Abstract
Background
The antiepileptic drug lamotrigine is effective in the treatment of focal epilepsies. It is thought to act by inhibition of glutamate release through blockade of voltage-sensitive sodium channels and stabilization of the neuronal membrane. Lamotrigine is also effective in the treatment of mood disorders such as bipolar disorder. However, its exact mechanism of action in these conditions remains unclear. The aim of the present study was to evaluate the antidepressant-like effect of lamotrigine in a mouse model of depression, namely the forced swimming test (FST). Association studies using specific and nonspecific ligands acting on serotonin (5-hydroxytryptamine; 5-HT1) receptor subtypes were undertaken to evaluate the potential role of these receptors in the anti-immobility effect of lamotrigine.
Methods
The mouse FST was performed after single administration of lamotrigine. Subactive doses of lamotrigine were administered in association with subactive doses of the following 5-HT1 receptor agonists or antagonists: 8-hydroxy-2-(di-n-propilamino)-tetralin (8-OH-DPAT, a standard 5-HT1A receptor selective agonist), pindolol (a presynaptic and postsynaptic 5-HT1A/1B receptor antagonist), NAN-190 (a 5-HT1A receptor antagonist), RU 24969 (a 5-HT1A/1B receptor agonist) and anpirtoline (5-HT1B agonist).
Results
Lamotrigine impaired spontaneous locomotor activity at doses of 4 mg/kg or greater, and activity decreased by more than 50% at the 16 mg/kg dose. When administered alone, lamotrigine (8 and 16 mg/kg) decreased immobility time in the FST. Only 8-OH-DPAT (1 mg/kg), pindolol (32 mg/kg) and RU 24969 (0.5 mg/kg) enhanced the antidepressant-like effect of lamotrigine in the FST.
Conclusions
These results suggest that postsynaptic 5-HT1A receptors might be involved in the activity of lamotrigine. Furthermore, they demonstrate that lamotrigine more closely resembles valproate and carbamazepine than lithium, with the advantage of an anti-immobility effect in the mouse FST when administered on its own.
Medical subject headings: lamotrigine; mice; models, animal; bipolar disorder; depression
Abstract
Contexte
La lamotrigine, anti-épileptique, est efficace dans le traitement de l'épilepsie focale. On croit que l'action de ce médicament fait appel à l'inhibition de la libération de glutamate par le blocage des canaux sodiques sensibles aux variations de tension et la stabilisation de la membrane neuronale. La lamotrigine est aussi efficace dans le traitement des troubles de l'humeur, comme le trouble bipolaire. Par ailleurs, on ne sait pas exactement quel mécanisme d'action est en jeu dans ces affections. La présente étude vise à évaluer l'effet antidépresseur de la lamotrigine dans un modèle de dépression chez la souris, nommément le test de la nage forcée (TNF). On a effectué des études d'association faisant appel à des ligants spécifiques et non spécifiques agissant sur les sous-types de récepteurs de la sérotonine (5-HT1) afin d'évaluer le rôle que pourraient jouer ces récepteurs dans l'effet anti-immobilité de la lamotrigine.
Méthodes
Le TNF chez la souris a été réalisé après administration d'une seule dose de lamotrigine à chaque animal. Des doses sous-actives de lamotrigine ont été administrées conjointement avec des doses sous-actives des agonistes ou antagonistes des récepteurs de la 5-HT1 que voici : la 8-hydroxy-2-(di-n-propilamino)-tétraline (8-OH-DPAT; agoniste sélectif standard des récepteurs de la 5-HT1A), le pindolol (antagoniste des récepteurs présynaptiques et post-synaptiques de la 5-HT1A/1B), le NAN-190 (antagoniste des récepteurs de la 5-HT1A), le RU 24969 (agoniste des récepteurs de la 5-HT1A/1B) et l'anpirtoline (agoniste de la 5-HT1B).
Résultats
Administrée à des doses d'au moins 4 mg/kg, la lamotrigine altère l'activité locomotrice spontanée. On a observé une diminution de l'activité de plus de 50 % après l'administration de la dose de 16 mg/kg. Administrée seule, la lamotrigine (8 et 16 mg/kg) a diminué le temps d'immobilité dans le TNF. Seuls le 8-OH-DPAT (1 mg/kg), le pindolol (32 mg/kg) et le RU 24969 (0,5 mg/kg) ont augmenté l'effet antidépresseur de la lamotrigine dans le TNF.
Conclusions
Ces résultats indiquent que les récepteurs post-synaptiques de la 5-HT1A pourraient jouer un rôle dans l'activité de la lamotrigine. De plus, ils démontrent que la lamotrigine, qui s'apparente davantage au valproate et à la carbamazépine qu'au lithium, offre l'avantage, administrée seule, d'avoir un effet anti-immobilité dans le TNF chez la souris.
