Abstract
Pregnancy and the postpartum period are recognized as times of vulnerability to mood disorders, including postpartum depression and psychosis. Recently, changes in sleep physiology and sleep deprivation have been proposed as having roles in perinatal psychiatric disorders. In this article we review what is known about changes in sleep physiology and behaviour during the perinatal period, with a focus on the relations between sleep and postpartum “blues,” depression and psychosis and on sleep-based interventions for the treatment and prevention of perinatal mood disorders. The interaction between sleep and perinatal mood disorders is significant, but evidence-based research in this field is limited. Studies that measure both sleep and mood during the perinatal period, particularly those that employ objective measurement tools such as polysomnography and actigraphy, will provide important information about the causes, prevention and treatment of perinatal mood disorders.
Medical subject headings: depression, postpartum; pregnancy; sleep; sleep deprivation
Abstract
Il est reconnu que les femmes sont vulnérables aux troubles de l'humeur, y compris la dépression postpartum et la psychose, au cours de la grossesse et en période postpartum. On a posé récemment une hypothèse selon laquelle les changements de la physiologie du sommeil et la privation de sommeil joueraient un rôle dans les troubles psychiatriques périnataux. Ce manuscrit passe en revue les connaissances actuelles au sujet des changements de la physiologie et du comportement du sommeil en période périnatale, avec accent sur le lien entre le sommeil et la dépression des premiers jours du postpartum, la dépression postpartum et la pychose pospartum, ainsi que sur les interventions axées sur le sommeil pour le traitement et la prévention des troubles de l'humeur périnataux. L'interaction entre le sommeil et les troubles de l'humeur périnataux est importante, mais peu de recherches factuelles ont été effectuées dans ce domaine. La réalisation d'études mesurant tant le sommeil que l'humeur au cours de la période périnatale, surtout si elles font appel à des outils de mesure objective comme la polysomnographie et l'actigraphie, produira des données importantes sur les causes, la prévention et le traitement des troubles de l'humeur périnataux.
Introduction
Pregnancy and the postpartum period are recognized as times of vulnerability to psychiatric disorders, including mood, anxiety and psychotic disorders. The causes of these conditions are far from clear, although numerous hypotheses have been put forward. Many of these focus on the dramatic changes in peripheral concentrations of sex steroids that occur during pregnancy and in the immediate postpartum period.1 Others draw attention to the predictive power of nonbiologic variables, such as lack of social support, marital or relationship conflict, and the stress of child care (see Robertson et al2 for review).
More recently, changes in sleep physiology and sleep deprivation have been proposed as having roles in perinatal psychiatric disorders. In this article, we first review what is known about changes in sleep physiology and behaviour during pregnancy and the early weeks and months post partum. We then review studies that have examined potential relations between sleep and postpartum mood disorders, including the postpartum “blues,” depression and psychosis. We close with a discussion of potential applications of this information to the prevention and treatment of perinatal mood disorders.
Methods
To identify relevant articles for this review, searches of the online databases MEDLINE and EMBASE were conducted using combinations of the search terms “sleep,” “pregnancy,” “puerperium,” “affect,” “mood,” “depression,” “psychosis” and “psychotic disorders.” Additional articles were identified by scanning the reference lists of the retrieved articles. All English- and French-language articles that reported original data related to maternal sleep and postpartum blues, depression or psychosis, as well as articles reporting original data for sleep-based interventions targeting one of these 3 conditions, were included in this review.
Why might sleep play a role in perinatal mood disorders? A review of sleep physiology
Sleep represents a dramatic change in physiologic state — one that occurs nightly. Sleep results from alterations in the balance of major neurotransmitter systems in the brain. Serotonin, norepinephrine, histamine, dopamine, melatonin, γ-aminobutyric acid and acetylcholine are all major players in the coordination of sleep and wake behaviours.3,4
Sleep can be defined and quantified with polysomnography (a basic type of sleep study), which collects physiologic information by various methods, including electroencephalography (EEG), electro-oculography and electromyography, and plots these variables against time. Additional physiologic measures including cardiac and respiratory function are commonly measured at the same time.
Not all time spent asleep is equal. Classically, sleep is characterized by an orderly progression of 4 non–rapid eye movement sleep states (numbered 1–4) followed by rapid eye movement (REM) sleep. This cycle generally takes about 90 minutes and repeats itself 4 or 5 times throughout the night, with an increasing amount of REM sleep in each successive cycle. A general summary of sleep stage characteristics is provided in Table 1.
Table 1
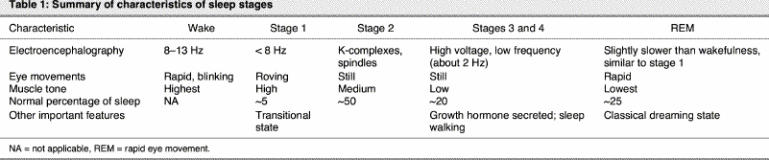
Stage 1 is typically thought of as a transitional sleep state, and excessive stage 1 sleep is a marker of poor-quality sleep. If something interrupts sleep (such as a baby crying), the person typically rises to wakefulness and then re-enters transitional stage 1 sleep. Stage 1 represents less than 5% of total sleep during the night, whereas stage 2 represents about 50%. Stage 2 sleep has a characteristic electroencephalographic signature, the K-complex, which consists of high-voltage negative and then positive discharges with a trailing spindle (high-frequency, low-voltage discharge of approximately 0.5 seconds).
