Abstract
Background:
The efficacy and safety of bimekizumab (BKZ), an inhibitor of interleukin (IL)-17F in addition to IL-17A, has been established in axial spondyloarthritis (axSpA). Early assessment of new bone formation is possible using 18F-fluoride positron emission tomography-computerised tomography (PET-CT) imaging to quantitatively monitor osteoblastic activity.
Objectives:
This exploratory study, initiated before phase IIb/III studies, assessed the efficacy and safety of BKZ in patients with radiographic (r-)axSpA and its effect on new bone formation.
Design:
Patients were randomised 2:1 to BKZ 160 mg every 2 weeks (Q2W; Weeks 0–10) then 320 mg Q4W (Weeks 12–44), or the reference drug: certolizumab pegol (CZP) 400 mg Q2W (Weeks 0–4), then 200 mg Q2W (Weeks 6–10), 400 mg Q4W (Weeks 12–44).
Methods:
Primary (Axial Spondyloarthritis Disease Activity Score (ASDAS) change from baseline (CfB)) and secondary endpoints (ASDAS-ID, ASDAS-MI) were assessed at Week 12. PET-positive axSpA lesion counts and osteoblastic activity quantification (mean SUVauc) were performed at baseline and Weeks 12 and 48 in the sacroiliac joints and spine (PET-CT substudy; not powered to evaluate differences).
Results:
In total, 76 patients were randomised; 26/76 entered the PET-CT substudy. At Week 12, the mean ASDAS CfB with BKZ was −2.1 (CZP: −1.8); ASDAS-ID and ASDAS-MI were achieved by 23.9% (11/46) (CZP: 20.8% (5/24)) and 60.9% (28/46) (CZP: 45.8% (11/24)) patients. Across treatments, clinical efficacy was maintained or increased further at Week 48. In the PET-CT substudy, the total number of PET-positive axSpA lesions and mean SUVauc were substantially reduced from baseline at Week 12 with BKZ and CZP, with reductions maintained or further reduced at Week 48. Treatments were well tolerated with no new safety signals.
Conclusion:
Dual inhibition of IL-17A and IL-17F with BKZ resulted in improved clinical outcomes and reduced osteoblastic activity in patients with r-axSpA, suggesting the potential of BKZ to reduce disease activity and new bone formation within 12 weeks of treatment. CZP findings were consistent with previous data. No new safety signals were identified.
Trial registration:
ClinicalTrials.gov, NCT03215277 (https://clinicaltrials.gov/study/NCT03215277).
Keywords: ankylosing spondylitis, axial spondyloarthritis, bDMARDs, bimekizumab, bone, IL-17 inhibitors, imaging, osteoblast, PET-CT, TNF inhibitors
Introduction
Radiographic axial spondyloarthritis (r-axSpA; also known as ankylosing spondylitis) 1 together with non-radiographic axSpA (nr-axSpA) comprise the disease spectrum of axSpA, a chronic inflammatory disease of the axial skeleton.2,3 New bone formation is a hallmark of axSpA, which can lead to syndesmophytes (bony outgrowths from spinal ligaments) 4 in the vertebral bodies of approximately 60%–70% of patients, and ankylosis of the sacroiliac joints (SIJ).5–7 Patients commonly experience restricted spinal mobility and impaired physical function as a result of this structural damage,2,3,8 and in r-axSpA, definitive structural damage to the spine and SIJ is visible on radiographs.2,3
Interleukin (IL)-17 inhibitors, a newer class of biologic disease-modifying antirheumatic drugs (bDMARDs), are an increasingly recognised alternative treatment option to tumour necrosis factor inhibitors (TNFis; first-line bDMARDs) for patients with axSpA who have an inadequate response to non-steroidal anti-inflammatory drugs (NSAIDs).9–11 Similar efficacy in musculoskeletal manifestations of axSpA across TNFis and IL-17 inhibitors has been assumed to date, with neither prioritised based on efficacy in treatment guidelines. 10 The IL-17 receptor complex binds to IL-17A and IL-17F homodimers and heterodimers and has been associated with promotion of inflammation and bone formation/damage, which are features of axSpA.12–15 Elevated concentrations of IL-17A and IL-17F have also been implicated in the pathogenesis of other immune-mediated inflammatory diseases, including plaque psoriasis and psoriatic arthritis. 12 Production of IL-17A and IL-17F by innate immune cells can occur independently of IL-23, which may explain the differing impacts of inhibiting the IL-17 versus IL-23 blockade in axSpA treatment. 12
Bimekizumab (BKZ) is a humanised monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A. 16 In trials covering the full spectrum of axSpA (r-axSpA: phase IIb BE AGILE trial and its open-label extension, phase III BE MOBILE 2; nr-axSpA: phase III BE MOBILE 1), dual inhibition of IL-17A and IL-17F with BKZ resulted in significant, rapid and sustained improvements in key efficacy outcomes, and was well tolerated.16–19 In the phase III trials, BKZ treatment also led to reductions in objective signs of inflammation, including high-sensitivity C-reactive protein (hs-CRP) and MRI assessments of the SIJ and spine.16,17
Conventional radiography is considered the gold standard technique for assessing structural damage in the SIJ and spine.6,20 However, reliable detection of radiographic changes is only possible after a minimum of 2 years. 6 MRI is a standard and effective technique for visualising active inflammatory changes in the axial skeleton and may detect the presence of active inflammation in the SIJ and spine prior to the appearance of radiographic changes.6,20,21 However, MRI may have limited specificity and sensitivity when used diagnostically to identify axSpA inflammatory lesions and comparative imaging studies suggest MRI is also less suitable for detecting new bone formation occurring in the absence of inflammation.22,23
MRI studies have demonstrated the effectiveness of TNFis at suppressing inflammation in the SIJ and spine and mitigating the long-term risk of developing fatty lesions in the spine.24,25 There are also well-documented X-ray data for the impact of long-term treatment with TNFis on new bone formation in axSpA, demonstrating the reduction of radiographic progression over extended treatment periods.26,27 The impact of IL-17 inhibitors on MRI-detected inflammation is well established,28–31 with evidence from the BE MOBILE trials highlighting the rapid and sustained anti-inflammatory effect of BKZ in the SIJ and spine.16,17 However, the effect of dual inhibition of IL-17A and IL-17F with BKZ on new bone formation is not yet established.
Investigating short-term (i.e. <1 year) efficacy on new bone formation requires a sensitive technique that can detect and quantify early changes in bone formation. 5 There is currently a lack of sensitive or specific systemic biomarkers available for detecting local osteoblastic activity. However, recent data in patients with r-axSpA have shown that fluorine-18-labelled fluoride ( 18 F-fluoride) positron emission tomography-computerised tomography (PET-CT) is a promising whole-body imaging technique that can assess and quantify the impact of treatment on new bone formation at different locations on the axial skeleton.5,32 PET enables non-invasive molecular imaging and quantification of tissue function and physiology, and the radiotracer 18F-fluoride can be used to detect and quantify ongoing bone mineralisation during bone formation by osteoblasts.5,32 18 F-fluoride is absorbed by hydroxyapatite crystals which comprise fluorapatite minerals in bone, especially at sites of bone remodelling. 5 Uptake of 18F-fluoride has been shown, and confirmed in biopsies, to identify lesions with osteoblastic activity and associated molecular new bone formation in the SIJ and spine. 5 Therefore, this imaging technique enables the assessment of pathologically increased bone mineralisation caused by disease activity and the quantitative assessment of the direct effects of pharmacological treatment on bone formation.5,32
Molecular new bone formation in anterior vertebral corners of the spine in r-axSpA may be detected up to 2 years earlier with 18F-fluoride PET-CT than detection of syndesmophytes with conventional radiography. 33 In a direct comparative study, 18F-fluoride PET-CT provided partially distinctive information about disease activity in patients with active r-axSpA compared with MRI or conventional radiography imaging techniques. 22 18 F-fluoride PET-CT imaging enabled visualisation of lesions with osteoblastic activity, including in those without bone marrow oedema and structural damage. 22 Consequently, PET-CT imaging may be useful for assessing treatment response in clinical practice and could offer opportunities for early detection of treatment impact on new bone formation.
This study aimed to assess the efficacy and safety of BKZ in patients with active r-axSpA, alongside the effect of BKZ on osteoblastic activity measured using 18F-fluoride PET-CT. Certolizumab pegol (CZP), a PEGylated, Fc-free TNFi approved for use in this indication,34–36 was included as a reference drug in this exploratory study to represent the TNFi drug class and contextualise results.
