to SpHE promoter activity by replacing the CGGAAC core sequence. Linearized constructs containing SpHE promoter elements driving CAT expression were microinjected into fertilized S. purpuratus eggs. The resulting embryos were analyzed for CAT activity and for exogenous template DNA content as described previously (10, 26). As shown in Fig. 6A, replacement of the Ets core binding site in the context of the −310 promoter results in reduced CAT enzymatic activity. When the −240 to −310 region is tested directly linked to the −90 to +20 basal promoter region, promoter activity resulting from replacement of the Ets site also is decreased to close to the basal promoter levels that we have observed in previous experiments (26). In a second set of experiments, we found that a 20-nucleotide fragment containing the Ets site linked to the SpHE basal promoter could reproducibly mediate a low level of activation (Fig. 6B). As shown in the transactivation experiment described below, one likely reason that this effect is modest is that the amount of SpEts4 protein in the embryo is limiting with respect to the transgenes, which are present at about 2,500 copies/expressing cell (18). It is also possible that the position of the Ets site with respect to the basal promoter is not optimal in the context of this artificial promoter. In any case, these results support the Ets site mutation data, which suggest that the SpHE SpEts cis element binds a factor with a transcriptional activating function. To test directly for transactivating activity of SpEts4, SpEts4 mRNA was coinjected with a SpHE promoter transgene into sea urchin one-cell zygotes. As shown in Fig. 6C, 0.2 pg of mRNA coinjected with the Ets site-basal promoter transgene increased CAT activity severalfold compared to that in embryos containing the transgene alone. This result indicates that endogenous SpEts4 protein is not sufficient to saturate the transgene target sites. These observations indicate that SpEts4 supplies a transcriptional activating activity in sea urchin embryos, as it does in yeast one-hybrid assays.
FIG. 6.
SpEts4 activates the SpHE promoter via the Ets element. (A) The effect of the Ets element on SpHE promoter activity in vivo was determined in the context of the intact −310 promoter (upper two constructs) and with a mutated version from which sequences between bp −90 and −240 had been deleted (lower two constructs). CAT reporter activity expressed from microinjected constructs from 75 embryos was assayed at the blastula stage. (B) CAT activity elicited by addition of the SpHE −250 Ets site (open box) to the SpHE basal promoter region (line) (bp −90 to +20). (C) Transactivation of the SpHE Ets site transgene by coinjection of SpEts4 mRNA. Levels of construct DNA were determined by slot blotting (left), using aliquots of the same embryo batches as assayed for CAT activity.
The early expression of the SpEts4 gene is appropriate for its encoding a SpHE regulator.
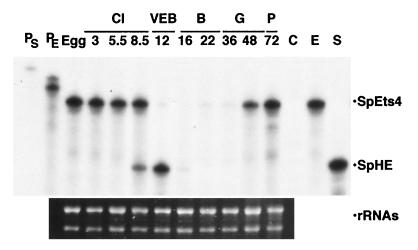
To determine whether expression of SpEts4 is consistent with its encoding a SpHE regulatory protein, an RNase protection assay was performed with combined probes representing SpEts4 and SpHE transcripts and total RNA from unfertilized eggs and selected embryo stages. To avoid possible cross-reaction and the resulting production of additional protected fragments, we used an SpEts4 probe representing sequence outside the conserved regions and likely to be gene specific. As shown in Fig. 7, fragments of the size expected for perfect duplexes were protected for each probe, indicating that these assays are specific for each mRNA, since, under the conditions used, RNase will cleave the hybridized probe at a single mismatched base pair. SpEts4 transcripts are relatively abundant and present at nearly equivalent levels in the unfertilized egg and in embryos through the first six cleavages (8.5 h; 64-cell stage). SpHE transcripts were first detectable in this assay at 8.5 h, reached peak levels at the very early blastula stage (12 h), and then rapidly decayed. In previous similar assays with probes that had higher specific activity, SpHE transcripts could be detected in the four- to eight-cell embryo (3 to 4 h) (20) and, as observed here, rapidly turned over after about 15 h postfertilization. We conclude that the relative patterns of accumulation of these mRNAs are consistent with SpEts4’s encoding a positive regulator of SpHE transcription.