Stages 3 and 4 are biologically similar and are sometimes referred to collectively as slow-wave sleep or delta sleep. They represent homeostatic or restorative sleep. For example, when someone is sleep deprived and has the opportunity to “pay back” sleep, he or she typically enters this stage readily.5 As the neurochemical content of the brain changes over the course of the night, slow-wave sleep is characterized by a reduction in amines such as norepinephrine and serotonin and an even greater relative reduction in acetylcholine.6 People tend to feel most rested after having ample slow-wave sleep.7
REM sleep is a distinct state associated with vivid dreaming. It is not the most restorative sleep state and is, in fact, physiologically unstable. Breathing tends to become more irregular in REM sleep, and cardiac arrhythmias may be more common. REM sleep is characterized by significant reductions in norepinephrine and serotonin and lesser decreases in acetylcholine in the brain relative to wakefulness, such that REM sleep is more cholinergic than slow-wave sleep. The REM sleep state most closely resembles wakefulness according to EEG and neurochemical criteria. Consequently, people often find it easier to waken out of REM sleep than other sleep states.8 Despite some similarities to wakefulness, however, significant regional differences in brain function occur in this state, with marked increases in limbic activity (“the emotional brain”) and reductions in activity in the prefrontal cortex (“the rational brain”), which might account for some features of dreams.9
Circadian (time-of-day) factors are also important for the expression of REM sleep. Thus, the sleep obtained in a daytime nap may not necessarily be of the same restorative value as sleep obtained at night. It is unusual to have REM sleep during daytime naps, for example, although this can occur in cases of significant sleep deprivation and in narcolepsy.10
Many of the major neurotransmitter systems involved in the regulation of sleep are responsible for multiple functions in the brain, including functions relevant to psychiatric disorders. It is therefore not surprising that significant interactions could occur between sleep and psychiatric disorders. As reviewed by Buysse et al,11 several notable research findings support such interactions, including the following:
Symptoms of insomnia are associated with significant risk for new-onset depression and anxiety disorders.
Sleep disturbances are among the most common symptoms of psychiatric disorders.
Manipulations of sleep and circadian rhythms are useful treatments for mood disorders.
Objective assessment of the characteristics of sleep in patients with depression, by polysomnographic methods, has revealed consistent differences from control populations in a number of sleep variables. One of the most robust findings has been that this disorder is characterized by a short REM latency (the time it takes to enter REM sleep from sleep onset).12 All antidepressant therapies, including psychotherapy, pharmacotherapy and electroconvulsive therapy, have demonstrated an improvement in depression associated with an increase in REM latency. Other characteristics of sleep that have been observed in patients with depression include difficulties initiating sleep, decreased sleep continuity (i.e., increased number of awakenings), decreased slow-wave sleep (i.e., decreased percentage of stage 3 or 4 sleep) and enhanced REM sleep.11
Sex differences in sleep physiology and behaviour have been identified, both in humans and in other species. In particular, higher amplitude of slow-wave activity and slower age-related decline in slow-wave activity have been reported in women.13 In addition, sleep is associated with hormonal changes.14 For example, thyroid-stimulating hormone is suppressed by sleep, whereas growth hormone is expressed during slow-wave sleep, and sleep deprivation blunts peaks in secretion of the latter. Cortisol follows a complex circadian rhythm, with increasing concentrations toward the end of the night. In contrast, prolactin generally peaks during sleep, but this peak can be reduced by remaining awake. Prolactin has also been noted to increase REM sleep in animals.14 Oxytocin appears to have dual function in sleep, at least in nonhuman animals. Under stress-free conditions, basal oxytocin may promote sleep, whereas under stress, oxytocin may increase wakefulness.15
Our understanding of hormonal influences on sleep regulation remains limited. The available data have been reviewed recently.13 It is notable that progesterone and its 5α-reduced metabolites, including allopregnanolone, have significant sedative properties.16 Estrogen, on the other hand, has generally excitatory effects in the nervous system and has been associated with a decrease in the expression of REM sleep.17 Concentrations of estrogen, progesterone and the 5α-reduced progesterone metabolites increase several-fold during pregnancy and drop to prepregnancy levels within a few days after childbirth;18 these changes could therefore be implicated in the fatigue and insomnia that have been observed in healthy pregnant women during the third trimester of pregnancy.19
Conflicting data about the role of sex hormones in sleep has likely resulted from differences in methodologic techniques. For example, some studies have assessed effects of pharmacologic hormonal therapy (e.g., with pregnenolone) on sleep in men;20 this is obviously much different from studying sleep on the basis of physiologic changes in the menstrual cycle in women.21,22 Similarly, the effect of exogenous administration of high doses of estrogen in the form of synthetic compounds may be very different from that of lower doses of naturally occurring compounds. In addition, it has been difficult to interpret the effects of estrogen in the context of progesterone co-administration in typical hormone therapy regimens.
Pregnancy-related changes in sleep physiology
Pregnancy and the postpartum state lend further variables to this complex equation. Given the high concentrations of endogenous progesterone typical of pregnancy, it is not surprising that a loss of its sedative effect may increase insomnia post partum, when progesterone concentrations drop. This increase in insomnia may also be related to the anxiolytic properties of progesterone and its metabolites. A helpful analogy might be that of benzodiazepine withdrawal, which is associated with a state of hyperarousal.
A recent systematic review of sleep in pregnancy identified significant knowledge gaps.23 Most of the data that have been collected about sleep in pregnancy have been devoid of objective physiologic measures. A number of studies have looked at the macroarchitecture of sleep using self-report surveys and have identified a greater incidence of sleepiness and insomnia relative to the nonpregnant state. Other studies, however, have contradicted these findings. Part of the problem with many of these studies has been the limited assessment of daytime napping behaviours, which may contribute significantly to total sleep in pregnancy. Furthermore, misperception of sleep state is common,24 and objective measures will probably be needed to provide clear answers. A full discussion of sleep disorders is beyond the scope of this article, but it is important to note that sleep disorders such as sleep disordered breathing25 and restless legs syndrome26 are surprisingly common in this population and may have consequences for both maternal and fetal health. In many cases, these disorders are amenable to simple therapeutic interventions.
Polysomnographic studies have identified a number of features that are characteristic of poor-quality sleep in pregnancy and post partum, including reduced sleep efficiency (time spent sleeping as a proportion of the total time spent in bed), an increase in stage 1 sleep and a reduction in slow-wave sleep.27,28,29,30,31
In an early study32 nightly awakenings increased progressively through pregnancy, but did not entirely resolve after delivery. This finding may not be surprising given the sleep disruption that a newborn may induce (including feeding requirements); however, the study found that the sleep disruption persisted even in a setting where the infant slept away from the mother.32
In another study of 33 pregnant women who underwent polysomnography in their own homes, total sleep time increased significantly in the first trimester but had decreased again by the third trimester, with less slow-wave sleep and more awakenings, which resulted in reduced sleep efficiency. The sleep difficulties were worst in the first month post partum.30 More recently, activity monitoring of 10 women from the 5th week before delivery to the 15th week post partum demonstrated a decrease in total sleep time, decreased sleep efficiency and increased time awake after sleep onset.24
Reported effects of pregnancy on REM sleep and REM latency have been variable, likely because of methodologic difficulties, including failure to account for the total duration of sleep and naps. Despite common reports of fatigue, no studies have quantified sleepiness with objective measures such as the multiple sleep latency test or the maintenance of wakefulness test. The issue of whether napping in the daytime contributes to a reduction in sleep “debt” caused by reduced sleep time at night has also not been addressed. However, taken together, the objective nocturnal sleep studies suggest a progressive worsening of sleep quality through pregnancy, with significant sleep problems after delivery, which may persist for at least the first 3 months post partum.