Methods
Study design and patients
This phase IIa, multicentre, randomised, double-blind, parallel-group, exploratory study (NCT03215277) was conducted at 38 sites in 8 countries (the United States and 7 across Europe). It included a 2- to 4-week screening period, a 48-week treatment period (12-week initial treatment period, followed by a 36-week treatment extension period), and a 20-week safety follow-up period after the final treatment dose at Week 44 (final efficacy assessment at Week 48) (Supplemental Figure S1). A PET substudy was conducted in a subset of patients in parallel, to assess imaging outcomes using 18F-fluoride PET-CT.
At screening, adults aged ⩾18 years with moderate to severe active r-axSpA, defined as Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ⩾4 and spinal pain (BASDAI Q2) ⩾4, were recruited. All patients fulfilled modified New York (mNY) classification criteria, 37 and were required to have ⩾3 months’ symptom duration and age of onset <45 years. In addition, hs-CRP levels >upper limit of normal (defined as 5 mg/L) were required at screening, as well as prior inadequate response, contraindication or intolerance to ⩾2 NSAIDs. Current NSAID therapy was permitted if the patient was receiving a stable dose from 2 weeks prior to baseline.
Patients were excluded if they had previously received >1 TNFi (for patients with 1 prior TNFi, a pre-baseline washout of 12 weeks was required for infliximab, adalimumab and golimumab and 28 days for etanercept) or had primary failure to any TNFi, defined as no response within the first 12 weeks of treatment. Those who had previously received BKZ, CZP or a biologic other than a TNFi were excluded. Patients with total ankylosis of the spine or inflammatory arthritic conditions other than r-axSpA were excluded; a diagnosis of inflammatory bowel disease (IBD) was permitted provided the patient had no active symptomatic disease at screening or baseline. Other exclusion criteria included active infection, history of chronic or recurrent infections, diagnosis of active tuberculosis (TB), high risk of acquiring TB or latent TB, presence of a secondary, non-inflammatory condition (e.g. fibromyalgia) with potential to interfere with study evaluations and significant neuropsychiatric disorder indicated by lifetime history of suicide attempt, suicidal ideation in the past 6 months (according to questions 4 and 5 of the Columbia-Suicide Severity Rating Scale (C-SSRS) at screening) or depression score ⩾10 or anxiety score ⩾15 on the Hospital Anxiety and Depression Scale (HADS) at screening.
After screening, patients were randomised 2:1 to BKZ or CZP. Patients in the BKZ group received the exploratory dose of BKZ 160 mg every 2 weeks (Q2W) for Weeks 0–10 and then BKZ 320 mg every 4 weeks (Q4W) for Weeks 12–44. As per the approved CZP dosing regimen,35,36 patients in the CZP group received a 400 mg loading dose Q2W during Weeks 0–4, followed by CZP 200 mg Q2W for Weeks 6–10 and then CZP 400 mg Q4W for Weeks 12–44 (Supplemental Figure S1). Randomisation was performed using interactive response technology. It should be noted that this exploratory study was designed and conducted prior to the investigation and establishment of the BKZ 160 mg Q4W dosing regimen, which has been used in recently published phase IIb and phase III studies in axSpA.16,19
BKZ and CZP were prepared and administered subcutaneously via a 2 mL vial or 1 mL prefilled syringe (BKZ) or a 1 mL prefilled syringe (CZP) by unblinded study personnel who were not involved in any other study aspects. BKZ-treated patients received one placebo injection (0.9% sodium chloride aqueous solution from a 10 mL vial or ampoule) Q2W to Week 4, in addition to their BKZ dose, to maintain blinding while the CZP group received the loading dose. All patients and designated sponsor and investigator site personnel were blinded to treatment assignment throughout the study; blinded site personnel did not discuss or have access to treatment-related information.
Clinical endpoints and safety
The primary efficacy endpoint was change from baseline (CfB) in Axial Spondyloarthritis Disease Activity Score (ASDAS)-CRP at Week 12. 38 The secondary efficacy endpoints, ASDAS inactive disease (ID; defined as ASDAS < 1.3) and ASDAS major improvement (MI; defined as reduction (i.e. improvement) in ASDAS of ⩾2.0 from baseline), were also assessed at Week 12. ASDAS, ASDAS-ID and ASDAS-MI were also assessed over time to Week 48. Other additional pre-defined efficacy variables included CfB in ASDAS over time, Assessment of SpondyloArthritis international Society 20% response (ASAS20), ASAS 40% response (ASAS40), ASAS partial remission and CfB in BASDAI and hs-CRP; all were assessed over time to Week 48. hs-CRP levels were also measured throughout to Week 64 (end of the safety follow-up period).
Incidence of treatment-emergent adverse events (TEAEs), treatment-emergent serious adverse events (SAEs) and TEAEs leading to discontinuation from the study were all pre-specified safety endpoints. TEAEs, SAEs and pre-specified safety topics of special monitoring are defined in the Supplemental Material and were assessed from Weeks 0–64. Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA Version 19.0).
PET imaging
PET imaging endpoints are presented for the subset of patients in the PET imaging substudy. Patients were eligible for the PET substudy if they were screened at one of the 11 study sites across Poland, Germany, Greece and The Netherlands that were able to perform 18F-fluoride PET-CT scans and provided separate informed consent. In the subset of patients enrolled in the PET substudy, scans of the SIJ and spine were performed at baseline (⩽14 days prior), Week 12 (⩽14 days after) and Week 48 (⩽14 days after). Additional details are provided in the Supplemental Material.
The PET scans were read visually with a qualitative assessment before a further, refined visual qualitative analysis was conducted. The refined analysis also enabled further classification of PET-positive lesions as axSpA, degenerative or uncertain/insecure by the independent reviewers, with an adjudicator in case of disagreement on axSpA versus degenerative classification. Only lesions classified as axSpA by both independent reviewers, or by agreement from the adjudicator, were analysed; PET-positive lesion refers to these axSpA lesions hereafter. Further details on the qualitative analysis are provided in the Supplemental Material.
PET-positive lesions were defined as lesions with 18F-fluoride uptake of ⩾1.5 times background levels; lesions with an 18F-fluoride uptake of >1.0 but <1.5 times were also included if intensity was asymmetrically higher than the locoregional and contralateral vertebral/sacroiliac sides, or uptake was present in an anatomical site highly suspected to be an axSpA location. Locations in the SIJ and spine were dichotomously scored for the presence or absence of PET-positive lesions (1 versus 0) at each timepoint.
Additionally, a quantitative analysis of 18F-fluoride uptake within PET-positive lesions was conducted in patients with PET-CT scans; further details are provided in the Supplemental Material. Standardised uptake values (SUV) normalised to the area under the individual integrated whole blood activity concentration curve (SUVauc) were assessed using Vrije University Medical Center’s in-house software (ACCURATE). 39 Change in bone formation, an exploratory endpoint, was determined using these SUVauc data (i.e. low mean SUVauc values correspond to low osteoblastic activity and therefore minimal pathological new bone formation).
Statistical analysis
Determination of sample size for the overall study and PET substudy are detailed in the Supplemental Material. This study was not powered to directly compare treatments with regards to secondary or exploratory endpoints, including endpoints assessed in the PET imaging substudy.
Analyses of the primary and secondary clinical efficacy endpoints, including the component variables, were performed on the per-protocol set (PPS), defined as all randomised patients who received ⩾1 treatment dose with a valid baseline and post-baseline measurement of ⩾1 efficacy variable and no important protocol deviation affecting the primary efficacy variable. Important protocol deviations were pre-defined and evaluated during a treatment-blinded data evaluation meeting; further details are provided in the Supplemental Material. Analyses from the PET substudy were performed on the PPS (PET-PPS), defined as all randomised patients who received ⩾1 treatment dose and had evaluable PET-CT or PET-MRI scan data at baseline and ⩾1 of the post-baseline assessments at Week 12 or Week 48. Safety data were summarised by treatment group for patients who had ⩾1 dose of BKZ or CZP, respectively (safety set).
Formal statistical testing was conducted for the primary efficacy endpoint at Week 12 following a Bayesian paradigm, performed using a linear regression model. Proof of concept (i.e. feasibility and initial evidence for targeted use of a treatment in a specific patient population) 40 was declared if the estimated posterior probability of a difference in mean CfB in ASDAS at Week 12 between treatment groups was ⩾97.5%. Further details on the Bayesian analysis are provided in the Supplemental Material.
Analyses of all other efficacy variables (i.e. not primary or secondary variables, or their components) were performed on the full analysis set (all randomised patients who received ⩾1 treatment dose with a valid baseline and post-baseline measurement for ⩾1 efficacy variable) and summarised descriptively by treatment group; no additional statistical testing was performed. Ninety-five percent confidence intervals for ASDAS and ASAS outcomes were calculated using a Wilson approximation.
