Abstract
Introduction
Type 1 diabetes mellitus (T1DM) is a disorder that arises following the selective autoimmune destruction of the insulin-producing beta cells. Beta-cell protective or beta-cell regenerative approaches have gained wider attention, and pharmacological approaches to protect the patient’s own insulin-producing beta-cell mass have been proposed. Verapamil is an L-type calcium channel blocker that has been reported to effectively lowers beta-cell thioredoxin-interacting protein expression in rodent beta cells and islets, as well as in human islets, and thus promotes functional beta-cell mass.
Methods and analysis
The trial is a multicentre, randomised, double-blind, placebo-controlled trial in participants with T1DM, investigating the effect of verapamil on preservation of beta-cell function (Ver-A-T1D). A total of 120 participants will be randomised in a 2:1 ratio between 360 mg verapamil and placebo, administered orally once daily. T1DM patients aged ≥18 and <45 years will be eligible for recruitment within 6 weeks of diagnosis (defined as day of starting insulin therapy). The primary objective will be to determine the changes in stimulated C-peptide response during the first 2 hours of a mixed meal tolerance test at baseline and after 12 months for 360 mg verapamil administered orally once daily versus placebo. Secondary objectives include the effects of 360 mg verapamil on (1) fasting C-peptide, (2) dried blood spot C-peptide, (3) glycated haemoglobin, (4) daily total insulin dose, (5) time in range by intermittent continuous glucose monitoring measures, (6) other biomarkers related to immunological changes and beta-cell death and (6) safety (vital signs, ECG).
Ethics and dissemination
Ethics approval was sought from the research ethics committee of all participating countries. All participants provided written informed consent before joining the study. Ver-A-T1D received first regulatory and ethical approvals in Austria. The publication policy is set in the innovative approach towards understanding and arresting type 1 diabetes grant agreement (www.innodia.eu).
Trial registration number
EudraCT, 2020-000435-45; ClinicalTrials.gov, NCT04545151. Protocol version: Version 8.0 (08 November 2021).
Keywords: DIABETES & ENDOCRINOLOGY, Randomized Controlled Trial, Clinical Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The investigational agent is a repurposed product with a well-established safety profile from over 50 years of use in different indications and, if effective, could be available at low cost.
In contrast to previous treatments developed to alter the disease course in this autoimmune condition, the agent targets the beta cell rather than the immune system directly, and therefore has the potential to be used in combination with immune-modulatory interventions in the future.
The trial is based on a master protocol with standard efficacy and mechanistic outcomes, which has been designed to form the basis of a future platform trial of combined interventions.
A limitation of the study is that it does not include children, which comprise around 40% of the newly diagnosed type 1 diabetes population.
The study will not establish the durability of the intervention, since it only spans 1 year of treatment.
Introduction
Background
Innovative approach towards understanding and arresting type 1 diabetes (INNODIA) is an Innovative Medicines Initiative consortium (IMI-2), established through Horizon 2020 initiative of the European Union, involving academic, industry and charitable partners. Recruitment to this INNODIA study is defined by the recruitment of subjects with a diagnosis of T1D identified within the first 6 weeks from diagnosis. INNODIA provides a standardised routine centralised assessment of critical immunological biological factors which determine the rate of progression of T1D with reference to declines in beta-cell function and the potential impact of investigational medicinal products (IMPs), which could alter these trajectories.
Rationale for the trial
Type 1 diabetes mellitus (T1DM) is a disorder that arises following the selective autoimmune destruction of the insulin-producing beta cells.1 2 A cure for T1DM would aim to ensure that the necessary endogenous functional beta-cell mass required for adequate insulin production is preserved or increased. The Diabetes Control and Complications Trial has shown that even a small amount of preserved endogenous insulin production has beneficial effects in terms of outcome, overall glycaemic control and prevention of severe hypoglycaemia.3,5 Beta-cell destruction is considered to be mainly immune mediated, and many efforts to stop or modify this destruction have focused on immunomodulatory, antigen-specific or anti-inflammatory interventions.6 Attempts to replace beta cells by pancreas or islet transplantation are associated with potentially severe side effects due to the necessary immunosuppression. Recently, beta-cell protective or beta-cell regenerative approaches have gained wider attention, and pharmacological approaches to protect the patient’s own insulin-producing beta-cell mass have been proposed.6
Pharmacokinetics and pharmacodynamics of verapamil
Verapamil (ATC: C08DA01) is an L-type calcium channel blocker that has been used as an antihypertensive compound for more than three decades and approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Verapamil effectively lowers beta-cell thioredoxin-interacting protein (TXNIP) expression in rodent beta cells and islets, as well as in human islets. This effect is based on the established mode of action of verapamil, blockade of L-type calcium channels and the resulting decrease in intracellular free calcium leading to inhibition of TXNIP transcription. In mouse models of diabetes, oral administration of verapamil promotes functional beta-cell mass and prevents and even reverses overt diabetes. In addition, downregulation of TXNIP also improves beta-cell function, including insulin production and secretion.7,13 In a randomised, double-blind, placebo-controlled phase II clinical trial, the efficacy and safety of oral verapamil added for 12 months to a standard insulin regimen in adult subjects with recent-onset T1D was assessed. Verapamil treatment, compared with placebo, was well tolerated and associated with an improved mixed meal-stimulated C-peptide area under the curve (AUC), a measure of endogenous beta-cell function, at 3 and 12 months, as well as with a lower increase in insulin requirements and fewer hypoglycaemic events.14 2-year follow-up of this study has since been published suggesting a continued effect,15 and the beneficial effect on C-peptide preservation has been replicated in a study in children and adolescents.16
Several retrospective studies have reported that verapamil use is associated with a lower risk of developing type 2 diabetes.17 18 Recently, it has been demonstrated that TXNIP, a cellular redox regulator, is overexpressed during hyperglycaemia and induces beta-cell apoptosis.10 As verapamil effectively lowers beta-cell TXNIP expression in rodent beta cells and islets, as well as in human islets, it therefore promotes functional beta-cell mass and prevents and even reverses overt diabetes.19
Verapamil inhibits the entry of calcium into smooth muscle cells of the systemic and coronary arteries and in the cells of cardiac muscle and the intracardiac conduction system. Verapamil lowers peripheral vascular resistance with little or no reflex tachycardia. Its efficacy in reducing both raised systolic and diastolic blood pressure is thought to be primarily due to this mode of action. Due to the effect on the movement of calcium in the intracardiac conduction system, verapamil reduces automaticity, decreases conduction velocity and increases the refractory period. This may cause the following cardiovascular side effects: bradycardic arrhythmias such as sinus bradycardia, sinus arrest with asystole, second-degree and third-degree atrioventricular (AV) block, bradycardia in atrial fibrillation, palpitations, tachycardia, development or aggravation of heart failure and hypotension.
After oral administration, verapamil is well absorbed (more than 90%), but undergoes extensive first-pass hepatic metabolism so that bioavailability is only 10%–23%. Verapamil is metabolised to several active and inactive metabolites. Most of the metabolites are excreted in bile. The most common side effects of verapamil are dose dependent and include constipation, dizziness, nausea, low blood pressure and headache. Other side effects seen include: oedema, congestive heart failure, pulmonary oedema, fatigue, elevated liver enzymes, shortness of breath, low heart rate, AV block, rash and flushing.
The target dose of 360 mg once daily and the minimum target dose of 240 mg were chosen according to the study of Ovalle et al.14 In this already published randomised, double-blind, placebo-controlled phase II clinical trial, the participants were randomly assigned to receive a once daily oral dose of sustained-release verapamil (titrated over the first 3 months from 120 to 360 mg) or placebo for a total of 12 months in addition to their insulin therapy. This dose was chosen according to its demonstrated tolerability and effectiveness in terms of calcium channel blockade and considering that the maximal recommended daily dose for verapamil is 480 mg. The rationale for the selected target dose in the current trial is based on the efficacy demonstrated in this trial.14
The Ver-A-T1D trial is conducted using the INNODIA Master Protocol within the INNODIA clinical trial network (www.INNODIA.eu).20 The aim of this trial is to confirm the effect of 360 mg verapamil administered orally once daily (titrated over the first 3 months from 120 to 360 mg) on the preservation of stimulated C-peptide at 12 months compared with placebo.
Methods and analysis
Objectives
The primary objective of Ver-A-T1D is to determine the changes in stimulated C-peptide response during the first 2 hours of a mixed meal tolerance test (MMTT) at baseline and after 12 months for 360 mg verapamil administered orally once daily versus placebo in adult people with new-onset T1D. The secondary objectives are to determine the effect of 360 mg verapamil administered orally once daily on (1) fasting C-peptide and dried blood spot (DBS) C-peptide measurements, (2) glycated haemoglobin (HbA1c), (3) daily total insulin dose, (4) continuous glucose monitoring (CGM) time in range over time, (5) to determine the effects of treatment on other biomarkers related to immunological changes and beta-cell death and survival in this population and (6) to determine the effects of 360 mg verapamil administered orally once daily on safety (vital signs, ECG). The tertiary objective will compare, between treatment arms and across the course of treatment, the patient-reported outcome measures (PROMs) scores completed by participants. Table 1 reports the specific trial objectives and related outcome measures.