Sleep and the “baby blues”
As many as 85% of childbearing women report mild and transient mood disturbances during the first week post partum, with a peak in negative mood on postpartum day 5.33 Although the “baby blues” have been associated with risk for a postpartum depressive episode,34 for most women, the symptoms do not impair functioning and are generally regarded as a normal consequence of the physical and emotional strains associated with labour and delivery.
Fatigue is nearly universal among women during the first days post partum, leading some to suggest that it may be associated with the mood lability and emotional reactivity observed in many women at this time.35 In a study of 30 primiparous postpartum mothers and 28 nonpostpartum mothers, the negative mood observed in the postpartum group during the first week after childbirth was no longer statistically significant after the data were controlled for time awake at night.36 This result suggests that the amount of time new mothers spend awake at night may determine whether or not they experience negative mood symptoms, which are often classified as the blues. One study of 35 psychologically healthy women who had undergone vaginal delivery found that participants' ratings of fatigue (as measured on a Likert-type scale ranging from not tired to total exhaustion) were significantly correlated with scores on the Beck Depression Inventory at both 2 days and 2 weeks post partum.37 There was no correlation between ratings of fatigue and depression at 6 weeks post partum. However, it is unclear whether scores on the 3 component items of the Beck Depression Inventory that relate to fatigue might be driving the early postpartum correlations.
Another line of research has examined a potential relation between loss of sleep in late pregnancy, loss of sleep due to nighttime labour and delivery, and negative mood in the first week post partum. In a prospective study of 63 women, those with a nighttime labour scored consistently higher on 2 scales of the postpartum blues during 8 of the first 9 postpartum days.38 Self-reported sleep disruption in the third trimester of pregnancy was also significantly associated with scores on various blues scales, although, as the authors noted, prenatal depression may be a confounding variable that could explain these findings. There was no relation between sleep disruption and mood scores on the subsequent day for any of the first 9 postpartum days. However, one might expect the effect of sleep loss to be cumulative, rather than apparent immediately after a night of lost sleep. Another study of 28 primiparous women failed to find a significant relation between sleep loss during the intrapartum period and negative mood in the first week post partum; however, this might have been the result of assessing mood too early in the postpartum period (postpartum day 1) or methodologic problems, including high attrition rates.39
In summary, there is limited evidence that sleep loss in late pregnancy, the intrapartum period and the first week post partum is associated with the “baby blues.” It is possible that chronic sleep deprivation mediates the relation between the blues and postpartum depression: it may be that for most women, mood improves in parallel with a gradual reduction in nighttime sleep disturbances. For those women who continue to experience disturbed sleep, negative mood may fail to remit and may develop into an episode of depression.36 Research is needed to test this hypothesis.
Sleep and postpartum depression
Although many women report negative mood during the first week post partum, only 10%–15% of mothers meet diagnostic criteria for an episode of major depression in the early weeks post partum. This more serious condition has come to be known as postpartum depression (PPD). Criteria set out in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), require that symptoms of major depression with postpartum onset begin within 4 weeks of childbirth;40 however, reports in the literature suggest that episodes of depression are common up to 2 years after delivery and that these later episodes should also be considered postpartum depression.41,42,43 Children of mothers with untreated PPD are more likely than children of nondepressed mothers to suffer from failure-to-thrive, behavioural problems and suboptimal cognitive, emotional and social development.44 These consequences for the children, together with consequences for the mother, in particular the high risk for relapse of depression,45 make PPD a serious mental health risk for perinatal women.
Maternal depression and the child's sleep problems
One line of evidence for a relation between sleep disruption and postpartum depression has focused on mood in the mothers of children with sleeping problems, including prolonged and frequent night waking and difficulty settling at night.46 An association between maternal depression and children's sleeping problems has been observed consistently,46,47,48,49,50 and improvements in the child's sleep have been associated with improvements in maternal depression.46,51 Although maternal sleep parameters have seldom been measured in these studies, it has been hypothesized that problem sleeping behaviour in the child could result in chronic sleep disruption for the mother, which could ultimately trigger maternal depression.
In a cross-sectional survey of 109 consecutive admissions to a mother–baby unit for infant feeding or settling problems, clinically significant fatigue (as measured with the Profile of Mood States Fatigue—Inertia subscale) was almost universal, but was distinct from clinical depression on the basis of cluster analysis.49 In one study that collected self-reported data on maternal sleep quality for a subset of participants, an intervention that successfully resolved infant sleep problems was associated with significantly better subjective maternal sleep quality.51 Possible relations between maternal depression scores and ratings of sleep quality were not discussed in that study. However, in a related study, when self-rated maternal sleep quality was controlled in the analysis, the association between infant sleep problems and maternal depressive symptoms was no longer statistically significant.48
While these data are suggestive, the “chicken and the egg” have not yet been clearly separated,46 and it is also possible that children of depressed mothers are simply more prone to sleeping problems, perhaps as a response to a perceived lack of emotional investment in the child or maternal difficulties in consistently handling resistance in settling or night awakenings.47 The children of depressed mothers may also be exhibiting early symptoms of genetically transmitted psychiatric or sleep disorders. Furthermore, infant sleeping problems may disrupt the sleep cycles of other family members, including the mother's partner and older children, and such disruptions could further affect maternal sleep. The direction of the relation between infant sleep problems and maternal depression is further muddied by the findings of Armstrong et al,46 who reported that children's sleep problems were associated with retrospectively assessed maternal sleep difficulties during the prenatal period (particularly the third trimester). The authors raised the following question: Are women who experience sleep disruption during both pregnancy and the postpartum period more likely to experience depression, or does sleep disruption in pregnancy alter fetal physiology and sleep cycles, leaving the infant predisposed to sleep problems early in life?