Imputation methods for missing ASDAS and ASAS component data are provided in the Supplemental Material. No imputation of missing data was performed for summaries of the ASDAS component efficacy variables or imaging assessments. All reported efficacy data in this manuscript are observed case.
This study was funded by UCB, who funded third-party medical writing and editorial assistance based on the authors’ input and direction.
Results
Patient disposition and baseline characteristics
From 4 October 2017 to 21 May 2020, 145 patients were screened and 76 were randomised in the overall study (BKZ: 51; CZP: 25). Overall, 50/51 (98.0%) BKZ-treated patients and 24/25 (96.0%) CZP-treated patients completed the initial treatment period to Week 12, and 47/51 (92.2%) and 22/25 (88.0%) patients completed to Week 48, respectively (Supplemental Figure S2(a)). The PPS comprised 72 (94.7%) patients, with 4 patients excluded due to important protocol deviations relating to incorrect treatment or dose of BKZ.
Baseline characteristics were similar between the BKZ and CZP groups, with the exception of age (⩾65 years), proportion of patients with ASDAS very high disease activity (ASDAS-vHD) and proportion of patients who had prior TNFi exposure, all of which were higher in the BKZ group (Table 1, Supplemental Table S1). Symptom duration was similar between the BKZ and CZP groups.
Table 1.
Patient demographics and baseline characteristics (PPS and PET-PPS).
| Mean (SD), unless otherwise stated | Overall study | PET substudy | ||
|---|---|---|---|---|
| BKZ n = 47 |
CZP n = 25 |
BKZ n = 15 |
CZP n = 8 |
|
| Sex, male, n (%) | 40 (85.1) | 21 (84.0) | 11 (73.3) | 6 (75.0) |
| Age, years | 41.1 (12.7) | 39.7 (8.2) | 45.6 (13.5) | 38.1 (6.7) |
| ⩾18 to <65 years, n (%) | 43 (91.5) | 25 (100.0) | 13 (86.7) | 8 (100.0) |
| ⩾65 to <85 years, n (%) | 4 (8.5) | 0 | 2 (13.3) | 0 |
| HLA-B27 positive, n (%) | 43 (91.5) | 22 (88.0) | 13 (86.7) | 8 (100.0) |
| BMI, kg/m2 | 27.1 (5.0) | 28.6 (6.0) | 26.5 (6.1) | 27.9 (6.6) |
| Time since diagnosis, years | 7.3 (8.4) | 6.5 (8.4) | 7.7 (8.8) | 2.3 (2.2) |
| Symptom duration, years | 14.1 (11.6) | 14.8 (8.2) | 16.2 (13.8) | 13.4 (6.9) |
| hs-CRP, mg/L, geometric mean (geometric CV, %) | 15.8 (102.3) | 11.5 (314.9) | 14.6 (83.8) | 6.6 (958.4) |
| ASDAS | 4.1 (0.7) | 4.0 (0.9) | 4.0 (0.8) | 3.5 (0.6) |
| ASDAS-HD, n (%) | 8 (17.0) | 9 (36.0) | 3 (20.0) | 5 (62.5) |
| ASDAS-vHD, n (%) | 39 (83.0) | 16 (64.0) | 12 (80.0) | 3 (37.5) |
| BASDAI | 6.6 (1.3) | 6.7 (1.4) | 6.4 (1.5) | 5.6 (0.5) |
| Total spinal pain | 7.2 (1.5) | 7.3 (1.7) | 7.2 (1.6) | 6.5 (1.7) |
| Nocturnal spinal pain | 7.1 (1.6) | 7.3 (1.7) | 7.0 (1.5) | 6.3 (1.3) |
| BASFI | 6.4 (1.7) | 6.4 (1.9) | 6.0 (1.4) | 5.1 (1.2) |
| PtGADA | 7.0 (1.5) | 7.0 (1.9) | 6.5 (1.5) | 5.6 (1.7) |
| Prior TNFi exposure, n (%) | 8 (17.0) | 1 (4.0) | 0 | 0 |
| History of uveitis, n (%) | 4 (8.5) | 0 | 2 (13.3) | 0 |
| Concomitant NSAID use at baseline, n (%) | 29 (61.7) | 14 (56.0) | 12 (80.0) | 6 (75.0) |
| Country, n (%) | ||||
| Poland | 21 (44.7) | 10 (40.0) | 10 (66.7) | 6 (75.0) |
| Czech Republic | 7 (14.9) | 4 (16.0) | 0 | 0 |
| Germany | 7 (14.9) | 3 (12.0) | 3 (20.0) | 1 (12.5) |
| Russia | 6 (12.8) | 3 (12.0) | 0 | 0 |
| Greece | 4 (8.5) | 2 (8.0) | 1 (6.7) | 1 (12.5) |
| Moldova | 0 | 3 (12.0) | 0 | 0 |
| The Netherlands | 1 (2.1) | 0 | 1 (6.7) | 0 |
| United States | 1 (2.1) | 0 | 0 | 0 |
PPS (overall study) and PET PPS (PET substudy). All patients were Caucasian.
ASDAS, Axial Spondyloarthritis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BKZ, bimekizumab; BMI, body mass index; CV, coefficient of variation; CZP, certolizumab pegol; HD, high disease activity; HLA-B27, human leukocyte antigen B27; hs-CRP, high-sensitivity C-reactive protein; PET, positron emission tomography; PET-PPS, positron emission tomography per-protocol set; PPS, per-protocol set; PtGADA, Patient’s Global Assessment of Disease Activity; SD, standard deviation; TNFi, tumour necrosis factor inhibitor; vHD, very high disease activity.
Clinical efficacy
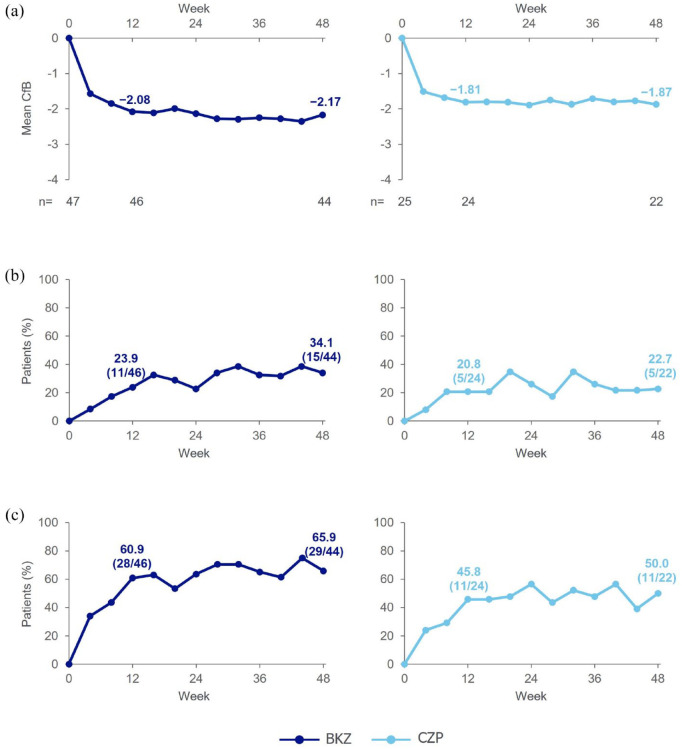
At Week 12, the mean CfB in ASDAS (primary efficacy endpoint) with BKZ was −2.1, and ASDAS-ID and ASDAS-MI (secondary efficacy endpoints) were achieved by 23.9% (11/46) and 60.9% (28/46) of BKZ-treated patients, respectively (Figure 1; Supplemental Table S2). Mean CfB in ASDAS observed at Week 12 was maintained at Week 48 (−2.2). The proportion of patients achieving ASDAS-ID increased from Week 12 to 34.1% (15/44) at Week 48, as did the proportion of patients achieving ASDAS-MI (65.9% (29/44)) (Figure 1; Supplemental Table S2).
Figure 1.
Primary and secondary clinical efficacy outcomes (OC).
Per-protocol set. Primary and secondary endpoints were assessed at Week 12. (a) ASDAS CfB (primary efficacy endpoint). (b) ASDAS-ID (secondary efficacy endpoint). (c) ASDAS-MI (secondary efficacy endpoint).
ASDAS, Axial Spondyloarthritis Disease Activity Score; BKZ, bimekizumab; CfB, change from baseline; CZP, certolizumab pegol; ID, inactive disease; MI, major improvement; OC, observed case.
In patients receiving CZP, mean CfB in ASDAS at Week 12 (−1.8) was maintained at Week 48 (−1.9). At Week 12, 20.8% (5/24) of patients achieved ASDAS-ID and 45.8% (11/24) of patients achieved ASDAS-MI. By Week 48, the proportions of patients achieving these levels slightly increased (ASDAS-ID: 22.7% (5/22); ASDAS-MI: 50.0% (11/22)) (Figure 1; Supplemental Table S2).
