Abstract
Introduction
Immune checkpoint blockers (ICBs) revolutionized the treatment of patients with advanced non-small cell lung cancer (NSCLC) but only a fraction of them obtain a response, and clinical benefit from these treatments is often difficult to predict. The aim of our study is to unveil the potential implications of antibody response to previous viral infections in predicting response to ICBs in patients with NSCLC.
Methods
Sera from patients treated with ICBs alone, chemotherapy (CT) or a combination of CT-ICBs were analyzed with VirScan (CDI Labs, USA), a high-throughput method that comprehensively analyzes epitope-level antiviral IgG antibodies via programmable phage display and immunoprecipitation sequencing.
Total number of unique positive peptides (tUP) was defined as the total number of non-overlapping positive “is a hit” peptides for each patient.
Results
Overall, 387 patients were included. Of them, 129 were treated with ICBs alone, 66 with CT-ICBs and 195 with CT alone. 90 out of 129 patients treated with ICBs alone received ICBs as a subsequent line of treatment, while CT-ICBs and CT were administered as upfront therapies.
A higher tUP was correlated with improved overall survival in patients treated with ICBs, and confirmed in the multivariate model (HR 0.43, 95% CI 0.24, 0.79, p=0.006), while it was not in those treated with CT-ICBs (p=0.8) and CT alone (p=0.1).
tUP was not correlated with programmed death-ligand 1 (PD-L1) expression, while at the transcriptome level it was correlated with several immune-related pathways, particularly involving B cells.
Conclusion
A higher number of viral peptides recognized by serum antibodies might reflect increased immune fitness, resulting in improved outcomes in ICBs treated patients with NSCLC.
Keywords: Immunization, Immune Checkpoint Inhibitor, Lung Cancer, Biomarker, Antibody
WHAT IS ALREADY KNOWN ON THIS TOPIC
Clinical benefit from immune checkpoint blockers (ICBs) is difficult to predict and patient’s-based biomarker are needed. We hypothesized that antibody response to previous viral infections might predict response to ICBs in patients with non-small cell lung cancer.
WHAT THIS STUDY ADDS
We found that the number of viral peptider recognized by serum antibodies and positivity for rhinovirus are correlated with improved response to ICBs.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Future research should be performed to further refine our findings and to better define the immune fitness of patients with cancer, based on antiviral immune response.
Introduction
While immune checkpoint blockers (ICBs) have changed the therapeutic landscape in advanced non-small cell lung cancer (NSCLC), leading to a substantial improvement in long-term outcome with an unprecedented rate of 5-year survivorship above 30% for selected cases,1 many patients still experience poor outcome under ICBs.
Programmed death-ligand 1 (PD-L1) represents the only biomarker that is widely approved by regulatory agencies, but its positive and negative predictive values are suboptimal.2
Moreover, there has recently been a question raised as to whether an intensification strategy involving the addition of chemotherapy (CT) could improve the efficacy of upfront treatment for advanced disease. However, little difference has been observed in unselected patients, while a CT-free regimen is associated with better tolerability.
Novel biomarkers to adapt treatment are therefore needed to improve tailoring for immune checkpoint-based therapies.
Apart from PD-L1, several other putative biomarker has been proposed, and many of them are based on tumor biology. For example, an increase in tumor mutation burden has been shown to be associated with an improved response, while the presence of particular mutations such as STK11 or KEAP1 seems associated with a worse prognosis under ICBs monotherapy. Other are based on tumor burden, such as metabolic tumor volume, circulating tumor DNA-based tumor fraction.3 Anyway, as ICBs exert an indirect action against the tumor by modulating the immune system, their efficacy depends on the immune system’s capacity to eliminate cancer cells. Despite the widespread use of ICBs in anticancer therapy, there remains an unmet need for an objective method to assess the immune system fitness before ICBs administration.4
VirScan is a single-well phage display immunoprecipitation and sequencing assay, designed for the comprehensive detection of antibodies against the viral epitopes associated with all viruses known to have human tropism.
We worked on the hypothesis that previous immune responses against virus may reflect the ability of the immune system to be activated by ICBs.