Table 1. Study objectives and outcomes.
| Objectives | Outcome measures | Timepoints(s) of evaluation of outcome measure |
| Primary objective | ||
| (1) To determine the changes in stimulated C-peptide response during the first 2 hours of a mixed meal tolerance test (MMTT) at baseline and after 12 months for 360 mg verapamil sustained release (SR) administered orally once daily versus placebo. | The area under the stimulated C-peptide response curve over the first 2 hours of an MMTT for the verapamil 360 mg and placebo arms. | 12 months |
| Secondary objectives | ||
| (2) To determine the effects of 360 mg verapamil SR administered orally once daily on fasting C-peptide and dried blood spot (DBS) C-peptide measurements over time. | Fasting C-peptide after 12 months therapy compared with placebo and home DBS for C-peptide. | Baseline, 1, 2, 3, 6, 9 and 12 months |
| (3) To determine the effects of 360 mg verapamil SR administered orally once daily on glycated haemoglobin (HbA1c), daily total insulin dose and continuous glucose monitoring (CGM) time in range. | Change in HbA1c baseline to 12 months, change in HbA1c baseline to 12 months, change in insulin requirements, baseline to 12 months as the daily total dose (3 days average) in units per kg body weight, CGM time in range (70–140 mg/dL, 3.9–7.8 mmol/L) and (70–180 mg/dL, 3.9–10.0 mmol/L), time above range (>180 mg/dL, >10.0 mmol/L), time below range (<70 mg/dL, <3.9 mmol/L) | Baseline and monthly from 1 to 12 months |
| (4) To determine the effects of treatment on other biomarkers related to immunological changes and beta-cell death and survival in this population. | ||
| (5) To determine the effects of 360 mg verapamil SR administered orally once daily on safety (vital signs, ECG). | Systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), pulse (bpm), ECG | Baseline, 1, 2, 3, 6, 9 and 12 months |
| Tertiary objective | ||
| (6) To compare between treatment arms and across the course of treatment, the patient-reported outcome measures scores completed by participants. | Diabetes Treatment Satisfaction Questionnaire (DTSQ), Audit of Diabetes-Dependent Quality of Life (ADDQoL), HypoFear questionnaires | Hypoglycaemia Fear Survey and DTSQ at 1, 6 and 12 months ADDQoL at 6 and 12 months |
Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines were used for this protocol.21
Study summary
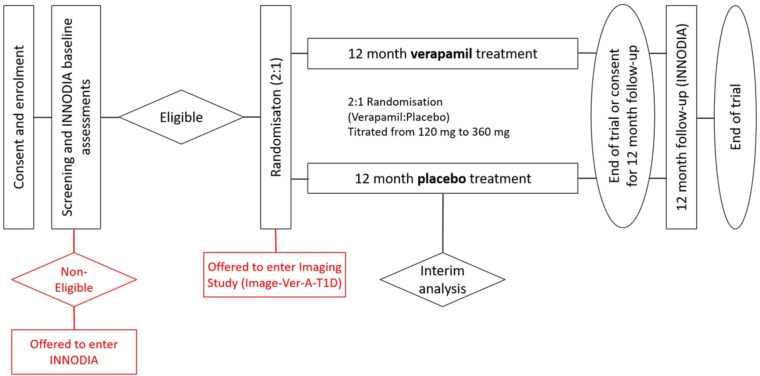
Ver-A-T1D is a multicentre, randomised, double-blind, placebo-controlled trial testing the efficacy of 360 mg verapamil administered orally once daily (titrated over the first 3 months from 120 to 360 mg) on protection of stimulated C-peptide decline in subjects with diagnosis of T1D within 6 weeks of diagnosis. The Ver-A-T1D trial design is shown in figure 1.
Figure 1. Ver-A-T1D trial design. INNODIA, innovative approach towards understanding and arresting type 1 diabetes.
A multicentre and multinational design has been chosen to ensure that the results are applicable for participants with different demographic characteristics. All sites are part of the existing INNODIA clinical network and are confirmed suitable for undertaking this specific study from the accreditation undertaken as part of INNODIA. However, to recruit the required number of participants, suitable INNODIA sites may work with their local existing network to identify and recruit potential participants. Additional sites in the UK that are part of the UK Type 1 Diabetes Research Consortium and can perform studies to appropriate standards consistent with the INNODIA platform will also be considered.
Further details on participating sites can be obtained from the Ver-A-T1D Coordinating team contact (ver-a-t1d@medunigraz.at) and via the INNODIA web page (innodia.eu - Clinical Trials).
Trial participants, study design and oversight
The aim is to randomise 138 participants in this trial to two arms, namely verapamil and placebo in a 2:1 allocation ratio, that will compensate for an estimated dropout rate of 15%. It is anticipated that approximately 230 participants will be required to be screened (approximately 60% consent rate), with 40 participants on the control arm and 80 on the experimental arm (a total of 120 subjects) are expected to complete the trial.
The rationale for the trial design is to investigate the effect of 360 mg verapamil administered orally once daily (titrated over the first 3 months from 120 mg to 360 mg) on preservation of beta-cell function compared with placebo at week 52 in adult subjects with newly diagnosed T1DM with residual beta-cell function. The duration of the trial with 52 weeks of exposure of 360 mg verapamil administered orally once daily (titrated over the first 3 months from 120 mg to 360 mg) has been chosen to align with the regulatory requirements from FDA and EMA. The FDA and EMA guidelines advise that studies of products aimed at preservation of beta-cell function in recent-onset T1DM with remaining endogenous insulin reserve, should evaluate metabolic outcomes, such as stimulated C-peptide levels. Therefore, the area under the stimulated C-peptide response curve over the first two hours of a mixed meal tolerance test (MMTT) has been chosen as the primary efficacy outcome.
The day-to-day management of the trial is the responsibility of the trial management group (TMG). The chief investigator will be responsible for the preparation and submission of annual safety reports and annual progress reports.
Trial steering committee (TSC)
The sponsor will constitute a TSC to provide the overall supervision of the trial. The TSC will monitor the trial progress, the safety data, the critical efficacy endpoints and conduct and advise on scientific credibility. The TSC will ultimately carry the responsibility for deciding whether a trial needs to be stopped on grounds of safety or efficacy. The TSC may recommend unblinding of any data for further analysis.
The TSC will consider recommendations from the independent data monitoring committee (IDMC). The TSC will decide whether to modify the trial or to seek additional data.
Independent data monitoring committee (IDMC)
The IDMC charter will detail the purpose of this committee including: the description of the membership, terms of reference, roles, responsibilities, authority, decision-making and relationships of the IDMC for this trial. The charter will further include the timing of meetings, methods of providing information to and from the IDMC, frequency and format of meetings, statistical issues and relationships with other committees. Briefly, it is planned that one interim analysis will be undertaken during this trial and considering these interim analyses and safety endpoints, the IDMC will advise the TSC of its recommendations regarding trial modification, continuation or termination of the trial. The IDMC charter will expand on the above.
Trial management group (TMG)
The TMG comprise investigators and individuals closely involved in running of the trial. The TMG aims to meet more frequently than the TSC to ensure that all practical details of the trial are progressing well.
Patient and public involvement
A clear priority of INNODIA is to keep the needs and concerns of patients with type 1 diabetes at the centre of the project. Ver-A-T1D involvement of patients is organised by a patient advisory committee (PAC). The specific activities of the PAC are to advise the Management Board of INNODIA on areas including informed consent (IC), clinical protocol review and relationships with regulatory authorities. In addition, the PAC members act as T1D ambassadors, helping to communicate results to the wider public across 15 European countries.
More information can be found at the INNODIA webpage (https://www.innodia.eu/pac/).
Inclusion and exclusion criteria
Box 1 lists the study’s inclusion and exclusion criteria. Potential participants may not enter the trial if any of the exclusion criteria listed in box 1 applies.
Box 1. Eligibility criteria.
Inclusion criteria
Have given written informed consent.
Age≥18 and <45 years at consent.
Must have a diagnosis of type 1 diabetes mellitus of within 6 weeks duration at screening (from date of the first insulin injection).
Must have at least one or more of the following diabetes-related autoantibodies present at screening: glutamic acid decarboxylase antibodies, IA-2 antibodies and/or Zinc transporter 8 antibody.
Must have fasting C-peptide levels≥100 pmol/L measured at screening.
Be willing to comply with intensive diabetes management.
Exclusion criteria
Be immunodeficient or have clinically significant chronic lymphopenia: Leucopenia (<3000 leucocytes /µL), neutropenia (<1500 neutrophils/µL), lymphopenia (<800 lymphocytes/µL) or thrombocytopenia (<100 000 platelets/µL).
Have active signs or symptoms of acute infection at the time of screening.
Be currently pregnant or lactating, or anticipate getting pregnant during the 12 months study period.
Require use of immunosuppressive agents, including chronic use of systemic steroids.
Have evidence of current or past HIV, hepatitis B or hepatitis C infection.
Have any complicating medical issues or abnormal clinical laboratory results that may interfere with study conduct, or cause increased risk to include pre-existing cardiac disease, chronic obstructive pulmonary disease, sickle cell disease, neurological or blood count abnormalities, as judged by the investigator.
Have persistent history of malignancies other than skin.
History of liver insufficiency or laboratory evidence of liver dysfunction with aspartate aminotransferase or alanine transaminase greater than three times the upper limits of normal.
History of renal insufficiency or evidence of renal dysfunction with creatinine greater than 1.5 times the upper limit of normal
Current or ongoing use of non-insulin pharmaceuticals that affect glycaemic control within prior 7 days of screening.
Use of any other investigational drug in the previous 30 days and/or intent on using any investigational drug for the duration of the trial.
Current use of verapamil or other calcium channel blockers.
Known hypersensitivity to verapamil or to any of its excipients.
Concomitant medication known for inducing or inhibiting CYP3A4 and/or P-glycoprotein metabolism.
Intake of grapefruit juice, liquorice, St. John’s Wort, cannabidiol, Ginkgo biloba.
Substrate intake of CYP3A4 and/or P-glycoprotein metabolism, as judged by the investigator.
Hypotension (of less than 100 mm Hg systolic), sick sinus syndrome (except patients with a functioning artificial pacemaker), uncompensated heart failure or severe left ventricular dysfunction; marked bradycardia (less than 50 beats/min), atrial flutter or atrial fibrillation in the presence of an accessory bypass tract (eg, Wolff-Parkinson-White syndrome), hypertrophic cardiomyopathy, acute myocardial infarction, attenuated neuromuscular transmission (eg, by myasthenia gravis, Lambert-Eaton syndrome, advanced Duchenne muscular dystrophy).
ECG second-degree or third-degree atrioventricular block.
Any condition that in the investigator’s opinion may adversely affect study participation or may compromise the study results.
Current use of beta-blockers.