The posterior probability of a difference between BKZ and CZP in the primary endpoint was 88.4% (95% credible interval for difference: −0.14, 0.60); therefore, the pre-specified threshold (i.e. posterior probability of ⩾97.5% for a greater improvement in ASDAS at Week 12 in the BKZ group compared with the CZP group), assessed using Bayesian analysis, was not met.
For other efficacy endpoints, the proportion of BKZ-treated patients achieving ASAS20, ASAS40 and ASAS partial remission at Week 12 was further increased at Week 48 (Supplemental Table S2). Reductions (i.e. improvements) from baseline in BASDAI were observed at Week 12, with further improvements at Week 48. Similarly, improvements in other efficacy endpoints were also observed at Week 12 in CZP-treated patients, with further improvements at Week 48 for most endpoints.
Mean levels (geometric) of hs-CRP were rapidly reduced below 4.0 mg/L by Week 4 with BKZ (from 15.8 mg/L at baseline to 3.5 mg/L at Week 4) and were sustained at a low level throughout the study to Week 48 (3.6 mg/L). Following the last treatment dose at Week 44 and the subsequent 20-week safety follow-up period, the mean hs-CRP value at Week 64 remained low (3.8 mg/L) in the group that had originally received BKZ (Supplemental Figure S3). This pattern of maintained, low hs-CRP levels to Week 64 remained the same when 2/47 patients who commenced another biologic after the last BKZ dose were excluded from the analysis (Week 64: 3.6 mg/L). In CZP-treated patients, similar reductions in hs-CRP were observed over time to Week 48. However, the mean hs-CRP value increased from 2.7 mg/L at Week 48 to 8.5 mg/L at Week 64, the end of the safety follow-up period (Supplemental Figure S3).
PET imaging
In total, 26 patients enrolled in the PET substudy; 18 patients were randomised to BKZ and 8 patients to CZP. The PET-PPS was made up of 15 BKZ-treated patients and 8 CZP-treated patients (Supplemental Figure S2(b)), which included 51.6% (16/31) of patients from Poland, 40.0% (4/10) from Germany, 33.3% (2/6) from Greece and 100.0% (1/1) from The Netherlands (Table 1). Baseline characteristics of patients in the PET substudy largely reflected those of the overall population in each group; however, BKZ-treated patients were older than CZP-treated patients (13.3% (2/15) versus 0% aged ⩾65 years, respectively). In the PET substudy, BKZ-treated patients also had a longer mean symptom duration than CZP-treated patients (16.2 versus 13.4 years) (Table 1).
Of patients in the PET-PPS, evaluable PET-CT scans from ⩾1 post-baseline assessment were available for 13/15 patients in the BKZ group and all 8 patients in the CZP group (Supplemental Figure S2(b)). PET-MRI scans were available for the remaining 2/15 patients in the BKZ group for visual, qualitative analysis only at the three assessed timepoints in the PET substudy; however, these images were not evaluated, and this study focused on the PET-CT imaging analysis (n = 13 (BKZ); n = 8 (CZP)). Among these 21 patients, Week 48 PET-CT scans were non-evaluable in one patient in each treatment group; all patients had evaluable Week 12 PET-CT scans. One CZP-treated patient had evaluable PET-CT scans at all timepoints when analysis sets were finalised and was therefore included in the PET-PPS, however the baseline scan of this patient was subsequently reassessed as non-evaluable during quantitative analysis. This patient’s baseline PET-CT scan data were treated as missing in PET-positive lesion counts, and the patient was excluded from SUVauc change from baseline analyses. In summary, PET-positive lesion assessments were available for 12/13 BKZ-treated patients at Week 48, and 7/8 CZP-treated patients at baseline and Week 48. For SUVauc at the patient level, CfB measurements were possible for 13/13 and 12/13 BKZ-treated patients and 7/8 and 6/8 CZP-treated patients at Week 12 and Week 48, respectively.
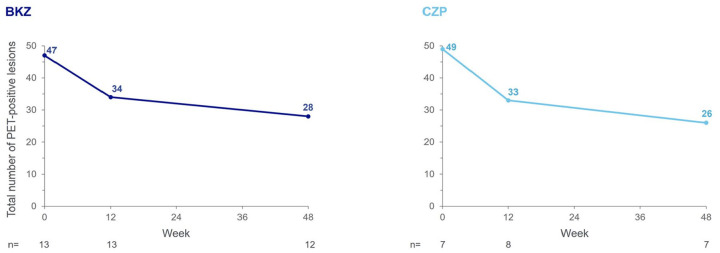
In the qualitative analysis of the BKZ group with evaluable PET-CT scans, the total number of PET-positive lesions (classified as axSpA) in the SIJ and spine across all patients at baseline was 47 (n = 13), decreasing by 27.7% to 34 lesions (n = 13) at Week 12 and by a further 17.6% to 28 lesions (n = 12) at Week 48 (Figure 2). Reductions in total number of PET-positive lesions were observed in 6/12 patients at Week 48, despite variation in the number of PET-positive lesions observed at baseline between patients (Supplemental Figure S4). Examples PET-CT images of PET-positive lesions over time are presented in Supplemental Figure S5.
Figure 2.
Total number of PET-positive lesions in the SIJ and spine.
PET-per protocol set, in patients with evaluable PET-CT scans. Measured across spine and SIJ regions. axSpA lesions as defined by agreement between both independent reviewers, or by agreement from the adjudicator in cases of disagreement, on axSpA versus degenerative classification. If no PET-positive lesions were observed on a scan during an initial visual qualitative analysis at baseline or Week 12, no further scans were performed at the subsequent visit(s). If a patient withdrew from the study early (i.e. prior to Week 48), a scan was performed at the early withdrawal visit if the previous examination showing PET-positive lesions was conducted ⩾12 weeks prior. Images from all available timepoints were assessed simultaneously by the patient in triplets (baseline, Week 12 and Week 48), with images presented side by side in chronological order and reviewers blinded to treatment only. One patient in the BKZ group was enrolled in The Netherlands; due to national requirements related to maximum annual radiation exposure in this location, the final PET-CT scan was performed 1 year after the baseline PET-CT scan (i.e. at Week 52). The data from this patient’s Week 52 assessments were pooled with Week 48 data from other patients.
axSpA, axial spondyloarthritis; BKZ, bimekizumab; CZP, certolizumab pegol; PET-CT, positron emission tomography-computerised tomography; SIJ, sacroiliac joints.
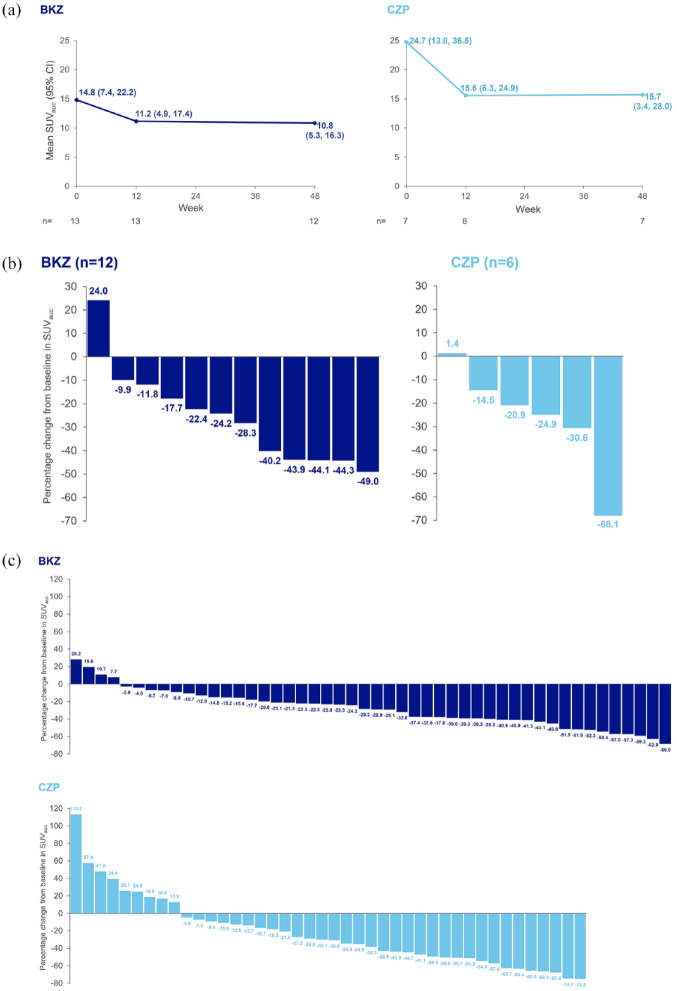
Mean quantitative lesional 18F-fluoride uptake (mean SUVauc) was also substantially reduced with BKZ. At baseline, the mean SUVauc in the spine and SIJ in BKZ-treated patients was 14.8, which decreased to 11.2 at Week 12 and slightly further to 10.8 at Week 48 (Figure 3(a)). These changes in SUVauc were also observed on the patient level, with most patients treated with BKZ (11/13) achieving reductions from baseline in SUVauc at Week 12, which were still evident in the majority of patients (11/12) at Week 48 (Figure 3(b)). Across all individual SIJ and spinal lesions assessed, the majority showed reductions from baseline in SUVauc at Week 12 (40/49) and Week 48 (44/48) with BKZ, with a percentage CfB of up to 68.5% at Week 48 (Figure 3(c)). When stratified anatomically by spinal location, reductions from baseline in mean SUVauc at Week 12, which were reduced slightly further at Week 48, were also observed in thoracic and lumbar regions with BKZ (baseline: 6.7 (thoracic), 3.6 (lumbar); Week 12: 5.3 (thoracic), 2.7 (lumbar); Week 48: 5.1 (thoracic), 2.3 (lumbar)); no change was observed in the cervical region (data not shown).