Introduction
The antiepileptic drug lamotrigine [3,5-diamino-6(2,3-dichlorophenyl)-1,2,4-triazine] is effective in the treatment of focal epilepsies with or without secondary generalization. Its mechanism of action is linked to blockade of voltage-sensitive sodium channels of the neuronal membrane, inhibition of release of excitatory amino acids such as glutamate and aspartate, and calcium-channel blockade.1,2
Lamotrigine is also effective in the treatment of mood disorders such as bipolar disorder.3,4,5,6 Over the past several decades, there has been continuing interest in established antiepileptic drugs such as carbamazepine and oxcarbazepine and on the new generation of antiepileptic drugs such as lamotrigine and topiramate for the treatment of bipolar disorder.7 Lamotrigine is superior to placebo in terms of prolonging time to intervention for depression in patients with mania or hypomania and in patients with depression.8,9
Certain antidepressants as well as the antiepileptic drug carbamazepine have calcium-channel–blocking properties which may be relevant to the physiopathology of epilepsies and affective disorders. Several clinical studies have evaluated the antimanic and antidepressant actions of calcium-channel blockers such as verapamil, diltiazem and nimodipine.10,11,12 The principal mechanism of action of these drugs in the treatment and prevention of bipolar I disorder may be blockade of sodium and calcium channels in presynaptic neurons and subsequent stabilization of the neuronal membrane.13
Furthermore, in a recent study,14 the effect of lamotrigine on serotonin (5-hydroxytryptamine, 5-HT)1A-receptor-mediated adenylcyclase responses in various regions of the rat brain was evaluated in vivo. The results suggested a possible mode of action of lamotrigine: downregulation of cortical 5-HT1A-receptor-mediated adenylcyclase responses. This pathway seems to be implicated in the therapeutic action of various classes of mood stabilizers. There is also new evidence for a role of 5-HT in the pathophysiology of manic–depressive illness. A recent study of subjects with a history of bipolar disorder, which used in situ hybridization to quantify 5-HT1A, 5-HT1B and 5-HT2A mRNA levels in the hippocampus and dorsal prefrontal cortex, showed that 5-HT receptor mRNA expression was altered in these subjects.15
Lithium, a classical mood stabilizer, is generally considered the treatment of choice for bipolar I disorder, decreasing the severity, length and recurrence of manic episodes, but it also has significant antidepressant properties in treating both bipolar and unipolar depression. A previous study16 demonstrated that lithium, carbamazepine and sodium valproate could act on certain 5-HT1 receptor subtypes in the forced swimming test (FST). Carbamazepine and sodium valproate are also effective in the treatment of several forms of affective disorders, such as treatment-resistant depression and manic depression.17,18
Animal models of bipolar disorders are a challenge to develop because of the complex interplay of mania, depression, euthymia and mixed states. Several animal models have been proposed, but they only partially match the illness, modelling either depression or manic behaviour, but not both.19 Many validated animal models of depression that fulfil the criteria of face, construct and predictive validity can be used. The FST is a useful model because it is quick and relatively simple to perform,20 and it has been used to investigate the mechanism of antidepressant action.16 The FST is also sensitive to compounds acting on the 5-HT system.21,22
To pursue our work on the role of 5-HT receptors in the mechanism of action of antiepileptic drugs in bipolar disorders, we used the FST to investigate the antidepressant-like effect of lamotrigine.
We also undertook several association studies involving lamotrigine. The use of association studies with 5-HT ligands in the field of depression has been widely reported. Certain preclinical studies have reported an interaction between antidepressants and 5-HT1A and/or 5-HT1B receptor ligands in animal models of depression.23,24 The anti-immobility effect of noradrenaline reuptake inhibitors (NRIs) may be mediated by postsynaptic 5-HT1A receptor subtypes, whereas the 5-HT1B receptor subtype in particular has been suggested to play a role in the anti-immobility effect of selective serotonin reuptake inhibitors (SSRIs) or imipramine.21,25 In addition, there is some clinical and preclinical evidence that augmentation with pindolol is effective in accelerating or enhancing the effects of antidepressants drugs such as the SSRIs and NRIs.26,27,28
Association studies with lamotrigine and the most specific brain-penetrating ligands for 5-HT1A and 5-HT1B receptors currently available would help in elucidating the mechanisms of action of the potential antidepressant-like effect of lamotrigine. We therefore undertook association studies with the following agents: 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT), a standard 5-HT1A receptor selective agonist;29 pindolol, a presynaptic and postsynaptic 5-HT1A/1B receptor antagonist;30 NAN-190, a 5-HT1A receptor antagonist;31 RU 24969, a 5-HT1A/1B receptor agonist;32 and anpirtoline, a highly potent 5-HT1B agonist that crosses the blood–brain barrier.33
Materials and methods
Animals
Naïve male Swiss mice (Elevage R. Janvier, Le Genest, France) were housed at constant room temperature (20°C, standard deviation [SD] 1°C) under a standard light–dark cycle, with free access to food and water. They were housed for at least 1 week before testing, in groups of 18 per cage (40 cm х 28 cm х 17 cm). Their average body weight on the day of the study was 20 (SD 2) g. Each mouse was used only once in a given experimental session. Mice were assigned at random to the treatment groups, each of which consisted of 10–12 mice. All experiments were performed within the guidelines of the French Ministry of Agriculture for experiments with laboratory animals (law 87 848) and with the approval of the research committee of the Université de Nantes.
Drugs
The following drugs were used: lamotrigine base; 8-OH-DPAT; (±) pindolol; NAN-190 [1-(2-methoxyphenyl)-4-[4-(2-phthalimido) butyl]piperazine]; RU 24969 [5-methoxy-3(1,2,3,6-tetrahydropyridin-4-yl)-1 H-indole]; and anpirtoline. All drugs were supplied by Tocris (Fisher Bioblock Scientific, Illkirch, France), except lamotrigine (supplied by GlaxoSmithKline, France). The 8-OH-DPAT, RU 24969 and anpirtoline were dissolved in distilled water, whereas the lamotrigine, (±) pindolol and NAN-190 were dissolved in a 1% aqueous solution of Tween 80. Each drug or the vehicle (distilled water) was administered intraperitoneally at a constant volume of 0.5 mL/20 g body weight.
