Abstract
OBJECTIVE/BACKGROUND:
The efficacy of laser interstitial thermal therapy (LITT) in recurrent glioblastoma (rGBM) is unknown. The goal of this study was to conduct a systematic review and pooled analysis of the literature for outcomes on patients with rGBM undergoing LITT.
METHODS:
A literature search was performed to retrieve all studies investigating overall survival, postprocedure survival, and progression-free survival outcomes of patients with rGBM undergoing LITT. Statistics were pooled together by meta-analysis of mean using a weighted random-effects or fixed-effect model.
RESULTS:
Eleven studies were included in the final cohort, representing a total of 134 patients with rGBM. The pooled mean age of the cohort at the time of recurrence was 56.7 ± 4.56 years; 41% of the cohort were female. For delivery of LITT, 2 studies used neodymium-yttrium aluminum-garnet laser (Nd:YAG laser), 3 studies used the Visualase system, 5 studies used the NeuroBlate system, and 1 study used both the NeuroBlate and the Visualase system. A total of 8 studies with 107 patients had available data for overall median survival. The pooled overall survival was found to be 18.6 months (95% confidence interval [CI] 16.2—21.1). A total of 6 studies with 93 patients had available data for post-LITT survival. The pooled post-LITT survival was found to be 10.1 months (95% CI 8.8—11.6). A total of 8 studies with 119 patients had available data for progression-free survival. Pooled progression free survival was found to be 6 months (95% CI 5.3—6.7).
CONCLUSIONS:
LITT is a novel minimally invasive procedure which, when used with optimal adjuvant therapy, may confer survival benefit for patients with rGBM.
Keywords: Glioblastoma, Laser interstitial therapy, Literature review, Pooled analysis, Recurrent glioblastoma, Survival outcomes, Thermal ablation
Glioblastoma (GBM) is the most aggressive primary intracranial tumor and constitutes 15%—20% of all primary intracranial tumors.1,2 Despite advances in therapies, the median survival ranges from 14 to 16 months and 2-year survival rate of ~30%, even with maximal therapy.3,4 The poor prognosis is largely due to tumor recurrence, with studies suggesting an inevitable outcome of recurrence after 32—36 weeks from initial multimodal treatment,5,6 and 6-month progression-free survival rate ranging from 5% to 15%.7,8 Median survival after recurrent GBM (rGBM) is approximately 30 weeks.9 In the context of rGBM, there is no standard of care, and current treatment options have limited efficacy.10 Only approximately 25% of recurrent glioblastomas are considered eligible for repeated surgery, and tumors in eloquent areas are often not eligible. In addition, the survival benefit of repeat surgery resection has been under revision. A retrospective cohort study failed to demonstrate survival benefit of second tumor resection and another study showed that repeated surgery provided only a 3-month benefit of survival. Upon second resection, the increased risk of neurologic deficits and wound-healing complications warrants a more thorough risk-benefit assessment.11 Other treatment options include lomustine,12 temozolomide rechallenge,12 bevacizumab with or without Irinotecan,13-15 intermediate-frequency electrical fields, or tumor treatment fields.15 Laser interstitial thermal therapy (LITT) is a minimally invasive cytoreductive option that is increasingly employed.
LITT is a novel treatment modality that has been used for a variety of intracranial lesions. The treatment typically features a stereotactically guided laser probe that is used to deliver controlled heat to surrounding tissues while using magnetic resonance thermography to monitor and conform the energy in real time.16 Potential advantages of LITT over repeat surgery include a decreased risk of wound-healing complications and cerebrospinal fistulae, decreased recovery time, as well as the ability to safely treat deep-seated lesions that may otherwise not be amenable to surgical resection.17
In the current manuscript, we conducted a systematic review of the available literature with quantitative pooling of overall survival and progression-free survival data to summarize outcomes of patients with rGBM undergoing LITT.
METHODS
Search Strategy
The current study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis.18 The search strategy was designed around the question: “What are the outcomes of patients with rGBM undergoing LITT as assessed by overall median survival, post-LITT survival and progression free survival?” An expert librarian first performed a comprehensive electronic search in PubMed, Embase, Scopus, evidence-based medicine, and Web of Science for studies published until April 13, 2020. We have attached the actual search strategy as a supplemental file (Supplementary Methods). Two investigators (A.M.-C. and M.A.A.) independently reviewed the identified articles. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart for search methodology and subsequent exclusions is provided in Figure 1. Institutional review board approval was neither sought nor required, as we only used published data for the study.