Materials and methods
Patients
Patients were retrospectively retrieved from two prospective trials, PREMIS study (NCT 03984318) for patients who received ICBs alone or in combination with CT and MSN study (NCT02105168) for those who received first-line platinum-based CT.
Serum was collected at treatment start and stored at −80°.
VirScan
VirScan complete methodology has been described elsewhere.5 6 Briefly, all viral proteins in the UniProt database with human tropism were collapsed on 90% identity and bioinformatically parsed into 56 amino-acid peptide sequences with 28 amino-acid overlaps between adjacent tiles to create a synthetic oligonucleotide library; this library was packaged into a T7 phage display vector and expanded in Escherichia coli. The expanded T7 phage library quality is confirmed by sequencing to have >90% of the library within one log of the overall average clonal frequency. An aliquot from this library is then reacted with diluted patient serum or other antibody-containing fluid. Bound antibodies are immunoprecipitated with protein A/G beads, the precipitate amplified by PCR, and the sequences quantified by a next-generation sequencing and analysis pipeline that compares patient-sample immunoprecipitation (IP) read counts to negative controls with no antibody input (mock-IPs) in the context of overall clonal frequency of individual peptides in the parent library. Output data are then created at both the peptide and whole-protein level, providing therefore a quantitative measure of the amount of each epitope-specific antibody.7 8
The total number of unique peptide seropositivity (tUP) was then calculated as the sum of the non-overlapping, significantly enriched “is a hit” peptides for each patient.
A second analysis was run based on AntiViral Antibody Response Deconvolution Algorithm (AVARDA), a software package developed to analyze VirScan data sets results, giving a probabilistic assessment of infection with species-level resolution by considering sequence alignment of all library peptides to each other and to all human viruses, accounting for antibody cross-reactivity among sequences shared by related viruses and for the disproportionate representation of individual viruses in the library, as previously described.9
RNA sequencing
RNA sequencing was carried out using fresh frozen tissue as previously described, as part of the MATCH-R study (NCT02517892).10 RNA sequencing data were processed using Trim Galore (V.0.4.4) for quality control and transcript quantification was performed using Kallisto (V.0.44.0) with GENCODE V.27 as a reference. Transcript-level estimates were aggregated to the gene level (58,288 genes) using TxImport (V.1.16.0), generating both raw counts and transcripts per million.
To analyze differentially expressed genes according to tUP, we filtered out genes with very low expression using a cut-off of 2 counts in 20% of samples and 50 counts in the whole cohort. A principal component analysis (PCA) was done on the top 500 variable genes. The R package DESeq2 V.1.34.0 was then used to generate PCA plots to detect and filter out outliers, then identify differentially expressed genes with respect to tUP, adjusted on the biopsy site. False discovery rates (FDR) were computed using the Benjamini-Hochberg method and an FDR cut-off of 0.05 was applied. Using log-fold change ranked values from differentially expressed genes, we performed enrichment analyses using the clusterProfiler package (V.4.2.2) with the Hallmark Gene Sets and the ontology gene sets from the Molecular Signatures Database using an FDR of 0.1%.
Statistical analysis
Continuous variables were described by their median and compared by a Student’s or Mann-Whitney test, when appropriate. Associations between continuous variables were analyzed with a Spearman test. Categorical variables are presented as percentages and compared using a χ2 or Fisher’s exact test when appropriate.
Overall survival (OS) is defined as the time from treatment initiation to death. Predictors of Progression Free Survival (PFS) and Overall Survival (OS) were analyzed using univariate and multivariable Cox models. Penalized smoothing splines approach was used to assess linearity in a multivariable cox models. Log transformation was applied for non-normally distributed variables.
For each test, a difference was considered significant if p<0.05.
Leiden clustering was used for dimensional reduction on peptides whose log-fold change was positive in at least 5% of the patients.11 For each of these clusters, the first principle component was extracted in order to get one feature per peptide-cluster, denoted as “group_X”, where X is the identifier of the group.
For machine learning, all input features were standardized. These features were used to train a Cox model from sksurv (l1 ratio=0.9) to predict the OS. Three different models were trained, based on three groups of patients depending on their treatment (CT, immunotherapy, or the combination of the two). The best alpha parameter of the Cox model is computed with 10-fold cross-validation.
