Abstract
Background
Stroke is the second leading cause of death and the third leading cause of disability worldwide. Motor dysfunction is a common sequela, which seriously affects the lives of patients. Theta burst stimulation (TBS) is a new transcranial magnetic therapy for improving motor dysfunction after stroke. However, there remains a lack of studies on the mechanism, theoretical model, and effectiveness of TBS in improving motor dysfunction following stroke.
Objective
This paper provides a comprehensive overview and assessment of the current impact of TBS on motor rehabilitation following stroke and analyzes potential factors contributing to treatment effect disparities. The aim is to offer recommendations for further refining the TBS treatment approach in subsequent clinical studies while also furnishing evidence for devising tailored rehabilitation plans for stroke patients.
Methods
This study was conducted following PRISMA guidelines. PubMed, Embase, Web of Science, and the Cochrane Library were searched systematically from the establishment of the database to February 2024. Relevant studies using TBS to treat patients with motor dysfunction after stroke will be included. Data on study characteristics, interventions, outcome measures, and primary outcomes were extracted. The Modified Downs and Black Checklist was used to assess the potential bias of the included studies, and a narrative synthesis of the key findings was finally conducted.
Results
The specific mechanism of TBS in improving motor dysfunction after stroke has not been fully elucidated, but it is generally believed that TBS can improve the functional prognosis of patients by regulating motor cortical excitability, inducing neural network reorganization, and regulating cerebral circulation metabolism. Currently, most relevant clinical studies are based on the interhemispheric inhibition model (IHI), the vicariation model, and the bimodal balance-recovery model. Many studies have verified the effectiveness of TBS in improving the motor function of stroke patients, but the therapeutic effect of some studies is controversial.
Conclusion
Our results show that TBS has a good effect on improving motor function in stroke patients, but more large-scale, high-quality, multicenter studies are still necessary in the future to further clarify the mechanism of TBS and explore the optimal TBS treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02170-2.
Keywords: Stroke, Theta-burst stimulation, Upper Extremity, Lower Extremity, Motor function, Systematic review
Introduction
Stroke is a global disease with high mortality and high disability rate. Globally, stroke is the second leading cause of death and the third leading cause of disability [1]. From 1990 to 2019, the number of strokes increased by 70.0%, and the number of stroke deaths increased by 43.0%, which poses a great threat to human health [2]. As a common and serious cerebrovascular disease, stroke often brings many pains and challenges to patient [3]. After stroke, patients often have severe motor dysfunction secondary to stroke. Common motor injuries include limb paralysis, muscle weakness, abnormal muscle tone, and somatosensory changes, etc. Upper limb injuries also involve the loss of hand fine motor function [4]. Lower-extremity dysfunction is characterized by poor balance, limited walking, postural instability, weak trunk control, and difficulty transferring weight [5]. Motor dysfunction not only affects the physical health of patients, but also has a considerable negative impact on the quality of life and social participation. Patients may be unable to participate in social activities, work and entertainment due to the inconvenience of mobility, leading to psychological loneliness, depression and loss, which seriously affects the mental health of patients. In addition, post-stroke care and treatment are expensive and time-consuming. The rehabilitation process requires professional medical equipment and personnel, and the cost is high. At the same time, the rehabilitation of patients also requires the family to invest a lot of time and energy to care for them, which brings a heavy burden to the family and society [6].
At present, drug therapy and conventional physical therapy (PT) are the main means of stroke rehabilitation, but the efficacy of these methods is limited [7]. Drug therapy mainly promotes rehabilitation by improving blood circulation and nerve nutrition, but its effect on repairing damaged nerve tissue is limited [8]. Although conventional PT, such as exercise therapy and physiotherapy, can improve the motor function of patients to a certain extent, it requires long-term adherence of patients, and the effect varies from person to person [9]. Even if patients receive traditional rehabilitation programs after stroke, there are still 50–60% of patients with varying degrees of motor function limitation [10]. Traditional treatment methods are difficult to meet the rehabilitation needs of patients in many cases. We need to constantly explore new rehabilitation methods and techniques to improve the rehabilitation effect of stroke patients.
Transcranial magnetic stimulation (TMS) is considered to be an effective adjuvant therapy for post-stroke rehabilitation, which can regulate the neural activity of the brain and enhance neuroplasticity, thereby restoring the motor function of patients[11]. Theta burst stimulation (TBS) is a new type of TMS mode that can be divided into intermittent theta burst stimulation (iTBS) and continuous theta burst stimulation (cTBS) [12]. Both can potentially modulate the excitability of the cerebral cortex and have the characteristics of low stimulation intensity and short stimulation time. ITBS can excite the neural activity of the cerebral cortex, while cTBS has an inhibitory effect [13]. At present, the research on TMS in the treatment of neurological diseases has achieved some results, but the mechanism, theoretical model and efficacy of TBS in improving motor dysfunction after stroke are still lacking. This article continues and updates the results of other previous reviews on this therapy, summarizes the effects of TBS on motor rehabilitation after stroke in current studies, and analyzes the possible reasons for the differences in therapeutic effects, in order to provide suggestions for further improvement of TBS treatment in follow-up clinical studies.
Materials and methods
Study enrollment and reporting
This study was conducted in accordance with the recently updated PRISMA 2020 (the preferred reporting items for systematic reviews and meta-analysis) statement [14] and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with ID code: CRD42024600859.
Inclusion criteria
The inclusion criteria for this review followed the PICOS [15] model.
Population (P): Adult patients with stroke diagnosed by CT or MRI and accompanied by limb motor dysfunction.
Intervention (I): TBS. There were no restrictions on the stimulation site, frequency, intensity, time, and duration of treatment, and any stimulation protocol would be included.
Comparison (C): In all experimental groups included in the study, TBS was used alone or in combination with other conventional treatment modalities, such as conventional neurorehabilitation, standardized motor training, robot-assisted training, conventional PT, occupational therapy (OT), and drug therapy. The control group received repeat transcranial magnetic stimulation (rTMS)/sham TBS alone or in combination with other conventional treatment modalities. The sham TBS refers to the ineffective stimulation with the stimulation coil perpendicular to the brain area, but the sound and treatment parameters of the stimulation are the same as those of the experimental group.