Trial procedures
The study procedures are reported in detail in online supplemental information 1, and the trial flowchart is shown in figure 2. The trial duration will be approximately 24 months, consisting of screening, randomisation, 12 months treatment period and an additional 12 months INNODIA follow-up. Throughout the trial, investigators work in accordance with ICH (International Council for Harmonisation) good clinical practice (GCP) and local regulations and ensure that trial procedures are performed as described in the protocol. Any discrepancies that result in protocol and/or GCP deviations, the investigator takes appropriate action to avoid recurrence of the detected discrepancies. If deviations do occur, the investigator must inform the monitor and the implications of the deviation must be reviewed and discussed. Deviations are documented and explained in a protocol deviation by stating the reason, date and the action(s) taken (if applicable).
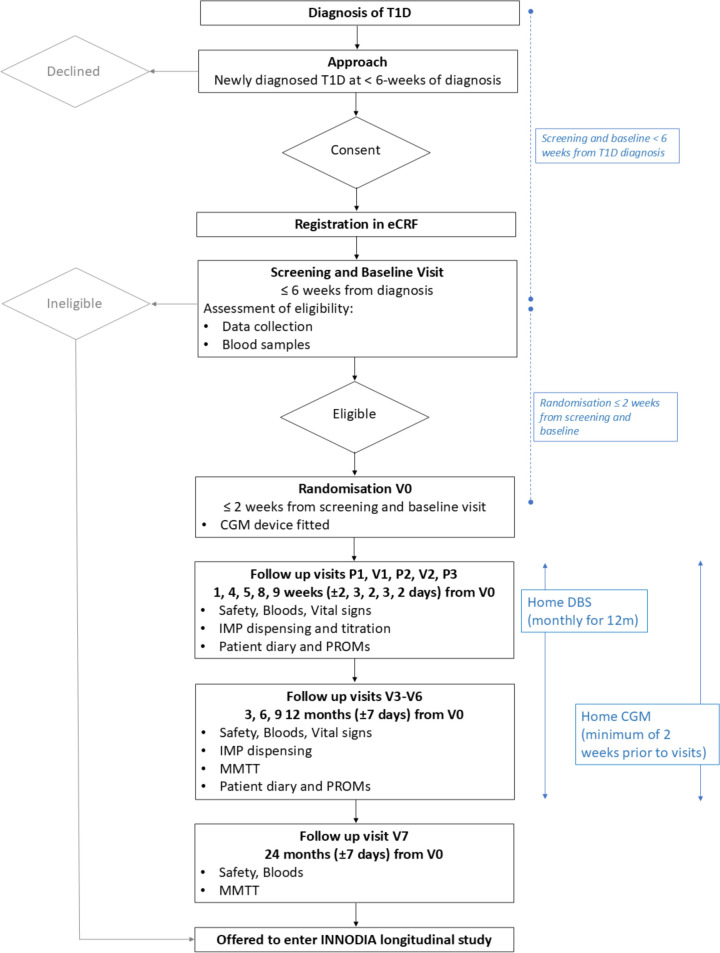
Figure 2. Ver-A-T1D trial flowchart. CGM, continuous glucose monitoring; DBS, dried blood spot; eCRF, electronic case report form; IMP, investigational medicinal product; MMTT, mixed meal tolerance test; INNODIA, innovative approach towards understanding and arresting type 1 diabetes; PROMs, patient-reported outcome measures; T1DM, type 1 diabetes mellitus.
The timing of the assessments and procedures are specified in the trial flowchart (figure 2) and detailed in online supplemental information 1. A subject screening log, a subject identification code list and a subject enrolment log are kept by the investigator and may be combined in one list. Additional logs kept include a prescreening log and staff and delegations of task(s) at sites. The investigator signs off the log of staff and the delegation of task(s) at site at the time of delegation. Protocol waivers or exemptions are not allowed. Immediate safety concerns should be discussed with the sponsor immediately on occurrence or awareness to determine if the participant should continue or discontinue study treatment.
Adherence to the study design requirements, including those specified in the trial flowchart (figure 2), is essential and required for study conduct. All screening evaluations must be completed and reviewed to confirm that potential participants meet all eligibility criteria. The investigator will maintain a screening log to record details of all participants screened and to confirm eligibility or record reasons for screening failure, as applicable. Procedures conducted as part of the participant’s routine clinical management (eg, blood count) and obtained before signing of the ICF may be used for screening or baseline purposes provided the procedures met the protocol-specified criteria and were performed within the time frame defined in the trial flowchart (figure 2). Throughout the trial, a maximum of 3 mL per kg of blood will be taken at each visit; no more than 204 mL per visit maximum. Repeated or unscheduled samples may be taken for safety reasons or for technical issues with the samples.
Participants who fail to satisfy eligibility criteria may be offered participation in the INNODIA longitudinal study (figures1 2).
Participant identification
Potentially eligible individuals are approached by healthcare professionals and/or local research teams during routine clinical appointment. The study is also advertised by poster and flyers in diabetes clinics, on social media, for example, Facebook and Twitter, via INNODIA or local sites own pages, on the INNODIA website, UK T1D Research Consortium website and other diabetes related websites and via newspaper if applicable. All potential individuals approached or that have contacted the research teams are provided with a verbal explanation of the study and written information sheets. Once they have been given sufficient time to consider their participation in the study, consent will be obtained.
Informed consent (IC)
The informed consent form (ICF) has been approved by the local ethics committee (EC) and complies with GCP, local regulatory requirements and legal requirements and can be found in the online supplemental information 2. The investigator or designee must ensure that each trial participant, or their legally acceptable representative, is fully informed about the nature and objectives of the trial and possible risks associated with their participation. The investigator or designee will obtain written IC from each participant or the participant’s legally acceptable representative before any trial-specific activity is performed. The ICF used for this trial and any change made during the trial, must be prospectively approved by the research ethics committee (REC). The investigator will retain the original of each participant signed ICF and a copy will be provided to the participant.
IC is sought on joining the study to confirm that participants are happy to be contacted to inform them of study results and future intervention and research diabetes studies organised by INNODIA. After completion of treatment at 12 months from diagnosis, participants will continue for a further 12 months in the observation part of the study. This will involve a single visit 24 months from randomisation. During IC, the investigator will explain the nature of the study to the participant and answer all questions regarding the study. Participants must be informed that their participation is voluntary and will be required to sign a statement of IC that meets the requirements of local regulations, ICH guidelines and the independent ethics committee (IEC) or study centre. The medical record must include a statement that written IC was obtained before the participant was enrolled in the study and the date the written consent was obtained. The authorised person obtaining the IC must also sign the ICF. A copy of the ICF(s) must be provided to the participant or the participant’s legally authorised representative.
Where participants require a verbal translation of the trial documentation by a locally approved interpreter/translator, it is the responsibility of the individual investigator to use locally approved translators. If the trial requires documentation in a different language (other than English) the translation and back translation documents need to be reviewed and approved by the sponsor prior to use with all sections of the approved documents must appear in the translation. The translated version must be appropriately dated and version controlled. Any new information that becomes available, that might affect the participant’s willingness to continue participating in the trial will be communicated to the participant as soon as possible. Participants must be reconsented to the most current version of the ICF(s) during their participation in the study.
Any new information which becomes available, which might affect the participant’s willingness to continue participating in the trial will be communicated to the participant as soon as possible and participants must be reconsented where an updated ICF(s) might impact on their decision to remain in the study.
Registration
Following IC, the participant will be registered on the INNODIA central database using deidentifiable information only and a participant ID generated. All identifiable information such as full name, contact details and date of birth will be registered locally following local policies and regulations. Participant eligibility for INNODIA Master protocol will be recorded at this stage, as well as gender and ethnicity.
Screening and baseline assessments for those who are eligible and have consented
The screening and baseline visit should be carried out within less than 6 weeks of the date of first insulin injection. Trial specific assessments will only be conducted after participants have given written IC and must be in place before the participant initiates fasting prior to the screening visit. Study procedures and their timing are summarised in figure 2.
Assessments performed at screening and baseline are as follows: demographics (age, gender, ethnicity), date of T1D diagnosis (date of first insulin dose), HbA1c at diagnosis, daily insulin regimen at time of visit, blood glucose at time of visit, physical examination (including height and weight), medical history, diabetes care, concomitant medication, including vaccinations in last 6 weeks, family medical history, ECG and vital signs. Additionally, for women of childbearing potential, if applicable, a pregnancy test will be performed according to local requirements (urine pregnancy test or serum pregnancy test). Blood will be collected for the following screening assessments: Fasting C-peptide, autoantibodies (glutamic acid decarboxylase antibodies, insulin auto-antibodies, IA-2 antibodies or Zinc transporter 8 antibody), safety lab (including full blood count, complete metabolic profile) and HIV, hepatitis B and C.
At the same visit, the following INNODIA baseline samples will be collected from all screened participants: DNA extraction, HbA1c, omics, beta-cell killing assay, whole blood RNA, microRNA (plasma omics), immune cells (peripheral blood mononuclear cell (PBMC)), urine (omics, including microbiome analysis), stool (omics, including microbiome and metabolome analysis).
Following review of the laboratory results from the screening samples by the local medical team, participants will be declared eligible or non-eligible for the clinical trial. If any inclusion criteria are answered no or any exclusion criteria are answered yes, the subject is a screening failure. For screening failures, the screening failure form in the electronic case report form (eCRF) must be completed with the reason for not continuing in the trial. Resampling or rescreening is not allowed if the subject has failed one of the inclusion criteria or meets one of the exclusion criteria related to laboratory parameters. However, if a lab test at the screening visit is inconclusive, a retest can be performed. The repeat test results must be available for evaluating the subject’s eligibility before randomisation. Eligible participants will be invited for the randomisation visit (V0) and asked to attend the visit fasting (from midnight). Non-eligible participants will be informed of the results of the screening visit and explained the reason for non-eligibility and invited to join the INNODIA longitudinal study.
Samples collected in the study as part of the INNODIA Clinical Trial Master Protocol will be stored and analysed as described in the Master Protocol and outlined in the online supplemental information 3. Additional samples collected that are in the same nature, for example samples from additional MMTT or for additional immune cell studies, will be stored and analysed according to the INNODIA Clinical Trial Master Protocol.