Methodologic difficulties also complicate interpretation of these data. Many studies have relied upon maternal reporting of children's sleep problems, and reporting or remembering bias associated with maternal depression is possible. Or, as Zuckerman et al52 have noted, it is possible that depressed mothers spend more time awake at night and therefore have more opportunity to observe child awakenings and other problems. Further research, including more objective assessments of maternal and fetal or neonatal sleep parameters, is required for a better understanding of the relation between an infant's or child's sleep problems and maternal depression.
Polysomnographic and EEG studies
More objective evidence for a possible relation between sleep disruption and depressive symptoms in the perinatal period comes from EEG studies, summarized in Table 2.
Table 2
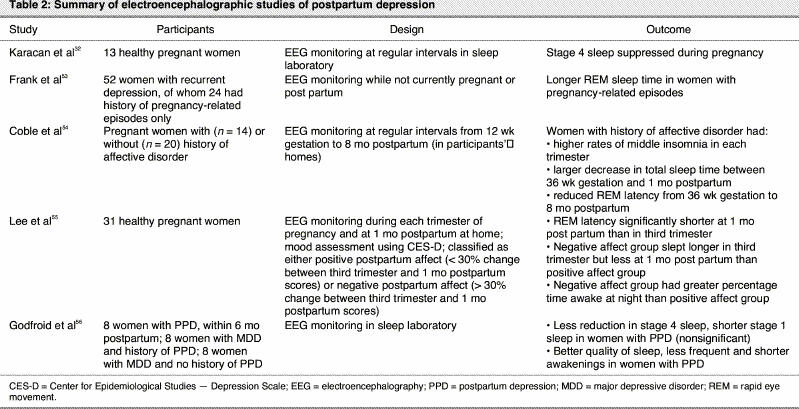
In 1969, Karacan et al32 first proposed that changes in EEG sleep patterns observed in pregnant and postpartum women could explain their vulnerability to emotional disturbances. On the basis of the suppression of stage 4 sleep observed in his studies of healthy pregnant patients, he suggested that subclinical depression might exist in pregnant women.
Twenty years later, Frank et al53 examined women with recurrent depression to identify any potential differences between those with pregnancy-related affective episodes and those with no episodes related to pregnancy or the postpartum period. The women with a history of pregnancy-related episodes showed longer REM sleep time, and this difference was largely accounted for by the women with a history of only postpartum episodes.
Other research has searched for potential changes in sleep physiology during pregnancy or the postpartum period and has associated these changes with mood disturbances. One study examined differences in EEG characteristics between perinatal women with a personal history of affective disorder and those with no psychiatric history.54 Although women in both groups experienced sleep disturbances, particularly in the first 2–3 months postpartum, women with a history of affective disorder experienced greater changes in total sleep time and in reduction of REM latency.54 Changes to total sleep time were apparent beginning early in pregnancy: women with a history of affective disorder reported higher rates of sleep maintenance problems or “middle” insomnia (waking in the night) in each trimester than did control subjects, despite the fact that women with a history of affective disorder spent more time in bed and had longer total sleep times. Between 36 weeks' gestation and 1 month postpartum, decreases in total sleep time were 2 to 3 times greater among women with a history of affective disorder. In those women, REM latency was reduced to values previously reported for young people with remitted depression (about 70 minutes). Surprisingly, REM latency remained reduced throughout the postpartum period, even after sleep continuity had improved. There were no differences in frequency of daytime napping during either pregnancy or the postpartum period.54 The authors suggested that among women with a history of affective disorder, the sleep system may be hypersensitive to the changes associated with normal pregnancy. Furthermore, since reduced REM latency is thought to be a marker for vulnerability to affective disorder, changes in sleep may explain in part the relation between psychiatric history and vulnerability to postpartum depressive episodes. However, they noted that chronic sleep disturbance may be a necessary but insufficient condition for the development of a postpartum affective disorder: despite the electroencephalographic changes observed in this study, only 1 of the 14 women with a history of an affective disorder experienced a postpartum depressive episode.
One other study has examined potential relations between polysomnography and mood in healthy postpartum women. Lee et al55 studied sleep during each trimester of pregnancy and at 1 month post partum. Self-reported mood state was most negative at 1 month post partum. Participants were classified as having either positive postpartum affect (< 30% change from third trimester depression scores) or negative postpartum affect (> 30% change from third trimester depression scores). It is unclear why the authors chose to separate the groups on the basis of change scores, rather than raw scores on the Center for Epidemiological Studies — Depression Scale (CES-D) at the postpartum assessment, and the mean CES-D scores for each group were not provided. Participants in both groups had shorter REM sleep latency at 1 month post partum than during the third trimester. However, the negative affect group had less REM sleep, whereas the positive affect group had more REM sleep at 1 month post partum than during the third trimester.55 Differences also emerged in total sleep time: the negative affect group slept on average 20 minutes longer in the third trimester, but 80 minutes less at 1 month post partum. Although time awake at night was similar between the groups, total time awake at 1 month post partum averaged 25% for the negative affect group and 16% for the positive affect group. The authors concluded that negative mood observed in the early postpartum period may be the result of fatigue, and suggested that these women might be better classified as clinically fatigued than clinically depressed.55
Only 1 study has specifically examined sleep in women currently suffering a postpartum depressive episode. That study compared EEG recordings for women currently in an episode of postpartum depression (onset within 4 weeks post partum and not more than 6 months post partum at the time of study), women currently in an episode of nonpostpartum major depression with a previous history of postpartum depression, and women currently in an episode of nonpostpartum major depression with no previous history of postpartum depression.56 The women in the nonpostpartum groups had not been pregnant within the previous 3 years, and the women in the postpartum group were not breast-feeding. EEG sleep was measured after 2 nights of habituation in a sleep laboratory; presumably, postpartum women were admitted without their infants. The women with postpartum depression showed less of a reduction in stage 4 sleep and shorter stage 1 sleep, although neither finding reached statistical significance after correction for multiple comparisons. The women with postpartum depression also reported better quality of sleep, with less frequent and shorter awakenings.56 The authors suggest that perhaps sleep is less disturbed in postpartum women with depression than in women with depression unrelated to pregnancy. Unlike the study of Frank et al,53 no differences were observed between the nonpostpartum women with and without previous history of postpartum depression.