Statistical analyses and figures were performed using R studio software V.2022.12.0+353 and Python, V.3.9.
Results
Overall, 387 patients were included. Of them, 129 were treated with ICBs alone, 66 with ICBs in combination with CT and 195 with CT alone. The main characteristics of the three cohorts are summarized in table 1.
Table 1. Baseline characteristics of the three cohorts of patients treated with immune checkpoint blockers alone (ICBs), ICBs in combination with chemotherapy (CT-ICBs) or CT alone (CT).
| Characteristic | ICBsN=126* | CT-ICBsN=66* | CTN=195* | P value† |
| Sex | 0.7 | |||
| Female | 49 (39) | 22 (33) | 74 (38) | |
| Male | 77 (61) | 44 (67) | 120 (62) | |
| Bone metastasis (yes) | 52 (41) | 33 (50) | 73 (38) | 0.2 |
| Liver metastasis (yes) | 17 (13) | 10 (15) | 24 (12) | 0.8 |
| Brain metastasis (yes) | 34 (27) | 24 (36) | 36 (19) | 0.010 |
| ECOG PS | 0.012 | |||
| 0–1 | 93 (74) | 46 (71) | 164 (85) | |
| ≥2 | 33 (26) | 19 (29) | 29 (15) | |
| Unknown | 0 | 1 | 1 | |
| Age | 67 (58–73) | 63 (56–70) | 61 (54–68) | 0.001 |
| Smocking habits | 0.6 | |||
| current/former | 113 (90) | 62 (94) | 179 (93) | |
| Never | 12 (9.6) | 4 (6.1) | 14 (7.3) | |
| Unknown | * | 0 | 3 | |
| PD-L1 TPS | 0.005 | |||
| 0% | 32 (27) | 24 (41) | 25 (53) | |
| 1–49% | 31 (26) | 19 (33) | 9 (19) | |
| ≥50% | 56 (47) | 15 (26) | 13 (28) | |
| Unknown | 7 | 8 | 149 | |
| LDH | >0.9 | |||
| ≤ULN | 82 (68) | 41 (67) | 106 (66) | |
| >ULN | 39 (32) | 20 (33) | 55 (34) | |
| Unknown | 5 | 5 | 33 | |
| Number of metastatic sites | 2.00 (1.00–3.00) | 3.00 (2.00–4.00) | 1.00 (1.00–2.00) | <0.001 |
| dNLR | 2.9 (2.0–4.5) | 4.0 (2.6–6.7) | 2.7 (1.7–7.3) | 0.005 |
| Histology | <0.001 | |||
| Non-squamous | 91 (72) | 56 (85) | 176 (91) | |
| Squamous | 35 (28) | 10 (15) | 18 (9.3) | |
| Line of treatment | ||||
| First line | 45 (36) | 66 (100) | 195 (100) | |
| Advanced lines | 81 (64) | 0 | 0 | |
| Unique peptide seropositivity count | 277 (219–368) | 261 (211–329) | 351 (260–450) | <0.001 |
n (%); Mmedian (IQR).
Pearson’s Chi-squaredχ2 test; Kruskal-Wallis rank sum test.
dNLR, derived neutrophil to lymphocyte ratioLDH, lactate dehydrogenase; PD-L1programmed death-ligand 1 PS, Eastern Cooperative Oncology Group performance status; TPS, tumor proportion score; ULN, upper limit of normality
Patients treated with ICBs had an older age at treatment start as compared with other treatment groups (67 years vs 63 and 61 for CT-ICBs combination and chemo, respectively), a higher proportion of PD-L1 high tumors and squamous subtype. Moreover, 90 out of 129 patients treated with ICBs alone received ICBs as a subsequent line of treatment, while CT-ICBs and CT were administered as upfront therapies.
Median follow-up was 40.1 months for ICBs (95% CI 35.9, 45.3), 31.4 months for combination of CT and ICBs (95% CI 31.2, 35.1) and 48.7 months for CT alone.
Median OS for patients treated with ICBs alone was 11.8 months (95% CI 7.9, 14.6) versus 24.4 months for CT-ICBs combination (95% CI 12.3, 30.9) versus 12.1 for CT alone (95% CI 8.6, 16.3).
