Abstract
Objective
This study aimed to identify variables that predict gout remission in people with erosive gout receiving urate‐lowering therapy.
Methods
We analyzed data from a two‐year, double‐masked randomized‐controlled trial of people with erosive gout, randomized to a serum urate target of <0.20 mmol/L or <0.30 mmol/L using oral urate‐lowering therapies. All participants had dual‐energy computed tomography (DECT) scans of the feet and ankles at baseline. The proportion of participants achieving gout remission according to the 2016 preliminary gout remission criteria and simplified gout remission criteria (without the patient reported outcomes) was analyzed. Logistic regression models were used to evaluate predictors of gout remission in year 2.
Results
The preliminary gout remission criteria were fulfilled in 11 of 97 participants (11%) at year 1 and 21 of 92 participants (23%) at year 2. The simplified criteria were fulfilled in 26 of 97 participants (27%) in year 1 and 40 of 92 participants (44%) in year 2. In multivariable regression models, baseline DECT monosodium urate crystal volume was the only significant independent predictor of gout remission at year 2, using either criteria. Each 1‐cm3 increase in the baseline DECT monosodium urate crystal volume decreased the odds of fulfilling the 2016 preliminary gout remission criteria (odds ratio [OR] 0.65, 95% confidence interval [CI] 0.46–0.93; P = 0.02) and the simplified gout remission criteria (OR 0.57, 95% CI 0.41–0.78; P < 0.001).
Conclusion
In people with erosive gout on urate‐lowering therapy, higher baseline DECT monosodium urate crystal volume is associated with lower odds of gout remission after two years of treatment, defined by either the preliminary gout remission criteria or simplified gout remission criteria.
INTRODUCTION
Gout is a chronic disease that typically presents with intermittent flares of inflammatory arthritis. Chronic gouty arthritis, tophi, and structural joint damage also occur in some people with gout. Dissolution of monosodium urate (MSU) crystals through the lowering of serum urate levels is the key long‐term management strategy. Allopurinol, febuxostat, probenecid, and benzbromarone, and the combination of these drugs, are widely used oral urate‐lowering therapies. 1 , 2 , 3
SIGNIFICANCE & INNOVATIONS.
Remission, which can be defined as “an absence of disease activity or a level of disease activity so low that it is not troublesome,” is an important goal of therapy in gout management. In 2016, preliminary remission criteria for gout were developed.
Variables associated with the achievement of gout remission are not well defined. This is the first study examining whether baseline dual‐energy computed tomography (DECT) measurement of monosodium urate crystal deposition can predict gout remission.
In this study, higher volumes of DECT monosodium urate crystal deposits at baseline predicted lower odds of remission after two years of urate‐lowering therapy.
In chronic rheumatic diseases, “remission” has been defined as “either a complete absence of disease activity or a level of disease activity so low that it is not troublesome to the patient.” 4 In 2016, a group of rheumatologists and researchers with expertise in gout established consensus for preliminary gout remission criteria using the outcome measures in rheumatology (OMERACT) core outcome domains for long‐term gout studies. 5 These remission domains were defined as absence of gout flares, absence of tophi, serum urate <0.36mmol/L, pain due to gout <2, and patient global assessment of gout disease activity score <2 on a 10‐cm visual analog scale (VAS) or 10‐point Likert scale. Gout remission requires all of these domains to be fulfilled over a provisional time frame of 12 months. 5 These criteria are presented in Supplementary Table 1.
Although the OMERACT core outcome domains for long‐term gout studies were developed with patient research partners, there was no patient contribution to development of the preliminary gout remission criteria. In a subsequent qualitative study, in which people with gout were asked about their perspective of the preliminary gout remission criteria, it was suggested that the “pain due to gout” domain and the “patient global assessment” domain may be redundant and overlap with the “gout flares” domain. 6 People with gout thought that if a person has an “absence of gout flares,” they would usually have no “pain due to gout” and, as such, would have favorable patient global assessment of their gout disease activity. Based on this understanding of the patient perspective, simplified gout remission criteria that measure gout remission without the pain due to gout and patient global assessment domains may be appropriate (Supplementary Table 1).
Since development of the preliminary gout remission criteria, only a few studies have investigated achievement of gout remission or the baseline clinical, laboratory, and demographic variables that are associated with achievement of gout remission. 7 , 8 , 9 , 10 We used data from a two‐year randomized‐controlled trial of 104 people with erosive gout who were randomly assigned to dose escalation oral urate‐lowering medication to achieve a serum urate target of <0.20 mmol/L or <0.30 mmol/L. 11 Our aim was to identify variables that predict gout remission in people with erosive gout receiving urate‐lowering therapy.
PATIENTS AND METHODS
Data were analyzed from participants in a two‐year double‐masked randomized‐controlled trial of people with erosive gout (ACTRN12615001219572). The inclusion criteria included gout according to the 2015 American College of Rheumatology/EULAR gout classification criteria, 12 at least one bone erosion on plain radiography of the feet, age >18 years, ability to provide informed consent, currently receiving treatment with an oral urate‐lowering agent, and having a serum urate concentration of ≥0.30 mmol/L. Ethical approval was obtained from the Southern Health and Disability Ethics Committee (approval no. 15/STH/108). All participants provided written informed consent. The full methods and results of the trial have been reported. 11 In brief, participants were randomized to an intensive serum urate target of <0.20 mmol/L or a standard target of <0.30 mmol/L. They attended monthly urate‐lowering therapy dose escalation visits until their serum urate target was reached and maintained for three months using the same standardized medication protocol; allopurinol doses were increased to a maximum dose of 900 mg daily, and if the treatment target was not achieved, probenecid was added at a maximum dose of 1 g twice daily. If this failed to achieve target serum urate, benzbromarone was prescribed at 100 mg daily in combination with allopurinol. If this was unsuccessful, febuxostat monotherapy was prescribed to a maximum dose of 120 mg daily.