Psychopharmacologic tests
Actimeter tests34 were performed to examine the effects of the various drugs on spontaneous locomotor activity. The animals were kept in the test room for 1 hour before the test for habituation. After injection of vehicle or drug, the mice were placed in holding cages. The spontaneous activity of naïve animals was recorded using a photoelectric actimeter (Osys, Changé, France). This apparatus consists of transparent cages from which the animal's activity is measured by light beams connected to a photoelectric cell. The total number of horizontal cage crossings was recorded over a period of 10 minutes. The actimeter test was performed independently of the FST test.
The FST was essentially the same as described in detail elsewhere.20 Mice were dropped individually into glass cylinders (height 25 cm, diameter 10 cm) containing 15 cm of water maintained at 23°C to 25°C and left there for 6 minutes. A mouse was judged immobile when it floated upright in the water and made only small movements to keep its head above water. The duration of immobility was recorded during the last 4 minutes of the 6-minute period by trained experimenters.
Procedure for single-administration studies
Actimeter tests were performed to examine the effect of lamotrigine on spontaneous locomotor activity. A wide range of doses was tested (0.5, 1, 2, 4, 8, 16 and 32 mg/kg), and a vehicle-treated group was included as a control.
Lamotrigine was tested alone in the FST to determine its potential antidepressant-like effect. In the first experiment, lamotrigine was administered 30 minutes before the test (at doses of 0.25, 0.5, 1, 2, 4, 8 and 16 mg/kg). In the second experiment, lamotrigine was administered 45 minutes before the test (at doses of 0.5, 1, 2, 4, 8, 16 and 32 mg/kg)
Procedure for association studies
Subactive doses of lamotrigine were defined as those that did not on their own reduce mobility in the FST. In the association studies, the lamotrigine was administered 45 minutes before testing in the FST, and the 5-HT ligands were administered 30 minutes before testing.
Two subactive doses of lamotrigine were administered in association with one subactive dose of each 5-HT receptor ligand. The subactive doses of the 5-HT receptor ligands, determined in previous studies, were as follows (data not shown): 8-OH-DPAT, 1 mg/kg; (±) pindolol, 32 mg/kg NAN-190; 0.5 mg/kg; RU 24969, 0.5 mg/kg; and anpirtoline, 1 mg/kg.
Data analysis
Results are expressed as percentage of the value observed in control animals or as means (and standard error of the mean [SEM]). The normal distribution of data was verified by a Kolmogorov–Smirnov test. The effect of lamotrigine on mouse behaviour in the actimeter test and in the FST was determined by a 1-way analysis of variance (ANOVA) and Dunnett's test for comparison with control groups and by a 2-way ANOVA followed by a Sidak test for association studies. All analyses were conducted using the SPSS program for IBM-compatible computers.
Results
Single administration of lamotrigine significantly decreased the spontaneous locomotor activity of mice in the actimeter test relative to the control group (control value 169.3 [SEM 10.7] crossings). With the 4 mg/kg dose, activity was 74% of control (p = 0.04), and a maximal effect was observed at the 32 mg/kg dose (25% of control) (p ≤ 0.001) (F7,72 = 17.47, p ≤ 0.001) (Fig. 1). At the 64 mg/kg dose, lamotrigine produced seizures leading to death of the mice.
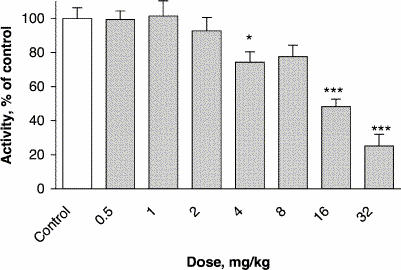
Fig. 1: Effect of lamotrigine on spontaneous locomotor activity. The results, in terms of percentage relative to the control group, are expressed as means and standard error of the mean (SEM) (n = 10 in each group). The asterisks indicate significant differences from the vehicle-treated (control) group, determined by 1-way analysis of variance (ANOVA) followed by Dunnett's test: *p ≤ 0.05, ***p ≤ 0.001.
Administration of lamotrigine at a dose of 8 mg/kg 30 minutes before the FST decreased the immobility time of mice to 88% of control values (p ≤ 0.001) (Fig. 2). At the 16 mg/kg dose, the immobility time was 83% of control (p ≤ 0.001) (F7, 72 = 7.82, p ≤ 0.001).
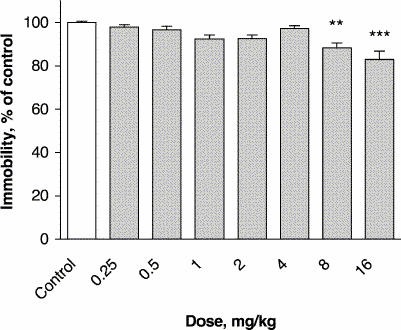
Fig. 2: Effect of lamotrigine administered 30 minutes before the test on immobility time in the forced swimming test (FST). The results, in terms of percentage relative to the control group, are expressed as means and SEM (n = 10 in each group). The asterisks indicate significant differences from the control group, determined by 1-way ANOVA followed by a Dunnett's test: **p ≤ 0.01, ***p ≤ 0.001.
Administration of lamotrigine at a dose of 32 mg/kg 45 minutes before the FST significantly decreased the immobility time of mice, to 59% of control values (F7,72 = 20.64, p ≤ 0.001) (Fig. 3).