Outcome (O): The primary outcome was the effect of TBS on the limitation of motor function of the patient's limbs. The secondary outcome was the occurrence of adverse reactions. The primary outcome measures included the results of clinical scales and neuro electrophysiological tests to assess limb motor function, and the secondary outcome measures included the recovery of activities of daily living and quality of life.
Study design (S): All published experimental studies, including randomized controlled trials and non-randomized controlled trials.
Exclusion criteria
The following types of studies were excluded: (1) animal experiments; (2) limb motor dysfunction caused by other causes, such as craniocerebral trauma, Alzheimer's disease, and Parkinson's disease, etc. (3) duplicate published studies; (4) studies for which the full text was not available through various approaches. (5) Missing experimental data.
Information sources and search strategy
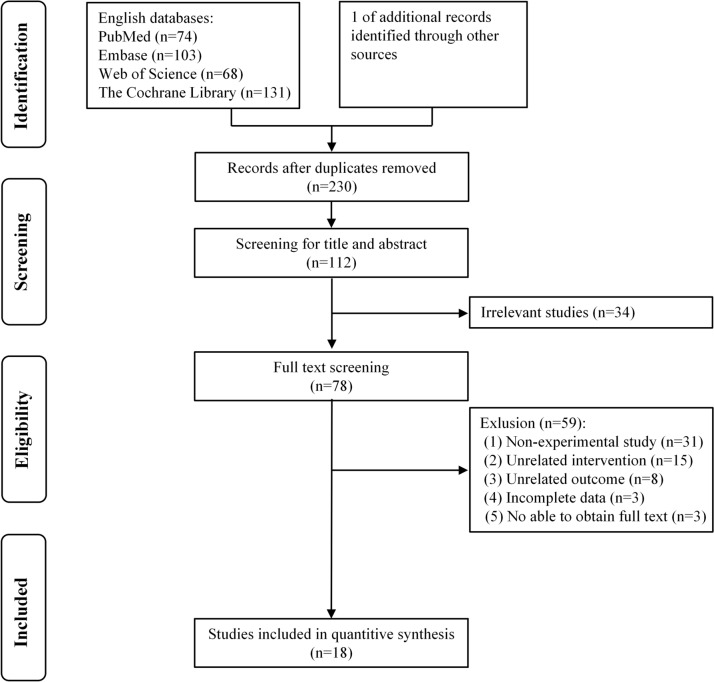
Two authors separately searched PubMed, Embase, Web of Science, and Cochrane Library for experimental studies on TBS improving limb motor function in stroke patients, including randomized controlled trials and non-randomized controlled trials. The search time limit was from the establishment of the database to February 29, 2024. A comprehensive search was carried out by combining medical subject headings and free words. The search terms included “Stroke”, “Cerebrovascular Accident,” “CVA,” “brain vascular accidents,” “hemiplegia,” “Upper Extremity,” “lower extremity,” “theta-burst stimulation,” “TBS,” “intermittent theta burst stimulation,” and “continuous theta burst stimulation.” In this systematic review (Fig. 1), a total of 18 studies were ultimately incorporated. Additional file 1: Table S1 presents the detailed search strategy.
Fig. 1.
Flowchart of included studies
Literature screening and data extraction
Two authors (LZ and AX) respectively searched the literature according to the established search strategy, imported all retrieved literature into the Endnote 20 document management system, and used the software management function to remove duplicate studies. They decided on the inclusion and exclusion of literature by sequentially reading the title, abstract, and text according to our previously established criteria. In case of disagreement during the screening process, the decision was made jointly based on the advice provided by the third author (DJ).
Two authors (YF and CW) separately extracted the following data from the included literature: The first author of the study, country, research type, intervention method (TBS stimulation site, TBS intensity, number of TBS pulses, stimulation coils, duration of treatment, and adjuvant therapy), age of subjects, course of disease, type of stroke, outcome indicators (primary outcome indicators and secondary outcome indicators), and adverse events occurred during the experiment. After all data were extracted, the results were cross-checked and the investigator (DJ) reviewed and corrected all discrepancies. Finally, the study conducted a narrative synthesis of the characteristics and contents of the included literature.
Quality evaluation
The Downs and Black checklist [16] is a methodological quality assessment tool for both randomized and nonrandomized studies. Given that some of the original checklist items did not apply to some of the included studies, we used a modified version of the Downs and Black checklist to assess the quality of the included studies. The modified checklist had 17 items and a maximum score of 17. For details, see Table 1. Two authors (YF and CW) scored the methodological quality of each article according to the actual situation of the included literature, and classified the quality of each study according to the percentage of the score in the total score of the item: low quality (< 50%), medium quality (50–74%) or high quality (≥ 75%) [17]. The evaluation results are shown in Table 3.
Table 1.
Quality modified checklist from Downs and Black
| Item | Scoring options | |||
|---|---|---|---|---|
| Yes | No | UTD | ||
| Q1 | Is the hypothesis/aim/objective of the study clearly described? | 1 | 0 | N |
| Q2 | Are the main outcomes to be measured clearly described in the Introduction or Methods section? | 1 | 0 | N |
| Q3 | Are the characteristics of the patients included in the study clearly described? | 1 | 0 | N |
| Q4 | Are the interventions of interest clearly described? | 1 | 0 | N |
| Q6 | Are the main findings of the study clearly described? | 1 | 0 | N |
| Q7 | Does the study provide estimates of the random variability in the data for the main outcomes? | 1 | 0 | N |
| Q8 | Have all important adverse events that may be a consequence of the intervention been reported? | 1 | 0 | N |
| Q9 | Have the characteristics of patients lost to follow-up been described? | 1 | 0 | N |
| Q10 |
Have actual probability values been reported(e.g. 0.035 rather than < 0.05) for the main outcomes except where the probability value is less than 0.001? |
1 | 0 | N |
| Q11 | Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | 1 | 0 | 0 |
| Q15 | Was an attempt made to blind those measuring the main outcomes of the intervention? | 1 | 0 | 0 |
| Q16 | If any of the results of the study were based on “data dredging,” was this made clear? | 1 | 0 | 0 |
| Q18 | Were the statistical tests used to assess the main outcomes appropriate? | 1 | 0 | 0 |
| Q20 | Were the main outcome measures used accurate (valid and reliable)? | 1 | 0 | 0 |
| Q21 | Were the patients in diverent intervention groups recruited from the same population? | 1 | 0 | 0 |
| Q23 | Were study subjects randomised to intervention groups? | 1 | 0 | 0 |
| Q26 | Were losses of patients to follow-up taken into account? | 1 | 0 | 0 |
Table 3.