Unique peptide seropositivity count
We first analyzed the tUP. Median tUP was 277 for ICBs (IQR 219–368), 261 for CT-ICBs (IQR 211–329) and 351 for CT alone (IQR 260–450).
tUP was inversely correlated with age (rho −0.28, p<0.0001) while no correlation was seen with other parameters such as lactate dehydrogenase (p=0.3), liver metastasis (p=0.14), bone metastasis (p=0.12), Eastern Cooperative Oncology Group Performance Status (ECOG PS) 2 or more (p=0.28), smocking status (p=0.24) or PD-L1 tumor proportion score (p=0.09).
We also analyzed, in a subset of 179 patients, the correlation of tUP with serum immune globulins concentration, finding none for IgG (p=0.876), IgM (p=0.611) and IgA (p=0.488).
In patients treated with ICBs, tUP was correlated with OS in univariate analysis, and confirmed in the multivariate model (HR 0.43, 95% CI 0.24, 0.79, p=0.006), while it was not in those treated with CT-ICBs (p=0.8) and CT alone (p=0.1), table 2 and online supplemental figure S1.
Table 2. Multivariate analysis for overall survival (OS) in immune checkpoint blockers (ICBs) alone, ICBs in combination with chemotherapy (CT-ICBs) or CT alone (CT).
| Characteristic | ICB OS | CT-ICBs OS | CT OS | ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| tUP(log) | 0.39 | 0.21, 0.74 | 0.004 | 2.48 | 0.38, 14.3 | 0.4 | 0.70 | 0.46, 1.06 | 0.10 |
| PD-L1 TPS | 0.98 | 0.97, 0.99 | <0.001 | 0.99 | 0.96, 1.01 | 0.3 | |||
| Histology (Sq vs nSq) | 1.37 | 0.77, 2.45 | 0.3 | 3.94 | 1.56, 16.8 | 0.007 | 0.88 | 0.47, 1.64 | 0.7 |
| Smocking (never vs current/former) | 1.24 | 0.56, 2.72 | 0.6 | 0.94 | 0.15, 2.17 | 0.4 | 2.12 | 1.28, 3.52 | 0.004 |
| ECOG PS (≥2 vs 0–1) | 1.81 | 1.05, 3.13 | 0.034 | 0.49 | 0.29, 8.17 | 0.6 | 0.75 | 0.42, 1.35 | 0.3 |
| Liver metastasis (yes vs no) | 1.14 | 0.49, 2.64 | 0.8 | 1.67 | 0.82, 12.0 | 0.093 | 1.66 | 1.10, 2.50 | 0.016 |
| Bone metastasis (yes vs no) | 1.15 | 0.67, 1.97 | 0.6 | 3.20 | 0.60, 6.61 | 0.3 | 2.33 | 1.53, 3.55 | <0.001 |
| Brain metastasis (yes vs no) | 1.50 | 0.87, 2.61 | 0.15 | 0.35 | |||||
| LDH high vs low | 2.36 | 1.39, 4.01 | 0.002 | 2.63 | 0.94, 1.05 | 0.8 | 0.99 | 0.97, 1.01 | 0.2 |
| Age | 1.01 | 0.98, 1.03 | 0.7 | 0.99 | 0.45, 14.9 | 0.3 | 1.31 | 0.76, 2.26 | 0.3 |
| dNLR (>3 vs ≤3) | 2.64 | 0.88, 7.90 | 0.082 | 4.75 | 0.70, 1.87 | 0.6 | 1.20 | 1.00, 1.43 | 0.049 |
| Number of metastatic sites | 0.90 | 0.72, 1.12 | 0.3 | 1.19 | 0.71, 1.99 | 0.5 | 1.10 | 0.71, 1.71 | 0.7 |
| Treatment line (second or more vs first) | 1.13 | 0.58, 2.16 | 0.7 | ||||||
ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; nSq, non-squamous; Sq, squamous; TPS, tumor proportion score; tUP, total number of unique peptide seropositivity
We then analyzed the UP for each species. We selected only those species with at least 5% positivity of the population (at least one peptide) and an average of two peptides or more in positive patients as previous work highlighted the need to improve specificity,5 and we did not find any significant enrichment after FDR correction, although a trend was seen with increased UP against common viruses such as coxsackievirus, rhinovirus and influenza virus being generally associated with improved prognosis under ICBs (online supplemental table S1).