Some limitations of this study should be noted. The design enabled the authors to separate the effects of the postpartum nature of the episode from the effects of current depression; however, the lack of a healthy postpartum control group limits the ability to isolate any effects potentially related to postpartum status. In addition, because women were presumably admitted to the sleep laboratory without their infants, it is possible that the sleep studied was not typical of their usual sleeping patterns (i.e., they would likely be wakened by the infant during most nights at home). As a result, the unusual sleep parameters, including better subjective quality of sleep reported for the postpartum group with depression, could be the result of sleep compensation in a group of women who were sleep deprived. The degree to which these data reflect sleep quality in postpartum women with depression in a more naturalistic setting (including in women who may be breast-feeding) is therefore questionable.
On the basis of these limited data, there do appear to be some consistent differences in EEG sleep and sleep time among women who are either at risk for postpartum depression or who report depressed mood in the postpartum period. Specifically, reduced REM latency, increased total sleep time during pregnancy and decreased total sleep time in the postpartum period appear to be characteristic of women who are at risk for an episode of postpartum depression.
An important question is whether there is a causal relation between EEG sleep parameters and postpartum depression, and if so, in which direction does it act? The studies of women with risk factors for postpartum depression (in particular, women with previous episodes of pregnancy-related affective disorder) suggest that these sleep changes may represent an underlying vulnerability to the disorder. This conclusion is congruent with studies of sleep disturbances and affective disorders in nonperinatal populations, which have found that insomnia and poor sleep quality are associated with risk for subsequent clinical depression.57
Several mechanisms have been proposed to account for the association between sleep and affective disorders in nonperinatal populations. One of these proposed mechanisms, the “social zeitgeber” hypothesis, seems particularly relevant to perinatal affective disorders. This hypothesis proposes that psychosocial changes that disrupt social routines can lead to disruption in biologic rhythms and, ultimately, depression.58 The significant psychosocial changes associated with giving birth to and caring for a new baby have been identified as social zeitgebers that could act in such a manner to trigger depression.59 Further longitudinal polysomnographic studies are therefore warranted to confirm the hypothesis that sleep disruptions during pregnancy and the early postpartum period are associated with future risk for postpartum depression.
Sleep and postpartum psychosis
Approximately 0.1%–0.2% of childbearing women experience psychosis during the first weeks post partum. Postpartum psychosis usually has severe effects on function and is associated with high risk of suicide and the possibility of infanticide.60 As such, early detection and rigorous treatment are of paramount importance. Many women who experience postpartum psychosis go on to have a recurrent illness consistent with bipolar disorder.61,62 Furthermore, approximately 20% of women with a pregravid diagnosis of bipolar disorder are reported to experience mania in the postpartum period,63 features of which often resemble a postpartum psychotic episode. As such, postpartum psychosis has been hypothesized as a subtype of bipolar affective illness.
Even historical accounts of postpartum psychosis or “puerperal insanity” noted that “the almost universal early symptom of puerperal cases is loss of sleep.”64,65 Insomnia has also been noted as a prominent symptom in contemporary cases of postpartum psychosis, with prevalence estimates of 42%–100% of cases.66,67 Recently, Sharma et al68 have argued that sleep loss may be the final common pathway in the development of postpartum psychotic episodes. Data from a chart review of 21 patients with postpartum psychosis who underwent vaginal delivery support this hypothesis: when matched for age and parity with a control group of women who delivered at the same hospital, women who experienced psychosis had significantly longer duration of labour and significantly more of them had a night-time delivery.68 Further research using a prospective design is required to confirm these findings; however, an association between insomnia, sleep loss and postpartum psychosis is consistent with what is known about sleep loss as a precipitant to mania in nonperinatal patients.69
Sleep interventions for prevention and treatment of perinatal psychiatric disorders
If sleep deprivation plays a causal role in the development of perinatal psychiatric disorders, then one might anticipate that reduction of sleep deprivation in new mothers could in turn reduce the risk for postpartum blues, depression and psychosis.70 Alternatively, modulation of sleep–wake patterns could offer relief to women in whom symptoms of these disorders have already developed. Little research to date has addressed this question.
Prevention
A number of practical strategies have been recommended to minimize sleep deprivation during the postpartum hospital stay, including demand feedings rather than routine feedings, maternal choice regarding whether the infant rooms-in or stays in the nursery, increased duration of the postpartum hospital stay and limited visiting hours.35,37 Using these strategies and others, the Women's Health Concerns Clinic at St. Joseph's Healthcare has developed a preventive intervention that is routinely offered to patients who present with high risk for postpartum depression (e.g., those with personal or family history of depression, those with subclinical symptoms of depression during pregnancy). These patients are offered a hospital stay of up to 5 days, a private hospital room and rooming-out of the infant at night. Women who choose to breast-feed are encouraged to pump milk during the day for nighttime feedings, use formula for night feedings or ask to be woken only when necessary to feed. Benzodiazepines are prescribed if required to encourage consistent nighttime sleep onset for the first postpartum week.
The effectiveness of this intervention has been evaluated in a chart review.71 The charts of all 179 antepartum patients seen in the clinic between 1996 and 2001 were reviewed, and the patients were contacted by telephone. Data on use of the intervention, the presence and severity of postpartum depression, use of antidepressant medication and the need for psychiatric postpartum admissions were gathered. The average score on the Edinburgh Postnatal Depression Scale was 11, lower than the cut-off usually required to establish the diagnosis of an episode of depression. Furthermore, only 6 mothers required a psychiatric admission.71 While these results are promising, further evidence is necessary, with greater emphasis on adherence to protocol, before this intervention can be routinely recommended. Plans for a randomized controlled trial are under way.
There is also some evidence, largely unpublished, for prevention of sleep deprivation as a prophylactic strategy for women at risk for postpartum psychosis. For these women (in particular those with bipolar disorder), early identification and treatment of sleep impairment has been recommended.72 This might involve adding a benzodiazepine as an adjunct to a mood stabilizer. Sharma and Mazmanian72 have reported preliminary success using olanzapine to prevent postpartum psychosis in women with bipolar disorder during pregnancy, though these data have not yet been published.