Quality appraisal of included studies
| Study/Item | 1 | 2 | 3 | 4 | 6 | 7 | 8 | 9 | 10 | 11 | 15 | 16 | 18 | 20 | 21 | 23 | 26 | Total score |
Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicolo et al. [18] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | High |
| Vink et al. [19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | High |
| Kuzu et al. [20] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | High |
| Kondo et al. [21] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 12 | Medium |
| Talelli et al. [22] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 13 | High |
| Ackerley et al. [23] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | High |
| Talelli et al. [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 15 | High |
| Chen et al. [25] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | High |
| Ackerley et al. [26] | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 12 | Medium |
| Chen et al. [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | High |
| Kim et al. [28] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | High |
| Zhang et al. [29] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | High |
| Liao et al. [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | High |
| Xie et al. [31] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | High |
| Koch et al. [32] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | High |
| Liao et al. [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | High |
| Lin et al. [34] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | High |
| Wang et al. [35] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | High |
UTD means Unable to determine. N means none.
Results
Study selection
We searched the above four databases strictly according to the inclusion and exclusion criteria formulated above, and the results of the literature search were 376. After using the Endnote 20 software management function to delete 146 duplicate articles, 230 remained. First, all the topics and abstracts were screened, and 152 were excluded. After full-text screening, non-experimental studies (n = 31), unrelated interventions (n = 15), unrelated outcomes (n = 8), unavailability of full-text (n = 3), and incomplete data (n = 3) were excluded. Finally, 18 articles met the inclusion criteria. Figure 1 shows the flow chart of the study.
Characteristics of the included studies
A total of 18 articles [18–35] from 9 different countries were included in this study, which were Switzerland (n = 1), the Netherlands (n = 1), Turkey (n = 1), Japan (n = 1), the United Kingdom (n = 2), New Zealand (n = 2), China (n = 8), South Korea (n = 1) and Italy (n = 1). The included literature included two non-randomized controlled trials [21, 24] and 16 randomized controlled trials [18–20, 22, 23, 25–35], of which one was a four-group trial [24], six were three-group trials [18, 20, 22, 26, 29, 30], and 11 were two-group trials [19, 21, 23, 25, 27, 28, 31–35]. There are 14 reports on iTBS [22–35], 7 reports on cTBS [18–22, 24, 26], and 3 reports on two TBS mode [22, 24, 26]. One article compared the effects of iTBS and cTBS on upper limb motor function [22], and another article combined iTBS and cTBS to improve upper limb motor dysfunction [29]. Table 2 shows the characteristics of the study in terms of basic information, intervention methods, outcome indicators and adverse events.
Table 2.
Characteristics of the included studies and outcome events
| References | Country | Research type | Adjuvant therapy | Type of intervention | Age (years) | Type of stroke | Type of motor function | Management of TBS | Outcomes | Adverse event |
|---|---|---|---|---|---|---|---|---|---|---|
| Nicolo et al. [18] | Switzerland | RCT |
1. Type: PT and active motor exercises of the upper extremity 2. Dosage of therapy: 60 min/d; 15 sessions, 3 weeks |
E1: cTBS-Contra-M1 (n = 14) E2: tDCS-Contra-M1 (n = 14) C: Sham cTBS/tDCS -Contra-M1 (n = 13) |
E1: 62.4 ± 12.3 E2: 68.5 ± 10.8 C: 64.3 ± 17.7 |
Subacute 33/41 ischemic; 8/41 hemorrhagic |
Upper extremity motor function | 1. Type of stimulation: a figure of eight coil, 600 pulses, 80% of RMT of nonparetic hand muscles 2. Duration of therapy: 9 sessions, 3 weeks |
Primary outcomes: 1. UE-FMA 2. BBT 3. NHPT 4. Jamar dynamometer |
N/A |
| Vink et al. [19] | Netherlands | RCT |
1. Type: PT and OT (consisting of individualized upper limb exercises and a daily training program) 2. Dosage of therapy: 60 min/d; 14 sessions, 2 weeks |
E: cTBS-Contra-M1 (n = 28) C: sham cTBS-Contra-M1 (n = 31) |
E: 56.8 ± 12 C: 63.4 ± 12 |
Acute; 50/59 ischemic; 9/59 hemorrhagic |
Upper extremity motor function | 1. Type of stimulation: an angulated 100 mm figure-of-eight coil, 600 pulses, 70% RMT of nonparetic FDI 2. Duration of therapy: 14 sessions, 2 weeks |
Primary outcomes: 1. ARAT Secondary outcomes: 1. mRS 2. FMA 3. BI 4. NHPT 5. JTT 6. EuroQol-5D 7. Stroke Impact Scale score—upper limb |
E: 10 cases of headache, 2 cases of Muscle pain C: 1 case of Nausea, 3 cases of headache, 1 case of Sensory Impairment, 2 cases of Slowed thinking |
|
Kuzu et al [20] |
Turkey | RCT |
1. Type: PT (consisting of range of motion, stretching, strengthening exercises and daily living activities) 2. Dosage of therapy: 60 min/d; 10 sessions |
E1: cTBS-Contra-M1 (n = 7) E2: LF-rTMS-Contra-M1 (n = 7) C: sham cTBS/LF-rTMS -Contra-M1 (n = 6) |