Baseline examinations
At baseline, participants attended a study visit in which the following variables were collected: age, gender, body mass index (BMI), ethnicity, disease duration, and comorbidities, as well as the OMERACT core outcome domains for long‐term gout studies: subcutaneous tophus count, serum urate concentration, and number of gout flares in the preceding three months. Pain was assessed by asking participants to “please mark the line at the point which best represents your level of pain today” on a 100‐mm VAS. For the patient global assessment, the general health EQ‐5D‐3L VAS, which records patient's self‐rated health on a 100‐mm VAS, was used.
At the baseline visit and annual follow‐up visits, dual‐energy computed tomography (DECT) scans of the feet and ankles were obtained on a dual x‐ray tube 128 detector row scanner (Somatom Definition Flash, Siemens Medical). 13 Participants were positioned feet first, supine, with their feet in a plantar flexed position. Both ankles and feet were scanned axially in one helical acquisition as previously described. 14 DECT MSU crystal deposition in the feet and ankles was measured by two researchers (CNS and KL) using automated volume assessment on a Siemens workstation using proprietary software (syngo MMWP VE 36A 2009, Siemens Medical). 15 Visible artifacts (nailbed, skin, beam‐hardening) were not included in the urate volume measurements. 16 The conventional computed tomography (CT) images were scored for bone erosion volume according to a gout CT bone erosion scoring method, based on the rheumatoid arthritis magnetic resonance imaging score (RAMRIS) for erosion 17 and validated for gout. 14 The gout CT bone erosion scoring system includes erosions of the following bones scored on a semiquantitative scale of 0 to 10 in each foot: first metatarsal head; second, third, and fourth metatarsal bases; cuboid; intermediate cuneiform; and distal tibia (maximum total score 140 points). The CT scans were scored by two independent readers (AJD and KB). Plain radiographs of the hands and both feet were also obtained at baseline and scored for erosions and joint space narrowing using a modified version of the Sharp/van der Heijde scoring method, 18 validated for gout. 19 The plain radiographs were scored by two independent readers (ND and KB). For each imaging assessment, the mean score from the two readers, who were masked to the treatment allocation, serum urate values, and each other's scores, were used in the analysis.
Outcomes
Over the two years, participants attended follow‐up visits in which serum urate concentration and gout flare frequency were measured every three months (13 weeks) and tophus measurements and patient reported outcomes were measured every six months (26 weeks). We assessed remission using the preliminary gout remission criteria which contained the domains mentioned previously (absence of gout flares, absence of tophus, serum urate <0.36 mmol/L, pain score <2, and patient global assessment score <2) 5 and simplified gout remission criteria, which contains the same domains without the patient reported outcomes. 6 Remission at year 1 was assessed using outcomes measured from baseline to week 52. For year 2, outcomes measured from week 52 through week 104 were used (Figure 1; Supplementary Table 1). Neither the pain questionnaire nor the patient global assessment questionnaires used in the study were specific to gout as recommended for the preliminary gout remission criteria. 5
Figure 1.
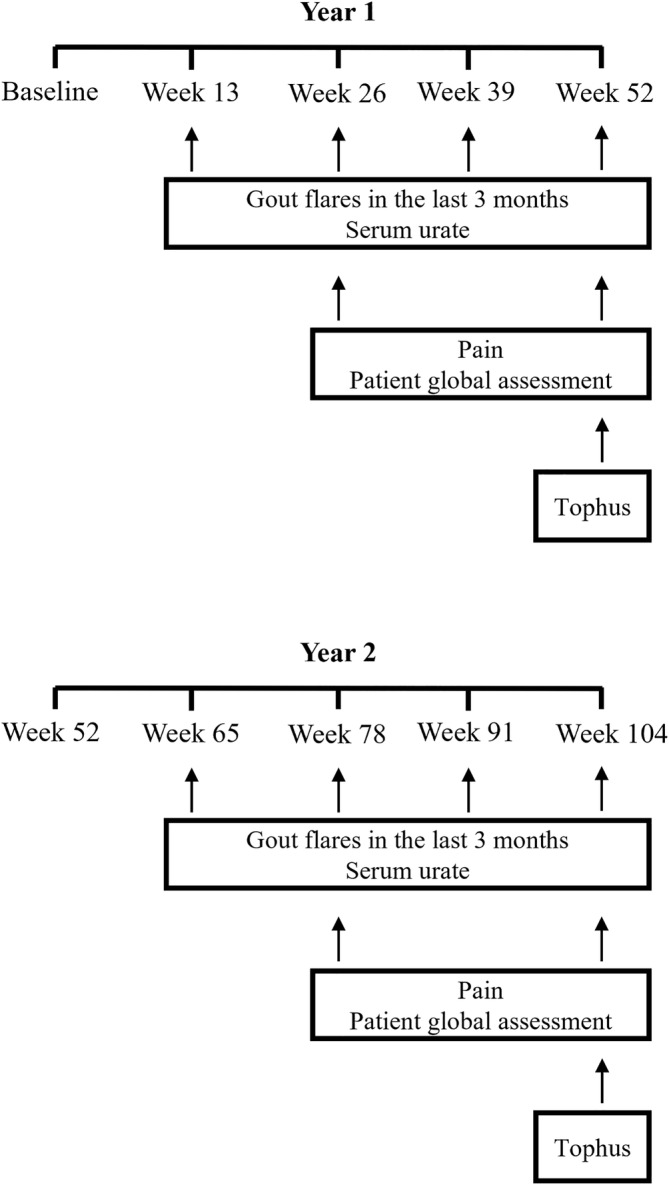
Measurement of individual remission domains. The gout flares and serum urate domains were computed using data collected every three months following baseline (between week 13 and week 52 during year 1 and week 65 and week 104 during year 2). The pain and patient global assessment (Ptga) domains were computed using data collected every six months (between week 26 and week 52 during year 1 and week 78 and week 104 during year 2). The tophus count at week 52 and month 104 were used to compute the tophus domain at year 1 and year 2, respectively.