Randomisation and blinding
The trial is double-blind, randomisation is carried out for all eligible participants using a web-based platform (Randomizer) at Medical University of Graz (MUG). At the randomisation visit (V0) participants meeting all inclusion criteria and none of the exclusion criteria will be assigned a unique participant ID number and centrally randomised to one of the two parallel treatment groups in a 2:1 ratio (verapamil 360 mg:placebo) titrated from: day 0 to week 4, 120 mg once daily; week 4 to week 8, 240 mg once daily; week 8 to month 12, 360 mg once daily. Placebo (matching verapamil 360 mg) will be titrated in the same manner. Trial participants and research teams are blinded to the treatment group for the duration of the trial. The double blinding will be achieved by providing verapamil-identical placebo tablets.
The randomisation software is programmed with blind-breaking instructions. In case of an emergency, an investigator has the responsibility for determining if unblinding of a participants’ treatment assignment is warranted. Participant safety must always be the first consideration in making such a determination. If the investigator decides that unblinding is warranted, the investigator should make every effort to contact the sponsor and medical monitor prior to unblinding a participant’s treatment assignment, unless this could delay emergency treatment of the participant. If a participant’s treatment assignment is unblinded, the sponsor must be notified within 24 hours after breaking the blind. The date and reason that the blind was broken must be recorded in the source documentation and eCRF, as applicable. When the code is broken, the treatment allocation will be accessible to the investigator and the TMG. If the code has been broken, the subject must be withdrawn from the trial and a withdrawal session must be completed in the eCRF.
Trial participants attend the randomisation visit fasted and have a 120 min MMTT with Ensure Plus for measuring C-peptide and glucose as a measurement of beta-cell response. Additional assessments include physical examination, vital signs and HbA1c. Capillary glucose and DBS will be collected at home before and 60 min after consumption of Ensure Plus, monthly, for the full 12-month follow-up for DBS C-peptide measurement. Participants will be set up with a continuous glucose monitor (CGM) and handed out a patient diary before leaving the clinical research facility.
Subsequent assessments: follow-up visits 1 and 2
The schematic representation of assessments at study visits can be found in online supplemental information 1. This table details assessments at all follow-up visits. Participants are assessed for adverse events (AEs), withdrawal criteria, concomitant medication including vaccination, safety lab, ECG, vital signs, pregnancy test (if applicable), DBS, IMP dispensing, dose titration to 240 mg at visit 1 and 360 mg at visit 2, CGM and patient diary reviews, diabetes care, PROMs, fasting C-peptide and HbA1c.
Subsequent assessments: follow-up visits 3–6
AE, withdrawal criteria, concomitant medication including vaccination, safety lab, ECG, DBS, IMP dispensing, CGM and patient diary review, diabetes care, vital signs, pregnancy test (if applicable), physical examination (height, weight). Additional INNODIA assessments include family medical history, MMTT (including fasting C-peptide and blood glucose), HbA1c, blood beta-cell killing, blood (omics), mircoRNA (plasma omics), immune cells (PBMC), urine (biomarkers) and stool (microbiome, metabolome).
Women of childbearing potential are required to use adequate contraception for the duration of the trial and for 7 days after the completion of last treatment (visit 6). This includes intrauterine device, hormonal based contraception (pill, contraceptive injection or implant etc), barrier contraception (condom or occlusive cap, eg, diaphragm or cervical cap with spermicide), true abstinence (where this is in accordance with the participant’s preferred and usual lifestyle). Men are required to use adequate contraception for the entire duration of the trial and for 7 days after the completion of the last treatment. This includes barrier contraception (condom and spermicide) or true abstinence (where this is in accordance with the participant’s preferred and usual lifestyle).
Subsequent assessments: phone visits 1–3
Phone visits 1–3 occur at 1 week ±2 days, 5 weeks ±2 days and 9 weeks ±2 days post treatment start. AEs, withdrawal and criteria and concomitant medication including vaccinations are recorded.
Long-term assessment: follow-up visit 7
At 24 months participants will be assessed for AEs, diabetes care and safety lab. Additional INNODIA assessments include MMTT (including fasting C-peptide and blood glucose), HbA1c, autoantibodies, blood beta-cell killing, blood (omics), whole blood RNA, RNA (plasma omics), immune cells (PBMC), urine (biomarkers) and stool (microbiome, metabolome).
End of trial participation
A participant is considered to have completed the study if he/she has completed all phases of the study, including the last visit. The end of the study is defined as the date of the last visit of the last participant in the study in the trial globally. Participants will be expected to continue normal standard of care during the trial period and following their participation in the trial.
Early discontinuation/withdrawal of participants
Participants may terminate participation in the study at any time. An investigator can stop the participation of a participant after consideration of the benefit/risk ratio. Possible reasons are (1) serious AEs (SAEs); (2) treatment emergent side effects, that do not allow dose escalation to 240 mg verapamil or placebo; (3) non-compliance with the study protocol; (4) technical grounds (eg, patient moves) or (5) early termination at the request of the chief investigator/principal investigator (PI) or principal coinvestigator.
Participants may withdraw without necessarily giving a reason, without any personal disadvantage and without affecting their usual patient care. Withdrawal and permission to retain samples and data already collected will be documented in the eCRF. Withdrawal by an investigator and the permission to retain sample and data already collected will be clearly documented in the eCRF and will not affect usual patient care. In rare instances, it may be necessary for a participant to permanently discontinue (definitive discontinuation) study intervention. If the study intervention is definitively discontinued, the participant will remain in the study to be evaluated for follow-up assessments.
A participant will be considered lost to follow-up if he or she repeatedly fails to return for scheduled visits and is unable to be contacted by the study site. The following actions must be taken if a participant fails to return to the clinic for a required study visit: the site must attempt to contact the participant and reschedule the missed visit as soon as possible and counsel the participant on the importance of maintaining the assigned visit schedule and ascertain whether the participant wishes to and/or should continue in the study. Before a participant is deemed lost to follow-up, the investigator or designee must make every effort to regain contact with the participant (where possible, two telephone calls or local equivalent methods). These contact attempts should be documented in the participant’s medical record. Should the participant continue to be unreachable, they will be considered to have withdrawn from the study. Participants who are withdrawn will not be replaced.
Verapamil 120 mg preparation, dose and administration
Verapamil is an L-type calcium channel blocker, that has been approved by the US FDA and the EMA.
Participants of the trial randomised to verapamil will receive verapamil 120 mg tablets at the following visits: V0, V1, V2, V3, V4, V5. Instruction on oral administration will happen at each visit. Participants will be instructed to take all IMP dose once daily at approximately the same time. Participants having mild side effects such as dizziness or hypotension may be advised to take it in the evening before sleep. Female participants are instructed to not dose IMP before a urine pregnancy test has been ruled out.
All participants will initiate 120 mg verapamil or 120 mg placebo treatment on the day of randomisation. As the target dose is 360 mg verapamil or placebo, the dose will be escalated in increments of 120 mg verapamil or placebo every month until 360 mg verapamil or placebo has been reached. In cases where participants suffer intolerable verapamil side effects related to the dose escalation, it is acceptable to maintain the current verapamil dose and postpone escalation by 1 month. If 360 mg verapamil or placebo is not tolerated due to side effects, the dose can be reduced to 240 mg verapamil or placebo, which is the lowest acceptable dose. In cases where 240 mg verapamil or placebo is not tolerated, the subject must be withdrawn.
The investigator or designee must confirm appropriate temperature conditions have been maintained during transit for all study medication received and any discrepancies are reported and resolved before use of the study medication. Only participants enrolled in the study may receive study medication and only authorised site staff may supply or administer study medication. All study medications must be stored in a secure, environmentally controlled and monitored (manual or automated) area in accordance with the labelled storage conditions with access limited to the investigator and authorised site staff. The investigator or designee must confirm appropriate temperature conditions have been maintained during transit for all study medication received and any discrepancies are reported and resolved before use of the study medication.
Further guidance and information for the final disposition of unused study interventions are provided in the pharmacy manual online supplemental information 4.
Placebo
Participants randomised to placebo will receive placebo tablets identical to verapamil that will be labelled as required per country requirement, labels will be blinded and provided centrally by the sponsor, MUG.
Known drug reactions and interaction with other therapies
Drug–drug interactions:
These known drug–drug interactions, selected for relevance of the Ver-A-T1D trial, see DRUGBANK Online for a complete list.
Atorvastatin: the serum concentration of verapamil can be increased when it is combined with atorvastatin.
Dasiglucagon: verapamil may increase the hypotensive activities of dasiglucagon.
Fenofibrate: the metabolism of fenofibrate can be decreased when combined with verapamil.
Fluvastatin: the metabolism of fluvastatin can be decreased when combined with verapamil.
Gemfibrozil: the metabolism of verapamil can be decreased when combined with gemfibrozil.
Insulin: the risk or severity of hypoglycaemia can be increased when verapamil is combined with insulin.
Lovastatin: the risk or severity of myopathy and rhabdomyolysis can be increased when verapamil is combined with lovastatin.
Magnesium: magnesium can cause a decrease in the absorption of verapamil resulting in a reduced serum concentration and potentially a decrease in efficacy.
Pravastatin: the serum concentration of pravastatin can be increased when it is combined with verapamil.
Rosuvastatin: the metabolism of rosuvastatin can be decreased when combined with verapamil.
Simvastatin: the risk or severity of myopathy and rhabdomyolysis can be increased when verapamil is combined with simvastatin.
Regarding interaction with HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase inhibitors (statins) via the CYP3A4 pathway, rosuvastatin, pravastatin and fluvastatin are labelled as ‘non-3A4 substrate’, thus have only minimal metabolism via the cytochrome P450 system.22 23
Advice on SARS-CoV-2/COVID-19 for the Ver-A-T1D trial
Patients may be exposed to the risk of COVID-19 transmission and infection in relation to site visits if an outbreak is ongoing in the country concerned at the time of trial conduct. The risk of COVID-19 transmission in relation to site visits is overall considered to be low. To minimise the risk, the following measures may be taken if appropriate:
The number of physical on-site visits has been limited to the extent possible.
On-site visits will be well prepared and as short as possible. Physical contact between study participants and site staff will be limited to the extent possible, and protective measures will be implemented (mouth and nose protectors will be used by both site staff and study participants).
Before entering the clinic, subjects will have a body temperature check and a symptom screening (coughing, shortness of breath, fever).