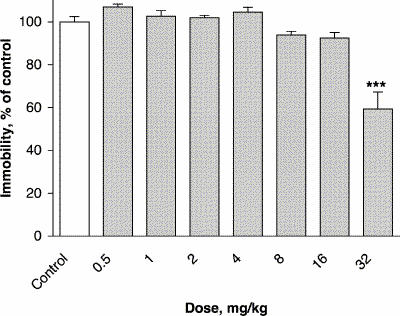
Fig. 3: Effect of lamotrigine administered 45 minutes before the test on immobility time in the FST. The results, in terms of percentage relative to the control group, are expressed as means and SEM (n = 10 in each group). The asterisks indicate significant differences from the control group, determined by 1-way ANOVA followed by a Dunnett's test: ***p ≤ 0.001.
Treatment with the subactive dose of 8-OH-DPAT (1 mg/kg) in combination with subactive doses of lamotrigine (2 and 4 mg/kg) reduced immobility time in the FST relative to lamotrigine alone, by 24% at 2 mg/kg lamotrigine and by 28% at 4 mg/kg lamotrigine (F5,54 = 11.44, p ≤ 0.001) (Fig. 4).
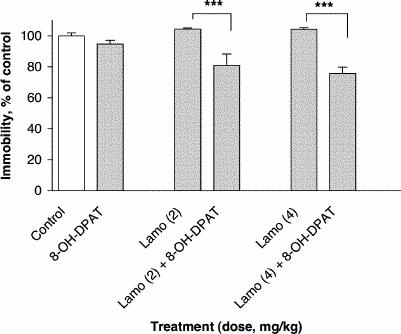
Fig. 4: Effect of lamotrigine (Lamo) administered 45 minutes before the test combined with 8-OH-DPAT administered 30 minutes before the test on immobility time in the FST. The results, in terms of percentage relative to the control group, are expressed as mean and SEM (n = 10 in each group). The asterisks indicate significant differences from the appropriate lamotrigine control group, determined by 2-way ANOVA followed by a Sidak test: ***p ≤ 0.001.
Treatment with the subactive dose of pindolol (32 mg/kg) in combination with subactive doses of lamotrigine (2 and 4 mg/kg) reduced immobility time in the FST relative to lamotrigine alone, by 13% at both doses of lamotrigine (F5,54 = 18.69, p ≤ 0.001) (Fig. 5).
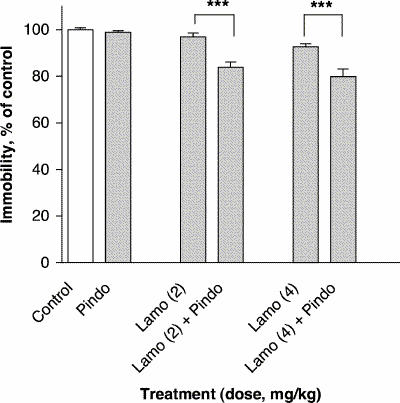
Fig. 5: Effect of lamotrigine (Lamo) administered 45 minutes before the test combined with pindolol (Pindo) administered 30 minutes before the test on immobility time in the FST. The results, in terms of percentage relative to the control group, are expressed as mean and SEM (n = 10 in each group). The asterisks indicate significant differences from the appropriate lamotrigine control group, determined by 2-way ANOVA followed by a Sidak test, ***p ≤ 0.001.
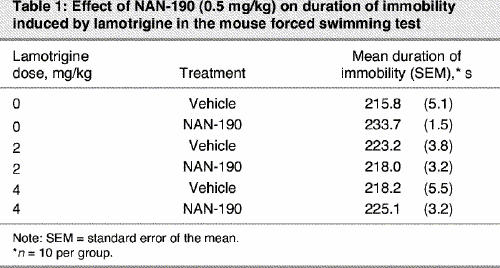
Treatment with the subactive dose of NAN-190 (0.5 mg/kg) in combination with subactive doses of lamotrigine (2 and 4 mg/kg) did not modify immobility time in the FST, despite a significant p value (F5,54 = 2.71, p ≤ 0.029) (Table 1).
Table 1
Treatment with a subactive dose of RU 24969 (0.5 mg/kg) in combination with the lower subactive dose of lamotrigine (2 mg/kg) did not modify immobility time in the FST (F5,54 = 6.36, p ≤ 0.001) (Fig. 6). However, with lamotrigine at 4 mg/kg, a 22% reduction in immobility time was observed (p ≤ 0.001).
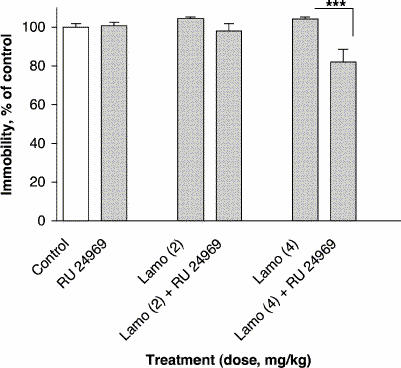
Fig. 6: Effect of lamotrigine (Lamo) administered 45 minutes before the test combined with RU 24969 administered 30 minutes before the test on immobility time in the FST. The results, in terms of percentage relative to the control group, are expressed as mean and SEM (n = 10 in each group). The asterisks indicate significant differences from the appropriate lamotrigine control group, determined by 2-way ANOVA followed by a Sidak test: ***p ≤0.001.