Statistical analysis
Results were described with median and interquartile range (IQR) for quantitative variables, and categorical variables were described using frequency and percentage. To assess the association between randomization group and fulfillment of the preliminary criteria and simplified criteria at year 1 and year 2, Pearson's chi‐square test was used. Agreement between the preliminary criteria and the simplified criteria at year 1 and year 2 was evaluated by the kappa coefficient. Complete‐case analysis methodology was used to account for missing data. Data were assumed to be missing at random, and there were no notable differences in the baseline variables of the participants included in the analysis and the overall group of N = 104 (Supplementary Table 2).
Potential baseline predictors of gout remission (selected based on expert clinical knowledge) were initially assessed using univariable and multivariable logistic regression analyses (Tables 1 and 2). To avoid overfitting of the logistic regression models, the number of variables to be used were reduced by selecting from candidate baseline variables with associations of P < 0.15 in univariable analyses. From these candidate variables, traditional iterative selection techniques (backward elimination, forward selection) and a modern selection strategy, lasso logistic regression, were used to identify predictor(s) for the achievement of gout remission. 20 , 21 , 22 From these approaches, multivariable logistic regression models were produced comprised of variables chosen on the basis of biologic plausibility, parsimony, and goodness of fit. Following this, models were also produced to evaluate significant predictor(s) adjusting for randomization group, baseline serum urate, baseline number of tophi, baseline number of gout flares, baseline pain, and patient global assessment. DECT MSU crystal volume scores were log transformed for regression analyses to normalize distribution, and a value of 0.005 was added to any DECT MSU crystal volume equaling zero before transformation. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported. Values of P < 0.05 were taken to indicate statistical significance. A receiver operator characteristic (ROC) curve analysis was used to evaluate the discriminatory ability of DECT MSU crystal volume for gout remission at year 2. Youden's index was used to estimate an optimal cutoff value for an outcome of not being in remission. Statistical analysis was performed using SPSS software version 28 and the “glmnet” package (R Foundation for Statistical Computing).
Table 1.
Baseline variables of those fulfilling the preliminary remission criteria and those not fulfilling the criteria at year 2*
| Total, N = 92 | Fulfilling criteria, n = 21 | Not fulfilling criteria, n = 71 | OR (95 CI) a | P value | |
|---|---|---|---|---|---|
| Age, median (IQR), y | 63 (55–71) | 66 (56–68) | 62 (54–72) | 1.00 (0.96–1.05) | 0.92 |
| Male, n (%) | 89 (97) | 20 (95) | 69 (97) | 0.58 (0.05–6.73) | 0.66 |
| Ethnicity, n (%) | |||||
| Māori | 8 (9) | 1 (5) | 7 (10) | 0.46 (0.05–3.94) | 0.48 |
| NZ European | 62 (67) | 15 (71) | 47 (66) | 1.28 (0.44–3.71) | 0.65 |
| Other | 8 (9) | 3 (14) | 5 (7) | 2.20 (0.48–10.09) | 0.31 |
| Pacific peoples | 14 (15) | 2 (10) | 12 (17) | 0.52 (0.11–2.52) | 0.42 |
| Age of first gout attack, median (IQR), y | 37 (30–52) | 38 (30–51) | 36 (29–55) | 1.00 (0.97–1.03) | 0.83 |
| Disease duration, median (IQR) | 20 (9–31) | 21 (11–26) | 20 (8–32) | 1.01 (0.98–1.04) | 0.65 |
| Body mass index median (IQR) | 31 (27–35) | 28 (27–31) | 31 (27–37) | 0.90 (0.82–1.00) | 0.051 |
| Baseline serum urate, median (IQR), mmol/L | 0.35 (0.31–0.40) | 0.36 (0.31–0.41) | 0.35 (0.31–0.40) | 0.10 (0.00–43.41) | 0.46 |
| Baseline subcutaneous tophus count, median (IQR) | 2 (0–4) | 1 (0–4) | 2 (0–5) | 0.95 (0.85–1.06) | 0.34 |
| Number of gout flares in preceding 3 mo at baseline, median (IQR) | 1 (0–2) | 0 (0–1) | 1 (0–2) | 0.72 (0.50–1.05) | 0.08 |
| Baseline pain, median (IQR), 10‐cm VAS | 0.35 (0.00–2.15) | 0 (0.00–0.60) | 0.50 (0.00–2.90) | 0.65 (0.43–0.99) | 0.04 |
| Baseline patient global assessment, median (IQR), 10‐cm VAS | 2.00 (1.00–3.50) | 1 (0.80–2.00) | 2.50 (1.00–3.50) | 0.82 (0.63–1.07) | 0.14 |
| Baseline serum creatinine, median (IQR), (μmol/L | 91 (82–105) | 90 (80–105) | 93 (84–105) | 1.00 (0.98–1.03) | 0.76 |
| Baseline creatinine clearance, median (IQR), mL/min | 72 (61–87) | 72 (61–82) | 73 (60–87) | 1.00 (0.97–1.02) | 0.69 |
| Comorbidities, n (%) | |||||
| Type 2 diabetes | 6 (7) | 1 (5) | 5 (7) | 0.66 (0.07–5.98) | 0.71 |
| High cholesterol | 40 (43) | 8 (39) | 32 (45) | 0.75 (0.28–2.03) | 0.57 |
| Hypertension | 45 (49) | 9 (43) | 36 (51) | 0.73 (0.27–1.95) | 0.53 |
| Cardiovascular disease | 11 (12) | 3 (14) | 8 (11) | 1.31 (0.32–5.47) | 0.71 |
| Kidney disease | 18 (20) | 3 (14) | 15 (21) | 0.62 (0.16–2.40) | 0.49 |
| Baseline DECT MSU crystal volume, median (IQR), cm3 | 0.12 (0.05–0.95) | 0.07 (0.03–0.10) | 0.18 (0.05–1.34) | 0.65 (0.47–0.90) | 0.01 |
| Baseline total radiographic damage score, median (IQR) | 5.70 (2.50–11.75) | 5.63 (3.13–11.38) | 5.88 (2.13–11.75) | 1.01 (0.96–1.06) | 0.75 |
| Baseline CT erosion score, median (IQR) | 7.00 (4.00–13.50) | 6.50 (4.00–11.50) | 7.00 (4.00–15.00) | 0.97 (0.90–1.05) | 0.46 |
| Randomization group, n (%) | |||||
| Intensive target group b | 46 (50) | 10 (48) | 36 (51) | 0.88 (0.33–2.34) | 0.80 |
CI, confidence interval; CT, computed tomography; DECT, dual‐energy computed tomography; IQR, interquartile range; MSU, monosodium urate; NZ, New Zealand; OR, odds ratio; VAS, visual analog scale.