Figure 3.
Total mean and range of SUVauc and percentage change from baseline in SUVauc over time in the SIJ and spine. (a) Total mean and range of SUVauc. (b) Percentage change from baseline in SUVauc at Week 48, by patient. (c) Percentage change from baseline in SUVauc at Week 48, in all individual lesions.
PET-per protocol set. Each bar represents an individual patient in panel (b) and an individual lesion in panel (c). Measured across spine and SIJ regions. axSpA lesions as defined by agreement between both independent reviewers, or by agreement from the adjudicator in cases of disagreement, on axSpA versus degenerative classification. One BKZ-treated patient did not have a Week 48 measurement, one CZP-treated patient had a baseline measurement but not a Week 48 measurement, and one CZP-treated patient did not have a baseline measurement but had a Week 48 measurement. Therefore, change from baseline was only calculable for 12 BKZ-treated patients and 6 CZP-treated patients. In panel (c), individual lesion data are presented for all 48 individual PET lesion locations with detectable PET-positive lesions in 12 patients in the BKZ group and all 42 individual PET lesion locations with detectable PET-positive lesions in 6 patients in the CZP group. If no PET-positive lesions were observed on a scan during an initial visual qualitative analysis at baseline or Week 12, no further scans were performed at the subsequent visit(s). If a patient withdrew from the study early (i.e. prior to Week 48), a scan was performed at the early withdrawal visit if the previous examination showing PET-positive lesions was conducted ⩾12 weeks prior. One patient in the BKZ group was enrolled in The Netherlands; due to national requirements related to maximum annual radiation exposure in this location, the final PET-CT scan was performed 1 year after the baseline PET-CT scan (i.e. at Week 52). The data from this patient’s Week 52 assessments were pooled with Week 48 data from other patients.
BKZ, bimekizumab; CZP, certolizumab pegol; PET-CT, positron emission tomography-computerised tomography; SIJ, sacroiliac joints; SUVauc, standardised uptake value by area under the curve.
In the qualitative analysis of patients receiving CZP, the total number of PET-positive lesions (classified as axSpA) in the SIJ and spine reduced by 32.7%, from 49 at baseline (n = 7; more PET-positive lesions per patient than in the BKZ group) to 33 at Week 12 (n = 8), and by a further 21.2% to 26 at Week 48 (n = 7) (Figure 2). The total number of PET-positive lesions at baseline in the CZP group was similar to the BKZ group, despite fewer patients being in this group. Reductions in number of PET-positive lesions were observed in 5/6 patients receiving CZP at Week 48, despite variation in the number of PET-positive lesions observed at baseline (Supplemental Figure S4). In the quantitative analysis, a reduction from baseline in mean SUVauc in the spine and SIJ of CZP-treated patients was observed at Week 12 (from 24.7 to 15.6) and maintained at Week 48 (15.7) (Figure 3(a)). These changes in SUVauc were also observed on the patient level at Week 12, with all patients achieving reductions from baseline (n = 7). Reductions from baseline were still evident in 5/6 patients at Week 48 (Figure 3(b)). Across all individual SIJ and spinal lesions assessed, most lesions had a reduction from baseline in SUVauc at Week 12 (43/49) and Week 48 (33/42) with CZP, with a percentage CfB of up to 75.3% at Week 48 (Figure 3(c)). When stratified anatomically by spinal location, reductions from baseline in mean SUVauc at Week 12, which were maintained at Week 48, were also observed in the thoracic region with CZP (baseline: 17.0, Week 12: 11.9, Week 48: 11.9); no change was observed in the lumbar region and there were no lesions in the cervical region at any timepoint (data not shown).
Safety
Safety data for the initial treatment period (Weeks 0–12) and overall study period (Weeks 0–64) are summarised in Table 2. In the initial treatment period, ⩾1 TEAE was reported for 28/51 (54.9%) patients receiving ⩾1 BKZ dose and 14/25 (56.0%) patients receiving ⩾1 CZP dose. For the overall study period, ⩾1 TEAE was reported for 42/51 (82.4%) BKZ-treated patients and 19/25 (76.0%) CZP-treated patients. TEAEs leading to study discontinuation during Weeks 0–64 occurred in 3/51 (5.9%) BKZ-treated patients and 3/25 (12.0%) CZP-treated patients (Table 2). Total treatment exposure was 10.5 patient-years at Week 12 and 58.0 patient-years at Week 64 with BKZ, and 4.7 patient-years and 22.9 patient-years with CZP, respectively.
Table 2.
Safety overview.
| n (%) | Initial treatment period Weeks 0–12 |
Overall study period (including SFU) Weeks 0–64 |
||
|---|---|---|---|---|
| BKZ n = 51 |
CZP n = 25 |
BKZ n = 51 |
CZP n = 25 |
|
| Overview | ||||
| Any TEAE | 28 (54.9) | 14 (56.0) | 42 (82.4) | 19 (76.0) |
| Severe TEAEs | 2 (3.9) | 2 (8.0) | 2 (3.9) | 2 (8.0) |
| TEAEs leading to study discontinuation | 2 (3.9) | 1 (4.0) | 3 (5.9) a | 3 (12.0) b |
| Drug-related TEAEs | 16 (31.4) | 4 (16.0) | 24 (47.1) | 9 (36.0) |
| Serious TEAEs | 3 (5.9) | 2 (8.0) | 5 (9.8) | 3 (12.0) |
| Death | 0 | 1 (4.0) c | 0 | 1 (4.0) c |
| Most frequently reported TEAEs d | ||||
| Nasopharyngitis | 4 (7.8) | 0 | 10 (19.6) | 6 (24.0) |
| Diarrhoea | 0 | 1 (4.0) | 0 | 3 (12.0) |
| Oral candidiasis | 0 | 0 | 6 (11.8) | 0 |
| Pharyngitis | 0 | 0 | 5 (9.8) | 0 |
| Worsening of AS e | 1 (2.0) | 0 | 4 (7.8) | 2 (8.0) |
| GGT increased | 1 (2.0) | 1 (4.0) | 3 (5.9) | 1 (4.0) |
| Upper respiratory tract infection | 1 (2.0) | 0 | 3 (5.9) | 1 (4.0) |
| Influenza | 0 | 0 | 3 (5.9) | 0 |
| Headache | 2 (3.9) | 2 (8.0) | 2 (3.9) | 2 (8.0) |
| ALT increased | 1 (2.0) | 2 (8.0) | 1 (2.0) | 2 (8.0) |
| AST increased | 1 (2.0) | 2 (8.0) | 1 (2.0) | 2 (8.0) |
| Tonsillitis | 0 | 0 | 1 (2.0) | 2 (8.0) |
| Periarthritis | 0 | 2 (8.0) | 0 | 2 (8.0) |
| Oral herpes | 0 | 0 | 0 | 2 (8.0) |
Safety set. MedDRA (Version 19.0).
TEAEs leading to discontinuation from the study in patients receiving BKZ were hepatitis E, relapsing pneumonia and acute renal failure.
TEAEs leading to discontinuation from the study in patients receiving CZP were suicide, myasthenia gravis and hard-to-heal left leg fracture.
One patient committed suicide, which was not considered related to treatment.
TEAEs >5% in any treatment group for the overall study period are reported by preferred term.
Assessed by preferred term of ankylosing spondylitis.
ALT, alanine aminotransferase; AS, ankylosing spondylitis; AST, aspartate aminotransferase; BKZ, bimekizumab; CZP, certolizumab pegol; GGT, gamma-glutamyltransferase; n, number of patients reporting ⩾1 TEAE in that category; SFU, safety follow-up; TEAE, treatment-emergent adverse event.