FIG. 7.
Accumulation of SpEts4 message precedes activation of the SpHE gene. Levels of SpEts4 and SpHE mRNAs were assayed by RNase protection with both probes combined in the same reactions and hybridized with total RNA isolated from eggs or embryos at the indicated stages of development. Hours postfertilization are indicated, as are the corresponding developmental stages (Cl, cleavage; VEB, very early blastula; B, blastula; G, gastrula; P, pluteus). Samples in the last two lanes serve as markers and are the RNase-resistant fragments for each single probe (E, SpEts4; S, SpHE) after hybridization to early-blastula-stage RNA. The left two lanes show unhybridized SpHE and SpEts4 probes (PS and PE, respectively). Lane C, negative control with both probes hybridized with yeast tRNA. The lower panel shows the ethidium bromide-stained gel of egg and embryo RNA samples, demonstrating that equal quantities of intact RNAs were used in these reactions.
SpEts4 is also expressed at much later stages, after gastrulation is complete and when SpHE is no longer transcribed. This observation implies that SpEts4 is also involved in the regulation of other genes in later stages of morphogenesis and histodifferentiation.
DISCUSSION
In this study we used a modification of the yeast one-hybrid system to clone a trans-acting factor that can activate the sea urchin SpHE gene. The one-hybrid approach has been successfully used to isolate other transcription factors, but virtually all of those studies utilized as bait a specific multimerized cis element. That kind of construct requires relatively precise mapping of the cis element by mutation and deletion, which is time-consuming. Furthermore, the SpHE regulatory region contains many positively acting sites, and multiple combinations of elements are able to drive expression to high levels in the correct cells (26, 27). Because of this functional redundancy, it was not possible for us to assign primary significance to any individual element. Therefore, we chose to use a major portion of the SpHE regulatory region, containing six different cis elements. To our knowledge, a similar approach has been used only once previously (16); however, in that case, the cis element was essentially naturally multimerized, since it appeared in the promoter several times. A related but converse approach, which utilized expression of DNA binding proteins in yeast to select single target cis elements from genomic DNA, has been successful (29).
Although the bait contained at least six cis elements (26), we only recovered clones encoding the one protein binding at an Ets motif, previously called site IC. Potential reasons for this include the possibilities that some proteins require interaction with other cofactors lacking in yeast for strong DNA binding, that mRNAs encoding some factors may be of relatively low abundance in eggs or underrepresented in our egg library, and that some factors may require posttranslational modification for binding that does not occur in yeast. However, an alternative explanation is that SpEts4 was more easily selected in the yeast screen because of its own transcriptional activation activity that resides in the N-terminal region. Furthermore, this activity appears to be quite strong, since it is nearly equivalent to that obtained with the positive control GAL4-p53 fusion protein.
The activating region of SpEts4 is characterized by a relatively high density of residues that are also found in the activation domains of other transcription factors, which include glutamine, serine, proline, and acidic residues. Different members of the Ets family also contain mapped activation domains that lack a conserved activation sequence but are characterized by similar enrichments for these amino acids (reviewed in reference 9). Which of these are responsible for transcriptional activation by SpEts4 is not known yet.
The selected protein, SpEts4, that binds to a SpHE promoter cis element is a member of the Ets transcription factor family, based on strong conservation in the DNA binding domain as well as similarity to a pointed domain outside this region. Based on the available sequences of the conserved Ets domain, this transcription factor is most closely related to Drosophila Ets4. Although sequence outside the Drosophila Ets4 DNA binding domain is not yet available, it is likely that it also will be more similar to SpEts4 than to other Ets proteins. Phylogenetic comparison of Ets domain sequences suggests that Drosophila Ets4 cannot be assigned to any of the nine known groups, including those with both invertebrate and vertebrate members (13). The identification of SpEts4 suggests that SpEts4 and Drosophila Ets4 constitute a new group within the Ets family. It is interesting that both factors are expressed in oocytes and early embryos (6), but determining whether they mediate conserved developmental functions will require identification of target genes in both species. It would be interesting to know if this group, currently with two invertebrate members, is conserved in vertebrates, as are some other groups of Ets factors. SpHE is a Zn2+-dependent metalloprotease of the stromelysin/collagenase family. Interestingly, Ets transcription factors have also been shown to be important regulators of genes encoding mammalian stromelysin (4, 24) and collagenase (14).