AVARDA algorithm
With the aim to determine if serum positivity against specific viruses was responsible for the observed correlation, we used the AVARDA algorithm to deconvolute VirScan results, obtaining a list of viruses for each patient with a high probability of serum positivity (p<0.05).
As expected, the most represented virus were Epstein-Barr virus (98%) followed by rhinovirus A (93%), rhinovirus B (80%).
As cytomegalovirus serology was available for 177 patients, 111 of which were positives, we first assessed the concordance between AVARDA Citomegalovirus (CMV) results and CMV serology for these patients, finding a high concordance between the two techniques (Cohen’s k=0.9, 95% CI 0.84, 0.97). No correlation was found between CMV serology and OS (p=0.6).
No correlation was found between the number of positive virus as detected by AVARDA and OS of ICBs (p=0.23).
Virus with a frequency lower than 5% of patients were excluded.
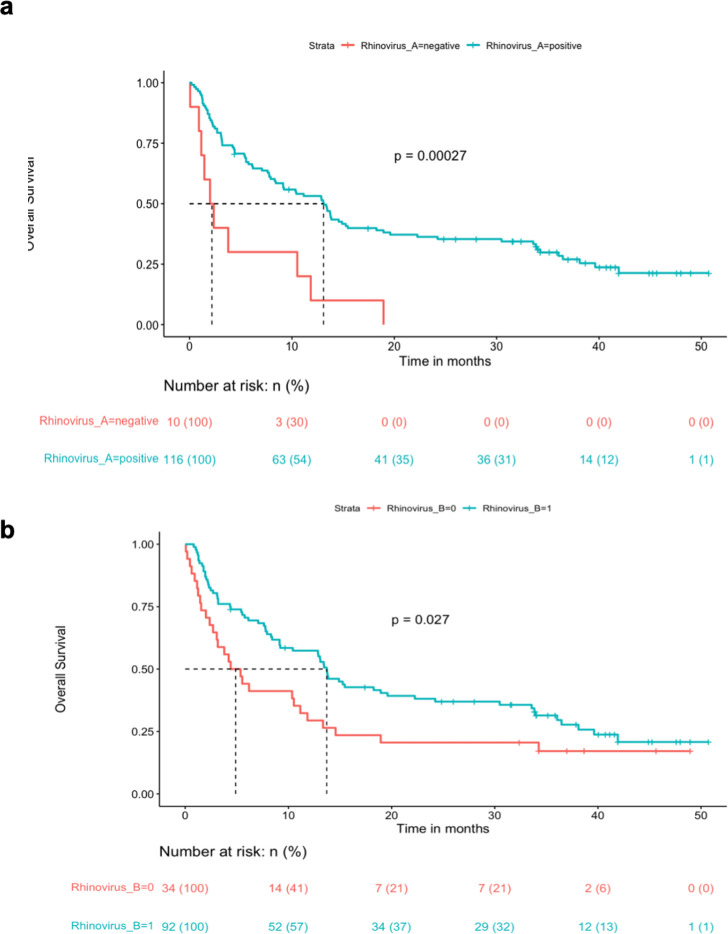
In univariate analysis, positivity for rhinovirus group A resulted significantly associated with improved OS after FDR correction (HR 0.31, 95% CI 0.16, 0.60, p=0.0006, p adjusted p=0.0346) and was confirmed in a multivariate model including other biomarkers (HR 0.37, 95% CI 0.18, 0.75, p=0.006). Positivity for rhinovirus group B was also associated with OS before FDR correction (p=0.029), but not after correction (figure 1). The trend was the same for the first line and pretreated ICBs (online supplemental figure S2).
Figure 1. Kaplan-Meier curves for overall survival according to AntiViral Antibody Response Deconvolution Algorithm algorithm positivity or negativity for rhinovirus A (a) and rhinovirus B (b).
No serum positivity was associated with OS in CT-ICBs or CT group after FDR correction.
Exploratory analysis of machine learning
In order to further explore the data, Leiden clustering was run on peptides to create 45 clusters of highly-correlated peptides (online supplemental figure S3 and table S2).