Continuous measures were analyzed as the odds of a one‐unit difference.
Standard target group set as reference.
Table 2.
Baseline variables of those fulfilling the simplified remission criteria and those not fulfilling the criteria at year 2
| Total, N = 92 | Fulfilling criteria, n = 40 | Not fulfilling criteria, n = 52 | OR (95% CI) a | P value | |
|---|---|---|---|---|---|
| Age, median (IQR), y | 63 (55–71) | 66 (55–71) | 62 (54–71) | 1.01 (0.98–1.05) | 0.46 |
| Male, n (%) | 89 (97) | 38 (95) | 51 (98) | 0.37 (0.03–4.26) | 0.43 |
| Ethnicity, n (%) | |||||
| Māori | 8 (9) | 3 (8) | 5 (10) | 0.76 (0.17–3.40) | 0.72 |
| NZ European | 62 (67) | 31 (78) | 31 (60) | 2.33 (0.92–5.89) | 0.73 |
| Other | 8 (9) | 4 (10) | 4 (8) | 1.33 (0.31–5.69) | 0.70 |
| Pacific peoples | 14 (15) | 2 (5) | 12 (23) | 0.18 (0.04–0.84) | 0.03 |
| Age of first gout attack, median (IQR) | 37 (30–52) | 40 (30–55) | 35 (29–51) | 1.01 (0.98–1.04) | 0.58 |
| Disease duration, median (IQR) | 20 (9–31) | 19 (8–25) | 20 (9–32) | 0.99 (0.96–1.02) | 0.59 |
| Body mass index, median (IQR) | 31 (27–35) | 28 (26–32) | 32 (28–37) | 0.88 (0.81–0.96) | 0.005 |
| Baseline serum urate, median (IQR), mmol/L | 0.35 (0.31–0.40) | 0.35 (0.30–0.40) | 0.35 (0.32–0.40) | 0.03 (0.00–5.33) | 0.19 |
| Baseline subcutaneous tophus count, median (IQR) | 2 (0–4) | 1 (0–4) | 2 (1–6) | 0.93 (0.85–1.01) | 0.10 |
| Number of gout flares in preceding 3 mo, median (IQR) | 1 (0–2) | 0 (0–1) | 1 (0–3) | 0.77 (0.60–0.98) | 0.04 |
| Baseline pain, median (IQR), 10‐cm VAS | 0.35 (0.00–2.15) | 0.20 (0.00–1.65) | 0.48 (0.00–3.10) | 0.86 (0.71–1.06) | 0.15 |
| Baseline patient global assessment, median (IQR), 10‐cm VAS | 2.00 (1.00–3.50) | 2.00 (1.00–3.00) | 2.50 (1.00–4.00) | 0.95 (0.80–1.13) | 0.55 |
| Baseline serum creatinine, median (IQR), μmol/L | 91 (82–105) | 93 (82–108) | 91 (82–104) | 1.01 (0.99–1.03) | 0.39 |
| Baseline creatinine clearance, median (IQR), mL/min | 72 (61–87) | 71 (58–82) | 74 (63–89) | 0.99 (0.96–1.01) | 0.20 |
| Comorbidities, n (%) | |||||
| Type 2 diabetes | 6 (7) | 2 (5) | 4 (7.7) | 0.63 (0.11–3.63) | 0.61 |
| High cholesterol | 40 (43) | 18 (45) | 22 (42) | 1.12 (0.49–2.56) | 0.80 |
| Hypertension | 45 (49) | 18 (45) | 27 (51.9) | 0.76 (0.33–1.73) | 0.51 |
| Cardiovascular disease | 11 (12) | 6 (15) | 5 (9.6) | 1.66 (0.47–5.88) | 0.43 |
| Kidney disease | 18 (20) | 8 (20) | 10 (19) | 1.05 (0.37–2.96) | 0.93 |
| Baseline DECT MSU crystal volume, median (IQR), cm3 | 0.12 (0.05–0.95) | 0.06 (0.03–0.11) | 0.37 (0.08–2.17) | 0.54 (0.40–0.73) | <0.001 |
| Baseline radiographic damage score, median (IQR) | 5.70 (2.50–11.75) | 5.196 (2.19–12.06) | 6.00 (2.50–11.75) | 1.00 (0.95–1.04) | 0.89 |
| Baseline CT erosion score, median (IQR) | 7.00 (4.00–13.50) | 6.25 (3.00–12.00) | 7.25 (5.00–15.50) | 0.96 (0.90–1.02) | 0.21 |
| Randomization group, n (%) | |||||
| Intensive target group b | 46 (50) | 20 (50) | 26 (50) | 1.00 (0.44–2.28) | >0.99 |
CI, confidence interval; CT, computed tomography; DECT, dual‐energy computed tomography; IQR, interquartile range; MSU, monosodium urate; NZ, New Zealand; OR, odds ratio; VAS, visual analog scale.