The use of a SARS-CoV-2 vaccine in patients treated with verapamil 120 mg has not been studied.
Given the risk posed by COVID-19 during the pandemic, however, decisions regarding the use of any vaccination, including approved/authorised for use SARS-CoV-2 vaccines, in patients treated with verapamil 120 mg should be made at the discretion of the investigator using their best clinical judgement and after careful consideration of risk benefit factors for the patient. The investigator must consult the vaccine product label for further information regarding associated risks and precautions, and also guidance from local regulatory agencies. Hypersensitivity events have been reported in association with certain vaccines, in close temporal relation to the application.
Applicable information regarding an individual’s receipt of vaccination(s) must be documented in the participant’s source documents and each administration date of the vaccine (each time) recorded as a concomitant medication in the eCRF. Any possible related AEs from the vaccination should be reported according to the AE/SAE reporting guidance and to the appropriate manufacture, according to local practice.
The sites must assess this situation on an ongoing basis and must provide real time feedback to the sponsor if there is the potential to impact clinical research operations or if conditions at the site have the potential to impact the ability to monitor either the safety of participants or the scientific integrity of study(ies) at the site.
Assessment of diabetes and appropriate attention to the standard of care treatment will be provided throughout the trial. It is therefore concluded that the potential benefits from the trial will outweigh the potential risks for the verapamil 120 mg, as well as placebo treated patients. Based on the risk assessment, the evaluation of COVID-19 and the implemented measures, the residual risk for study participants is considered low.
Concomitant treatments
Concomitant medications will be assessed and recorded at each trial and phone visit. Participants will continue their current insulin treatment after they have been randomised, and it is preferred that participants continue the same type of insulin treatment throughout the trial. Participants will be trained in diabetes self-care including carbohydrate counting before and at randomisation and whenever needed during the trial to achieve the most optimal diabetes control according to local standard of care. During the trial participants will receive insulin treatment to achieve metabolic control according to the local insulin titration guideline. Bolus and/or basal insulin can be stopped or paused at all times during the trial at the discretion of the investigator. The participant’s need for bolus insulin will be documented in the eCRF.
During the MMTT no rapid or short-acting insulin will be given, the use of rapid-acting insulin is acceptable up to 2 hours before the MMTT and the use of short-acting insulin up to 6 hours before the MMTT, to correct hyperglycaemia. Long-acting insulin and basal rates on an insulin pump will not be discontinued during the MMTT. During the DBS home collection, short-acting or rapid-acting insulin should not be used until the end of the collection.
Any permitted medication or vaccine (including over the counter or prescription medicines, vitamins and/or herbal supplements) that the subject is receiving at the time of enrolment or receives during the study are recorded along with, reason for use, dates of administration including start and end dates and dosage information (dose and frequency). The use of non-insulin pharmaceuticals that affect glycaemic control, alpha-blockers, beta-blockers, cardiac glycosides, antiarrhythmics, ivabradine, lithium, sulfinpyrazone, almotriptan and acetylsalicylic acid is prohibited for the trial duration. Concomitant medication known for inducing or inhibiting CYP3A4 and/or P-glycoprotein metabolism are also prohibited.
Compliance with trial treatment
Compliance with study intervention will be assessed at each visit by the investigator or designee and is assessed by counting returned tablets during the site visits and documented in the source documents and eCRF. Deviation(s) from the prescribed dosage regimen are recorded in the eCRF. A record of the number of study tablets dispensed and taken by each participant is maintained and reconciled with study drug accountability and compliance records. Treatment start and stop dates, including dates for treatment delays and/or dose reductions, will also be recorded in the eCRF.
Missed and unscheduled visits
If a visit is missed, every effort is made to ensure information is collected and participants will be invited for the next scheduled visit according to the visit schedule. An unscheduled visit can be scheduled at any time at the discretion of the investigator, for example, in case additional blood samples must be performed for safety reasons. This should be reported on the unscheduled visit form in the eCRF stating the reason for the visit. If the subject attends the clinic due to resampling of visit-related assessments, including MMTT, this is not considered an unscheduled visit. The date of the assessments for a specific visit must be updated in the eCRF accordingly. Likewise, coming to the site for additional trial products or ancillary supplies is not considered as an unscheduled visit.
Evaluation of AEs
The sponsor expects that AEs are recorded from the point of IC regardless of whether a participant has yet received a medicinal product. Individual AEs should be evaluated by the investigator. This includes the evaluation of its seriousness, and any relationship between the IMP(s) and/or concomitant therapy and the AE (causality). Additional information on the definitions for assessments of safety in Ver-A-T1D can be found in online supplemental information 3.
Seriousness is assessed against the criteria outlined in online supplemental information 3. This defines whether the event is an AE, SAE or a serious adverse reaction (SAR). Assessment of causality is categorised as: (1) definitely: a causal relationship is clinically/biologically certain. This is therefore an adverse reaction (AR); (2) probable: a causal relationship is clinically/biologically highly plausible and there is a plausible time sequence between onset of the AE and administration of the IMP and there is a reasonable response on withdrawal. This is therefore an AR; (3) possible: a causal relationship is clinically/biologically plausible and there is a plausible time sequence between onset of the AE and administration of the IMP. This is therefore an AR; (4) unlikely: a causal relation is improbable and another documented cause of the AE is most plausible. This is therefore an AE; (5) unrelated: a causal relationship can be definitely excluded and another documented cause of the AE is most plausible. This is therefore an AE. Unlikely and unrelated causalities are considered not to be IMP related. Definitely, probable and possible causalities are considered to be IMP related. A pre-existing condition must not be recorded as an AE or reported as an SAE unless the condition worsens during the trial and meets the criteria for reporting or recording in the appropriate section of the eCRF.
All events should be graded for severity according to the NCI-CTCAE Toxicity Criteria (V.5.0). AEs and ARs are recorded in the medical notes and the appropriate section of the eCRF at all phone and site visits. SAEs and SARs are to be reported to the sponsor as detailed below.
Expected AEs/SAEs
The following are (S)AEs that could be reasonably expected for this trial population during the trial: (1) hypoglycaemia and (2) diabetic ketoacidosis. These events must be recorded in the eCRF. Episodes not fulfilling the criteria for an SAE are not to be reported as AEs. If one of the above-mentioned episodes fulfils the criteria for an SAE then in addition to the above, an SAE form must also be filled in. The events are exempt from being reported as SAEs only if the causalities are not considered to be trial drug related.
Reporting of SAEs
AEs and ARs should be recorded in the medical notes and the appropriate section of the eCRF and/or AE/AR log. SAEs and SARs should be reported to the sponsor. Each PI needs to record all AEs and report serious AEs to the chief investigator using the trial specific SAE form within 24 hours of their awareness of the event. The chief investigator is responsible for ensuring the assessment of all SAEs for expectedness and relatedness is completed and the onward notification of all SAEs to the sponsor immediately but not more than 24 hours of first notification. The sponsor has to keep detailed records of all SAEs reported to them by the trial team.
The chief investigator is also responsible for prompt reporting of all SAE findings to the competent authority of each concerned member state if they could: (1) adversely affect the health of participants, (2) impact the conduct of the trial, (3) alter the risk to benefit ratio of the trial or (4) alter the competent authority’s authorisation to continue the trial in accordance with Directive 2001/20/EC. SAEs are reported to the chief investigator at MUG.
Reporting of suspected unexpected serious adverse reactions (SUSARs)
All suspected ARs related to an IMP (the tested IMP and comparators) that occur in the concerned trial and are both unexpected and serious (SUSARs) are subject to expedited reporting. The sponsor delegates the responsibility of notification of SUSARs to the chief investigator. The chief investigator must report all the relevant safety information previously described, to the sponsor, competent authorities in the concerned member states and EC in the concerned member states. The chief investigator shall inform all investigators concerned of relevant information about SUSARs that could adversely affect the safety of participants.
All parties must be notified of fatal or life-threatening SUSARs as soon as possible, but no later than seven calendar days after the trial team and sponsor has first knowledge of the minimum criteria for expedited reporting. In each case, relevant follow-up information should be sought and a report completed as soon as possible. It should be communicated to all parties within an additional 8 calendar days. Non-fatal, non-life-threatening SUSARs and safety issues must be reported to all parties as soon as possible, but no later than 15 calendar days after first knowledge of the minimum criteria for expedited reporting. Further relevant follow-up information should be given as soon as possible. Information on the final description and evaluation of an AR report may not be available within the required time frames for reporting. For regulatory purposes, initial expedited reports should be submitted within the time limits as soon as the minimum following criteria are met: (1) a suspected IMP, (2) an identifiable participant (eg, trial participant code number), (3) an AE assessed as serious and unexpected, and for which there is a reasonable suspected causal relationship and (4) an identifiable reporting source. When available and applicable, a unique clinical trial identification (EudraCT number or in case of non-European Community trials the sponsor’s trial protocol code number) and a unique case identification (ie, sponsor’s case identification number) should also be reported.
In case of incomplete information at the time of initial reporting, all the appropriate information for an adequate analysis of causality should be actively sought from the reporter or other available sources. Further available relevant information should be reported as follow-up reports. In certain cases, it may be appropriate to conduct follow-up of the long-term outcome of a particular reaction. Electronic reporting is the expected method for expedited reporting of SUSARs to the competent authority. The format and content as defined by the competent authority should be adhered to.
Pregnancy reporting
All pregnancies within the trial in female trial participants will be collected after the start of study intervention and until 7 days after the last dose and should be reported to the chief investigator and the sponsor using the relevant pregnancy reporting form within 14 days of notification. Details of pregnancies in female participants will be collected after the first trial-related activity after obtaining IC and until pregnancy outcome. If a pregnancy is reported in a female participant, the investigator should inform the chief investigator and sponsor within 14 calendar days of learning of the pregnancy and pregnancy outcome should be documented in the participant’s medical record. Participants will be followed to determine the outcome of the pregnancy. The investigator will report information on the participant and the pregnancy outcome until the newborn infant is 1 month of age in accordance with EMA.24 Abnormal pregnancy outcome (eg, spontaneous abortion, foetal death, stillbirth, congenital anomalies and ectopic pregnancy) is considered an SAE.