SpEts4 binds effectively to an Ets site in the SpHE promoter and to the Drosophila E74 site from the E74 promoter (22) but not to motifs recognized by the PU.1 Ets class or a sea urchin Ets2-like factor (8). The exact sequence features that determine binding affinities of different Ets factors are not known. However, consistent with our in vitro binding data is the observation that in the sequences immediately flanking the GGAA core, the SpHE SpEts4 site is more similar to the Drosophila E74 competitor sequence than to those of the PU.1 and Ets2 binding site competitors. Random oligonucleotide site selection assays with either E74 (22) or its mammalian homolog, Elf-1 (15), generated a consensus recognition site, AAC/TCC/AGGAAGT, which is an excellent match (10 of 11) to the SpHE SpEts4 site (AACCCGGAACTA) (Fig. 5), although closely related, but somewhat more degenerate, consensus sites have also been obtained for other Ets subfamily members (reviewed in reference 13). As is the case for all Ets factors, binding of SpEts4 to the SpHE promoter results in DNase I hypersensitivity near the core recognition motif (12, 26, 28).
Many Ets proteins interact with other transcription factors, and in some cases (e.g., AP1, SRF, CBF, Sp-1, and myb), this interaction facilitates binding and/or activity (reviewed in references 9 and 25). Candidate regions for binding interacting proteins reside on either side of the SpEts4 binding site in the SpHE promoter. About 15 nucleotides 5′ of the SpEts4 site in the SpHE promoter is a consensus motif for binding the NF-κB class of transcription factors (GGGTAATCC) (3), which are known to interact with Ets family members (2). Immediately downstream of the Ets site is a large DNase I-protected region produced by a cis element(s) and factor(s) that remain to be defined (26, 28).
The site with which SpEts4 interacts confers positive activity in the SpHE promoter, as demonstrated by assays in vivo on wild-type and mutated SpHE promoter-driven transgenes. Mutation of the Ets site in a partial promoter containing only two other identified upstream cis elements reduces activity nearly to basal levels, indicating that it is at least essential, if not sufficient, for promoter activity in this construct. That the function conferred by the SpEts4 cis element is positive is strongly supported by the observation that transcription activated through the SpHE Ets site could be significantly upregulated by coinjected SpEts4 mRNA. The finding that SpEts4 protein activates SpHE transcription agrees well with our previous in vivo genomic footprinting assays. There is within the cis element to which SpEts4 binds a strong dimethylsulfate-sensitive site at early stages when the SpHE promoter is active but not at later stages when it is inactive (28). Since this site maps to the exact nucleotide position observed in similar assays of an Ets-DNA interaction (1), it likely reflects an in vivo SpEts4-DNA interaction that occurs when the SpHE gene is active. The demonstration that SpEts4 confers positive activity is consistent with our model that it is one of a set of positive factors regulating SpHE transcription.
The timing of SpEts4 gene expression is consistent with its serving as an activator of SpHE transcription. SpEts4 transcripts, which accumulate during oogenesis, persist only until about the 64-cell stage (8 h postfertilization) and turn over rapidly during the next several hours, corresponding to one or two cleavages. SpHE transcription begins in the 4- to 8-cell embryo, mRNA levels are maximal at about 12 h postfertilization (170-cell stage), and then transcription is repressed and mRNA levels decay within the next several hours. SpHE is not transcribed at late embryonic stages when SpEts4 mRNAs reappear, implying that SpEts4 protein regulates other genes during sea urchin embryogenesis.