Continuous measures were analyzed as the odds of a one‐unit difference.
Standard target group set as reference.
RESULTS
Clinical features
Baseline clinical features of participants are displayed in Tables 1 and 2. The median age was 63 years and most participants were male (97%), with a median disease duration of 20 years.
Fulfillment of individual remission domains
Of the 97 participants in the first year of the study, fulfillment of the serum urate domain was reached by 90 participants (93%). This was the most frequently fulfilled domain, followed by 75 participants (77%) fulfilling the tophi domain, 60 participants (62%) fulfilling the pain domain, 34 participants (35%) fulfilling the patient global assessment domain, and 30 participants (31%) fulfilling the gout flares domain (Figure 2). Of the 92 participants in the second year of the study, fulfillment of the serum urate domain was reached by 80 participants (87%), followed by 76 participants (83%) fulfilling the tophi domain, 67 participants (73%) fulfilling the pain domain, 54 participants (59%) fulfilling the gout flares domain, and 33 participants (36%) fulfilling the patient global assessment domain (Figure 2).
Figure 2.
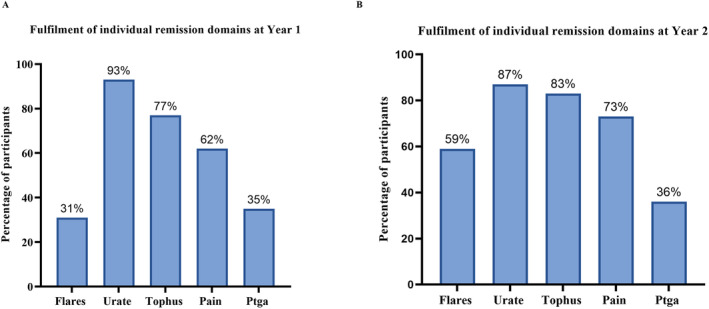
Fulfillment of individual remission domains (A) at year 1 and (B) year 2 in the following domains: gout flares, serum urate, tophus, pain, patient global assessment (Ptga).
Fulfillment of preliminary gout remission criteria and simplified gout remission criteria
Of the 97 participants in year 1, 11 participants (11%) fulfilled the preliminary gout remission criteria, including five in the standard target group and six in the intensive target group (P > 0.99). Of the 92 participants in year 2, the preliminary gout remission criteria were fulfilled in 21 participants (23%), including 11 in the standard target group and 10 in the intensive target group (P > 0.99).
In year 1, the simplified gout remission criteria were fulfilled in 26 participants (27%), including 13 in both the standard target and intensive target groups (P > 0.99). In year 2, the simplified criteria were fulfilled in 40 participants (44%), including 20 in both the standard target and intensive target groups (P > 0.99).
In year 1 there was moderate agreement between the preliminary gout remission criteria and simplified gout remission criteria (κ = 0.54, 95% CI 0.34–0.74; P < 0.001). Similarly, there was moderate agreement between these criteria in year 2 (κ = 0.56, 95% CI 0.40–0.71; P < 0.001). The preliminary gout remission criteria were fulfilled by 9 of 92 participants (10%) at both years, and the simplified gout remission criteria were fulfilled by 16 of 92 participants (17%) at both years.
Comparison of baseline variables between those fulfilling and not fulfilling the preliminary gout remission criteria and simplified gout remission criteria
In univariable logistic regression analyses, lower DECT MSU crystal volume and pain at baseline were associated with the fulfillment of the preliminary gout remission criteria at year 2 of the study (Table 1). The median (IQR) baseline DECT MSU crystal volume for those who fulfilled the criteria was 0.07 cm3 (0.03–0.10) compared to 0.18 cm3 (0.05–1.34) in those who did not (OR 0.65, 95% CI 0.47–0.90; P = 0.01). The median (IQR) baseline pain score for those who fulfilled the preliminary criteria was 0.00 (0.00–0.60) compared to a score of 0.50 (0.00–2.90) in those who did not (OR 0.65, 95% CI 0.43–0.99; P = 0.04).
Compared to those who fulfilled the simplified criteria at year 2, those that did not fulfill the criteria were more likely to be Pacific Peoples (OR 0.18, 95% CI 0.04–0.84; P = 0.03), have higher BMI at baseline (OR 0.88, 95% CI 0.81–0.96; P = 0.005), have a higher number of gout flares at baseline (OR 0.77, 95% CI 0.60–0.98; P = 0.04), and have higher DECT MSU crystal volume at baseline (OR 0.54, 95% CI 0.40–0.73; P < 0.001) (Table 2).
Baseline DECT MSU crystal volume is a significant predictor of gout remission
To identify independent predictors for fulfillment of the preliminary gout remission criteria, baseline BMI, patient global assessment score, pain score, number of gout flares, and DECT MSU crystal deposition were candidate variables entered into regression models for selection. As shown in Table 3, among these variables, only baseline DECT MSU crystal deposition was identified as a significant predictor in the final model, with an OR of 0.65 (95% CI 0.46–0.93; P = 0.02). Baseline DECT MSU crystal deposition was also a significant predictor when controlling for the baseline preliminary remission variables that were all forced in the model (serum urate, tophus count, number of gout flares, pain, patient global assessment) and randomization group; (OR 0.65, 95% CI 0.44–0.96; P = 0.029) (Supplementary Table 3).
Table 3.