Collection of pregnancy information—female participants who become pregnant will adhere to the following steps:
Investigator will collect pregnancy information on any female participant, who becomes pregnant while participating in this trial.
Information will be recorded on the appropriate form and submitted to the chief investigator and sponsor within 14 calendar days of learning of a participant’s pregnancy.
Participant will be followed to determine the outcome of the pregnancy. The investigator will collect follow-up information on participant and neonate, which will be forwarded to the sponsor. Generally, follow-up will not be required for longer than 1 month beyond the delivery date.
Any termination of pregnancy will be reported, regardless of foetal status (presence or absence of anomalies) or indication for procedure.
While pregnancy itself is not considered to be an AE or SAE, any pregnancy complication or elective termination of a pregnancy will be reported as an AE or SAE.
A spontaneous abortion is always considered to be an SAE and will be reported as such.
Any SAE occurring because of a post-trial pregnancy which is considered possibly/probably related to the trial product by the investigator will be reported to the sponsor.
Any female participant who becomes pregnant while participating in the trial will discontinue trial product.
Toxicity management—emergency procedures
Verapamil has a vasodilating action on the vascular system. Toxic effects occur usually after a delay of 1–5 hours following ingestion. The main cardiovascular symptoms are: bradycardia and AV block (in 82% of cases) hypotension and cardiogenic shock (in 78% of cases) cardiac arrest (in 18% of cases). First-degree AV block is treated as outlined in the online supplemental information 3. Pulmonary oedema may occur. Impairment of consciousness and seizures may occur and are related to a low cardiac output. Nausea and vomiting may be observed. Metabolic acidosis due to shock and hyperglycaemia may occur. Verapamil is a calcium channel blocker and inhibits the entry of calcium through calcium channels into cardiovascular cells. Verapamil reduces the magnitude of the calcium current entry and decreases the rate of recovery of the channel. Verapamil decreases peripheral vascular and coronary resistance, but it is a less potent vasodilator than nifedipine. In contrast, its cardiac effects are more prominent than those of nifedipine. At doses necessary to produce arterial vasodilatation, verapamil has much greater negative chronotropic, dromotropic and inotropic effects than nifedipine. At toxic doses, calcium channel inhibition by verapamil results in three principal effects: hypotension due to arterial vasodilatation, cardiogenic shock secondary to a negative inotropic effect, bradycardia and AV block. The therapeutic effects of verapamil on hypertension and angina pectoris are due to arterial systemic and coronary vasodilatation. The antiarrhythmic activity of verapamil is due to a delay in impulse transmission through the AV node by a direct action. Toxicity may occur after ingestion of 1 g. verapamil was tested on human peripheral lymphocytes in vitro using micronucleus (MN) test. The MN frequencies showed increase after all treatment. The results of FISH (Fluorescence in situ hybridization) analysis suggest that verapamil, separately or combined with ritodrine, shows to a larger extent aneugenic than clastogenic effect. Verapamil hydrochloride may increase blood alcohol (ethanol) concentrations and prolong its effects.
Toxicity management—mild-to-moderate toxicity
Patients who have asymptomatic bradycardia can be admitted and observed with telemetry if judged reasonable by the investigator. Obtain peripheral intravenous access and monitor ECG. Mild hypotension may only require treatment with intravenous fluid administration.
Toxicity management—severe toxicity
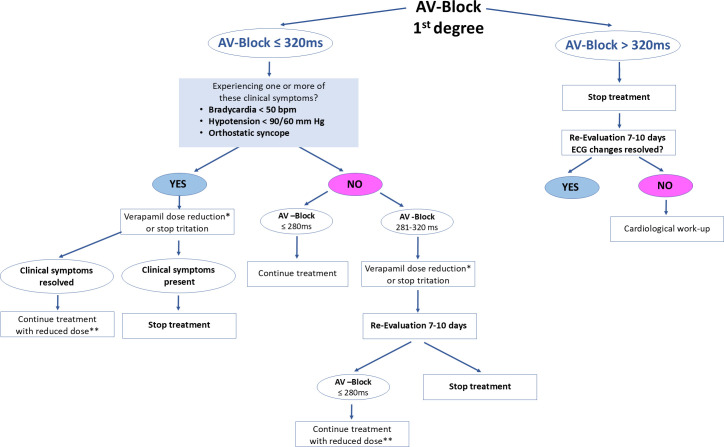
Patients with bradycardia and hypotension require standard advanced cardiac life support treatment. Place a central line and consider placement of an arterial line. Standard first-line treatment includes atropine for bradycardia, although in a serious poisoning, it is rarely effective. High-dose insulin and dextrose have been effective in animal studies and multiple case reports in patients with hypotension refractory to other modalities and should be considered early in patients with significant hypotension. Use intravenous calcium in severe poisonings, although in these cases, beneficial effects of calcium infusion (calcium chloride is preferred) may be very minimal or short lived. Repeat bolus doses or a continuous intravenous infusion are often needed. Standard vasopressors should be administered to maintain blood pressure. Lipid emulsion has been successful in animal studies and several case reports of patients with hypotension refractory to other therapies. Intravenous glucagon has been used with variable success. In a patient whose haemodynamic status continues to be refractory despite the treatment described above, extracorporeal membrane oxygenation or cardiopulmonary bypass should be considered. Treat seizures with intravenous benzodiazepines; barbiturates or propofol may be needed if seizures persist or recur. First-degree AV block is treated as per figure 3.
Figure 3. Ver-A-T1D atrioventricular (AV) block management. *Dose reduction should be 120 mg less. **Minimal dose should be 240 mg.
Storage and analysis of samples
Samples collected in the study as part of the INNODIA Clinical Trial Master Protocol will be stored and analysed as described in the Master Protocol. Additional samples collected that are in the same nature, for example, samples from additional MMTT or for additional immune cell studies, will be stored and analysed according to the INNODIA Clinical Trial Master Protocol.20
Statistics overview
The trial is a multicentre, randomised, double-blind, placebo-controlled trial in subjects with T1D within 6 weeks of diagnosis. A total sample size of 120 participants will be randomised in a 2:1 ratio between 360 mg verapamil and placebo. The primary endpoint of interest is the area under the stimulated C-peptide response curve over the first 2 hours of an MMTT after 12 months therapy compared with placebo.
One interim analysis will be performed using data available when approximately 50 participants have been randomised to determine whether the trial should be stopped for futility.
All analyses will be performed on an intention-to-treat approach that will include all randomised participants, irrespective of protocol compliance.
Evaluation of results (definitions and response/evaluation of outcome measures)
Statistical methods for primary analyses
All model assumptions will be checked via graphical means such as plotting residuals versus dose and fitted values. The primary outcome, AUC C-pep value will be transformed to ln(AUC C-pep+1) as recommended by.5 The transformed AUC C-pep value is assumed to be normally distributed; this distributional assumption will be assessed using a Q–Q plot and whether the residuals of the model deviate from a straight line. If the residuals are not normally distributed, then the outcome will be transformed to improve the assumption. If no transformation is available, then non-parametric methods will be used.
The primary endpoint, the area under the C-peptide curve over the first 2 hours (using all available measurements within the first 2 hours) of an MMTT (AUC C-pep) at 12 months (after transformation AUC C-pep →ln(AUC C-pep+1)) will be analysed using a linear mixed model at the end of the trial. The model will have fixed effects of treatment and time, and the random effect will be participant ID. Additionally, autocorrelations will be included within participants and ideally an unstructured matrix will be fitted stratified by treatment group, however, if the data do not allow such complexity then an AR(1) autocorrelation pattern will be estimated. The contrast of interest is the mean difference in AUC C-pep between verapamil 360 mg and placebo at 12 months.
The transformed AUC C-pep value is assumed to be normally distributed and this distributional assumption will be assessed using a Q–Q plot and whether the residuals of the model deviate from a straight line. Additionally, departures from normality will be assessed by using normality tests, such as for instance the Shapiro-Wilk test. If model assumptions are violated for the AUC C-pep →ln(AUC C-pep+1) values, then log or square root transformations will be applied. If none of these transformations yields normally distributed residuals, then treatments will be compared by means of non-parametric methods using the change from baseline as the dependent variable.
Statistical methods for secondary analyses
The secondary endpoints will also be analysed via a mixed effects models with fixed effects of treatment and time, and the random effect will be participant ID. If required, the models may include additional covariates, which may be potential factors that are confounding the relationship between treatment and outcomes.
Subgroup analyses will be considered for a select list of potential covariates, the subgroup treatment effect will be analysed using an interaction test and additional factors will be included in the model to conduct this test.
A detailed statistical analysis plan (SAP) will be completed before the final database lock and will be based on top of the INNODIA Master SAP within the INNODIA clinical trial network (www.innodia.eu).
Interim analyses
The interim analysis should be carried out after 10 months from the start of the trial.
As with the primary analysis, the endpoint, the area under the C-peptide curve over the first 2 hours of an MMTT (AUC-C-pep) will be analysed using a linear mixed model. The model will have fixed effects of treatment and time, and the random effect will be participant ID. Additionally, autocorrelations will be included within participants and ideally an unstructured matrix will be fitted stratified by treatment group; however, if the data do not allow such complexity, then an AR(1) autocorrelation pattern will be estimated. The analysis will be an intention to treat analysis. The contrast of interest is the mean difference in AUC-C-pep between verapamil 360 mg and placebo at 6 months, as there will be no one who has completed the 12 month follow-up visit at 10 months. The statistical test will be the z-test and the trial will be recommended to stop if the z-statistics is less than −0.5, that is, where treatment is marginally worse than placebo.
Given a recruitment rate of five participants per month, the interim analysis at 10 months should have 15 people with 3 months of follow-up data, 15 people with 6 months of follow-up data and 15 people with 9 months of follow-up data. Different recruitment rates will alter the operating characteristics of the trial, but the type 1 error at the final analysis is controlled, though power may vary. If recruitment is faster than the timing of the interim analysis will be reassessed, if there is sufficient information the interim may proceed earlier than 10 months.
Model assumptions will be checked via graphical means such as plotting residuals versus dose and fitted values. The AUC-C-pep is assumed to be normally distributed, this distributional assumption will be assessed using a Q–Q plot and whether the residuals of the model deviate from a straight line. If the residuals are not normally distributed, then the outcome will be transformed to improve the assumption.