E1: 61.3 ± 9.8 E2: 56.3 ± 11.5 C: 65.0 ± 4.6 |
Chronic; 20/20 ischemic |
Upper extremity motor function | 1. Type of stimulation: an eight-shaped 70 mm coil, 600 pulses, 80% AMT of nonparetic APB 2. Duration of therapy: 10 sessions |
Primary outcomes: 1. MAS 2. UE-FMA Secondary outcomes: 1. FIM 2. MAL 3. Brunnstrom upper extremity and hand motor recovery stage |
N/A |
| Kondo et al. [21] | Japan | NRCT |
1. Type: Intensive OT (consisting of two one-to-one training sessions and Two self-training exercises) 2. Dosage of therapy: 240 min/d; 12 sessions, 15 days |
E1: cTBS-Contra-the nonlesional hemisphere (n = 32) E2: LF-rTMS-Contra- the nonlesional hemisphere (n = 71) |
E1: 60.0 ± 14.2 E2: 62.3 ± 12.5 |
Chronic; 41/103 ischemic; 62/103 hemorrhagic |
Upper extremity motor function |
1. Type of stimulation: a 70-mm figure-8 coil, 2400 pulses, 80% AMT of FDI 2. Duration of therapy: 12 sessions, 15 days |
Primary outcomes: 1. FMA 2. WMFT | N/A |
| Talelli et al. [22] | UK | RCT (A crossover study) | N/A |
E1: cTBS-Contra-the motor hotspot E2: iTBS-Ipsil-the motor-hotspot E3: sham cTBS/Itbs-Contra/Ipsil-the motor hotspot |
57.7 ± 14.9 |
Chronic; 6/6 hemorrhagic |
Upper extremity motor function |
1. Type of stimulation: a 70 mm figure of eight coil, 600 pulses, 80% AMT 2. Experiments were conducted at intervals of at least 10 days, and the order was randomized |
Primary outcomes: 1. SRT 2. Simple reaction grip strength 3. VAS 4. I/O curves 5. RMT 6.AMT |
N/A |
| Ackerley et al. [23] | New Zealand | RCT |
1. Type: PT (consisting of strengthening, task-specific and functional tasks) 2. Dosage of therapy: 45 min/d; 10 sessions, 10 days |
E: iTBS-Ipsil-M1 (n = 9) C: sham iTBS-Ipsil-M1 (n = 9) |
E: 61(median) C: 71(median) |
Chronic; N/A |
Upper extremity motor function |
1. Type of stimulation: 600 pulses, 90% AMT of nonparetic FDI 2. Duration of therapy: daily, 10 days |
Primary outcomes: 1. ARAT 2. UE-FMA 3. Paretic FDI MEP 4. CE asymmetry 5. FA asymmetry |
N/A |
| Chen et al. [27] | China | RCT |
1. Type: Conventional PT (including limb positioning, postural training, stretching, task-oriented therapy, and sensory stimulation) 2. Dosage of therapy:50 min/d; 10 sessions, 2 weeks |
E: iTBS-Ipsil-Cerebellar (n = 16) C: sham iTBS-Ipsil-Cerebellar (n = 16) |
E: 57.38 ± 8.04 C: 51.44 ± 9.19 |
Subacute; 18/32 ischemic; 14/32 hemorrhagic |
Upper extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 600 pulses, 80% AMT of APB 2. Duration of therapy: 10 sessions, 2 weeks |
Primary outcomes: 1. MAS 2. MTS Secondary outcomes: 1. Hmax/Mmax ratio 2. MEP potential latency and amplitude 3. CMCT 4. BI |
N/A |
| Kim et al. [28] | South Korea | RCT (A crossover study) | N/A |
E1: iTBS-Ipsil-FCR hot pot E2: sham iTBS-Ipsil-FCR hot pot |
60.7 ± 8.7 |
Chronic; 12/15 ischemic; 3/15 hemorrhagic |
Upper extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 600 pulses, 80% AMT of paretic FCR 2. The two experiments were performed in a randomized order and separated by 1 week |
Primary outcomes: 1. MAS 2. MTS 5.PTA 3. H/M ratio 4. PT 5. PTA 6. work and rectified integrated EMG activity of wrist flexor |
N/A |
| Zhang et al. [29] | Chinese Hong kong | RCT |
1. Type: Robot-assisted training (consisting of the upper limb proximal joint training and distal joint training) 2. Dosage of therapy: 40 min/d; 10 sessions, 3 weeks |
E1: priming iTBS-Contra/Ipsil-the motor hotspot (n = 14) E2: nonpriming iTBS-Contra/Ipsil-the motor hotspot (n = 14) C: sham stimulation-Contra/Ipsil -the motor hotspot (n = 14) |
E1: 58.21 ± 9 E2: 59.5 ± 8.56 C: 64 ± 5.39 |
Chronic; 24/42 ischemic; 18/42 hemorrhagic |
Upper extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 600 pulses, 70% RMT 2. Duration of therapy: 10 sessions, 3 weeks |
Primary outcomes: 1. UE-FMA 2. ARAT 3. The mean velocity of movement |
N/A |
| Liao et al. [30] | China | RCT |
1. Type: PT (including trunk control training, sit-to-stand training, balance exercises, and gait training) 2. Dosage of therapy: 90 min/d; 15 sessions, 3 weeks |
E1: iTBS-Ipsil-M1 (n = 12) E2: iTBS-Ipsil-cerebellar (n = 12) C: sham iTBS-Ipsil-M1/cerebellar (n = 12) |
E1: 58.25 ± 14.63 E2: 57.17 ± 11.63 C: 57.08 ± 11.28 |
Subacute; 12/36 ischemic; 24/36 hemorrhagic |
Lower extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 1200 pulses, 80% RMT of FDI 2. Duration of therapy: 15 sessions, 3 weeks |
Primary outcomes: 1. BBS Secondary outcomes: 1. FMA-LE 2. TIS 3. BI 4. mRS 5. FAC 6. HAMD 7. HAMA |
E1: 1 case of mild headache E2: 1 case of mild vertigo |
| Xie et al. [31] | China | RCT |
1. Type: Conventional PT (including transfer, balance, and ambulation training 2. Dosage of therapy: 50 min/d; 10 sessions, 2 weeks |
E: iTBS-Ipsil-cerebellar (n = 18) C: sham iTBS-Ipsil-cerebellar (n = 18) |
E: 52.35 ± 8.62 C: 54.41 ± 7.01 |
Subacute; 20/36 ischemic; 16/36 hemorrhagic |
Lower extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 600 pulses, 80% AMT of nonparetic APB 2. Duration of therapy: 10 sessions, 2 weeks |
Primary outcomes: 1. FMA-LE Secondary outcomes: 1. 10 MWT 2. TUG 3. FAC 4. RMT 5. MEP amplitude |
N/A |
| Koch et al. [32] | Italy | RCT |
1. Type: PT (including muscle stretching, active-assisted mobilizations, progressive neuromuscular facilitation training balance exercises, and gait training) 2. Dosage of therapy: 90 min/d; 21 sessions, 3 weeks |