Although conventional sedative hypnotic medications do not produce normal sleep (in that restorative slow-wave sleep is typically severely restricted with most such agents), these drugs may be helpful in kick-starting a normal circadian sleep cycle. Newer ultra-short-acting non-benzodiazepine agents such as zopiclone, zolpidem and zaleplon have been proposed as better options in nonperinatal populations73 and are thought to have less effect on sleep architecture. Considerations about the safety of exposure to these medications during breast-feeding (and interaction with birth control, as well as future potential teratogenicity) have yet to be adequately addressed. Furthermore, the safety of the infant must be ensured, as sedative agents may limit the mother's ability to respond to infant care needs. Despite these limitations, short-term use of these medications in certain situations may provide significant benefit, particularly where there is prominent anxiety.
It has been suggested that for women at very high risk for postpartum psychosis (e.g., those with a history of psychotic episodes associated with previous pregnancies) daytime labour may be preferable, and therefore induction or surgical delivery may be considered.72 Controlled research is required to support these invasive interventions.
Treatment
The therapeutic use of critically timed sleep deprivation (particularly at the end of the night, when REM sleep is more prominent) temporarily improved mood in a pilot study of 9 patients with postpartum mood disorders.74 This finding stands in contrast to the data presented here, which identify sleep deprivation as a potential risk factor for depressed mood, and requires replication. However, it may be that sleep deprivation late in the night is effective in resetting a new mother's circadian clock and in allowing restorative “catch-up” sleep when sleep is later allowed. Sleep deprivation therapies may have little utility in clinical practice, however, since in populations of nonpregnant women with depression, the effects of sleep deprivation on symptoms of depression have been short-lived, with recurrence of symptoms within 1 week.75 In the study of perinatal patients,74 the response to sleep deprivation in 1 woman was successfully prolonged by administering lithium for 2 months after the sleep deprivation treatment, and another patient remained well for 5 months of follow-up with no supplemental pharmacotherapy; follow-up data on the other 7 participants were not reported. Recently, adjunctive treatment with transcranial magnetic stimulation has been proposed to prolong the effect of sleep deprivation therapy;76 this method may be safe in pregnancy,77 although further study is required.
In one case series, a partial sleep deprivation therapy was applied to 3 women with postpartum psychosis. In contrast to the study of sleep deprivation in depression,74 the patients' condition worsened in response to this protocol: 2 of the 3 patients experienced mania, and the third experienced hypomania. All 3 patients improved after recovery sleep, but required further treatment with mood stabilizers.78 The results of this small study provide support for the hypothesis that sleep deprivation may be a precipitant of mania and should be avoided in women at risk for postpartum psychosis.
Light is important in the circadian regulation of sleep, and light therapies have been proposed as a treatment option for perinatal women. An open study found that in 16 pregnant patients with major depression who were treated with phototherapy for 3–5 weeks, mood scores on the Hamilton Rating Scale for Depression improved.79 These findings were recently replicated in a small double-blind placebo-controlled trial (n = 10): although the difference between the active treatment and placebo groups was not statistically significant at the end of the 5-week trial, women who received the active treatment had significantly lower scores on the Hamilton Rating Scale for Depression after an additional 5 weeks of light therapy.80 Further research is warranted to determine whether light therapies might be suitable for patients who are unable or unwilling to take medications.
Treatment of underlying sleep disorders is beyond the scope of this review, but if an underlying sleep disorder is identified, directed therapy may improve sleep quality and quantity. Important examples of sleep disorder treatment issues in pregnancy include the use of continuous positive airway pressure for sleep apnea syndrome81 and the potential use of iron supplementation in restless legs syndrome.30
Limitations
There were methodologic weaknesses in many of the studies included in this review that could limit the extent to which their findings can be generalized. The limitations of self- reported measures of sleep quantity and quality have been noted above. However, polysomnography studies typically place significant burden upon research participants, particularly those who are already burdened with caring for a new infant. Therefore, the women who feel able and agree to participate in perinatal polysomnography studies are unlikely to be representative of all perinatal women. Finally, we have focused here on the relation between sleep and postpartum blues, depression and psychosis. The extent to which a relation exists between sleep and other perinatal psychiatric disorders, including bipolar disorder and anxiety disorders, requires further research.
Conclusions
The literature reviewed here indicates that the interaction between sleep and perinatal mood disorders is significant and worthy of further study. Reduction of sleep deprivation during the perinatal period may offer a cost-effective method for the prevention, and potentially treatment, of postpartum depression and psychosis. Studies that measure both sleep and mood during the perinatal period, particularly those that employ objective measurement tools such as polysomnography or actigraphy, will provide important information about the causes, prevention and treatment of perinatal mood disorders.
Acknowledgments
The authors wish to thank Patricia Donoghue for her assistance in the preparation of this manuscript. This work was supported in part by a postdoctoral fellowship to Lori E. Ross from the Father Sean O'Sullivan Research Centre, St. Joseph's Healthcare, Hamilton.
Footnotes
Contributors: The review was conceived and critically revised by Dr. Steiner. Drs. Ross and Murray conducted the literature search, reviewed the articles and drafted the review. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Lori E. Ross, Women's Mental Health and Addiction Research Section, Centre for Addiction and Mental Health, 250 College St., Rm. 601A, Toronto ON M5T 1R8; fax 416 979-6811; l.ross@utoronto.ca
Submitted Aug. 27, 2004; Revised Dec. 15, 2004; Accepted Jan. 4, 2005
References
- 1.Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity — adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res 2001;133:1-38. [DOI] [PubMed]
- 2.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 2004;26:289-95. [DOI] [PubMed]
- 3.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24:726-31. [DOI] [PubMed]
- 4.Jones B. Basic mechanisms of sleep–wake states. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 2nd ed. Philadelphia: WB Saunders; 1994. p. 145-62.