For each of these clusters, the first principle component was extracted in order to get one feature per peptide-cluster, denoted as “group_X”, where X is the identifier of the group.
A model was trained that showed no specific cluster was associated with improved OS in none of the cohorts (online supplemental figure S3).
RNA sequencing analysis
In order to evaluate the biological correlates of the tUP at tumor level, we retrieved the transcriptome data for 27 patients treated with ICBs that were included in the MATCH-R study, for whom RNA sequencing was available.
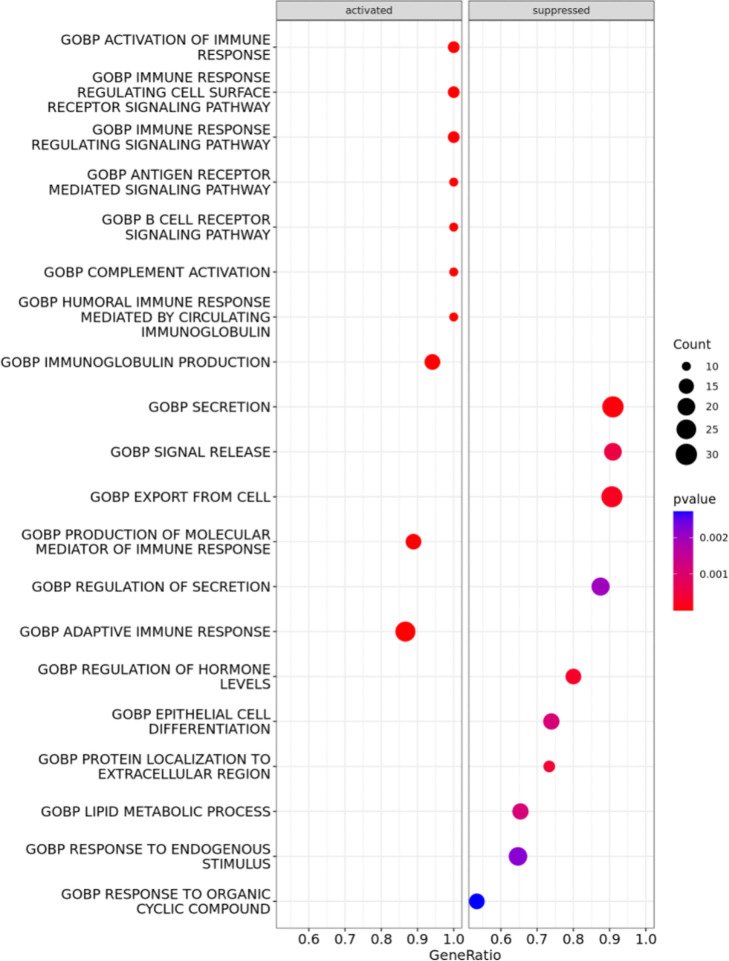
Enrichment analysis showed that different Gene Ontology Biological Process pathways were increasingly activated with increasing tUP (figure 2). Among these pathways, were listed the activation of the immune response, immune response regulating cell surface-receptor signaling pathway, immune response signaling pathway, antigen receptor-mediated signaling pathway, B-cell receptor signaling pathway, complement activation, humoral immune response mediated by circulating immunoglobulin, immune globulin production, production of molecular mediator of immune response and adaptive immune response.
Figure 2. Transcriptomic correlates of total number of unique peptide seropositivity (tUP). Gene Ontology pathways are suppressed or activated with the increase of tUP. The size of the circle depends on the number of genes found in the pathway, the colors on the statistical significance. GOBP, Gene Ontology Biological Process.
Discussion
In this paper, we described for the first time how an immune response against common viral infections might be used to predict the response to cancer immunotherapy.