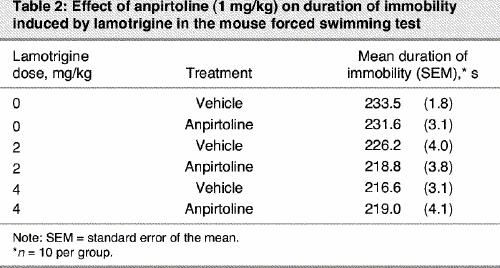
Treatment with the subactive dose of anpirtoline (1 mg/kg) in combination with subactive doses of lamotrigine (2 and 4 mg/kg) did not modify immobility time in the FST (F5,54 = 4.51, p = 0.002) (Table 2).
Table 2
Discussion
For patients with bipolar disorder, the antiepileptic drug lamotrigine has been shown to be more effective in treating depression than in treating hypomania or mania.35 Recently, a number of studies have reported that this drug is effective in the treatment of major depressive disorders, including refractory unipolar depression,36,37,38 which suggests promise for the use of lamotrigine as an augmentation drug. Pilot studies have suggested that lamotrigine augmentation of SSRIs such as fluoxetine39 and paroxetine40 is well tolerated and provides efficacy superior to that of SSRI monotherapy. Lamotrigine may also have antidepressant properties in patients with unipolar depression and may accelerate the onset of action when given in combination with classical antidepressants.40
The aim of the present study was to evaluate the antidepressant-like effect of lamotrigine in the FST. Despite many years of research, no valid and satisfactory animal model of bipolar depression has been developed to evaluate the mechanism of action of mood stabilizers. Animal models mimic only limited aspects of the behaviours seen in this disorder, either mania or depression. Here, we have evaluated the anti-immobility effect of lamotrigine in mice using a simple but robust model of depression, the FST.20,21,22 Furthermore, the FST enables investigation of mechanisms of action of drugs through the use of association studies with receptor-specific ligands. The present work focuses mainly on 5-HT1 subtype receptors.
In this study, lamotrigine impaired spontaneous locomotor activity, with some effect at a dose of 4 mg/kg and a decrease in activity of more than 50% at 16 mg/kg. In addition, lamotrigine at doses of 8 and 16 mg/kg, administered 30 minutes before the FST, decreased immobility time, thus demonstrating antidepressant-like effects despite the drug's sedative effects at these doses. In a previous study using the FST in mice,41 lamotrigine, at doses of 15 and 25 mg/kg, was found to be inactive relative to saline. This discrepancy might be due to differences in methodology. Indeed, in the previous study,41 lamotrigine was administered 60 minutes before testing instead of 30 minutes as in our study. Furthermore, the size of the glass cylinder was larger (16 cm v. 10 cm). Standardization of the FST is required to avoid difficulties in replicating experiments between laboratories and to ensure reproducibility of results.
Lamotrigine administered alone seems to be more effective than lithium, valproate or carbamazepine in decreasing immobility time, with an effect of about 17% at the upper dose of 16 mg/kg. Lithium and sodium valproate have been previously evaluated with the FST and were found to be without effect,42 whereas carbamazepine was weakly effective at a dose of 32 mg/kg.16
Recently, large double-blind, placebo-controlled trials comparing lamotrigine with lithium in the maintenance treatment of patients with bipolar I disorders have been performed.43 Both drugs were effective as maintenance treatment, and they had potentially complementary mood-stabilizing properties. These data are consistent with the results of the present experimental study and suggest that lamotrigine is more effective in treating depression, whereas lithium is more effective in treating mania.8,9,43 According to the newly available nomenclature for bipolar disorder, which distinguishes “above baseline,” for mania and hypomania and mixed states, from “below baseline,” for depression and subsyndromal depression, lithium would be the prototype of class A drugs in treatment of “above baseline” moods and lamotrigine might belong to class B drugs in treatment of “below baseline” moods.5,8,9 Valproate and carbamazepine also belong to class A.5
Alteration of serotonin transmission is implicated in many mood disorders including bipolar disorders. Lopez-Figueroa et al15 have recently shown alteration in 5-HT1A, 5-HT1B and 5-HT2A mRNA levels using post mortem in situ hybridization in the brains of subjects with a history of major depressive disorder or bipolar disorder.
In the present study, lamotrigine was coadministered with specific 5-HT1A and 5-HT1B receptor agonists and antagonists to investigate the role of these receptors in vivo in the mechanism of action of lamotrigine in the FST, as reported previously in our laboratory for the other mood stabilizers (lithium, carbamazepine and sodium valproate).16 Only 8-OH-DPAT (a postsynaptic 5-HT1A receptor agonist), pindolol (a presynaptic and postsynaptic 5-HT1A/1B receptor antagonist) and to a lesser extent RU 24969 (a 5-HT1A/1B receptor agonist that acts essentially via postsynaptic receptors) were able to enhance the antidepressant-like effect of lamotrigine in the FST.
5-HT1 subtype receptors are believed to exist at the presynaptic level as autoreceptors controlling 5-HT release and as heteroreceptors controlling the release of other neurotransmitters44 and at the postsynaptic level (coupled to adenylate cyclase).
8-OH-DPAT, like other high-affinity agonists for 5-HT1A receptors, has been shown to decrease immobility time in the FST on its own.21 Treatments with PCPA (p-chlorophenylalanine, inhibitor of 5-HT synthesis) causing serotonergic depletion or with the neurotoxin 5,7-DHT (5,7-di-hydroxytryptamine) do not affect the immobility response to 8-OH-DPAT in the mouse FST, which suggests that this effect is mediated by postsynaptic 5-HT1A receptors.45 In the present study, the observed additive effect of a subactive dose of 8-OH-DPAT in combination with lamotrigine may suggest that postsynaptic 5-HT1A receptors play a role in the ability of these drug to reduce immobility in the FST.