- 5.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res 1993;626:190-9. [DOI] [PubMed]
- 6.Stickgold R. Sleep: off-line memory reprocessing. Trends Cogn Sci 1998;2:484-92. [DOI] [PubMed]
- 7.Heinzer R, Gaudreau H, Decary A, Sforza E, Petit D, Morisson F, et al. Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: contribution to daytime sleepiness. Chest 2001;119:1807-13. [DOI] [PubMed]
- 8.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002;25:120-38. [DOI] [PubMed]
- 9.Braun AR, Balkin TJ, Wesensten NJ, Gwadry F, Carson RE, Varga M, et al. Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science 1998;279:91-5. [DOI] [PubMed]
- 10.Mitler MM, Van den Hoed J, Carskadon MA, Richardson G, Park R, Guilleminault C, et al. REM sleep episodes during the Multiple Sleep Latency Test in narcoleptic patients. Electroencephalogr Clin Neurophysiol 1979;46(4):479-81. [DOI] [PMC free article] [PubMed]
- 11.Buysse DJ, Nofzinger EA, Keshavan MS, Reynolds CF, Kupfer DJ. Psychiatric disorders associated with disturbed sleep and circadian rhythms. In: Turek FW, Zee PC, editors. Regulation of sleep and circadian rhythms. New York: Marcel Dekker Inc.; 1999. p. 597-641.
- 12.Riemann D, Berger M, Voderholzer U. Sleep and depression — results from psychobiological studies: an overview. Biol Psychol 2001;57:67-103. [DOI] [PubMed]
- 13.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep 1999;22:540-55. [PubMed]
- 14.Steiger A. Sleep and endocrinology. J Intern Med 2003;254:13-22. [DOI] [PubMed]
- 15.Lancel M, Kromer S, Neumann ID. Intracerebral oxytocin modulates sleep–wake behaviour in male rats. Regul Pept 2003;114:145-52. [DOI] [PubMed]
- 16.Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, et al. Menstrual cycle effects on cortical excitability. Neurology 1999;53:2069-72. [DOI] [PubMed]
- 17.Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res 1996;734:275-85. [PubMed]
- 18.Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev 1990;11:124-50. [DOI] [PubMed]
- 19.Zib M, Lim L, Walters WA. Symptoms during normal pregnancy: a prospective controlled study. Aust N Z J Obstet Gynaecol 1999;39:401-10. [DOI] [PubMed]
- 20.Steiger A, Trachsel L, Guldner J, Hemmeter U, Rothe B, Rupprecht R, et al. Neurosteroid pregnenolone induces sleep-EEG changes in man compatible with inverse agonistic GABAA-receptor modulation. Brain Res 1993;615:267-74. [DOI] [PubMed]
- 21.Parry BL, Mendelson WB, Duncan WC, Sack DA, Wehr TA. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res 1989;30:285-303. [DOI] [PubMed]
- 22.Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol 2001;530:565-74. [DOI] [PMC free article] [PubMed]
- 23.Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med 2001;134:396-408. [DOI] [PubMed]
- 24.Kang MJ, Matsumoto K, Shinkoda H, Mishima M, Seo YJ. Longitudinal study for sleep–wake behaviours of mothers from pre-partum to post-partum using actigraph and sleep logs. Psychiatry Clin Neurosci 2002;56:251-2. [DOI] [PubMed]
- 25.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest 2000;117:137-41. [DOI] [PubMed]
- 26.Goodman JD, Brodie C, Ayida GA. Restless leg syndrome in pregnancy. BMJ 1988;297:1101-2. [DOI] [PMC free article] [PubMed]
- 27.Karacan I, Heine W, Agnew HW, Williams RL, Webb WB, Ross JJ. Characteristics of sleep patterns during late pregnancy and postpartum periods. Am J Obstet Gynecol 1968;101:579-86.
- 28.Hertz G, Fast A, Feinsilver SH, Albertario CL, Schulman H, Fein AM. Sleep in normal late pregnancy. Sleep 1992;15:246-51. [DOI] [PubMed]
- 29.Driver HS, Shapiro CM. A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep 1992;15:449-53. [DOI] [PubMed]
- 30.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol 2000;95:14-8. [DOI] [PubMed]
- 31.Schorr SJ, Chawla A, Devidas M, Sullivan CA, Naef RW 3rd, Morrison JC. Sleep patterns in pregnancy: a longitudinal study of polysomnography recordings during pregnancy. J Perinatol 1998;18:427-30. [PubMed]
- 32.Karacan I, Williams RL, Hursch CJ, McCaulley M, Heine MW. Some implications of the sleep patterns of pregnancy for postpartum emotional disturbances. Br J Psychiatry 1969;115:929-35. [DOI] [PubMed]
- 33.Kendell RE, McGuire RJ, Connor Y, Cox JL. Mood changes in the first three weeks after childbirth. J Affect Disord 1981;3:317-26. [DOI] [PubMed]
- 34.Cox JL, Connor Y, Kendell RE. Prospective study of the psychiatric disorders of childbirth. Br J Psychiatry 1982;140:111-7. [DOI] [PubMed]
- 35.Errante J. Sleep deprivation or postpartum blues? Top Clin Nurs 1985;6:9-18. [PubMed]
- 36.Swain AM, O'Hara MW, Starr KR, Gorman LL. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol 1997;90:381-6. [DOI] [PubMed]
- 37.Gardner DL. Fatigue in postpartum women. Appl Nurs Res 1991;4:57-62. [DOI] [PubMed]
- 38.Wilkie G, Shapiro CM. Sleep deprivation and the postnatal blues. J Psychosom Res 1992;36:309-16. [DOI] [PubMed]
- 39.Mead-Bennett E. The relationship of primigravid sleep experience and select moods on the first postpartum day. J Obstet Gynecol Neonatal Nurs 1990;19:146-52. [DOI] [PubMed]
- 40.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 41.Kendell RE, Wainwright S, Hailey A, Shannon B. The influence of childbirth on psychiatric morbidity. Psychol Med 1976;6:297-302. [DOI] [PubMed]
- 42.Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. Br J Psychiatry 1995;166:191-5. [DOI] [PubMed]
- 43.Steinberg SI, Bellavance F. Characteristics and treatment of women with antenatal and postpartum depression. Int J Psychiatry Med 1999;29:209-33. [DOI] [PubMed]
- 44.Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Women Ment Health 2003;6:263-74. [DOI] [PubMed]
- 45.Bell AJ, Land NM, Milne S, Hassanyeh F. Long-term outcome of post-partum psychiatric illness requiring admission. J Affect Disord 1994;31:67-70. [DOI] [PubMed]
- 46.Armstrong KL, Van Haeringen AR, Dadds MR, Cash R. Sleep deprivation or postnatal depression in later infancy: separating the chicken from the egg. J Paediatr Child Health 1998;34:260-2. [DOI] [PubMed]
- 47.Stoleru S, Nottelmann ED, Belmont B, Ronsaville D. Sleep problems in children of affectively ill mothers. J Child Psychol Psychiatry 1997;38:831-41. [DOI] [PubMed]
- 48.Hiscock H, Wake M. Infant sleep problems and postnatal depression: a community-based study. Pediatrics 2001;107:1317-22. [DOI] [PubMed]
- 49.Fisher JR, Feekery CJ, Rowe-Murray HJ. Nature, severity and correlates of psychological distress in women admitted to a private mother–baby unit. J Paediatr Child Health 2002;38:140-5. [DOI] [PubMed]
- 50.Morrell J, Steele H. The role of attachment security, temperament, maternal perception, and care-giving behavior in persistent infant sleeping problems. Infant Ment Health J 2003;24:447-68.