E: iTBS-Ipsil-cerebellar (n = 18) C: sham iTBS-Ipsil-cerebellar (n = 18) |
E: 63 ± 11 C: 65 ± 12 |
Chronic 36/36 ischemic |
Lower extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 1200 pulses, 80% AMT 2. Duration of therapy: daily, 3 weeks |
Primary outcomes: 1. BBS Secondary outcomes: 1. FMA 2.BI 3. Gait analysis(step length, step width) 4. GMFP 5. oscillatory activity |
N/A |
| Liao et al. [33] | China | RCT |
1. Type: PT (including trunk control training, sit-to-stand training, balance exercises, and gait training) 2. Dosage of therapy: 50 min/d; 15 sessions, 3 weeks |
E: iTBS-Ipsil-cerebellar (n = 15) C: sham iTBS-Ipsil-cerebellar (n = 15) |
E: 51.53 ± 9.22 C: 55.40 ± 8.10 |
Subacute and Chronic; 15/30 ischemic; 15/30 hemorrhagic |
Lower extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 600 pulses, 80% AMT 2. Duration of therapy: 10 sessions, 2 weeks |
Primary outcomes: 1. BBS Secondary outcomes: 1. TIS 2.FMA-LE 3. BI 4. CSP 5. MEP amplitude |
E: 1 case of a mild headache C: N/A |
| Lin et al. [34] |
Chinese Taiwan |
RCT |
1. Type: PT (including transfer, balance, and ambulation training) 2. Dosage of therapy: 45 min/d;10 sessions, 5 weeks |
E: iTBS-Ipsil-LE-M1 (n = 10) C: sham iTBS-Ipsil-LE-M1 (n = 10) |
E: 60.8 ± 8.1 C: 61.1 ± 9.7 |
Chronic; 16/20 ischemic; 4/20 hemorrhagic |
Lower extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 1200 pulses,100% MT 2. Duration of therapy: 10 sessions, 5 weeks |
Primary outcomes: 1. NIHSS 2. mRS 3. BRS 4. TUG 5. 10 MWT 6. FMA-LE 7. BI |
N/A |
| Wang et al. [35] | China | RCT |
1. Type: Conventional PT (including muscle strength training, balance and coordination training, walking training, and activities of daily living training) 2. Dosage of therapy: 30 min/d; 20 sessions, 4 weeks |
E: iTBS-Ipsil-cerebellar (n = 21) C: sham iTBS-Ipsil-cerebellar (n = 21) |
E: 52.62 ± 8.61 C: 54.62 ± 7.85 |
Chronic; 25/42 ischemic; 17/42 hemorrhagic |
Lower extremity motor function |
1. Type of stimulation: a 70-mm Fig. 8 coil, 600 pulses,80% AMT 2. Duration of therapy: 20 sessions, 4 weeks |
Primary outcomes: 1. FMA-LE 2. BBS 3. MBI 4. MEP potential latency |
N/A |
Acute stroke means stroke < 1 month. Subacute stroke means stroke from 1 to 6 months. Chronic stroke means stroke more than 6 months. N/A means not available.
non-randomized controlled trial, NRCT; randomized controlled trial, RCT; MT, motor threshold; RMT, rest motor threshold; UE-FMA, Upper-Extremity Fugl-Meyer Assessment; WMFT, the Wolf Motor Function Test; FAS, the functional ability score; PT, Physical therapy; tDCS, transcranial direct current stimulation; BBT, Box and Block test; NHPT, Nine Hole Peg Test; OT, Occupational therapy; JTT, Jebsen Taylor Test; FDI, First dorsal interosseous; ARAT, the Action Research Arm Test score; mRS, the modified Rankin Scale; BI, the Barthel Index; APB, Abductor pollicis brevis muscle; MAS, Modified Ashworth Scale; FIM, Functional Independence Measure; MAL, Motor Activity Log; AMT, active motor threshold; SRT, simple reaction time; VAS, the visual analogue scale; I/O curves, Input–Output curves; PF, Preload force; PD, Preload duration; MTS, the modified Tardieu scale; SWV, the shear wave velocity; Hmax/Mmax ratio, the H-maximum wave/M-maximum wave amplitude ratio; MEP, motor-evoked potential; CMCT, central motor conduction time; CE, corticomotor excitability; FA, fractional anisotropy of the posterior limb of the internal capsule; PT, peak torque; PTA, peak torque angle; EMG, Electromyography; FCR, flexor carpi radialis; BBS, Berg balance scale; FMA-LE, Fugl-Meyer assessment scale for lower extremities; TIS, The trunk impairment scale; HAMD, Hamilton depression scale; HAMA, Hamilton anxiety scale; 10 MWT, The ten-meter walking test; TUG, The Timed Up and Go test; FAC, The functional ambulation category scale; GMFP, global mean field power; CSP, the cortical silent period; MT,midline motor threshold; NIHSS, National Institute of Health Stroke Scale; BRS, Brunnstrom Stage; MBI, modified Barthel Index.
Quality evaluation
In this study, the Modified Downs and Black Checklist was used to assess the potential bias of the included studies. Two articles were of medium quality [21, 26], 16 articles were of high quality[18–20, 22–25, 27–35], and the methodological quality of the included studies was relatively high. The evaluation results are shown in Table 3.
Basic characteristics of iTBS related studies
TBS can be divided into iTBS and cTBS according to the different pulse stimulation modes. 14 studies used iTBS [22–35], of which 8 studies reported the effects of iTBS on upper limb motor function in stroke patients [22–29]. Six articles explored the effects of iTBS on lower limb motor function in stroke patients [30–35], including 13 randomized controlled trials [22, 23, 25–35] and one non-randomized controlled trial [24].
All stroke patients included in the study were accompanied by varying degrees of limb motor dysfunction. The average age of the patients was over 50 years old, but there were differences in the average age of the patients among different studies. The course of the disease included subacute phase and chronic phase. Most studies selected stroke patients in the chronic phase, including 134 patients with hemorrhagic stroke and 183 patients with ischemic stroke.