Figure 1.
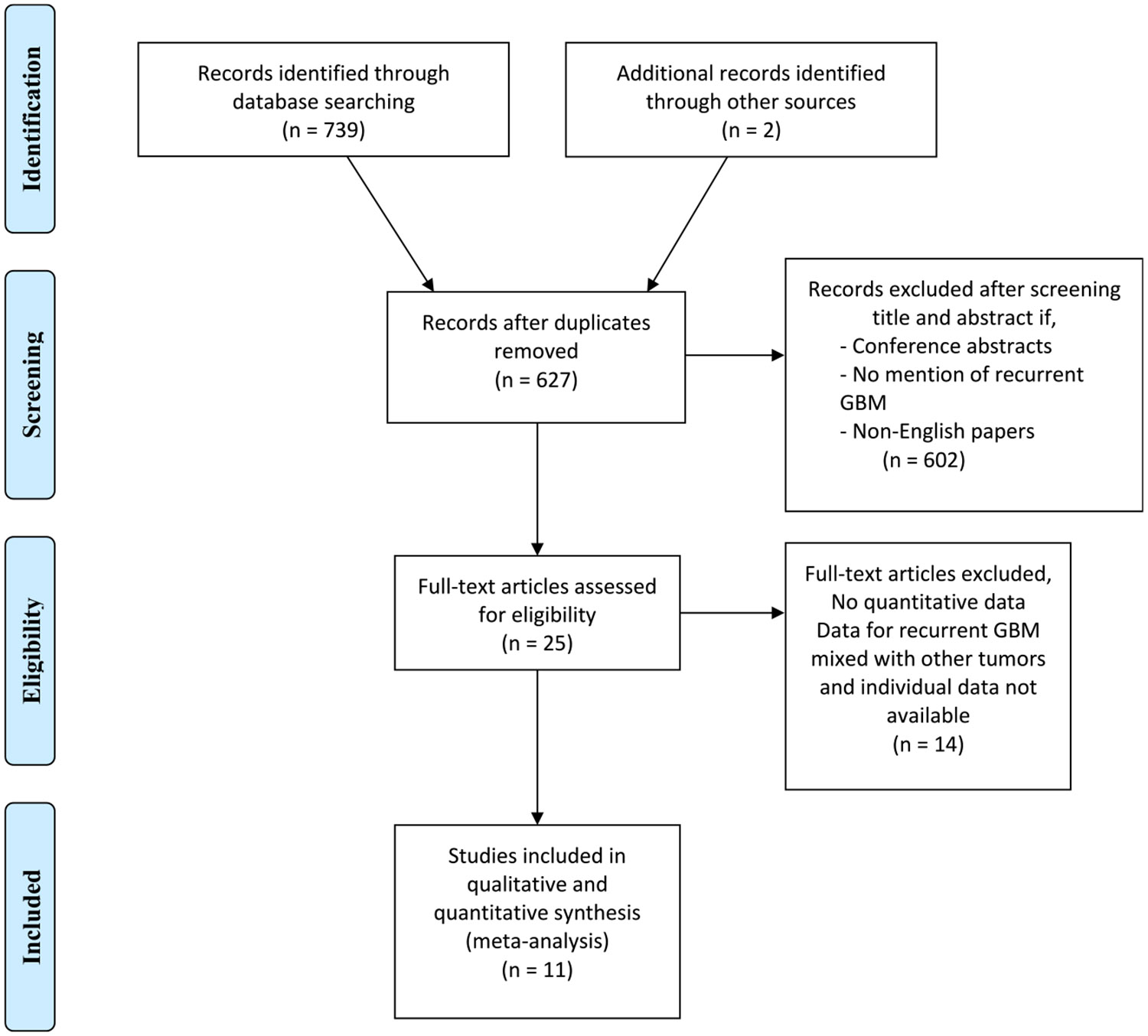
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) study selection flowchart.
Selection Criteria
We included studies if they matched the following criteria: 1) documented recurrence of GBM after initial therapy; and 2) provided quantitative data on overall survival, post-treatment survival, or progression-free survival after LITT. When studies included patients with multiple tumor pathologies, only patients with rGBM were included. Studies with pooled data without distinction of rGBM cases were excluded. Survival metrics were calculated from studies that reported individual data for patients with rGBM but not overall median or progression-free survival.