- 51.Hiscock H, Wake M. Randomised controlled trial of behavioural infant sleep intervention to improve infant sleep and maternal mood. BMJ 2002;324:1062-5. [DOI] [PMC free article] [PubMed]
- 52.Zuckerman B, Stevenson J, Bailey V. Sleep problems in early childhood: continuities, predictive factors, and behavioral correlates. Pediatrics 1987;80:664-71. [PubMed]
- 53.Frank E, Kupfer DJ, Jacob M, Blumenthal SJ, Jarrett DB. Pregnancy-related affective episodes among women with recurrent depression. Am J Psychiatry 1987;144:288-93. [DOI] [PubMed]
- 54.Coble PA, Reynolds CF 3rd, Kupfer DJ, Houck PR, Day NL, Giles DE. Childbearing in women with and without a history of affective disorder. II. Electroencephalographic sleep. Compr Psychiatry 1994; 35:215-24. [DOI] [PubMed]
- 55.Lee KA, McEnany G, Zaffke ME. REM sleep and mood state in childbearing women: Sleepy or weepy? Sleep 2000;23:877-85. [PubMed]
- 56.Godfroid IO, Hubain PP, Dramaix M, Linkowski P. Le sommeil durant la dépression du post-partum. Encephale 1997;23:262-6. [PubMed]
- 57.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol 1997;146:105-14. [DOI] [PubMed]
- 58.Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch Gen Psychiatry 1988;45:948-52. [DOI] [PubMed]
- 59.Monk TH, Essex MJ, Smider NA, Klein MH. The impact of the birth of a baby on the time structure and social mixture of a couple's daily life and its consequences for well-being. J Appl Soc Psychol 1996;26(14):1237-58.
- 60.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry 1987;150:662-73. [DOI] [PubMed]
- 61.Steiner M, Yonkers KA. Evidence-based treatment of mood disorders in women. Ment Fitness 2003;2:34-67.
- 62.Sharma V. Role of sleep loss in the causation of puerperal psychosis. Med Hypotheses 2003;61(4):477-81. [DOI] [PubMed]
- 63.Reich T, Winoker G. Postpartum psychoses in patients with manic depressive disease. J Nerv Ment Dis 1970;151:60-8. [DOI] [PubMed]
- 64.Jones R. Puerperal insanity. BMJ 1902;1:579-85. [DOI] [PMC free article] [PubMed]
- 65.Davidson GM. Concerning schizophrenia and manic-depressive psychosis associated with pregnancy and childbirth. Am J Psychiatry 1936;92:1336-46.
- 66.Brockington IF, Oates M, Rose G. Prepartum psychosis. J Affect Disord 1990;19:31-5. [DOI] [PubMed]
- 67.Hunt N, Silverstone T. Does puerperal illness distinguish a subgroup of bipolar patients? J Affect Disord 1995;34:101-7. [DOI] [PubMed]
- 68.Sharma V, Smith A, Khan M. Does sleep loss lead to postpartum psychosis? [abstract]. 156th annual meeting of American Psychiatric Association; 2003 May 17-22; San Francisco.
- 69.Wehr TA. Sleep loss: a preventable cause of mania and other excited states. J Clin Psychiatry 1989;50(Suppl):8-16. [PubMed]
- 70.Bozoky I, Corwin EJ. Fatigue as a predictor of postpartum depression. J Obstet Gynecol Neonatal Nurs 2002;31:436-43. [DOI] [PubMed]
- 71.Steiner M, Fairman M, Jansen K, Causey S. Can postpartum depression be prevented? [abstract]. Marcé Society International Biennial Scientific Meeting; 2002 Sep 25–27; Sydney, Australia.
- 72.Sharma V, Mazmanian D. Sleep loss and postpartum psychosis. Bipolar Disord 2003;5:98-105. [DOI] [PubMed]
- 73.Terzano MG, Rossi M, Palomba V, Smerieri A, Parrino L. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem and zaleplon. Drug Saf 2003;26:261-82. [DOI] [PubMed]
- 74.Parry BL, Curran ML, Stuenkel CA, Yokimozo M, Tam L, Powell KA, et al. Can critically timed sleep deprivation be useful in pregnancy and postpartum depressions? J Affect Disord 2000;60:201-12. [DOI] [PubMed]
- 75.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev 2002;6:361-77. [PubMed]
- 76.Eichhammer P, Kharraz A, Wiegand R, Langguth B, Frick U, Aigner JM, et al. Sleep deprivation in depression stabilizing antidepressant effects by repetitive transcranial magnetic stimulation. Life Sci 2002;70:1741-9. [DOI] [PubMed]
- 77.Nahas Z, Bohning DE, Molloy MA, Oustz JA, Risch SC, George MS. Safety and feasibility of repetitive transcranial magnetic stimulation in the treatment of anxious depression in pregnancy: a case report. J Clin Psychiatry 1999;60:50-2. [DOI] [PubMed]
- 78.Strouse TB, Szuba MP, Baxter LR. Response to sleep deprivation in three women with postpartum psychosis. J Clin Psychiatry 1992; 53: 204-6. [PubMed]
- 79.Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666-9. [DOI] [PubMed]
- 80.Epperson CN, Terman M, Terman JS, Hanusa BH, Oren DA, Peindl KS, et al. Randomized clinical trial of bright light therapy for antepartum depression: preliminary findings. J Clin Psychiatry 2004;65:421-5. [DOI] [PubMed]
- 81.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med 2000;162:252-7. [DOI] [PubMed]