The TBS treatment protocol uses 600 pulses 11 items [22–29, 31, 33, 35], 1200 pulses 3 items [30, 32, 34], 20 or more sessions 2 items [32, 35], 10 to 15 sessions 9 items (15 sessions 1 item, 10 sessions 8 items) [23–25, 27, 29–31, 33, 34], 1 time 3 terms [22, 26, 28]. One study did not specify the type of stimulation coil for iTBS [23], and the remaining 13 studies all used figural 8 coils [22, 24–35]. Regarding the stimulation site of iTBS to improve upper limb motor function, 3 studies were ipsilateral M1 [23, 25, 26], 1 was contralateral cerebellum [27], 1 was the motor hot spot corresponding to FDI [24], and 1 was the motor hot spot corresponding to FCR on the affected side [28]. Two studies did not explain in detail the location of the stimulation motor hot spot [22, 29]. FDI was selected to determine motor threshold in 4 studies [23–26], APB in 1 study [27], FCR in 1 study [28], and 2 studies were not reported [22, 29]. As for the stimulation site of iTBS to improve the motor function of the lower limbs, one item is the motor hot spot corresponding to the contralateral rectus femoris muscle [34], four items are the contralateral cerebellum [31–33, 35], and one item compares the difference between the stimulation of the contralateral cerebellum and the ipsilateral M1 [30]. Among them, FDI was selected for motor evoked potential (MEP) measurement in one study [30], APB in one study [31], rectus femoris in one study [34], and three studies were not reported [32, 33, 35].
Regarding adjuvant therapy combined with iTBS, 10 studies were PT [23, 24, 26, 27, 30–35], 1 was PT and OT [19], 1 was robot-assisted training [29], and 2 studies did not take any adjuvant therapy [22, 28]. The duration of daily adjuvant therapy was more than 50 min in 3 cases [25, 30, 32], between 40 and 50 min in 6 cases [23, 27, 29, 31, 33, 34], less than 40 min in 2 cases [26, 35], and 1 case was not reported [24]. Adjuvant therapy was given for 20 or more courses in 2 cases [32, 35], 15 courses in 2 cases [30, 33], 10 courses in 7 cases [23–25, 27, 29, 31, 34], and 1 case was not specified [26]. There were differences in the specific adjuvant treatment modalities in each study, as detailed in Table 2.
Basic characteristics of cTBS related studies
Seven articles reported the effect of cTBS on upper limb motor function in stroke patients [18–22, 24, 26], but no literature on the effect of cTBS on lower limb motor function was retrieved. There were five randomized controlled trials [18–20, 22, 26] and two non-randomized controlled trials [21, 24].
All the stroke patients included in the study were middle-aged and elderly people with different degrees of limb motor dysfunction, including acute stage, subacute stage and chronic stage. Among them, 85 patients had hemorrhagic stroke, 144 patients had ischemic stroke, and the stroke type of 50 patients was not specified in the study.
Six items of 600 pulses [18–20, 22, 24, 26], 1 item of 2400 pulses [21], 4 items of 10 to 15 courses [19–21, 24], 1 item of 9 courses [18], and 2 items of 1 course [22, 26]were used in the TBS treatment regimen. All seven studies used figure-of-eight coils. Regarding the stimulation site of cTBS to improve upper limb motor function,4 studies were on the contralateral M1 [18–20, 26], 1 was on the nonlesional hemisphere [21], 1 was on the motor hot spot corresponding to FDI [24], and 1 study did not explain the location of the motor hot spot of the stimulation in detail [22]. FDI was selected to measure MEP in four studies [19, 21, 24, 26], APB in one study [20], hand muscles in one study [18], and one study was not reported [22].
Regarding adjuvant therapy in combination with iTBS,4 studies were PT [18, 20, 24, 26], 1 was OT [21], 1 was both PT and OT [19], and 1 study did not take any adjuvant therapy [22]. The duration of daily adjuvant therapy was more than 60 min in 1 item [21], 30 to 60 min in 3 items [18–21], less than 30 min in 1 item [26], and 1 item was not reported [24]. Five courses of adjuvant therapy were 10 or more [18–21, 24], and one was not specified [26]. There were differences in the specific adjuvant treatment modalities in each study, as detailed in Table 2.
Adverse events
Numerous clinical trials have established the safety and efficacy of TBS in treating stroke. In one of the studies included in this article, headache was the most common side effect after cTBS treatment, with a higher incidence in the experimental group, while other side effects, such as muscle pain and nausea, were relatively rare [19]. Treatment with cTBS was safe and well tolerated in this study, with no severe adverse events. One study reported that after iTBS treatment, only one patient in the experimental group had a mild headache but did not require treatment to resolve the headache [33]. Another experiment showed that after iTBS stimulation of the cerebellum or M1, one patient reported mild headache and one patient experienced mild vertigo after the first intervention [30]. In addition, the other experiments did not show any adverse events. In general, the use of TBS in the treatment of stroke is safe and reliable, but the relevant safety guidelines of TBS still need to be gradually improved to reduce the occurrence of adverse events.
Discussion
Limb dysfunction is one of the common sequelae of stroke. Limitation of upper limb function can lead to difficulties in daily activities such as eating, dressing, and personal care, and a decline in quality of life [36]. Lower limb motor dysfunction is often accompanied by a decline in balance function, usually due to poor proprioception, decreased motor control ability, and abnormal integration of the nervous system. In addition, balance problems may be the main reason for poor walking ability and increased risk of falls in stroke patients [37]. Therefore, the improvement of upper limb and lower limb motor disorders is the key to the rehabilitation of stroke.
Mechanism of TBS in improving motor dysfunction after stroke
The common rTMS commonly used in clinical practice can be divided into high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) and low-frequency repetitive transcranial magnetic stimulation (LF-rTMS). HF-rTMS can produce the same excitatory effect as iTBS and improve the excitability of the cerebral cortex, while LF-rTMS has the same inhibitory effect as cTBS. It can reduce the excitability of cortical neurons in stimulated brain areas [38]. The mechanism of rTMS in improving motor dysfunction after stroke is similar to that of TBS. By regulating calcium channels, RTMS induces long-term potentiation (LTP) and long-term depression (LTD) effects on synaptic structure and function [39]. It can regulate the activity of cerebral cortex, promote the release of a variety of neurotransmitters and the secretion of neurotrophic factors [40, 41], regulate gene expression, inhibit the secretion of pro-inflammatory cytokines, reduce neuroinflammatory response [42], induce microcirculation repair of necrotic nerve tissue, improve cerebral blood flow [43], and promote nerve growth and regeneration. It can also intervene in the remodeling of nerve tissue structure through neuromodulation to improve motor function [44]. Compared with common repetitive transcranial magnetic therapy, TBS can regulate the excitability of cerebral cortex in a shorter time, produce corresponding physiological changes and nerve repair effects, and promote the recovery of motor function. In addition, stroke patients are often accompanied by anxiety and depression [45]. TBS treatment can improve the compliance of patients during treatment and improve the efficiency of clinical treatment.