Data Extraction
The following data were extracted from the included studies: 1) first author and year of publication, 2) sample size of overall patients and those with rGBM, 3) time to progression/recurrence, 4) type of LITT system used, 5) adjuvant treatments, 6) complications, 7) overall survival 8) post-LITT survival, and 9) progression-free survival post-LITT. All data points were abstracted directly from article manuscripts, tables, and figures by 3 investigators (M.R., A.M.-C., and M.A.A.).
Statistical Analysis
The primary outcomes of the study were 1) median overall survival 2) median post-LITT survival, and 3) progression-free survival. All statistics were pooled together by a meta-analysis of means using weighted random-effects or fixed-effect model depending on heterogeneity.19 When a study did not report any variance metric (95% confidence interval [CI], standard error, or standard deviation) for continuous outcomes of interest, we imputed these using established methods.20 Heterogeneity was assessed using the I2 statistics, with values >50% denoting significant heterogeneity.21 We also performed sensitivity analyses, by excluding from each analysis studies that used older LITT systems (other than the Visualase or the NeuroBlate system).
Quality Assessment of Included Studies
We assessed the quality of each study using the modified Newcastle—Ottawa Scale for noncomparable studies.22 Since none of the outcomes had more than 10 studies available, we did not generate funnel plots to observe publication bias (Table 1).33
Table 1.
Assessment of the Quality of Included Studies According to the Modified Newcastle—Ottawa Quality Assessment Scale
| Study | Quality Assessment Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Representativeness of Exposed Cohort |
Representativeness of Nonexposed Cohort |
Ascertainment of Exposure |
Outcome of Interest |
Assessment of Outcome |
Adequate Duration of Follow-up |
Adequate Follow-up of Cohorts |
|
| Beaumont et al., 201823 | *** | — | *** | *** | ** | *** | *** |
| Carpentier et al., 201224 | ** | — | *** | ** | ** | ** | *** |
| Hawasli et al., 201325 | *** | — | *** | * | *** | *** | *** |
| Kamath et al., 201926 | *** | — | *** | *** | ** | *** | *** |
| Mohammadi et al., 201427 | *** | — | *** | * | ** | * | * |
| Reimer et al., 199828 | ** | — | *** | * | ** | *** | *** |
| Schwarzmaier et al., 200629 | *** | — | *** | ** | ** | * | * |
| Shah et al., 202030 | *** | — | *** | ** | ** | *** | *** |
| Ali et al., 201831 | ** | — | *** | *** | ** | *** | *** |
| Sloan et al., 201332 | *** | — | *** | ** | *** | *** | *** |
| Thomas et al., 201617 | *** | — | *** | *** | *** | *** | *** |
Representativeness of exposed cohort: very representative, retrospective institutional cohort (***); somewhat representative, selected patients (**); not representative (*).
Representativeness of nonexposed cohort: not available.
Ascertainment of exposure: all patients in cohort received treatment (***).
Outcome of interest: study reports all of the outcomes evaluated in this analysis (***); study reports most of the outcomes evaluated in this analysis (**); study reports some of the outcomes evaluated in this analysis (*).
Adequate duration of follow-up: study reports complete lifespan of all patients with GBM (***); study reports complete follow-up for most patients with GBM (**); study reports complete follow-up for some patients with GBM (*).
GBM, glioblastoma.
RESULTS
Search Strategy and Study Characteristics
The initial comprehensive search identified 739 articles, describing 627 unique studies. After screening the abstracts and applying the exclusion criteria, we identified 25 eligible studies that underwent full-text screening. Eleven studies met all inclusion and exclusion criteria and were used for data extraction. These studies described a total of 134 patients with rGBM.17,23-32 Most studies were published during the last decade, between 2012 and 2019,17,23-31 with the exception of Reimer et al. (1998)17,23-31 and Schwarzmaier et al. (2006).17,23-31
Demographics and Clinical Characteristics of the Cohort
The pooled mean age and variance of the cohort at the time of recurrent diagnosis was 56.7 ± 4.56 years of age. Based on 10 studies, 41% (50/121) of the pooled cohort was female. The most commonly involved region was the frontal lobe (42.5%), followed by the parietal lobe (29%), the temporal lobe (26.1%), corpus callosum (20.9%), and thalamus (14.2%). For delivery of LITT, the 2 older studies28,29 both used neodymiumdoped yttrium aluminum garnet (Nd:YAG laser; Domier, Friedrichshafen, Germany), 3 studies17,23-31 used the Visualase Thermal Therapy System (Visualase, Inc., Houston, Texas, USA), 5 studies17,23-31 used the NeuroBlate system (Monteris Medical Corporation, Plymouth, Minnesota, USA), whereas 1 study17,23-32 used both the Visualase and the NeuroBlate systems (Table 2).