Our data suggests that patients whose serum antibodies are capable of recognizing a higher number of unique viral peptides have an improved prognosis when treated with ICBs. When going to the species level, this correlation was especially seen with more common viruses, such as rhinovirus, coxsackievirus and enterovirus. We believe that our results indicate that having antibodies against a higher number of viral peptides is a sign of immune system fitness which, in turn, affects the immune response against cancer. On the other side, the effect seems to be absent in the CT cohort, supporting that our observation is linked to immune fitness, and in the CT-ICB cohort, suggesting that an intensified regime might overcome it. This is consistent with previous evidences on antibiotic therapy, for example, that is known to be associated with reduced immune response against tumor but its detrimental effect can be compensated by the synergistic interaction between ICBs and cytotoxic CT.12
Antibodies are indeed secreted by B cell and constitute their receptor, and therefore the polyclonality of antibodies against specific viruses is the reflection of distinct antigen-specific B cells populations.
B cells have been long considered as secondary in the development of antitumor responses, while recent evidences are highlighting their importance in the context of ICB for cancer treatment. For instance, B-cell transcriptomic markers have been described as the most differentially expressed genes in discriminating between responders versus non-responders in the context of neoadjuvant ICBs, with B cells being especially localized within tertiary lymphoid structures (TLS).13 In turn, TLS are associated with augmented response in advanced diseases.14
Moreover, while T cells are the key player in anticancer immunotherapy, being those actively killing the tumor,15 it has been shown that there is a strong link between T-cell and B-cell response, as basically there is no T-cell response without the corresponding B-cell response.16 17 Therefore, it is conceivable that a higher plasticity of B-cell reflects a higher plasticity of T-cell repertoire.
This is consistent also with the correlation found with age, showing a decreasing tUP for elderly patients.
As the most significant peptides come from rhinovirus, coxsakievirus and enterovirus, our hypothesis is that the frequency of infection from these viruses could balance the variable and unknown time between the infection and blood sample taken, which creates a ground noise in VirScan data due to the retrospective nature of the study and lack of information about viral infections or vaccinations timing.
When we went to the transcriptome level, we found that tUP correlated with several pathways implicated in immune response and especially B-cell response, thus further corroborating this interpretation of our findings.
Among the limitations of this work, the retrospective nature did not allow to collect the data about the time of infection or vaccination. It is known that antibodies typically reach a peak in weeks after infection and then decrease in months. For influenza, for instance, the peak is reached after 4–7 weeks and drop to pre-infection levels after around 1 year.18
As the antibody profile may depend on the time between infection and sample collection, this could constitute a confounder. Moreover, different exposure and severity of the infection can also play a role.
Finally, due to these limitations and to the lack of a validation cohort, it is essentially a proof-of-concept study.
In perspective, future studies should overcome this limitation by studying the response to a particular virus in the context of a controlled exposure, as it might be the case of vaccination. In this sense, a correlation between a higher humoral response after COVID-19 vaccination and improved outcome under ICBs was seen in a small cohort of patients, thus reinforcing the idea of deducing the immune fitness of the immune system against cancer by studying the immune response to viruses.19 Otherwise, this approach could be of interest in those cancer that are mediated by viral infections, such as Human Papilloma Virus (HPV) positive cancers.
supplementary material
Footnotes
Funding: This study was supported by Fondation Dassault. This work benefited from a French State grant managed by the Agence Nationale de la Recherche, under the third program of investments for the future (PIA), integrated into France 2030, with reference ANR-21-RHUS-0013 (RHU REVEAL).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Project number 08-027, ethical commettee of Kremlin Bicetre for MSN; 1-18-48 / 18.01167.011848-MS01, ethical committee “Sud Ouest et Outre Mer 1”, Toulouse for PREMIS. Participants gave informed consent to participate in the study before taking part.
Data availability free text: Data are available upon request to the corresponding author, after discussion with steering committee of the two studies.
Contributor Information
Filippo G Dall’Olio, Email: Filippogustavo.DALL-OLIO@gustaveroussy.fr.
Wael Salem Zrafi, Email: Wael_Salem.ZRAFI@gustaveroussy.fr.
Quentin Blampey, Email: quentin.blampey@centralesupelec.fr.
Francois-Xavier Danlos, Email: Francois-xavier.danlos@gustaveroussy.fr.
Matthieu Roulleaux-Dugage, Email: Matthieu.ROULLEAUX-DUGAGE@gustaveroussy.fr.
Gabriel Roman, Email: gabriel.roman@cdi-lab.com.
Charles Naltet, Email: cnaltet@ghpsj.fr.
Paul-Henry Cournède, Email: paul-henry.cournede@centralesupelec.fr.