Although the specific mechanism of TBS in improving motor dysfunction after stroke has not been fully clarified, it is generally believed that TBS can improve the functional prognosis of patients by regulating the excitability of motor cortex, inducing the reorganization of neural network and regulating the circulation and metabolism in the brain [46]. The current mechanism explanations are as follows.
Firstly, from the perspective of synaptic plasticity, TBS can improve the plasticity of the brain by promoting or inhibiting synaptic transmission [47]. Synaptic plasticity occurs in the form of LTP and LTD. Cortical LTP and LTD are commonly mediated by N-methyl-D-aspartate receptors (NMDA-R) receptor activation [48] and are associated with glutamate receptors (Glu-R) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPA-R) are also closely related [47, 49].TBS can regulate the activity of neurotransmitter receptors, change the concentration of postsynaptic calcium ions, reduce or increase the entry of intracellular calcium ions, and affect the postsynaptic response mediated by neurotransmitter receptors, thereby producing LTD or LTP-like effects and promoting the recovery of brain nerve function.
Secondly, from the point of gene and protein level, TBS plays an important role in the recovery of brain function after stroke by affecting gene expression and protein synthesis and changing synaptic remodeling. The early gene proteins c-Fos and zif268 are associated with synaptic connectivity and remodeling, and some studies have shown that iTBS enhances c-Fos protein expression in the limbic cortex and zif286 expression in most cortical regions [50]. Thimm et al. [51] also found that cortical c-Fos and zif268 expression was enhanced, while parvalbumin (PV) and glutamic acid decarboxylase (GAD67) expression was decreased in the cortex after iTBS treatment. In addition, a study by Benali et al. [52] showed that TBS affected the expression of calbindin PV and calbindin D-28 k (CB) in the cortex, and the change of cortical activity after TBS treatment was related to the change of inhibitory system activity. ITBS primarily affects the inhibitory control of pyramidal output activity by decreasing the expression of PV in rapidly firing interneurons, whereas cTBS more likely affects the dendritic integration of synaptic inputs controlled by other types of inhibitory interneurons by decreasing the expression of CB [52]. ITBS and cTBS regulate the expression of various gene proteins in different ways, regulate the activity of different types of inhibitory cells, affect the change of cortical activity, and ultimately promote the recovery of brain function after brain injury.
Thirdly, from the perspective of cortical excitability regulation, TBS can affect the excitability of the cerebral cortex, induce the reorganization of neural network, and promote the recovery of motor function. The amplitude of MEP is an important index for evaluating cortical excitability. Huang et al. [53] used a transcranial magnetic stimulator to deliver TBS to human primary motor cortex (M1). They found that cTBS acting on the contralateral hemisphere M1 could increase the amplitude of MEP in that hemisphere, while iTBS acting on the affected hemisphere M1 could decrease the amplitude of MEP in that hemisphere. In other words, iTBS could enhance the excitability of the stimulated side M1, while cTBS had the opposite effect. In addition to using TBS to stimulate M1, other studies have explored different brain targets [27, 28, 30–35]. The effect of TBS on cortical excitability in different brain regions has shown the same results as Huang et al. [53], which promotes the recovery of motor function in stroke patients.
Fourthly, from the perspective of motor pathway reconstruction, TBS may play an important role in the structural and functional recovery of descending white matter conduction tracts. White matter is mainly composed of nerve fibers, which are responsible for the transmission of nerve signals, and the descending fiber bundles are responsible for the transmission of motor signals. The corticospinal tract (CST) is a descending white matter conduction pathway, which is an important fiber bundle connecting the cerebral cortex and the spinal cord, and plays a key role in motor function. The degree of injury after focal brain injury is closely related to motor recovery [54, 55]. After stroke, the interruption of cerebral blood flow leads to ischemia and hypoxia, which causes nerve cell death and tissue damage, and leads to a series of pathological changes in white matter fiber tracts, especially CST, which may cause axon swelling and fracture, demyelination, reduce nerve conduction velocity, and affect the transmission of nerve signals [56]. Relevant studies have found that when TBS acts on the motor area of cerebral cortex, it can increase the postsynaptic potential between CST neurons, enhance the synaptic transmission efficiency by inducing LTP/LTD and synaptic plasticity changes, thereby enhancing motor signal transmission and improving the motor function of stroke patients [53]. Fujiki et al. [57] used MEP to reflect the integrity and excitability of CST and found that after TBS intervention, the driving effect of cerebral cortex on spinal motor neurons was enhanced, and the corticospinal excitability of patients was significantly improved, which could activate muscles more effectively and improve motor performance. In contrast to conventional techniques for functional assessment of white matter, diffusion tensor imaging (DTI) allows qualitative and quantitative assessment of white matter tracts and their microstructural integrity [58, 59]. Wadden et al. [60] used cTBS to stimulate M1 or primary somatosensory cortex on the healthy side of stroke patients and used DTI to observe the microstructure changes of white matter. It was found that the fractional anisotropy (FA) of white matter tracts in the experimental group was significantly different from that in the control group, the microstructural integrity of white matter was significantly improved, and the patients' motor performance was also significantly improved after the intervention. However, only one study has used DTI to explore the effect of TBS on white matter tracts after stroke. Future research should focus on quantitative morphometric measurements of white matter tracts after stroke to further explore its specific mechanisms.