Antagonist activity at presynaptic 5-HT1A receptors, which blocks the negative feedback mechanism involved in 5-HT neurotransmission via autoreceptors,46 has been implicated in the potentiating effects of pindolol in the FST.21 Furthermore, it has been demonstrated that pindolol acts at 5-HT1B presynaptic receptors in the FST with antidepressant-like activity.47 In this study, the combination of subactive doses of lamotrigine and pindolol also decreased immobility time in the FST, which supports presynaptic activity, but the effect was less pronounced than with 8-OH-DPAT.
RU 24969 is believed to act via postsynaptic 5-HT1B and 5-HT1A receptor activation.32 Only the higher subactive dose of lamotrigine was potentiated by RU 24969. Given that anpirtoline was without effect on lamotrigine-induced immobility time, whereas 8-OH-DPAT potentiated the effect, it seems more likely that a 5-HT1A agonist activity might be involved in the potentiation induced by RU 24969.
Taking these data together, and considering the effect size of the decrease in immobility time of lamotrigine in association with the various ligands (i.e., 8-OH-DPAT > pindolol > RU 24969), we can speculate that postsynaptic 5-HT1A receptors might be involved in the activity of lamotrigine. Other neurotransmitters regulated by postsynaptic 5-HT1A heteroreceptors might also participate in the antidepressant-like effect of lamotrigine.
Although there is a large clinical literature reporting the use of lamotrigine in bipolar disorders or treatment-resistant depression, few studies have addressed the possible neurochemical basis of its therapeutic effect, and these have yielded conflicting results. Some researchers have found that lamotrigine has little or no effect on 5-HT transmission,6,48 whereas Ahmad et al49 recently found that single administration of lamotrigine (10 and 20 mg/kg) decreased extracellular 5-HT in the hippocampus of freely moving rats (determined by in vivo microdialysis). In contrast, carbamazepine and lamotrigine were the only anticonvulsant drugs found to inhibit 5-HT uptake both in vitro and in vivo,50 which would result in elevated extracellular 5-HT concentrations. However, the relevance of these findings to bipolar depression is unclear. A recent study in rats demonstrated significantly less inhibition of forskolin-stimulated adenylcyclase activity by [3H]8-OH-DPAT in cortical membranes but not in the hippocampus of rats treated with lamotrigine, relative to control animals. This suggests that one mechanism of action of lamotrigine might be the downregulation of responses mediated by cortical 5-HT1A receptors.14
The role of 5-HT1A receptors in the mechanism of action of lamotrigine in bipolar disorders has already been examined in healthy human male volunteers51 using the selective 5-HT1A agonist ipsapirone. Treatment with lamotrigine for 1 week did not significantly modify the hypothermic responses or cortisol responses to ipsapirone. However, that study was limited by the small sample size and the lack of a placebo control, so further investigations are required in humans. Furthermore, it is difficult to conclude anything about the localization of the pharmacologic activity from such studies, as there is considerable heterogeneity between species and technical protocols. For example, the hypothermic response involves only postsynaptic receptors in rats and both presynaptic and postsynaptic receptors in mice.52,53,54
In a previous study, we examined the antidepressant-like activity of lithium, sodium valproate and carbamazepine in the FST using inactive or subactive doses of these drugs in association with the 5-HT1A/B ligands. The results suggested that 5-HT1B receptors may play a role in the potentiating effect of lithium in the mouse FST. Lithium demonstrated anti-immobility effects in association with RU 24969 and with anpirtoline.16 Conversely, pretreatment with carbamazepine increased mobility when administered in combination with 8-OH-DPAT and RU 24969, which suggests a role for 5-HT1A receptors. Sodium valproate in association with 8-OH-DPAT, pindolol and RU 24969 induced antidepressant-like activity in comparison to saline control animals in the FST.
In the present study, strong potentiation of lamotrigine activity with 8-OH-DPAT suggested a 5-HT1A receptor mechanism, but the role of the 5-HT1B receptor was less clear, as anpirtoline, a more specific 5-HT1B receptor agonist, failed to enhance lamotrigine-induced antidepressant-like activity. To define the mechanism further, it would be of interest to antagonize active doses of lamotrigine with NAN 190. The intracortical administration of selective compounds would also help in understanding the possible implication of 5-HT1A/1B receptors.
The results presented here demonstrate that lamotrigine activity is more closely related to valproate and carbamazepine than to lithium, with the advantage of an anti- immobility effect in the mouse FST when administered on its own. These results are consistent with the finding that lamotrigine was more effective in treating bipolar disorders in patients in whom depressive symptoms predominated over mania or hypomania.55
Acknowledgments
The authors thank Marie-Claude Colombel and Marie-Noël Hervé for their technical help.
Footnotes
Contributors: All authors contributed to the conception and design of the research article and to acquiring and interpreting the data. Drs. Bourin and Hascoët drafted and revised the article. All authors gave final approval for the version to be published.
Competing interests: None declared.