Daniel Gautheret, Email: daniel.gautheret@universite-paris-saclay.fr.
Mihaela Aldea, Email: Mihaela.ALDEA@gustaveroussy.fr.
David Planchard, Email: david.planchard@gustaveroussy.fr.
Fabrice Barlesi, Email: Fabrice.BARLESI@gustaveroussy.fr.
Aurelien Marabelle, Email: aurelien.marabelle@gustaveroussy.fr.
Tyler Hulett, Email: tylerhulett@gmail.com.
Nathalie Chaput-Gras, Email: CHAPUT-GRAS.Nathalie@gustaveroussy.fr.
Benjamin Besse, Email: benjamin.besse@gustaveroussy.fr.
Data availability statement
Data are available upon reasonable request.
References
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50% JCO . 2021;39:2339–49. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takada K, Toyokawa G, Shoji F, et al. The Significance of the PD-L1 Expression in Non-Small-Cell Lung Cancer: Trenchant Double Swords as Predictive and Prognostic Markers. Clin Lung Cancer. 2018;19:120–9. doi: 10.1016/j.cllc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Dall’Olio FG, Marabelle A, Caramella C, et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2022;19:75–90. doi: 10.1038/s41571-021-00564-3. [DOI] [PubMed] [Google Scholar]
- 4.Verster JC, Kraneveld AD, Garssen J. The Assessment of Immune Fitness. J Clin Med. 2022;12:22. doi: 10.3390/jcm12010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu GJ, Kula T, Xu Q, et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science. 2015;348:aaa0698. doi: 10.1126/science.aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan D, Wansley DL, Sie BM, et al. PhIP-Seq characterization of serum antibodies using oligonucleotide-encoded peptidomes. Nat Protoc. 2018;13:1958–78. doi: 10.1038/s41596-018-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larman HB, Zhao Z, Laserson U, et al. Autoantigen discovery with a synthetic human peptidome. Nat Biotechnol. 2011;29:535–41. doi: 10.1038/nbt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mina MJ, Kula T, Leng Y, et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. 2019;366:599–606. doi: 10.1126/science.aay6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monaco DR, Kottapalli SV, Breitwieser FP, et al. Deconvoluting virome-wide antibody epitope reactivity profiles. EBioMedicine. 2022;75:103747. doi: 10.1016/j.ebiom.2021.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradat Y, Viot J, Yurchenko AA, et al. Integrative Pan-Cancer Genomic and Transcriptomic Analyses of Refractory Metastatic Cancer. Cancer Discov. 2023;13:1116–43. doi: 10.1158/2159-8290.CD-22-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traag VA, Waltman L, van Eck NJ. From Louvain to Leiden: guaranteeing well-connected communities. Sci Rep. 2019;9:5233. doi: 10.1038/s41598-019-41695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortellini A, Ricciuti B, Facchinetti F, et al. Antibiotic-exposed patients with non-small-cell lung cancer preserve efficacy outcomes following first-line chemo-immunotherapy. Ann Oncol. 2021;32:1391–9. doi: 10.1016/j.annonc.2021.08.1744. [DOI] [PubMed] [Google Scholar]
- 13.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature New Biol. 2020;577:549–55. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhersecke L, Brunet M, Guégan J-P, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer . 2021;2:794–802. doi: 10.1038/s43018-021-00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Presti D, Dall’Olio FG, Besse B, et al. Tumor infiltrating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit Rev Oncol Hematol. 2022;177:103773. doi: 10.1016/j.critrevonc.2022.103773. [DOI] [PubMed] [Google Scholar]
- 16.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnjatic S, Atanackovic D, Jäger E, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci U S A. 2003;100:8862–7. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Ning Y, Chen MIC, et al. Individual and Population Trajectories of Influenza Antibody Titers Over Multiple Seasons in a Tropical Country. Am J Epidemiol. 2018;187:135–43. doi: 10.1093/aje/kwx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelli F, Giannarelli D, Fabbri A, et al. Immune-related adverse events and disease outcomes after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients receiving immune checkpoint inhibitors. Cancer Immunol Immunother . 2023;72:3217–28. doi: 10.1007/s00262-023-03489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.