Number of participants to be enrolled (sample size calculation)
From the randomised, double-blind, placebo-controlled phase II clinical trial the SD for the AUC C-peptide endpoint after an MMTT over 2 hours at 12 months was 0.27 nmol/L/min as per Ovalle et al.14 With this SD, 90% power, 5% significance level then 40 participants on the control arm and 80 on the treatment arm will be needed to detect a change of 0.18 nmol/L/min in C-peptide. All tests are for superiority tests and the tests are two-sided tests.
Criteria for the premature termination of the trial
The sponsor designee/INNODIA reserves the right to close the study site or terminate the study at any time for any reason at the sole discretion of the sponsor. Study sites will be closed on study completion. A study site is considered closed when all required documents and study supplies have been collected, and a study site closure visit has been performed.
The investigator may initiate study site closure at any time, provided there is reasonable cause and sufficient notice is given prior to the intended termination.
Reasons for the early closure of a study site by the sponsor or investigator may include, but are not limited to:
Failure of the investigator to comply with the protocol, the requirements of the IRB/IEC or local health authorities, the sponsor’s procedures, or GCP guidelines.
Inadequate recruitment of participants by the investigator.
Discontinuation of further study intervention development.
If the study is prematurely terminated or suspended, the sponsor shall promptly inform the investigators, the IECs/IRBs, the regulatory authorities and any contract research organisation(s) used in the study of the reason for termination or suspension, as specified by the applicable regulatory requirements. The investigator shall promptly inform the subject and should assure appropriate subject therapy and/or follow-up.
Procedure to account for missing or spurious data
All participants who are randomised will be included in this analysis and the model will have fixed effects for time and treatment and participant is a random effect. All available measurements over time will be included in the analysis. The estimates assume that the missing data are missing at random. If the missing data are non-ignorable, then a sensitivity analysis will be performed.
The secondary endpoints will also be analysed using a mixed effects model similar to the one described for the primary outcome. Again, model assumptions and distributional assumptions will be inspected graphically.
Definition of the end of the trial
The end of the trial is defined as the date of the last visit of the last participant in the trial.
Data management and eCRF
All data will be transferred into an eCRF which will be anonymised. All trial data in the eCRF must be extracted from and be consistent with the relevant source documents. The eCRFs must be completed, dated and signed by the investigator or designee in a timely manner. It remains the responsibility of the investigator for the timing, completeness, legibility and accuracy of the eCRF pages. The eCRF will be accessible to trial coordinators, data managers, the investigators, clinical trial monitors, auditors and inspectors as required.
All subject data relating to the trial will be recorded on eCRFs. The investigator is responsible for verifying that data entries are accurate and correct by electronically signing the eCRF.
Corrections to the eCRF data may be made by the investigator or the investigator’s delegated staff. An audit trail will be maintained in the eCRF application containing as a minimum: the old and the new data, identification of the person entering the data, date and time of the entry and reason for the correction. If corrections are made by the investigator’s delegated staff after the date when the investigator signed the eCRF, the eCRF must be signed and dated again by the investigator.
The investigator must be able to access his/her trial documents without involving the sponsor in any way. If applicable, electronic CRF and other subject data will be provided in an electronic readable format to the investigator before access is revoked to the systems supplied by the sponsor. Site-specific CRFs and other subject data (in an electronic readable format or as paper copies or prints) must be retained by the trial site. If the provided electronic data (eg, the CD-ROM) is not readable during the entire storage period, the investigator can request a new copy. A copy of all data will be stored by the sponsor.
Data will be collected using INNODIA (e)CRFs. Suitably qualified personnel designated by the PI and listed on the delegation of responsibility log will be responsible for completing the eCRF. Each clinical centre will be responsible for managing collected data and for generating and resolving data queries.
Source data
Source documents provide evidence for the existence of the participant and substantiate the integrity of the data collected. Source documents are filed at the investigator’s site. Data reported on the source data form or entered in the eCRF that are transcribed from source documents must be consistent with the source documents or any discrepancies must be explained. The investigator may need to request previous medical records or transfer records, depending on the study. Also, current medical records must be available.
To enable peer review, monitoring, audit and/or inspection the investigator must agree to keep records of all participating participants (sufficient information to link records for example, eCRFs, hospital records and samples), all original signed ICFs and copies of the eCRF in an electronic readable format.
Definition of what constitutes source data can be found in a source document agreement at each trial site. There will only be one source document defined at any time for any data element.
Data protection and participant confidentiality
All investigators and trial site staff involved in this trial must comply with the requirements of the Data Protection Act 1998, General Data Protection Regulation (EU) 2016/679, local data protection laws and Trust Policy with regard to the collection, storage, processing, transfer and disclosure of personal information and will uphold the act’s core principles.
Participants will be assigned a unique study identifier as agreed with the sponsor. Any participant records or datasets that are transferred to the sponsor will contain the identifier only; participant names or any information which would make the participant identifiable will not be transferred.
The participant must be informed that his/her personal study-related data will be used by the sponsor in accordance with local data protection law. The level of disclosure must also be explained to the participant.
The participant must be informed that his/her medical records may be examined by clinical quality assurance auditors or other authorised personnel appointed by the sponsor, by appropriate IEC members, and by inspectors from regulatory authorities.
Data may also be sent out to non-European countries.
Protocol compliance and breaches of GCP
Prospective, planned deviations or waivers to the protocol are not allowed and must not be used. All participating sites must ensure that any substantial amendment is approved before implementation by an accredited EC.
However, deviations from the protocol should be avoided. If deviations do occur, the investigator must inform the monitor and the implications of the deviation must be reviewed and discussed. Protocol deviations must be adequately documented on the relevant forms and reported to the chief investigator and sponsor immediately.
Deviations must be documented and explained in a protocol deviation by stating the reason, date and the action(s) taken. Some deviations, for which corrections are not possible, can be acknowledged and confirmed via edit checks in the eCRF or via listings from the protocol deviation forms.
Deviations from the protocol which are found to occur repeatedly will not be accepted and will require immediate action and could potentially be classified as a serious breach. Any potential/suspected serious breaches of GCP must be reported immediately to the sponsor without any delay.
The trial master file (TMF) will be kept up to date by the coordinating centre, and each participating site will be responsible for maintaining their investigator site files (ISFs). These files need to be complete at the end of the trial and archived for 25 years. No records may be destroyed or transferred to another location or party without written notification of the sponsor during the retention period.
The sponsor will be responsible for archiving the TMF. Other participating sites will be responsible for archiving their ISF. Records and documents including source data will be stored at each participating site. The investigator must be able to access his/her trial documents without involving the sponsor in any way. If applicable, electronic CRF and other subject data will be provided in an electronic readable format to the investigator before access is revoked to the systems supplied by the sponsor.
All essential and trial documentation will be securely archived after the last analysis of the study data has been completed and the final study report has been submitted to the relevant bodies.
The investigator must make all trial documentation and related records available should an MHRA or EMA inspection or any regulatory authority inspection occur. Should a monitoring visit or audit be requested, the investigator must make the trial documentation and source data available to the sponsor’s representative. All participant data must be handled and treated confidentially.
The sponsor’s monitoring frequency will be determined by an initial risk assessment performed prior to the start of the trial. A detailed monitoring plan will be generated detailing the frequency and scope of the monitoring for the trial. Throughout the course of the trial, the risk assessment will be reviewed and the monitoring frequency adjusted as necessary. The scope and frequency of the monitoring will be determined by the risk assessment and detailed in the monitoring plan for the study.
Ethics and dissemination
Ethical committee review
Before the start of the trial and on implementation of any amendment approvals of the trial protocol, protocol amendments, ICFs and other relevant documents, for example, advertisements and general practitioner information letters if applicable were obtained from the REC. Ethics approval was sought from the following ECs: MUG ethics committee (ID: 32-664 ex 19/20), Commissie voor Medische Ethiek ZNA, p/a ZNA Koningin Paola Kinderziekenhuis Antwerpen (ID: 5494), Comité de Protection des Personnes Est II, CHRU—Hôpital Saint Jacques (ID: 21.01.26.73506), Comitato Etico dell’IRCCS Ospedale San Raffaele di Milano (ID: 9/05/22), Comitato Etico Regione Toscana—Area Vasta Sud Est (ID: 19709), Medizinische Hochschule Hannover Ethikkommission (Nr. 9465_AMG_mono_2020), Medizinische Hochschule Hannover Ethikkommission und Ethikkommission der Landesärztekammer Baden-Württemberg (Nr. 9465_AMG_M_2020) and NHS Health Research Authority, London—City & East REC (reference: 20/LO/1295). Thus, all procedures were conducted in compliance with the ethical standards established by the institutional ethics and research committees. All participants provided written IC prior to enrolment in the study.
All correspondence with the REC is retained in the TMF/ISF and annual reports are submitted to the REC in accordance with national requirements. It is the chief investigator’s responsibility to produce the annual reports as required.
Regulatory compliance
The trial will not commence until a clinical trial authorisation is obtained from the applicable regulatory authorities. The protocol and trial conduct will comply with the Medicines for Human Use (Clinical Trials) Regulations 2004 and any relevant amendments. Regulatory authorities will receive the clinical trial application, protocol amendments, reports on SAEs and the clinical trial report (CTR) according to national requirements.
Development safety update reports will be submitted to the regulatory authorities in accordance with national requirements. It is the chief investigators’s responsibility to produce the annual reports as required.
Protocol amendments
Protocol amendments must be reviewed and agreement received from the sponsor for all proposed amendments prior to submission to the HRA, REC and/or MHRA.
The only circumstance in which an amendment may be initiated prior to HRA, REC and/or MHRA approval is where the change is necessary to eliminate apparent, immediate risks to the participants (urgent safety measures). In this case, accrual of new participants will be halted until the HRA, REC and/or MHRA approval has been obtained.
In the event of an important safety measure, the PI or suitable qualified delegate at the participating site will be informed within 48 hours by the chief investigator or suitable qualified member of the study team.
Peer review
This study protocol has been peer reviewed by the sponsor, TMG and principal statistician.