Table 2.
Demographic and Clinical Characteristics of Patients in Each Study
| Study | N | Patients with rGBM |
Age at Recurrence Diagnosis, Mean ± SD |
Female | Intracranial Region of Recurrence* |
Median KPS |
Outcomes Assessed |
LITT System |
|---|---|---|---|---|---|---|---|---|
| Beaumont et al., 201823 | 15 | 6 | 52.16 ± 10.9 | 66.70% | Corpus callosum (15) | 80 | OS, post-LITT survival, PFS | NeuroBlate |
| Carpentier et al., 201224 | 4 | 4 | 50.25 ± 7.8 | 25% | Temporal (2), frontal (2) | N/A | OS, post-LITT survival | Visualase |
| Hawasli et al., 201325 | 11 | 4 | 71 ± 4.7 | 25% | Thalamus (4), frontal (1), corpus callosum (1), insular (1). basal ganglia (1), parietal (2) | N/A | PFS | NeuroBlate |
| Kamath et al., 201926 | 54 | 41 | 58.8 ± 10.8 | 31.50% | Frontal (14), temporal (8), parietal (9), occipital (1), parieto-occipital (4), temporo-parietal (4), corpus callosum (8), insular (2), thalamic (8) | N/A | OS, post-LITT survival, PFS | NeuroBlate |
| Mohammadi et al., 201427 | 34 | 19 | 56 ± 4.56 | 38% | Frontal (15 lesions), thalamic (7), parietal (5), temporal (5), insular (2), corpus callosum (1) | N/A | PFS | NeuroBlate |
| Reimer et al., 199828 | 4 | 4 | 59 ± 4.24 | 50% | Temporal (1), frontal (3) | N/A | PFS | Nd:YAG laser |
| Schwarzmaier et al., 200629 | 16 | 16 | 62 ± 4.56 | 37.50% | Frontoparietal (1) Frontal (3) parieto-occipital (3) temporo-parietal (3) occipital (1) parietal (1) temporal (1) corpus callosum (1) frontotemporal (1) parasagittal (1) | 70 | OS, post-LITT survival | Nd:YAG laser |
| Shah et al., 202030 | 91 | 14 | 54 ± 4.56 | 50% | Frontal (4), frontoparietal (2), parietal (1), temporal (6), occipital (1), | 91 | OS, PFS | Visualase |
| Ali et al., 201831 | 3 | 3 | 52 ± 12.6 | 66.70% | Frontotemporal (2), parietal (1), | Post-LITT survival, PFS | Visualase | |
| Sloan et al., 201332 | 10 | 10 | 55 ± 4.56 | 20% | Frontal (3), temporal (2), parietal (3), temporoparietal (1), temporo-occipital (1), | 80 | OS, post-LITT survival | NeuroBlate |
| Thomas et al., 201617 | 13 | 13 | 48.9 ± 4.56 | N/A | Frontal (6), temporal (1), corpus callosum (2), cingulate (2), insular (2) | 80 | OS, post-LITT survival, PFS | Visualase and NeuroBlate |
rGBM, recurrent glioblastoma; SD, standard deviation; KPS, Karnofsky Performance Score; LITT, laser interstitial thermal therapy; OS, overall survival; N/A, not available; PFS, progression-free survival.
Overall Median Survival
A total of 8 studies with 107 patients had available data for overall median survival. The pooled overall survival was 18.6 months (95% CI 16.2—21.1) with an overall I2 of 98.7% (Table 3).
Table 3.