One death occurred in the CZP group in Weeks 0–12 due to suicide; however, this event was not considered treatment-related by the investigator and the patient’s HADS-D scores, C-SSRS assessment and investigator assessment at prior study visits did not raise any concerns. During Weeks 0–64, SAEs (summarised in Supplemental Table S3) occurred in 5/51 (9.8%) BKZ-treated patients and 3/25 (12.0%) CZP-treated patients. Most frequently reported TEAEs (>5%) with BKZ and CZP during Weeks 0–64 are presented in Table 2.
Fungal infections were reported in 10/51 (19.6%) BKZ-treated patients and no CZP-treated patients. The majority of these were oral candidiasis infections (6/10), with the remaining 4 patients reporting fungal infections not elsewhere classified. All fungal infections were mild to moderate, non-systemic, localised and resolved with antifungal medications; none led to discontinuation from the study or were classified as serious.
Among pre-specified safety topics of interest for Weeks 0–64, no opportunistic infections (including TB), malignancies, IBD or anaphylactic reactions occurred with BKZ or CZP. No major adverse cardiovascular events (MACE) were identified by the pre-defined MACE search criteria; however, as an additional measure, reported cardiovascular events were adjudicated by an external committee. A serious TEAE of coronary artery stenosis reported in a BKZ-treated patient (Supplemental Table S3) was subsequently adjudicated as a MACE.
Hypersensitivity reactions occurred in six BKZ-treated patients (11.8%; eight occurrences) and one CZP-treated patient (4.0%; one occurrence); almost all were skin and subcutaneous tissue disorders (one BKZ-treated patient had an occurrence of an eye disorder) and all were mild to moderate. There were no injection site reactions in BKZ-treated patients and one mild reaction occurred in a CZP-treated patient. Hepatic events occurred in 4/51 (7.8%) BKZ-treated patients and 3/25 (12.0%) CZP-treated patients, the majority of which were transient, mild to moderate liver enzyme elevations and not considered treatment-related by the investigators. All liver enzyme elevations considered related to treatment were resolved and did not lead to discontinuation from the study.
Uveitis, the most common extra-musculoskeletal manifestation of axSpA, 2 occurred in 1/51 (2.0%) BKZ-treated patients and 1/25 (4.0%) CZP-treated patients (not a pre-specified safety topic of interest); neither patient had a history of uveitis. None of the patients in the BKZ group with a history of uveitis (4/51) had a uveitis flare.
Discussion
In this phase IIa study, the pre-specified Bayesian analysis threshold for the primary endpoint was not met, and hence proof of concept could not be declared. However, treatment with an exploratory dose of BKZ (160 mg Q2W, then 320 mg Q4W) resulted in improvements in all assessed stringent efficacy outcomes from baseline to Week 12, which were maintained or further improved at Week 48. Results were consistent with the rapid and sustained long-term clinical responses observed in the phase IIb (BE AGILE) and phase III (BE MOBILE) trials.16–19 Improvements in assessed efficacy outcomes were also observed at Week 12 and largely maintained or further improved at Week 48 with CZP, the reference drug, which were consistent with previous phase III trial data and the known effect of CZP in r-axSpA.34,41
Rapid and sustained reduction of systemic inflammation over time, measured by hs-CRP levels, was also observed with BKZ and CZP to Week 48, consistent with findings from the BE MOBILE 2 trial and the known long-term anti-inflammatory effect of CZP.16,17,25,41 Inhibition of proinflammatory cytokines and decreased local (MRI) and systemic (CRP) inflammation with BKZ have been linked to improvement in clinical signs and symptoms associated with axSpA.16,17,42 Notably, mean hs-CRP remained low in the BKZ group at Week 64, 20 weeks after administration of the last treatment dose, unlike in the CZP group. This suggests that BKZ may have a prolonged anti-inflammatory effect in patients with r-axSpA. However, this could also result from BKZ and CZP concentrations falling below the levels required to suppress CRP in patients with axSpA at different times due to the different elimination half-lives of BKZ and CZP (~3 and 2 weeks, respectively),15,35,42 or potential differences in biological half-lives.
PET-CT imaging data demonstrate that, in addition to reducing systemic inflammation, BKZ also has an inhibitory effect on osteoblastic activity in patients with r-axSpA, as assessed at 12 and 48 weeks of treatment. 18F-fluoride PET-CT is a measure of bone mineralisation, which occurs as a result of osteoblastic activity during bone formation.5,32 Therefore, the reductions from baseline in the number of PET-positive lesions and mean SUVauc observed at Week 12 and Week 48 with BKZ treatment correspond to reductions in osteoblastic activity and bone mineralisation. These data are suggestive of an early impact of BKZ on new bone formation (i.e. reduction in syndesmophytes and ankyloses); however, long-term studies with conventional radiography are ongoing and will be needed to confirm this. Findings in this study are consistent with pre-clinical data that demonstrated the potentially beneficial effect of dual inhibition of IL-17A and IL-17F on pathological new bone formation in r-axSpA. 13 The upstream causal process of this reduction in osteoblastic activity is not well defined and has been linked to a variety of factors, including reductions in MRI bone inflammation. 43 Reductions from baseline in both the number of PET-positive lesions and mean SUVauc across the SIJ and spine with BKZ were also more pronounced between baseline and Week 12 than between Week 12 and Week 48, suggesting treatment with BKZ has an early effect on pathological bone formation. Overall, these 18F-fluoride PET-CT imaging data show that targeted inhibition of IL-17A and IL-17F with BKZ reduces osteoblastic activity and has the potential to reduce new bone formation in patients with r-axSpA at risk of structural disease progression.
Reductions in osteoblastic activity (i.e. reductions from baseline in the number of PET-positive lesions and mean SUVauc) were also observed with CZP at Week 12 and Week 48, in line with the known effect of TNFi treatment in reducing spinal structural progression in patients with r-axSpA.5,24,44 The findings for CZP are also consistent with a previous PET-CT imaging study that detected decreases in bone formation after 12 weeks of TNFi treatment.5,22
Although the baseline characteristics of patients in the PET substudy largely reflected the overall patient population in each treatment group, on average BKZ-treated patients in the PET substudy were older, had symptoms for longer, had more established structural damage and a greater proportion had previous TNFi exposure, compared with those who received CZP. Due to the smaller number of patients in the PET substudy, the imbalances in baseline characteristics between treatment groups were more pronounced than in the overall population and the potential impact on the observed inhibitory activity of BKZ on osteoblasts may have been greater. Despite the baseline characteristics in the BKZ group suggesting, on average, patients had more established disease, substantial reductions in osteoblastic activity were still observed and almost all lesions had a quantitative decrease in signal with BKZ. It is possible that further reduction of pathological osteoblastic activity could be achieved with BKZ, or completely prevented, if patients are treated in the early stages of axSpA when little or no structural damage is present, although additional studies are needed to investigate this. Additionally, there were differences between treatment groups in baseline imaging assessments, with more PET-positive axSpA lesions per patient and higher mean SUVauc in the CZP group compared with the BKZ group. Differences in baseline characteristics between treatment groups, and the potential effect of these baseline differences on treatment response, were not evaluated, as the study was not powered to assess this. Although of interest, comparison of baseline SUVauc values against another measure of osteoblastic activity was not possible, given this is a novel molecular quantification assessment technique at the tissue level.
Both BKZ and CZP were well tolerated and their respective safety profiles were consistent with previous studies, with no new or unexpected safety signals observed.16–19,34,41 Occurrence of fungal infections with BKZ, particularly oral candidiasis, aligns with other clinical studies and was expected due to the known role of the IL-17 in host defence against Candida at the mucosal barrier.45–49
Incidence of uveitis was low with both BKZ and CZP, comparable with uveitis data from the phase IIb BE AGILE trial to Week 156 (r-axSpA) and pooled data from phase IIb and III BKZ trials across the spectrum of axSpA,18,50 as well as CZP data from the phase III RAPID-axSpA trial to Week 204 (r-axSpA) and the phase IV C-VIEW trial.41,51
A key strength of this exploratory study was the quantification of lesional 18F-fluoride uptake using PET-CT imaging to objectively assess osteoblastic activity and evaluate the impact of dual IL-17A and IL-17F inhibition, and TNF inhibition, on the process of bone formation in patients with r-axSpA. Additional strengths were the inclusion of CZP as a reference drug to represent the standard of care (TNFis), triplet readings of PET-CT images per patient across timepoints to reduce data variability between readings, reporting of long-term (48-week) outcomes and the selection of stringent efficacy outcomes (e.g. ASDAS-ID, ASDAS-MI) to assess treatment response.