Final logistic regression model showing baseline predictors for the fulfillment of gout remission criteria at year 2
| OR (95% CI) | P value | |
|---|---|---|
| Preliminary gout remission a | ||
| Baseline DECT MSU crystal volume | 0.65 (0.46–0.93) | 0.02 |
| Baseline pain VAS | 0.70 (0.47–1.04) | 0.10 |
| Simplified gout remission b | ||
| Baseline DECT MSU crystal volume | 0.57 (0.41–0.78) | <0.001 |
| Baseline BMI | 0.92 (0.84–1.01) | 0.08 |
BMI, body mass index; CI, confidence interval; DECT, dual‐energy computed tomography; MSU, monosodium urate; OR, odds ratio; VAS, visual analog scale.
X2 (2) =13.01, P = 0.001; Nagelkerke R 2 = 0.21.
X2 (2) =25.09, P < 0.001; Nagelkerke R 2 = 0.33.
To identify independent predictors for the fulfillment of the simplified gout remission criteria, baseline BMI, tophus count, number of gout flares, ethnicity, and DECT MSU crystal volume were candidate variables entered into regression models for selection. As shown in Table 3, DECT MSU crystal volume was the only significant predictor in the final model (OR 0.57, 95% CI 0.41–0.78; P < 0.001). Baseline DECT MSU crystal deposition again remained a significant predictor for fulfillment of the simplified gout remission criteria when controlling for baseline serum urate, tophus count, number of gout flares, and randomization group (OR 0.56, 95% CI 0.39–0.77; P < 0.001) (Supplementary Table 4).
For the ROC curve analysis, the area under the curve for the discriminative ability of baseline DECT MSU crystal deposition in predicting fulfillment of the preliminary remission criteria at year 2 was 0.68 (95% CI 0.56–0.79; P = 0.003) and for the simplified remission criteria at year was 0.77 (95% CI 0.68–0.87; P < 0.001) 2 (Supplementary Figure 1).
We determined a cutoff value to estimate the probability of “no remission” in our cohort (Supplementary Figure 2). Participants with a DECT MSU crystal volume >0.11 cm3 at baseline were more likely to not be in remission at year 2. The probability that a person was not in remission when they had a baseline DECT MSU crystal volume above the cutoff value (positive predictive value) was 0.91 (95% CI 0.79–0.98) for the preliminary remission criteria and 0.79 (95% CI 0.66–0.91) for the simplified remission criteria (Table 4).
Table 4.
DECT MSU crystal deposition volume cutoff for not fulfilling gout remission criteria*
| Criteria | ROC curve analysis | Cut point, cm3 | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Youden's Index (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | P value | |||||||
| Preliminary criteria | 0.68 (0.56–0.79) | 0.003 | >0.11 | 0.59 (0.47–0.70) | 0.80 (0.58–0.92) | 0.91 (0.79–0.98) | 0.36 (0.22–0.51) | 0.39 (0.18–0.60) |
| Simplified criteria | 0.77 (0.68–0.87) | <0.001 | >0.11 | 0.71 (0.58–0.82) | 0.74 (0.59–0.85) | 0.79 (0.66–0.91) | 0.66 (0.51–0.80) | 0.46 (0.29–0.65) |
AUC, area under the curve; CI, confidence interval; DECT, dual‐energy computed tomography; MSU, monosodium urate; PPV, positive predictive value; NPV, negative predictive value; ROC, receiver operator characteristic.
In an exploratory analysis, serial DECT MSU crystal volumes were analyzed. DECT urate volumes reduced over two years of treatment. Compared to those not fulfilling the remission criteria, people fulfilling the remission criteria had generally lower DECT MSU crystal volumes at year 1 and year 2 (Supplementary Table 5). The median differences between groups were greater when using the simplified remission criteria (Supplementary Table 5).
DISCUSSION
In this study, we aimed to identify variables that predict gout remission in people receiving intensive urate‐lowering therapy. Across the intervention groups, there were no differences in reaching gout remission, defined by either the preliminary gout remission criteria or by the simplified gout remission criteria, between those in the intensive target group and those in the standard target group. Higher DECT MSU crystal volume at baseline was also associated with lower odds of gout remission, using either criteria. People with high DECT MSU crystal volumes at baseline may need more intensive urate‐lowering therapies to clear deposits, may need other treatments including more anti‐inflammatory therapy to prevent gout flares, and may need other health care services to improve pain and global assessment including allied health, pain management, or nurse support.
In this analysis, lower baseline MSU volume measured by DECT was associated with gout remission using both preliminary and simplified criteria. These findings indicate that the simplified remission definition has similar properties to the preliminary gout remission criteria and that gout remission defined by the simplified criteria may be sufficient. This is important as simplified criteria would be more feasible in clinical research compared to the preliminary remission criteria. However, further analysis is required, particularly the concurrent validity of the simplified criteria with other gout outcomes.
The overall preliminary gout remission criteria were fulfilled in 11% of participants in year 1 and 23% in year 2 of the study. These rates can be compared with other studies, which vary from 9.1% of participants in the Alvarado‐de la Barrera et al 9 study to 48.2% of participants reported by Schlesinger et al. 8 The differences in the frequency of remission across these studies is likely to reflect disease severity at baseline, as well as the study duration. For individuals with severe and long‐standing gout, such as those in our cohort, remission is difficult to achieve over two years. Participants in this study had a median disease duration of 20 years and erosive gout at baseline. However, even with an intensive urate‐lowering schedule using oral medications and serum urate targets of <0.20 mmol/L or <0.30 mmol/L over the two‐year study, a low proportion of participants reached gout remission at either year, with only 10% of participants fulfilling the preliminary gout remission criteria at both year 1 and year 2. Alvarado‐de la Barrera et al 9 also observed in their study that people with severe gout (which they defined as five or more tophi at baseline) did not fulfill the preliminary gout remission criteria over five years, with one of the main obstacles to remission being the size and number of tophi at baseline. In our study, the higher baseline DECT MSU crystal volume is a likely indicator of more‐severe disease. However, baseline gout disease duration, serum urate, tophus count, and patient global assessment did not predict future gout remission, so we believe our findings indicate that DECT MSU crystal volume does provide more information about future remission than clinical variables alone.