Overall Survival for Recurrent GBM
| Study | Sample Size | Overall Survival (95% CI) |
|---|---|---|
| Beaumont et al., 201825 | 6 | 27.78 months (16.35—39.22) |
| Kamath et al., 201928 | 41 | 22.30 months (17.19—27.41) |
| Ali et al., 201824 | 3 | 15.00 months (9—21) |
| Sloan et al., 201331 | 10 | 20.56 months (10.64—30.48) |
| Shah et al., 202030 | 14 | 23.00 months (17.89—28.11) |
| Thomas et al., 201617 | 13 | 23.00 months (17.89—28.1) |
| Carpentier et al., 201226 | 4 | 27.25 months (24.21—30.29) |
| Schwarzmaier et al., 200632 | 16 | 9.40 (8.57—10.23) |
| Overall (pooled) | 107 | 18.62 months (16.18—21.06) |
GBM, glioblastoma; CI, confidence interval.
We also performed a sensitivity analysis to include only those studies that used either the NeuroBlate or the Visualase, thereby excluding the 2 older studies with Nd:YAG laser. This analysis revealed the pooled overall survival to be 20.2 months (95% 17.4e23.1).
Post-LITT Survival
A total of 6 studies with 93 patients had available data for post-LITT survival. The pooled post-LITT survival was 10.2 months (95% CI 8.8—11.6). The overall I2 of the analysis was 91% (Table 4).
Table 4.
Overall Survival Post-LITT for Recurrent GBM
| Study | Sample Size | Survival Post-LITT (95 %CI) |
|---|---|---|
| Beaumont et al., 201823 | 6 | 27.78 months (16.35—39.22) |
| Kamath et al., 201926 | 41 | 11.80 months (9.29—14.31) |
| Ali et al., 201831 | 3 | 9 months (2.8—15.22) |
| Sloan et al., 201332 | 10 | 10.53 months (6.89—14.17) |
| Thomas et al., 201617 | 13 | 7.00 months (4.01—9.99) |
| Carpentier et al., 201224 | 4 | 10.75 months (7.71—13.79) |
| Schwarzmaier et al., 200629 | 16 | 6.9 months (6.07—7.73) |
| Overall (pooled) | 93 | 10.19 months (8.76—11.62) |
LITT, laser interstitial thermal therapy; GBM, glioblastoma; CI, confidence interval.
We again performed a sensitivity analysis to include only those studies that used either the NeuroBlate or the Visualase, thereby excluding the sole study that used a Nd:YAG laser. This analysis revealed the pooled post-LITT survival to be 10.9 months (95% 9.1—12.6).
Progression-Free Survival
A total of 8 studies with 119 patients had available data for progression-free survival. The pooled progression-free survival was 6.2 months (95% CI 5.37—6.95). The overall I2 of the analysis was 80.3% (Table 5).
Table 5.
Progression-Free Survival Post-LITT for Recurrent GBM
| Study | Sample Size | Progression-Free Survival (95% CI) |
|---|---|---|
| Beaumont et al., 201823 | 6 | 4.50 months (2.40—6.60) |
| Kamath et al., 201926 | 41 | 7.30 months (5.46—9.14) |
| Ali et al., 201831 | 3 | 4.25 months (1.70—6.80) |
| Shah et al., 202030 | 14 | 5.60 months (4.34—6.86) |
| Mohammadi et al., 201427 | 34 | 5.10 months (4.29—5.91) |
| Thomas et al., 201617 | 13 | 5.00 months (3.70—6.30) |
| Hawasli et al., 201325 | 4 | 8.26 months (7.28—9.24) |
| Reimer et al., 199828 | 4 | 7.00 months (5.63—8.37) |
| Overall (pooled) | 119 | 6.16 months (5.37—6.95) |
LITT, laser interstitial thermal therapy; GBM, glioblastoma; CI, confidence interval.
We also performed a sensitivity analysis to include only those studies that used either the NeuroBlate or the Visualase, thereby excluding one study that used Nd:YAG laser. This analysis revealed a pooled progression-free survival of 6 months (95% CI 5.2—6.7).