Declaration of Helsinki and GCP
The trial will be performed in accordance with the spirit and the letter of the Declaration of Helsinki, the conditions and principles of GCP, the protocol and applicable local regulatory requirements and laws.
GCP training
All trial staff must hold evidence of appropriate GCP training or undergo GCP training prior to undertaking any responsibilities on this trial. This training should be updated every 2 years or in accordance with your Trust’s policy.
Authorisation of participating sites
Prior to initiating a participating site, the following documentation will be in place: (1) ISF, (2) ethics approval from each country in addition and following home country approval, (3) competent authority approval, (4) all relevant local institutional approvals (eg, local hospital institution), (5) signed participating site agreement when required, (6) insurance statement, (7) protocol signed and dated by PI, (8) confirmation of receipt of investigator’s brochure by PI, (9) patient information leaflets including ICF and any other study material for participants to be provided in English and translated to home country language, (10) delegation of responsibility and signature log, (11) PI signed and dated CV, (12) signed and dated CVs from everyone listed on the delegation of responsibility log, (13) GCP certificate from PI and everyone listed on the delegation of responsibility log, (14) final eCRF, (15) study manual and standard operating procedures, (16) signed source data verification agreement form and (17) local laboratory accreditation (or equivalent) and reference ranges for the protocol-specified parameters.
Procedure for initiating/opening a new site
The study manager and/or monitor will organise the initiation meeting on behalf of the CI and invite all the participating site study members. The CI or delegate, study manager and/or monitor and PI will present throughout the meeting. The PI’s legal responsibilities will be listed in the participating site agreement, if applicable, but each recruiting site will have a nominated PI who will be expected to:
Read the protocol and agree to follow it and future amended protocols in accordance with ICH GCP guidelines, legal and regulatory requirements.
Providing oversight of the conduct of the trial at the site and adherence to requirements of ICH guidelines, the IRB/IEC and all other applicable local regulations.
Provide written summaries of the status of the trial in accordance with the requirements, policies and procedures established by the IRB/IEC and/or regulatory authorities.
Notify the IRB/IEC of SAEs or other significant safety findings, as required by IRB/IEC procedures.
Ensuring submission of the CTR synopsis to the IRB/IEC.
Attend initiation meeting and subsequent study meetings or delegate to a suitable qualified team member.
Adhere to safety reporting timelines.
Have overall responsibility of data collection and responsibility of maintaining ISF.
Be thoroughly familiar with the appropriate use of the investigational product(s), as described in the protocol, in the current investigator’s brochure, in the product information and in other information sources provided by the sponsor.
Permit monitoring and auditing by the sponsor, and inspection by the appropriate regulatory authority(ies).
Maintain a list of appropriately qualified persons to whom the investigator has delegated significant trial-related duties.
Publications policy
Ownership of the data arising from this study are owned by the beneficiary/ beneficiaries of the INNODIA HARVEST consortium who generated them. On completion of the study, the data will be analysed and tabulated and a final study report prepared. Participating local investigators will have no rights to publish any of the study data without the permission of the chief investigator.
As outlined in the consortium agreement, each INNODIA partner (participant) has a maximum of 30 days to approve a publication or submit an objection after they have received the draft version in writing to the coordination team (publication@innodia.eu). If no response is gathered by day 30, then approval is assumed to be granted.
Nevertheless, the beneficiaries acknowledge that some kind of publications may require shorter approval times due to given submission timelines (table 2).
Table 2. Reporting timeframes.
| Type of communication | Period for approval | Reminder sent out after | Approval required from |
| Full research paper | 30 days | 20 days | All INNODIA and INNODIA partners |
| Abstracts/posters | 7 days | 5 days | |
| Press releases* | 5 days | -- | |
| Public communication | 5 days | -- |
Where national media releases are made, key messages and INNODIA researchers mentioned in the release should be circulated in English for approval.
INNODIAinnovative approach towards understanding and arresting type 1 diabetes
In case of exceptional urgency, the coordination team can grant permission to submit an abstract or a manuscript sub condition, meaning that the manuscript or abstract will have to be withdrawn from the review and publication process in case an INNODIA beneficiary objects.
Participants and legal representatives will be notified of the outcome of this study by a specifically designated newsletter, after the study has been published.
Trial status
Ver-A-T1D closed recruitment on 3 May 2024. Date of first enrolment was 8 February 2021 in Medical University of Graz (MUG). Ver-A-T1D received regulatory and ethics approval in Graz from the EC of the MUG on 24 August 2020. Regulatory approval has also been granted by the Austrian competent authority on 11 February 2021. Planned last patient last visit is 20 May 2025, with final reporting due in May 2026.
supplementary material
Footnotes
Funding: This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement number 115797 (INNODIA) and number 945268 (INNODIA HARVEST). This Joint Undertaking receives support from the Union’s Horizon 2020 research and innovation programme, ‘EFPIA’, ‘JDRF’ and ‘The Leona M. and Harry B. Helmsley Charitable Trust’. The IMP is supplied by Medical University of Graz (MUG). The CE-marked CGM devices are provided by DexCom (USA) and supported by IMI2-JU under grant number 945268 (INNODIA HARVEST). Any dissemination of results must indicate that it reflects only the author’s view and that the JU is not responsible for any use that may be made of the information it contains. This study was sponsored by MUG, Department of Internal Medicine, Division of Endocrinology and Diabetology, Austria. MUG will accept full financial liability for harm caused to participants in the clinical trial caused through the negligence of its employees and honorary contract holders. There are no specific arrangements for compensation should a participant be harmed through participation in the trial without any negligence. MUG will arrange insurance for negligent harm caused as a result of protocol design and for non-negligent harm arising through participation in the clinical trial. Participants or their legal representatives of this study will not receive any payment for participating in this study; however, all reasonable travel costs incurred while travelling to the recruiting centre for each study visit will be reimbursed to the participant or legal representative by the coordinating centre.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-091597).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Contributor Information
Julie Wych, Email: wychj@cardiff.ac.uk.
Martina Brunner, Email: martina.brunner@medunigraz.at.
Rachel Stenson, Email: StensonR@cardiff.ac.uk.
Piotr Jaroslaw Chmura, Email: piotr.chmura@cpr.ku.dk.
Thomas Danne, Email: danne@hka.de.
Adrian Paul Mander, Email: adrian.x.mander@gsk.com.
Chantal Mathieu, Email: chantal.mathieu@uzleuven.be.
Colin Dayan, Email: DayanCM@cardiff.ac.uk.
Thomas R Pieber, Email: thomas.pieber@medunigraz.at.
Data availability statement
No data are associated with this article. No data are associated with this article. Data will be available in accordance with the data sharing plan and can be found in the online supplemental information (Ver-A-T1D_Data Sharing Plan_18.09.2024).
References
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–62. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørensen JS, Johannesen J, Pociot F, et al. Residual β-Cell function 3-6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care. 2013;36:3454–9. doi: 10.2337/dc13-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Lachin JM, McGee P, Palmer JP, et al. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014;63:739–48. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen LA, Dayan CM. Immunotherapy for type 1 diabetes. Br Med Bull. 2021;140:76–90. doi: 10.1093/bmb/ldab027. [DOI] [PubMed] [Google Scholar]
- 7.Zhou R, Tardivel A, Thorens B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 8.Eissa LD, Ghobashy WA, El-Azab MF. Inhibition of thioredoxin-interacting protein and inflammasome assembly using verapamil mitigates diabetic retinopathy and pancreatic injury. Eur J Pharmacol. 2021;901:174061. doi: 10.1016/j.ejphar.2021.174061. [DOI] [PubMed] [Google Scholar]
- 9.Domingues A, Jolibois J, Rougé P, et al. The Emerging Role of TXNIP in Ischemic and Cardiovascular Diseases. A Novel Marker and Ther Target Int J Mol Sci. 2021;22 doi: 10.3390/ijms22041693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Saxena G, Mungrue IN, et al. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57:938–44. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Hui ST, Couto FM, et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008;22:3581–94. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Cha-Molstad H, Szabo A, et al. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296:E1133–9. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X, He W, Pang Y, et al. Redox-dependent and independent effects of thioredoxin interacting protein. Biol Chem. 2020;401:1215–31. doi: 10.1515/hsz-2020-0181. [DOI] [PubMed] [Google Scholar]
- 14.Ovalle F, Grimes T, Xu G, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med. 2018;24:1108–12. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu G, Grimes TD, Grayson TB, et al. Exploratory study reveals far reaching systemic and cellular effects of verapamil treatment in subjects with type 1 diabetes. Nat Commun. 2022;13:1159. doi: 10.1038/s41467-022-28826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forlenza GP, McVean J, Beck RW, et al. Effect of Verapamil on Pancreatic Beta Cell Function in Newly Diagnosed Pediatric Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2023;329:990–9. doi: 10.1001/jama.2023.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin T, Kuo S-C, Chang Y-Y, et al. Verapamil Use Is Associated With Reduction of Newly Diagnosed Diabetes Mellitus. J Clin Endocrinol Metab. 2017;102:2604–10. doi: 10.1210/jc.2016-3778. [DOI] [PubMed] [Google Scholar]
- 18.Cooper-Dehoff R, Cohen JD, Bakris GL, et al. Predictors of development of diabetes mellitus in patients with coronary artery disease taking antihypertensive medications (findings from the INternational VErapamil SR-Trandolapril STudy [INVEST]) Am J Cardiol. 2006;98:890–4. doi: 10.1016/j.amjcard.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Xu G, Chen J, Jing G, et al. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848–56. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunger DB, Bruggraber SFA, Mander AP, et al. INNODIA Master Protocol for the evaluation of investigational medicinal products in children, adolescents and adults with newly diagnosed type 1 diabetes. Trials. 2022;23:414. doi: 10.1186/s13063-022-06259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostapanos MS, Milionis HJ, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs. 2010;10:11–28. doi: 10.2165/13168600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Rowan CG, Brunelli SM, Munson J, et al. Clinical importance of the drug interaction between statins and CYP3A4 inhibitors: a retrospective cohort study in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2012;21:494–506. doi: 10.1002/pds.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agency EM Guideline on the exposure to medicinal products during pregnancy: need for postauthorisation data. 2005