In the context of a partial SpHE promoter transgene, this Ets cis element mediates binding of an essential positive activity. The same partial promoter also drives expression of a reporter gene appropriately only in nonvegetal cells, as can several different subsets of cis elements in the SpHE regulatory region (27). These results have led us to propose that restriction of SpHE transcription to nonvegetal cells of the early blastula reflects the partitioning of multiple positive activities to this early embryonic domain. The results presented here suggest that SpEts4 is one of those activities. Experiments to characterize the expression patterns of SpEts4 during sea urchin embryogenesis and to test whether SpEts4 function is restricted to cells in nonvegetal blastomeres of the early sea urchin embryo are under way.
ACKNOWLEDGMENTS
We thank Geoff Childs for Ets motif oligonucleotides and for sharing information about their properties, Eric Howard for advice on genetic screening in yeast, and Xiaomei Pan for technical assistance.
This work was supported by an NIH grant (NIGMS 25553) to R.C.A.
REFERENCES
- 1.Ahne B, Stratling W H. Characterization of a myeloid-specific enhancer of the chicken lysozyme gene. Major role for an Ets transcription factor-binding site. J Biol Chem. 1994;269:17794–17801. [PubMed] [Google Scholar]
- 2.Bassuk A G, Anandappa R T, Leiden J M. Physical interactions between Ets and NF-κB/NFAT proteins play an important role in their cooperative activation of the human immunodeficiency virus enhancer in T cells. J Virol. 1997;71:3563–3573. doi: 10.1128/jvi.71.5.3563-3573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulikas T. A compilation and classification of DNA binding sites for protein transcription factors from vertebrates. Crit Rev Eukaryot Gene Expr. 1994;4:117–321. doi: 10.1615/critreveukargeneexpr.v4.i2-3.10. [DOI] [PubMed] [Google Scholar]
- 4.Buttice G, Kirkinen M. A polyoma virus enhancer A-binding protein-3 site and Ets-2 protein have a major role in the 12-O-tetradecanoylphorbol-13-acetate response of the human stromelysin gene. J Biol Chem. 1993;268:7196–7204. [PubMed] [Google Scholar]
- 5.Calzone F J, Thèzè N, Thiebaud P, Hill R L, Britten R J, Davidson E H. Developmental appearance of factors that bind specifically to cis-regulatory sequences of a gene expressed in the sea urchin embryo. Genes Dev. 1988;2:1074–1088. doi: 10.1101/gad.2.9.1074. [DOI] [PubMed] [Google Scholar]
- 6.Chen T, Buntine M, Karim F D, Thummel C S. Isolation and characterization of five Drosophila genes that encode an ets-related DAN binding domain. Dev Biol. 1992;151:176–191. doi: 10.1016/0012-1606(92)90225-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z-Q, Kan M C, Pribyl L, Lautenberger J A, Moudrianakis E, Papas T. Molecular cloning of the ets protooncogene of the sea urchin and analysis of its developmental expression. Dev Biol. 1988;125:432–440. doi: 10.1016/0012-1606(88)90224-2. [DOI] [PubMed] [Google Scholar]
- 8.Childs, G. 1996. Personal communication.