Correspondence to: Dr. Michel Bourin, EA 3256, Neurobiologie de l'anxiété et de la dépression, Faculté de Médecine, BP 53508, 1, rue Gaston Veil, 44035 Nantes cedex, France; fax 33 02 40 41 28 56; michel.bourin@univ-nantes.fr
Submitted July 16, 2004; Revised Jan. 18, 2005; Accepted March 14, 2005
References
- 1.Cheung H, Kamp D, Harris E. An in vitro investigation of the action of lamotrigine on neuronal voltage-activated sodium channels. Epilepsy Res 1992;13:107-12. [DOI] [PubMed]
- 2.Cunningham MO, Jones RSG. The anticonvulsivant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacology 2000;39:2139-46. [DOI] [PubMed]
- 3.Weisler R, Risner ME, Acher J, Houser T. Use of lamotrigine in the treatment of bipolar disorder [abstract]. American Psychiatric Association annual meeting; 1994 May 21–26; Philadelphia.
- 4.Walden J, Hesslinger BJ, Van Calker D, Berger M. Addition of lamotrigine to valproate may enhance efficacy in the treatment of bipolar affective disorder. Pharmacopsychiatry 1996;29:193-5. [DOI] [PubMed]
- 5.Ketter TA, Manji HK, Post RM. Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J Clin Psychopharmacol 2003;23:484-94. [DOI] [PubMed]
- 6.Goldsmith DR, Wagstaff AJ, Ibbotson T, Perry CM. Lamotrigine: a review of its use in bipolar disorder. Drugs 2003;63:2029-50. [DOI] [PubMed]
- 7.Grunze H, Walden J. Relevance of new and newly rediscovered anticonvulsants for atypical forms of bipolar disorder. J Affect Disord 2002;72 Suppl 1:S15-21. [DOI] [PubMed]
- 8.Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003;64:1013-24. [DOI] [PubMed]
- 9.Calabrese JR, Vieta E, Sheldon MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol 2003;13 Suppl 2:S57-66. [DOI] [PubMed]
- 10.Brunet G, Cerlich B, Robert P, Dumas S, Souetre E, Darcourt G. Open trial of a calcium antagonist, nimodipine, in acute mania. Clin Neuropharmacol 1990;13:224-8. [DOI] [PubMed]
- 11.Dubovsky S. Calcium antagonists in manic-depressive illness. Neuropsychobiology 1993;27:184-92. [DOI] [PubMed]
- 12.Walden J, Fritze J, Van Calker D, Berger M, Grunze H. A calcium antagonist for the treatment of depressive episodes: single case reports. J Psychiatr Res 1995;29:71-6. [DOI] [PubMed]
- 13.von Wegerer J, Hesslinger B, Berger M, Walden J. A calcium antagonistic effect of the new antiepileptic drug lamotrigine. Eur Neuropsychopharmacol 1997;7:77-81. [DOI] [PubMed]
- 14.Vinod KY, Subhash MN. Lamotrigine induced selective changes in 5-HT1A receptor mediated response in rat brain. Neurochem Int 2002;40:315-9. [DOI] [PubMed]
- 15.Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, et al. Serotonin 5-HT1A, 5-HT1B and 5-HT2A, receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry 2004;55:225-33. [DOI] [PubMed]
- 16.Redrobe JP, Bourin M. Evidence of the activity of lithium on 5-HT1B receptors in the mouse forced swimming test: comparison with carbamazepine and sodium valproate. Psychopharmacology (Berl) 1999;141:370-7. [DOI] [PubMed]
- 17.Calabrese JR, Delucchi GA. Phenomenology of rapid cycling manic depression and its treatment with valproate. J Clin Psychiatry 1989;50:30-4. [PubMed]
- 18.Kramlinger KG, Post RM. The addition of lithium to carbamazepine. Antidepressant efficacy in treatment-resistant depression. Arch Gen Psychiatry 1989;46:794-800. [DOI] [PubMed]
- 19.Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:209-24. [DOI] [PubMed]
- 20.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977;229:327-36. [PubMed]
- 21.Redrobe JP, McSweeney CP, Bourin M. The role of 5-HT1A and 5-HT1B receptors in antidepressant drug actions in the mouse forced swimming test. Eur J Pharmacol 1996;318:213-20. [DOI] [PubMed]
- 22.Redrobe JP, Bourin M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol 1997;325:129-35. [DOI] [PubMed]
- 23.Redrobe JP, Bourin M. Augmentation of antidepressant pharmacotherapy: a preclinical approach using the forced swimming test. CNS Spectr 1999;4:73-81.