DISCUSSION
Laser interstitial therapy has emerged as a minimally invasive alternative to surgery for recurrent glioblastoma. LITT results in heat-induced protein denaturation propagated through an ablation radius of 20 mm. Visualase and NeuroBlate are the 2 systems approved by the Food and Drug Administration that are available in the United States and appear equivalent to each other with regard to efficacy as determined by laser wavelength and exposure time. The incorporation of a CO2—saline cooling system permits power up to 15 W with maximum temperature threshold of 90°C and 50°C at the ablation and periphery zones, respectively.34 The addition of visible helium to the invisible CO2 laser increased efficiency, precision, and applicability in neurosurgical procedures. LITT developed after the use of Nd:YAG laser in an experimental brain model by Bown in 1983.35 Through LITT, tumor necrosis occurs with temperatures between 50°C and 80°C. The incorporation of magnetic resonance imaging software for the Arhenius thermal dose model allows appropriate feedback-control for increased preservation of surrounding tissue but also increased predictability of tissue necrosis.36 Real-time extent of thermal ablation can be calculated through a software incorporated within the NeuroBlate System capable of predicting tissue damage based on temperature and exposure time, which determines the thermal damage threshold (TDT).32 Mohammadi et al.27 demonstrated that the extent of tumor coverage by TDT lines has an impact on overall survival on patients with high-grade glioma. The authors suggest that the concept of tumor coverage by TDT lines may be analogous to “extent of resection,” which is discussed in open surgical resection literature.
LITT has surged as a viable option to radiotherapy or repeated surgery for recurrent GBM. In our study, to address possible selection bias introduced by different LITT modalities, we performed a sensitivity analysis comparing outcomes from studies using modern LITT modalities such as NeuroBlate versus Visualase,17,23-32 and the Nd:YAG laser.28,29 We observed that difference in survival time was around 1 month. This confirms previous results of no superiority between LITT modalities.37 In a recent retrospective review, preoperative Karnofsky Performance Score (KPS; ≤70) before LITT was associated with increased number of motor deficit and overall survival.38 However, data regarding LITT improving quality of life in recurrent GBM have yet to be discussed.39
Heterogeneity in LITT timing during different disease stages poses a challenge in terms of evaluating the role of this treatment modality on patients with recurrent disease. Throughout the study, we provide a framework that seeks to increase decision-making capacity on the management of recurrent GBM. Patients with recurrent tumors might be more frequently eligible for this procedure as an alternative to avoid a second open surgery. A recent systematic review reported an overall survival of 22 months in patients undergoing a second surgery with chemotherapy at recurrence.40 Our analysis indicates an overall survival of 18.6 months from diagnosis. Following recurrence, a second surgery and chemotherapy was associated with an extended survival of 9.6 months, whereas our analysis reports a post-LITT survival of 10.9 months. In addition, we found a post-LITT PFS of 6 months, which is slightly superior to the 4.8 months following repeated surgery and chemotherapy in this retrospective study.40 According to these data, one of the advantages associated to the LITT option is possible chemotherapy avoidance at recurrence. Furthermore, for inoperable cases such as deep-seated or near-to-eloquent-areas gliomas, where biopsy might be the only option, LITT is associated with an additional survival of 7 months.41 Continued improvement in the technology and reduction in the steepness of the learning curve over time have also been thought to contribute to improved survival.27,29,38,41 However, the study by Schwarzmaier et al.29 showed that not only the learning curve but also longer interval between recurrence and LITT, lower KPS, and larger tumor volume might have influenced the poorer outcomes of the early years. Appropriate patient selection is an important factor in safety and efficacy, with more favorable outcomes among patients with lesions <3 cm diameter, in those with a predicted achievable ablation ≥80%, and in those with KPS ≥70.27,29,38,41
Complications do occur and inversely correlate with overall survival.41 Among the most common reported serious complications are brain edema,23 hydrocephalus,23,25,26 transient and permanent neurologic deficit,23,25 seizure,17 as well as possibility of leptomeningeal spread and seeding.32 Patients with larger and newly diagnosed lesions have a greater risk of complications than those with recurrent, smaller tumors.23 Compared with open surgery, LITT is associated with significantly reduced postoperative hospital stay and overall cost.41 From this study, we can conclude that LITT is a safe and effective method in this population that could be used as an alternative to surgery in selected cases. The numbers of catheters used during the LITT procedure is a determinant factor when assessing both safety and cost and making accurate comparisons with surgery from an overall effectiveness point of view. However, given limited data available for these variables, further research is required to make a closer effectiveness comparison with surgery.