A limitation of this study was the use of a different BKZ dosing regimen (160 mg Q2W then 320 mg Q4W) to the EU-approved dosage for patients with r-axSpA (160 mg Q4W) based on the recent phase IIb and phase III studies.16,19,42 This is due to this exploratory study being designed and conducted prior to the investigation and establishment of the BKZ 160 mg Q4W dosing regimen. Therefore, BKZ data from this study should not be generalised to the 160 mg Q4W dose and direct comparisons to those later studies should not be made. The small sample size, in both the overall study and PET substudy, also limited evaluation of the effects of BKZ, particularly in the substudy where there is notable variation in imaging data between patients within each treatment group. This study was not powered to directly compare treatments with regards to secondary or exploratory endpoints, including endpoints assessed in the PET imaging substudy. Similarly, while of interest, association analyses between clinical outcomes and PET imaging outcomes were not possible due to the small sample sizes in the study. Additional study limitations included the subjective nature of qualitative image analysis when evaluating PET-positive lesions, the absence of a placebo comparator group and the late stage of disease in most patients potentially resulting in no or reduced ongoing bone formation. The inclusion of serological biomarker assessments for osteoblastic activity in this study may have enabled further analyses to strengthen conclusions on new bone formation. However, the ability of 18F-fluoride PET-CT imaging to assess new bone formation at the local level, enabling different responses to treatment across clinically relevant sites to be captured, 32 provides highly sensitive data on osteoblastic activity which may not be picked up by serological biomarkers.
Conclusion
In conclusion, our study demonstrates that dual inhibition of IL-17A and IL-17F with BKZ results in improvements in stringent clinical outcomes, and may lead to reductions in osteoblastic activity in the SIJ and spine, assessed using 18F-fluoride PET-CT. These imaging data suggest that BKZ may have the potential to reduce new bone formation in patients with r-axSpA at risk of structural disease progression; however, further investigation with larger patient populations and long-term radiological follow-up is required before definitive conclusions can be drawn. The data also provide further evidence for improvements in stringent clinical outcomes and reductions in osteoblastic activity with CZP, consistent with the known effects of TNFi treatment. Upcoming 2-year analyses of the BE MOBILE trials will assess radiographic progression in patients treated with BKZ and further elucidate the impact of BKZ treatment on new bone formation in r-axSpA.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X241293944 for Impact of bimekizumab and certolizumab pegol on efficacy, safety and osteoblastic activity in radiographic axial spondyloarthritis: results from a phase IIa, multicentre, randomised, double-blind, exploratory study with PET-CT imaging by Xenofon Baraliakos, Jerney de Jongh, Denis Poddubnyy, Gerben J. C. Zwezerijnen, Robert Hemke, Sophie Glatt, Stevan Shaw, Lucian Ionescu, Assem el Baghdady, Joanne Mann, Ralph Paul Maguire, Tom Vaux, Natasha de Peyrecave, Marga Oortgiesen, Dominique Baeten and Conny van der Laken in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank the patients and their caregivers in addition to all the investigators and their teams who contributed to these studies. The authors also acknowledge Raphael Teichmann, PhD, UCB, Monheim, Germany, for global clinical project management of the study, Celia Menckeberg, PhD, UCB, Breda, The Netherlands, for publication coordination and editorial assistance and Evelyn Turner, BSc, Costello Medical, Cambridge, UK, for medical writing and editorial assistance. This study was funded by UCB.
Footnotes
ORCID iDs: Xenofon Baraliakos  https://orcid.org/0000-0002-9475-9362
https://orcid.org/0000-0002-9475-9362
Denis Poddubnyy  https://orcid.org/0000-0002-4537-6015
https://orcid.org/0000-0002-4537-6015
Ralph Paul Maguire  https://orcid.org/0000-0003-3937-6622
https://orcid.org/0000-0003-3937-6622
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xenofon Baraliakos, Rheumazentrum Ruhrgebiet Herne, Ruhr-University Bochum, Herne, Germany.
Jerney de Jongh, Department of Rheumatology, Amsterdam Rheumatology and Immunology Center, Amsterdam UMC, Amsterdam, The Netherlands.
Denis Poddubnyy, Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany; Department of Epidemiology, German Rheumatism Research Centre, Berlin, Germany.
Gerben J. C. Zwezerijnen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, The Netherlands
Robert Hemke, Department of Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, The Netherlands.
Sophie Glatt, UCB, Brussels, Belgium.
Stevan Shaw, UCB, Slough, UK.
Lucian Ionescu, UCB, Brussels, Belgium.
Assem el Baghdady, UCB, Slough, UK; Centre for Pharmaceutical Medicine Research, Institute of Pharmaceutical Science, King’s College London, London, UK.
Joanne Mann, UCB, Slough, UK.
Ralph Paul Maguire, UCB, Brussels, Belgium.
Tom Vaux, UCB, Slough, UK.
Natasha de Peyrecave, UCB, Brussels, Belgium.
Marga Oortgiesen, UCB, Raleigh, NC, USA.
Dominique Baeten, Department of Rheumatology, Amsterdam Rheumatology and Immunology Center, Amsterdam; UMC, Amsterdam, The Netherlands UCB, Slough, UK.
Conny van der Laken, Department of Rheumatology, Amsterdam Rheumatology and Immunology Center, Amsterdam UMC, De Boelelaan 1117, 1081 HV Amsterdam, Amsterdam, The Netherlands.
Declarations
Ethics approval and consent to participate: This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. Ethical approvals were obtained from the relevant institutional review boards (national, regional or Independent Ethics Committee (IEC), or Institutional Review Board (IRB)) at participating sites. The full list of IEC/IRBs, including IEC/IRB name and ethical approval numbers, is provided in the Supplemental Material. All patients provided written informed consent in accordance with local regulations, the International Council for Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.
Consent for publication: All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study. Therefore, patient consent for publication was not required.
Author contributions: Xenofon Baraliakos: Conceptualization; Investigation; Methodology; Writing – review & editing.
Jerney de Jongh: Investigation; Writing – review & editing.
Denis Poddubnyy: Investigation; Writing – review & editing.
Gerben J.C. Zwezerijnen: Investigation; Writing – review & editing.
Robert Hemke: Investigation; Writing – review & editing.
Sophie Glatt: Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing.
Stevan Shaw: Conceptualization; Methodology; Writing – review & editing.
Lucian Ionescu: Conceptualization; Methodology; Writing – review & editing.
Assem el Baghdady: Methodology; Project administration; Supervision; Writing – review & editing.
Joanne Mann: Formal analysis; Validation; Writing – review & editing.
Ralph Paul Maguire: Formal analysis; Investigation; Methodology; Project administration; Validation; Writing – review & editing.
Tom Vaux: Formal analysis; Validation; Writing – review & editing.
Natasha de Peyrecave: Methodology; Writing – review & editing.
Marga Oortgiesen: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Dominique Baeten: Conceptualization; Methodology; Writing – review & editing.
Conny van der Laken: Conceptualization; Investigation; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This article was based on the NCT03215277 study, which was sponsored by UCB. Support for third-party medical writing assistance for this article, provided by Evelyn Turner, BSc, Costello Medical, Cambridge, UK, was funded by UCB in accordance with Good Publication Practice (GPP) 2022 guidelines (https://www.ismpp.org/gpp-2022).
X.B.: Speakers bureau from AbbVie, BMS, Chugai, Eli Lilly, Galapagos, MSD, Novartis, Pfizer, UCB; paid instructor for AbbVie, BMS, Chugai, Eli Lilly, Galapagos, MSD, Novartis, Pfizer, UCB; consultant of AbbVie, BMS, Chugai, Eli Lilly, Galapagos, Gilead, Novartis, Pfizer, UCB; grant/research support from: Novartis, UCB; J.d.J.: Employee of Teva Pharmaceuticals since May 2023; D.P.: Speaker for AbbVie, BMS, Eli Lilly, MSD, Novartis, Pfizer and UCB; consultant for AbbVie, Biocad, Eli Lilly, Gilead, GSK, MSD, MoonLake, Novartis, Pfizer, Samsung Bioepis and UCB; grant/research support from: AbbVie, Lilly, MSD, Novartis and Pfizer; G.J.C.Z.: No potential conflicts of interest declared; R.H.: No potential conflicts of interest declared; S.G., S.S., L.I., J.M., R.P.M., T.V. and N.d.P.: Employees of UCB; A.e.B. and D.B.: Former employees of UCB; M.O.: Employee and stockholder of UCB; C.v.d.L.: Consultant or advisor for Lilly, Galapagos, GSK, UCB; research support from AbbVie, GSK, Novartis, Pfizer, UCB.
Availability of data and materials: Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after study completion. Investigators may request access to anonymised individual patient-level data and redacted study documents which may include: analysis-ready datasets, study protocols, annotated case report forms, statistical analysis plans, dataset specifications and clinical study reports. Prior to the use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password-protected portal.