- 24.Tatarczynska E, Klodzinska A, Stachowicz K, Chojnacka-Wojcik E. Effect of combined administration of 5-HT1A or 5-HT1B/D receptor antagonists and antidepressants in the forced swimming test. Eur J Pharmacol 2004;487:133-42. [DOI] [PubMed]
- 25.O'Neill MF, Conway MW. Role of 5-HT1A and 5-HT1B receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology 2001;24:391-8. [DOI] [PubMed]
- 26.Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry 1994;51:248-51. [DOI] [PubMed]
- 27.Artigas F, Celeda P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends Pharmacol Sci 2001;22:224-8. [DOI] [PubMed]
- 28.Blier P, Bergeron R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J Clin Psychopharmacol 1995;15:217-22. [DOI] [PubMed]
- 29.Middlemiss DN, Fozard JR. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol 1983;90:151-3. [DOI] [PubMed]
- 30.Clifford EM, Gartside SE, Umbers V, Cowen PJ, Hajos M, Sharp T. Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A autoreceptor in vivo. Br J Pharmacol 1998;124:206-12. [DOI] [PMC free article] [PubMed]
- 31.Williams AR, Dourish CT. Effect of the putative 5-HT1A receptor antagonist NAN 190 on free feeding and on feeding induced by the 5-HT1A receptor agonist 8-OHDPAT in the rat. Eur J Pharmacol 1992;219:105-12. [DOI] [PubMed]
- 32.Martinez-Price DL, Geyer MA. Subthalamic 5-HT1A and 5-HT1B receptor modulation of RU 24969-induced behavioral profile in rats. Pharmacol Biochem Behav 2002;71:569-80. [DOI] [PubMed]
- 33.Schlicker E, Werner U, Hamon M, Gozlan H, Nickel B, Szelenyi I, et al. Anpirtoline, a novel, highly potent 5-HT1B receptor agonist with antinociceptive/antidepressant-like actions in rodents. Br J Pharmacol 1992;105:732-8. [DOI] [PMC free article] [PubMed]
- 35.Bourin M, Lambert O, Guitton B. Treatment of acute mania — from clinical trials to recommendations for clinical practice. Hum Psychopharmacol Clin Exp 2005;20:15-26. [DOI] [PubMed]
- 34.Boissier JR, Simon P. Action de la caféine sur la motilité spontanée de la souris. Arch Int Pharmacodyn Ther 1965;158:212-21. [PubMed]
- 36.Rocha FL, Hara C. Lamotrigine augmentation in unipolar depression. Int Clin Psychopharmacol 2003;18:97-9. [DOI] [PubMed]
- 37.Barbee JG, Jamhour NJ. Lamotrigine as an augmentation agent in treatment-resistant depression. J Clin Psychiatry 2002;63:737-41. [DOI] [PubMed]
- 38.Frye MA, Ketter TA, Kimbrell TA, Dunn RT, Speer AM, Osuch EA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14. [DOI] [PubMed]
- 39.Barbosa L, Berk M, Vorster M. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J Clin Psychiatry 2003;64:403-7. [DOI] [PubMed]
- 40.Normann C, Hummel B, Scharer LO, Horn M, Grunze H, Walden J. Lamotrigine as adjunct to paroxetine in acute depression: a placebo-controlled, double-blind study. J Clin Psychiatry 2002;63:337-44. [DOI] [PubMed]
- 41.Szymczyk G, Zebrowska-Lupina I. Influence of antiepileptic on efficacy of antidepressant drugs in forced swimming test. Pol J Pharmacol 2000;52:337-44. [PubMed]
- 42.Nixon MK, Hascoët M, Bourin M, Colombel M. Additive effect of lithium and antidepressants in the forced swimming test: further evidence for involvement of the serotoninergic system. Psychopharmacology (Berl) 1994;115:59-64. [DOI] [PubMed]
- 43.Keck PE, Nelson EB, McElroy SL. Advances in the pharmacologic treatment of bipolar depression. Biol Psychiatry 2003;53:671-9. [DOI] [PubMed]
- 44.Sarhan H, Fillion G. The therapeutic potential of 5-HT1B autoreceptors and heteroreceptors and 5-HT moduline in CNS disorders. CNS Spectr 1998;3:50-8.
- 45.Luscombe GP, Martin KF, Hutchins LJ, Gosden J, Heal DJ. Mediation of the antidepressant-like effect of 8-OHDPAT in mice by postsynaptic 5-HT1A receptors. Br J Pharmacol 1993;108:669-77. [DOI] [PMC free article] [PubMed]
- 46.Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 1996;19:378-83. [DOI] [PubMed]
- 47.Bourin M, Redrobe JP, Baker G. Pindolol does not act only on 5-HT1A receptors in augmenting antidepressant activity in mouse forced swimming test. Psychopharmacology 1998;136:226-34. [DOI] [PubMed]
- 48.Leach MJ, Randall AD, Stefani A, Hainsworth AH. Lamotrigine: mechanism of action. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. 5th ed. Philadelphia: Lippincott William & Wilkins; 2002. p. 363-415.
- 49.Ahmad S, Fowler LJ, Whitton P. Effect of acute and chronic lamotrigine on basal and stimulated extracellular 5-hydroxytryptamine and dopamine in the hippocampus of the freely moving rat. Br J Pharmacol 2004;142:136-42. [DOI] [PMC free article] [PubMed]
- 50.Southam E, Kirkby D, Higgins GA, Hagan RM. Lamotrigine inhibits monoamine uptake in vitro and modulates 5-hydroxytryptamine uptake in rats. Eur J Pharmacol 1998;358:19-24. [DOI] [PubMed]
- 51.Shiah IS, Yatham LN, Lam RW, Zis AP. Effects of lamotrigine on the 5-HT1A receptor function in healthy human males. J Affect Disord 1998;49:157-62. [DOI] [PubMed]
- 52.Bill DJ, Knight M, Forster EA, Fletcher A. Direct evidence for an important species difference in the mechanism of 8-OHDPAT- induced hypothermia. Br J Pharmacol 1991;103:1857-64. [DOI] [PMC free article] [PubMed]
- 53.Millan MJ, Rivet JM, Canton H, Le Marouille-Girardon S, Gobert A. Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J Pharmacol Exp Ther 1993;264:1364-76. [PubMed]
- 54.Meller E, Chalfin M, Bohmaker K. Serotonin 5-HT1A receptor-mediated hypothermia in mice: absence of spare receptors and rapid induction of tolerance. Pharmacol Biochem Behav 1992;43:405-11. [DOI] [PubMed]
- 55.Bourin M, Lambert O, Guitton B. Treatment of acute mania — from clinical trials to recommendations for clinical practice. Hum Psychopharmacol 2005;20:15-26. [DOI] [PubMed]