- 9.Crepieux P, Coll J, Stehelin D. The Ets family of proteins: weak modulators of gene expression in quest for transcriptional partners. Crit Rev Oncogenet. 1994;5:615–638. [PubMed] [Google Scholar]
- 10.Gan L, Zhang W, Klein W H. Repetitive DNA sequences linked to the sea urchin Spec genes contain transcriptional enhancer-like elements. Dev Biol. 1990;139:186–196. doi: 10.1016/0012-1606(90)90287-s. [DOI] [PubMed] [Google Scholar]
- 11.Golub T R, Goga A, Barker G F, Afar D E, McLaughlin J, Bohlander S K, Rowlet J D, Witte O N, Gilliland D G. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol Cell Biol. 1996;16:4107–4116. doi: 10.1128/mcb.16.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graves B J, Gillespie M E, McIntosh L P. DNA binding by the ETS domain. Nature. 1996;384:322. doi: 10.1038/384322a0. [DOI] [PubMed] [Google Scholar]
- 13.Graves B J, Petersen J M. Specificity within the ets family of transcription factors. Adv Cancer Res. 1998;75:1–55. doi: 10.1016/s0065-230x(08)60738-1. [DOI] [PubMed] [Google Scholar]
- 14.Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John S, Marais R, Child R, Light Y, Leonard W G. Importance of low affinity Elf-1 sites in the regulation of lymphoid-specific inducible gene expression. J Exp Med. 1996;183:743–750. doi: 10.1084/jem.183.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S Y, Chung H J, Thomas T L. Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J. 1997;11:1237–1251. doi: 10.1046/j.1365-313x.1997.11061237.x. [DOI] [PubMed] [Google Scholar]
- 17.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon A P, Flytzanis C N, Hough-Evans B R, Katula K S, Britten R J, Davidson E H. Introduction of cloned DNA into sea urchin egg cytoplasm: replication and persistence during embryogenesis. Dev Biol. 1985;108:420–430. doi: 10.1016/0012-1606(85)90045-4. [DOI] [PubMed] [Google Scholar]
- 19.Rao, S. K., and G. Childs. 1993. GenBank accession no. LL19541.
- 20.Reynolds S D, Angerer L M, Palis J, Nasir A, Angerer R C. Early mRNAs, spatially restricted along the animal-vegetal axis of sea urchin embryos, include one encoding a protein related to tolloid and BMP-1. Development. 1992;114:769–786. doi: 10.1242/dev.114.3.769. [DOI] [PubMed] [Google Scholar]
- 21.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urness L D, Thummel C S. Molecular interactions within the ecdysone regulatory hierarchy: DNA binding properties of the Drosophila ecdysone-inducible E74A protein. Cell. 1990;63:47–61. doi: 10.1016/0092-8674(90)90287-o. [DOI] [PubMed] [Google Scholar]
- 23.Wang M M, Reed R R. Molecular cloning of the olfactory neuronal transcription factor Olf-1 by genetic selection in yeast. Nature. 1993;364:121–126. doi: 10.1038/364121a0. [DOI] [PubMed] [Google Scholar]
- 24.Wasylyk B, Gutman A, Nicholson R, Wasylyk B. The c-ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoproteins. EMBO J. 1991;10:1127–1134. doi: 10.1002/j.1460-2075.1991.tb08053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasylyk B, Hahn S L, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 26.Wei Z, Angerer L M, Gagnon M L, Angerer R C. Characterization of the SpHE promoter that is spatially regulated along the animal-vegetal axis of the sea urchin embryo. Dev Biol. 1995;171:195–211. doi: 10.1006/dbio.1995.1271. [DOI] [PubMed] [Google Scholar]
- 27.Wei Z, Angerer L M, Angerer R C. Multiple positive cis-elements regulate the asymmetric expression of the SpHE gene along the sea urchin embryo animal-vegetal axis. Dev Biol. 1997;187:71–78. doi: 10.1006/dbio.1997.8603. [DOI] [PubMed] [Google Scholar]
- 28.Wei Z, Angerer L M, Angerer R C. The SpHE gene is down-regulated in sea urchin late blastulae despite persistence of multiple positive factors sufficient to activate its promoter. Mech Dev. 1997;67:171–178. doi: 10.1016/s0925-4773(97)00118-4. [DOI] [PubMed] [Google Scholar]
- 29.Wilson T E, Fahrner T J, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1299. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- 30.Yang Q, Angerer L M, Angerer R C. Structure and tissue-specific developmental expression of a sea urchin arylsulfatase gene. Dev Biol. 1989;135:53–65. doi: 10.1016/0012-1606(89)90157-7. [DOI] [PubMed] [Google Scholar]