References
- 1. Boel A, Molto A, van der Heijde D, et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019; 78: 1545. [DOI] [PubMed] [Google Scholar]
- 2. Navarro-Compán V, Sepriano A, El-Zorkany B, et al. Axial spondyloarthritis. Ann Rheum Dis 2021; 80: 1511–1521. [DOI] [PubMed] [Google Scholar]
- 3. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390: 73–84. [DOI] [PubMed] [Google Scholar]
- 4. National Library of Medicine (NIH). Syndesmophyte, https://www.ncbi.nlm.nih.gov/medgen/780898 (accessed April 2023).
- 5. Bruijnen STG, Verweij NJF, van Duivenvoorde LM, et al. Bone formation in ankylosing spondylitis during anti-tumour necrosis factor therapy imaged by 18F-fluoride positron emission tomography. Rheumatology (Oxford) 2018; 57: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009; 60: 93–102. [DOI] [PubMed] [Google Scholar]
- 7. Poddubnyy D, Sieper J. Mechanism of new bone formation in axial spondyloarthritis. Current Rheumatol Rep 2017; 19: 55. [DOI] [PubMed] [Google Scholar]
- 8. Baraliakos X, Listing J, Rudwaleit M, et al. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther 2008; 10: R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 10. Ramiro S, Elena N, Alexandre S, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis 2023; 82: 19. [DOI] [PubMed] [Google Scholar]
- 11. Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019; 71: 1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navarro-Compán V, Puig L, Vidal S, et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front Immunol 2023; 14: 1191782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah M, Maroof A, Gikas P, et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 2020; 6: e001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology 2011; 134: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018; 77: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Heijde D, Deodhar A, Baraliakos X, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis 2023; 82: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baraliakos X, Deodhar A, van der Heijde D, et al. Bimekizumab treatment in patients with active axial spondyloarthritis: 52-week efficacy and safety from the randomised parallel phase 3 BE MOBILE 1 and BE MOBILE 2 studies. Ann Rheum Dis 2024; 83: 199–213. [DOI] [PubMed] [Google Scholar]
- 18. Baraliakos X, Deodhar A, Dougados M, et al. Safety and efficacy of bimekizumab in patients with active ankylosing spondylitis: 3-year results from a phase 2b randomized controlled trial and its open-label extension study. Arthritis Rheumatol 2022; 74: 1943–1958. [DOI] [PubMed] [Google Scholar]
- 19. van der Heijde D, Gensler LS, Deodhar A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 2020; 79: 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baraliakos X, Heldmann F, Callhoff J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis 2014; 73: 1819–1825. [DOI] [PubMed] [Google Scholar]
- 21. Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005; 52: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 22. de Jongh J, Verweij NJF, Yaqub M, et al. 18F-Fluoride PET provides distinct information on disease activity in ankylosing spondylitis as compared to MRI and conventional radiography. Eur J Nucl Med Mol Imaging 2023; 50: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lukas C, Cyteval C, Dougados M, et al. MRI for diagnosis of axial spondyloarthritis: major advance with critical limitations ‘Not everything that glisters is gold (standard)’. RMD Open 2018; 4: e000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Heijde D, Baraliakos X, Hermann KG, et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018; 77: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baraliakos X, Kruse S, Auteri SE, et al. Certolizumab pegol treatment in axial spondyloarthritis mitigates fat lesion development: 4-year post-hoc MRI results from a phase 3 study. Rheumatology 2021; 61: 2875–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torgutalp M, Rios Rodriguez V, Dilbaryan A, et al. Treatment with tumour necrosis factor inhibitors is associated with a time-shifted retardation of radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis 2022; 81: 1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baraliakos X, Listing J, Rudwaleit M, et al. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor alpha antibody infliximab. Ann Rheum Dis 2005; 64: 1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology 2019; 58: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braun J, Blanco R, Marzo-Ortega H, et al. Two-year imaging outcomes from a phase 3 randomized trial of secukinumab in patients with non-radiographic axial spondyloarthritis. Arthritis Res Ther 2023; 25: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maksymowych WP, van der Heijde D, Baraliakos X, et al. Tofacitinib is associated with attainment of the minimally important reduction in axial magnetic resonance imaging inflammation in ankylosing spondylitis patients. Rheumatology 2018; 57: 1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol 2019; 71: 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frost ML, Moore AE, Siddique M, et al. 18F-fluoride PET as a noninvasive imaging biomarker for determining treatment efficacy of bone active agents at the hip: a prospective, randomized, controlled clinical study. J Bone Miner Res 2013; 28: 1337–1347. [DOI] [PubMed] [Google Scholar]
- 33. Park EK, Pak K, Park JH, et al. Baseline increased 18F-fluoride uptake lesions at vertebral corners on positron emission tomography predict new syndesmophyte development in ankylosing spondylitis: a 2-year longitudinal study. Rheumatol Int 2017; 37: 765–773. [DOI] [PubMed] [Google Scholar]
- 34. Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014; 73: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. European Medicines Agency (EMA). Certolizumab pegol summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/cimzia-epar-product-information_en.pdf (2010, accessed May 2023).
- 36. Food and Drugs Administration (FDA). Certolizumab pegol prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125160s270lbl.pdf (2017, accessed July 2023).
- 37. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 38. van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009; 68: 1811–1818. [DOI] [PubMed] [Google Scholar]
- 39. Boellaard R. Quantitative oncology molecular analysis suite: ACCURATE (Abstract only). J Nucl Med 2018; 59: 1753. [Google Scholar]
- 40. Campbell CM, Gilron I, Doshi T, et al. Designing and conducting proof-of-concept chronic pain analgesic clinical trials. Pain Rep 2019; 4: e697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Heijde D, Dougados M, Landewé R, et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology (Oxford) 2017; 56: 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. European Medicines Agency (EMA). Bimekizumab summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf (2021, accessed July 2023).
- 43. Nakamura A, Talukdar A, Nakamura S, et al. Bone formation in axial spondyloarthritis: is disease modification possible? Best Pract Res Clin Rheumatol 2019; 33: 101491. [DOI] [PubMed] [Google Scholar]
- 44. Braun J, Baraliakos X, Hermann KG, et al. Effect of certolizumab pegol over 96 weeks of treatment on inflammation of the spine and sacroiliac joints, as measured by MRI, and the association between clinical and MRI outcomes in patients with axial spondyloarthritis. RMD Open 2017; 3: e000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021; 397: 487–498. [DOI] [PubMed] [Google Scholar]
- 46. Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med 2021; 385: 142–152. [DOI] [PubMed] [Google Scholar]
- 47. Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med 2021; 385: 130–141. [DOI] [PubMed] [Google Scholar]
- 48. Merola JF, McInnes IB, Ritchlin C, et al. Bimekizumab in patients with active psoriatic arthritis: 16-week efficacy & safety from BE COMPLETE, a phase 3, multicentre, randomised, placebo-controlled study. Ann Rheum Dis 2022; 81: 167–169. [Google Scholar]
- 49. McInnes IB, Coates LC, Landewé R, et al. Bimekizumab in bDMARD-naïve patients with psoriatic arthritis: 24-Week efficacy & safety from BE OPTIMAL, a phase 3, multicentre, randomised, placebo-controlled, active reference study. Ann Rheum Dis 2022; 81: 206–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rudwaleit M, Brown M, Van Gaalen FA, et al. POS0668 Low uveitis rates in patients with axial spondyloarthritis treated with bimekizumab: pooled results from phase 2b/3 trials. Ann Rheum Dis 2023; 82: 614–615. [DOI] [PubMed] [Google Scholar]
- 51. van der Horst-Bruinsma IE, van Bentum RE, Verbraak FD, et al. Reduction of anterior uveitis flares in patients with axial spondyloarthritis on certolizumab pegol treatment: final 2-year results from the multicenter phase IV C-VIEW study. Ther Adv Musculoskelet Dis 2021; 13: 1759720x211003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X241293944 for Impact of bimekizumab and certolizumab pegol on efficacy, safety and osteoblastic activity in radiographic axial spondyloarthritis: results from a phase IIa, multicentre, randomised, double-blind, exploratory study with PET-CT imaging by Xenofon Baraliakos, Jerney de Jongh, Denis Poddubnyy, Gerben J. C. Zwezerijnen, Robert Hemke, Sophie Glatt, Stevan Shaw, Lucian Ionescu, Assem el Baghdady, Joanne Mann, Ralph Paul Maguire, Tom Vaux, Natasha de Peyrecave, Marga Oortgiesen, Dominique Baeten and Conny van der Laken in Therapeutic Advances in Musculoskeletal Disease