Fulfillment of the individual domains varied within our cohort. The serum urate domain was the most fulfilled domain in year 1 (93%) and year 2 (87%). This was unsurprising particularly as participants had been randomized at baseline to a serum urate target of <20 mmol/L or <0.30 mmol/L. However, this did not fully translate to the fulfillment of the “gout flares” domain, which only 31% of participants achieved in year 1 and 59% of participants achieved in year 2. This discrepancy between serum urate and gout flare frequency has also been noted in clinical trials that have demonstrated that suppression of gout flares often takes several years of low serum urate levels, even when target serum urate of <0.36 mmol/L is achieved. 23 , 24
A key result of our study was that higher baseline DECT MSU crystal volume is associated with lower odds of achieving gout remission over two years. We determined a DECT MSU crystal volume cut point of >0.11 cm3 was optimal to predict those who would have a higher probability of not reaching gout remission over two years. Although this is a low volume, the cut point of 0.11 cm3 is well above the submillimeter artifact that can be visible on DECT scanning. Of note, visible artifact was removed before volume measurement by the DECT readers.
In a previous cross‐sectional study, DECT MSU crystal volume was lower in those experiencing remission, as defined by the preliminary gout remission criteria. 25 DECT MSU crystal volume has been shown to positively correlate with serum urate, gout flare frequency, and tophi. 15 , 26 , 27 , 28 , 29 Similarly, Pascart et al 29 found in a 12‐month observational study that DECT measurement of urate deposits predicted the risk of gout flares. When controlling for the baseline outcomes that contribute to the preliminary remission criteria and simplified gout remission criteria (serum urate, tophi, gout flares, pain, patient global assessment) as well as randomization group, DECT MSU crystal volume remained an independent predictor of gout remission.
Strengths of this study include the systematic approach to data collection as part of the double‐masked clinical trial study design. There were some limitations to this analysis. The pain and patient global assessment questionnaires were not specific to gout, which may have impacted on the assessment of the preliminary gout remission criteria. Participants’ scoring of the pain VAS may have been based on the experience of nongout pain. Furthermore, the patient global assessment questionnaire was not an objective measure of gout disease activity as it related more to overall health. These nonspecific questionnaires may also account for the lower proportion of participants fulfilling the preliminary gout remission criteria compared to the simplified criteria that did not include the questionnaires. Due to the small sample size and the infrequent fulfillment of remission in our cohort at both year 1 and year 2, few predictor variables could be included in regression models relative to the number of outcomes. 30 , 31 It is possible that this could have led to false‐negative findings of other possible predictors of gout remission. A further limitation is that these findings may not be generalizable to people with gout who have shorter disease duration or less severe disease.
In conclusion, gout remission is difficult to achieve for individuals with erosive gout, even with intensive oral urate‐lowering medication over a two‐year period. Baseline DECT MSU crystal volume predicts gout remission after two years.
AUTHOR CONTRIBUTIONS
All authors contributed to at least one of the following manuscript preparation roles: conceptualization AND/OR methodology, software, investigation, formal analysis, data curation, visualization, and validation AND drafting or reviewing/editing the final draft. As corresponding author, Dr Dalbeth confirms that all authors have provided the final approval of the version to be published, and takes responsibility for the affirmations regarding article submission (eg, not under consideration by another journal), the integrity of the data presented, and the statements regarding compliance with institutional review board/Helsinki Declaration requirements.
Supporting information
Disclosure form
Figure S1:
Figure S2:
Supplementary Table 1: Preliminary gout remission criteria [5] and simplified gout remission criteria [6]
Supplementary Table 2: Comparison of baseline variables between those included in complete case analyses and the original number of participants.
Supplementary Table 3: Logistic regression model showing DECT MSU crystal deposition volume as an independent predictor for the fulfilment of the preliminary gout remission criteria at Year 2 when controlling for baseline remission components and randomisation group.
Supplementary Table 4: Logistic regression model showing DECT MSU crystal deposition volume as an independent predictor for the fulfilment of the simplified gout remission criteria at Year 2 when controlling for baseline remission components and randomisation group
Supplementary Table 5: MSU deposition detected by DECT at baseline, Year 1 and Year 2 between patients who fulfilled remission criteria vs patients who did not at Year 2.
ACKNOWLEDGMENTS
Open access publishing facilitated by The University of Auckland, as part of the Wiley ‐ The University of Auckland agreement via the Council of Australian University Librarians.
ACTRN: 12615001219572.
Ms Tabi‐Amponsah's work was supported by a University of Auckland Health Research Doctoral Scholarship.
Additional supplementary information cited in this article can be found online in the Supporting Information section (http://onlinelibrary.wiley.com/doi/10.1002/acr.25414).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr.25414.