The current study is not a pooled analysis, as the absence of hazard ratios pre-cluded a true meta-analysis. The study is limited by the risk of selection bias due to lack of stratification for known survival prognostic factors in glioblastoma such as isocitrate dehydrogenase mutation status,42 or Ki67 index,43 subventricular zone location,44 presentation at younger than 50 years of age,45 or O6-methylguanine-DNA methyl-transferase methylation status.46 Future studies will be necessary to better define the risks and efficacy of LITT in specific biomolecular tumor contexts.29 The study is also limited by the small cohort, with only 10 studies meeting eligibility criteria for inclusion in our analysis. In one of the studies, deaths among nearly one-half of the patients were due to non—tumor-related causes, and 4 patients did not complete follow-up by the time the study ended (follow-up less than 85%).29 Specific information regarding patients with rGBM in relation to progression-free survival, survival after LITT, and overall median survival could not be extrapolated from 324,29,32 and 425,27,28,30 studies, respectively. Another limitation accounts for nonaccoutrements of outcomes defined in relation to optimal thermal ablation technique defined per the TDT lines coverage. In addition, this analysis includes patients who may have not be eligible for surgical resection and therefore underwent LITT; this could have introduced a selection bias to our study that should be acknowledged. Similarly, these findings could as well emphasize how LITT is a safe alternative to surgery when surgery resection of recurrent GBM is not feasible. We would like to acknowledge the intrinsic limitation when interpreting survival analysis, given a lack of specific distinction between treatment-related pseudoprogression and actual disease progression. Although our data are based on best-available literature, any of the herein included studies specifically address characterization of real recurrence versus pseudoprogression. For example, we previously observed that 16% of patients treated for presumed tumor progression were indeed cases of pseudoprogression due to treatment-related radiographic changes. This observation might serve as a call for improving accuracy of outcomes reported on rGBM-related research.
CONCLUSIONS
Results in our study show that LITT provides a survival at least comparable with that of open surgery for patients with rGBM. LITT is a safe and effective treatment comparable with the current standard of care for recurrent GBM that can be used as an alternative to surgery. While no clear benefit over open surgery in terms of survival can be established yet, additional advantages such as decreased morbidity and hospital costs make LITT an attractive alternative in this population of patients. In patients where surgery is not amenable, LITT is associated with extended benefit on survival. Safety and effectiveness in LITT rely on appropriate patient selection and center experience.
Supplementary Material
Abbreviations and Acronyms
- CI
Confidence interval
- GBM
Glioblastoma
- KPS
Karnofsky Performance Score
- LITT
Laser interstitial thermal therapy
- Nd:YAG laser
Neodymium-doped yttrium aluminum garnet
- rGBM
recurrent glioblastoma
- TDT
Thermal damage threshold
Footnotes
Conflict of interest statement:
Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008685.
REFERENCES
- 1.Birk HS, Han SJ, Butowski NA. Treatment options for recurrent high-grade gliomas. CNS Oncol. 2017;6:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesfin FB, Al-Dhahir MA. Gliomas. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021; 2020. [Google Scholar]
- 3.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 5.Ammirati M, Galicich JH, Arbit E, Liao Y. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987;21:607–614. [DOI] [PubMed] [Google Scholar]
- 6.Choucair AK, Levin VA, Gutin PH, et al. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg. 1986;65:654–658. [DOI] [PubMed] [Google Scholar]
- 7.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. [DOI] [PubMed] [Google Scholar]
- 9.Lamborn KR, Yung WKA, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Linde ME, Brahm CG, de Witt Hamer PC, et al. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol. 2017;135:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringel F, Group SS, Pape H, et al. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2015;18:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandes AA, Tosoni A, Basso U, et al. Second-line chemotherapy with irinotecan plus carmustine in glioblastoma recurrent or progressive after first-line temozolomide chemotherapy: a phase II study of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). J Clin Oncol. 2004;22: 4779–4786. [DOI] [PubMed] [Google Scholar]
- 13.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27: 4733–4740. [DOI] [PubMed] [Google Scholar]
- 14.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012;19: 1636–1640. [DOI] [PubMed] [Google Scholar]
- 16.Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus. 2015;38:E13. [DOI] [PubMed] [Google Scholar]
- 17.Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016;41:E12. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, PRISMA-P Group, Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.st: metan and other meta-analysis commands in Stata. Available at: https://www.stata.com/statalist/archive/2006—12/msg00735.html. Accessed May 10, 2020.
- 20.16.1.3.1 Imputing standard deviations. Available at: https://handbook-5—1.cochrane.org/chapter_16/16_1_3_1imputing_standard_deviations.htm. Accessed May 11, 2020.