REFERENCES
- 1. Kim JW, Kwak SG, Park SH. Prescription pattern of urate‐lowering therapy in Korean gout patients: data from the national health claims database. Korean J Intern Med (Korean Assoc Intern Med) 2018;33(1):228–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen‐Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007‐2016. Arthritis Rheumatol 2019;71(6):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dehlin M, Drivelegka P, Sigurdardottir V, et al. Incidence and prevalence of gout in Western Sweden. Arthritis Res Ther 2016;18(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Felson D. Defining remission in rheumatoid arthritis. Ann Rheum Dis 2012;71(suppl 2):i86–i88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Lautour H, Taylor WJ, Adebajo A, et al. Development of preliminary remission criteria for gout using Delphi and 1000Minds consensus exercises. Arthritis Care Res (Hoboken) 2016;68(5):667–672. [DOI] [PubMed] [Google Scholar]
- 6. Tabi‐Amponsah AD, Stewart S, Hosie G, et al. The patient experience of gout remission: a qualitative study. ACR Open Rheumatol 2023;5(8):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cipolletta E, Di Battista J, Di Carlo M, et al. Sonographic estimation of monosodium urate burden predicts the fulfillment of the 2016 remission criteria for gout: a 12‐month study. Arthritis Res Ther 2021;23(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlesinger N, Edwards NL, Khanna PP, et al. Evaluation of proposed criteria for remission and evidence‐based development of criteria for complete response in patients with chronic refractory gout. ACR Open Rheumatol 2019;1(4):236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvarado‐de la Barrera C, López‐López CO, Álvarez‐Hernández E, et al. Are target urate and remission possible in severe gout? A five‐year cohort study. J Rheumatol 2020;47(1):132–139. [DOI] [PubMed] [Google Scholar]
- 10. Dalbeth N, Frampton C, Fung M, et al. Concurrent validity of provisional remission criteria for gout: a dual‐energy CT study. Arthritis Res Ther 2019;21(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalbeth N, Doyle AJ, Billington K, et al. Intensive serum urate lowering with oral urate‐lowering therapy for erosive gout: a randomized double‐blind controlled trial. Arthritis Rheumatol 2022;74(6):1059–1069. [DOI] [PubMed] [Google Scholar]
- 12. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2015;74(10):1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalbeth N, Aati O, Gao A, et al. Assessment of tophus size: a comparison between physical measurement methods and dual‐energy computed tomography scanning. J Clin Rheumatol 2012;18(1):23–27. [DOI] [PubMed] [Google Scholar]
- 14. Dalbeth N, Doyle A, Boyer L, et al. Development of a computed tomography method of scoring bone erosion in patients with gout: validation and clinical implications. Rheumatology (Oxford) 2011;50(2):410–416. [DOI] [PubMed] [Google Scholar]
- 15. Rajan A, Aati O, Kalluru R, et al. Lack of change in urate deposition by dual‐energy computed tomography among clinically stable patients with long‐standing tophaceous gout: a prospective longitudinal study. Arthritis Res Ther 2013;15(5):R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mallinson PI, Coupal T, Reisinger C, et al. Artifacts in dual‐energy CT gout protocol: a review of 50 suspected cases with an artifact identification guide. AJR Am J Roentgenol 2014;203(1):W103–W109. [DOI] [PubMed] [Google Scholar]
- 17. Ostergaard M, Edmonds J, McQueen F, et al. An introduction to the EULAR‐OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 2005;64(suppl 1):i3–i7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three‐year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35(1):26–34. [DOI] [PubMed] [Google Scholar]
- 19. Dalbeth N, Clark B, McQueen F, et al. Validation of a radiographic damage index in chronic gout. Arthritis Care Res 2007;57(6):1067–1073. [DOI] [PubMed] [Google Scholar]
- 20. Sauerbrei W, Perperoglou A, Schmid M, et al. State of the art in selection of variables and functional forms in multivariable analysis‐outstanding issues. Diagn Progn Res 2020;4(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Community Health 2020;8(1):e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinze G, Wallisch C, Dunkler D. Variable selection ‐ a review and recommendations for the practicing statistician. Biom J 2018;60(3):431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stamp LK, Chapman PT, Barclay ML, et al. A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout. Ann Rheum Dis 2017;76(9):1522–1528. [DOI] [PubMed] [Google Scholar]
- 24. Stamp LK, Chapman PT, Barclay M, et al. Allopurinol dose escalation to achieve serum urate below 6 mg/dL: an open‐label extension study. Ann Rheum Dis 2017;76(12):2065–2070. [DOI] [PubMed] [Google Scholar]
- 25. Bursill D, Taylor WJ, Terkeltaub R, et al. Gout, Hyperuricaemia and Crystal‐Associated Disease Network (G‐CAN) consensus statement regarding labels and definitions of disease states of gout. Ann Rheum Dis 2019;78(11):1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gamala M, Linn‐Rasker SP, Nix M, et al. Gouty arthritis: decision‐making following dual‐energy CT scan in clinical practice, a retrospective analysis. Clin Rheumatol 2018;37(7):1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalbeth N, Nicolaou S, Baumgartner S, et al. Presence of monosodium urate crystal deposition by dual‐energy CT in patients with gout treated with allopurinol. Ann Rheum Dis 2018;77(3):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dalbeth N, Billington K, Doyle A, et al. Effects of allopurinol dose escalation on bone erosion and urate volume in gout: a dual‐energy computed tomography imaging study within a randomized, controlled trial. Arthritis Rheumatol 2019;71(10):1739–1746. [DOI] [PubMed] [Google Scholar]
- 29. Pascart T, Grandjean A, Capon B, et al. Monosodium urate burden assessed with dual‐energy computed tomography predicts the risk of flares in gout: a 12‐month observational study: MSU burden and risk of gout flare. Arthritis Res Ther 2018;20(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49(12):1373–1379. [DOI] [PubMed] [Google Scholar]
- 31. Harrell FE Jr, Lee KL, Matchar DB, et al. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer Treat Rep 1985;69:1071–1077. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Figure S1:
Figure S2:
Supplementary Table 1: Preliminary gout remission criteria [5] and simplified gout remission criteria [6]
Supplementary Table 2: Comparison of baseline variables between those included in complete case analyses and the original number of participants.
Supplementary Table 3: Logistic regression model showing DECT MSU crystal deposition volume as an independent predictor for the fulfilment of the preliminary gout remission criteria at Year 2 when controlling for baseline remission components and randomisation group.
Supplementary Table 4: Logistic regression model showing DECT MSU crystal deposition volume as an independent predictor for the fulfilment of the simplified gout remission criteria at Year 2 when controlling for baseline remission components and randomisation group
Supplementary Table 5: MSU deposition detected by DECT at baseline, Year 1 and Year 2 between patients who fulfilled remission criteria vs patients who did not at Year 2.