- 21.Higgins JPT. Measuring inconsistency in metaanalyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid-Based Med. 2018;23:60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaumont TL, Mohammadi AM, Kim AH, Barnett GH, Leuthardt EC. Magnetic resonance imaging-guided laser interstitial thermal therapy for glioblastoma of the corpus callosum. Neurosurgery. 2018;83:556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44: 361–368. [DOI] [PubMed] [Google Scholar]
- 25.Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions. Neurosurgery. 2013;73:1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reimer P, Bremer C, Horch C, Morgenroth C, Allkemper T, Schuierer G. MR-monitored LITT as a palliative concept in patients with high grade gliomas: preliminary clinical experience. J Magn Reson Imaging. 1998;8:240–244. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzmaier H-J, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol. 2006;59:208–215. [DOI] [PubMed] [Google Scholar]
- 30.Shah AH, Semonche A, Eichberg DG, et al. The role of laser interstitial thermal therapy in surgical neuro-oncology: series of 100 consecutive patients. Neurosurgery. 2020;87:266–275. [DOI] [PubMed] [Google Scholar]
- 31.Ali SC, Basil GW, Diaz RJ, Komotar RJ. The safety of bevacizumab administered shortly after laser interstitial thermal therapy in glioblastoma: a case series. World Neurosurg. 2018;117:e588–e594. [DOI] [PubMed] [Google Scholar]
- 32.Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma. J Neurosurg. 2013;118:1202–1219. [DOI] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 34.Krivosheya D, Borghei-Razavi H, Barnett GH, Mohammadi AM. Laser interstitial thermal therapy in glioblastoma. In: Glioma—Contemporary Diagnostic and Therapeutic Approaches. Ibrahim Omerhodžić and Kenan Arnautović, IntechOpen. Available at: https://www.intechopen.com/books/glioma-contemporary-diagnostic-and-therapeutic-approaches/laser-interstitial-thermal-therapy-in-glioblastoma. Accessed October 17, 2020. [Google Scholar]
- 35.Salem U, Kumar VA, Madewell JE, et al. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging. 2019;19:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yung JP, Shetty A, Elliott A, et al. Quantitative comparison of thermal dose models in normal canine brain. Med Phys. 2010;37:5313–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagman C, Chung LK, Pelargos PE, et al. Laser neurosurgery: A systematic analysis of magnetic resonance-guided laser interstitial thermal therapies. J Clin Neurosci. 2017;36:20–26. [DOI] [PubMed] [Google Scholar]
- 38.Shao J, Radakovich NR, Grabowski M, et al. Lessons learned in using laser interstitial thermal therapy for treatment of brain tumors: a case series of 238 patients from a single institution. World Neurosurg. 2020;139:e345–e354. [DOI] [PubMed] [Google Scholar]
- 39.Wright J, Chugh J, Wright CH, et al. Laser interstitial thermal therapy followed by minimal-access transsulcal resection for the treatment of large and difficult to access brain tumors. Neurosurg Focus. 2016;41:E14. [DOI] [PubMed] [Google Scholar]
- 40.Wann A, Tully PA, Barnes EH, et al. Outcomes after second surgery for recurrent glioblastoma: a retrospective case-control study. J Neurooncol. 2018;137:409–415. [DOI] [PubMed] [Google Scholar]
- 41.Voigt JD, Barnett G. The value of using a brain laser interstitial thermal therapy (LITT) system in patients presenting with high grade gliomas where maximal safe resection may not be feasible. Cost Eff Resour Alloc. 2016;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Combs SE, Rieken S, Wick W, et al. Prognostic significance of IDH-1 and MGMT in patients with glioblastoma: one step forward, and one step back? Radiat Oncol. 2011;6:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W-J, He D-S, Tang R-X, Ren F-H, Chen G Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015;16:411–420. [DOI] [PubMed] [Google Scholar]
- 44.Adeberg S, Bostel T, König L, Welzel T, Debus J, Combs SE. A comparison of long-term survivors and short-term survivors with glioblastoma, sub-ventricular zone involvement: a predictive factor for survival? Radiat Oncol. 2014;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J, Lv X, Lu C, et al. Prognostic factors of patients with gliomas—an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer. 2020;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radke J, Koch A, Pritsch F, et al. Predictive MGMT status in a homogeneous cohort of IDH wildtype glioblastoma patients. Acta Neuropathol Commun. 2019;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