Fifthly, from the perspective of changes in regional cerebral blood flow perfusion, the recovery of neurological function may be associated with the improvement of cerebral blood flow [61]. TBS can also promote the recovery of motor function by improving the cerebrovascular function of stroke patients. After stroke, the integrity of the blood–brain barrier (BBB) structure is compromised, leading to increased permeability, abnormal cerebral blood flow regulation and substance metabolism [62, 63]. Relevant studies have shown that TBS can reduce the degradation of BBB tight junction components (ZO-1, cludin-5, occludin and caveolin-1) on the one hand, significantly reduce BBB permeability in stroke patients, promote reoxygenation and reperfusion of microcerebral vessels, and maintain normal vascular morphology [64]. On the other hand, it can enhance the expression of HIF-1α, regulate the hypoxia response, and increase the surface area and volume of astrocytes related to the vascular system, and transform the pro-inflammatory A1 state into the anti-inflammatory A2 phenotype, repair the structure and perfusion damage of blood vessels, and improve the vascular prognosis [64]. At the same time, TBS also plays an important role in the regulation of cerebral blood flow. ITBS can increase the cerebral blood flow of the cerebral cortex, while cTBS can reduce the cerebral blood flow, and this change in cerebral blood flow may be related to the regulation of TBS on the excitability of the cerebral cortex [65]. Pichiorri et al. [66] used transcranial Doppler ultrasound monitoring to find that after receiving iTBS in healthy subjects, the responsiveness of bilateral vasodilation to Carbon dioxide increased, and the cerebral blood flow under the stimulation coil increased temporarily, indicating that iTBS may affect the microcirculation of the brain and improve the perfusion of cerebral blood flow by regulating the relaxation and contraction function of cerebral vessels. Cho et al. [67] found that cTBS could reduce the cerebral blood flow in the ipsilateral dorsolateral prefrontal cortex (BA46) and the rostral part of the prefrontal cortex (BA10) in normal people, indicating that the effect of cTBS on the brain is related to the change of cerebral blood flow. In order to ensure the safety of subjects, Pichiorri and Cho's study is based on normal people, which can also produce corresponding improvement effect on stroke patients, and the subsequent clinical application needs to adopt corresponding TBS treatment strategies according to the specific conditions of patients.
Theoretical model of TBS in improving motor dysfunction after stroke
At present, the use of TBS to improve the level of motor function after stroke is mainly based on the interhemispheric inhibition (IHI) model [68], the vicariation model and the bimodal balance-recovery model [69], and most relevant clinical studies are based on these three models.
The IHI model assumes balanced mutual inhibition between the two hemispheres of the healthy brain, and also predicts that once damaged, inhibition from the affected hemisphere will be reduced [68]. After stroke, the mutual inhibition between the two hemispheres is no longer balanced, and the inhibition effect of the contralateral cortex on the affected cortex is stronger, resulting in the phenomenon of excessive excitation of the contralateral side and excessive inhibition of the affected side, which hinders the remapping and re-learning of the neuroplasticity of the reserved structure of the affected hemisphere and is not conducive to the recovery of the motor function of the patients [70]. Studies have shown that restoring the balance of the excitability of the interhemispheric cortex can improve the overall prognosis [71]. According to this theory, stroke can be treated by inhibiting the contralateral cortex’s excitability or enhancing the affected cortex’s excitability. Recent proof-of-principle studies have shown that using specific transcranial magnetic stimulation patterns to stimulate the affected hemisphere to up-regulate excitability, or to stimulate the contralateral hemisphere to down-regulate excitability, can cause significant behavioral improvement in convalescent stroke patients [72]. It is worth noting that this model is based on the upper limb motor system, and whether the IHI model can be applied to the recovery of lower limb motor function is still controversial. Chieffo et al. [68] believe that the IHI model may not be applicable to the lower limb, but so far, there is also some evidence that the IHI model is suitable for the recovery of lower limb motor function[30–33, 35], which still needs to be proved by a large number of clinical studies in the future.
The vicariation model assumes that the reorganization of brain regions after brain injury can replace the function of nearby injured regions[73, 74], that is, the healthy side of the brain can compensate for the function of the affected side of the brain, and the activity of the healthy hemisphere may contribute to the functional recovery after stroke [75]. Considering this compensatory neural plasticity, promoting stimulation should be applied to the healthy hemisphere to enhance the excitability of compensatory neurons [74]. According to this model, facilitation in the contralateral hemisphere may stimulate brain tissue reorganization and restore the balance of motor cortex excitability between the two sides of the brain, thereby promoting functional recovery of the affected limb. The IHI model and the vicariation model suggest opposite predictions for optimal neuromodulation strategies in stroke patients. The IHI model predicts that inhibiting the excitability of the contralateral hemisphere will benefit stroke recovery because it improves the abnormal inhibition of the contralateral hemisphere by the affected hemisphere. The vicariation model predicted that such an intervention would be counterproductive because it would interfere with the compensatory activity of the contralateral hemisphere.
Di Pino et al. [69] considered that neither the IHI model nor the vicariation model is sufficient and not suitable for the treatment of all stroke patients, so they put forward a new neural rehabilitation model, the bimodal balance-recovery model, which links the interhemispheric balance and the structural reserve after the lesion. It is believed that the integrity of the remaining function of the motor area and corticospinal tract in the affected hemisphere determines the recovery mechanism after stroke, which perfects the above two theories. The mechanism of the bimodal balance-recovery model can be explained by the inhibitory effect on the contralateral hemisphere based on the IHI model in the patients with less damage and the promoting effect on the contralateral hemisphere based on the vicariation model in the patients with more damage. Subsequent related studies have also demonstrated that the healthy hemisphere should be inhibited for patients with lower injury, and on the contrary, the healthy hemisphere should be promoted for patients with higher injury [76, 77].
Application of cTBS in improving upper limb motor function after stroke
Studies on the improvement of upper limb motor function by cTBS have shown different results, and the therapeutic effect is somewhat controversial. At present, there are two randomized controlled studies using cTBS combined with conventional exercise training to improve upper limb hemiplegia after stroke, and the results show that the upper limb motor function of patients is significantly improved after treatment [19, 20]. However, Nicolo et al. [18] reported that cTBS in the contralateral M1 of the subject’s brain could not improve the upper limb motor function level of stroke patients and improve the clinical motor gain. In addition, the trial also assessed the brain function of patients before and after treatment by electroencephalogram (EEG), and found that if noninvasive brain stimulation (NIBS) started within the first 4 weeks after stroke, it would increase the functional connectivity (FC) of ipsilateral resection of motor nodes, and early intervention seemed to enhance the recovery of motor function better. van Lieshout et al. [78] demonstrated in a meta-analysis that the use of TMS to improve upper limb motor function should be given as early as possible and more beneficial starting in the first month after stroke. A recent study achieved early intervention. cTBS on the contralateral M1 combined with upper limb training started within 3 weeks after the onset of stroke, promoted the recovery of upper limb motor function, reduced disability and dependence, shortened the length of hospital stay in the rehabilitation center, and also had a long-term effect on the recovery of upper limb motor function [19]. At present, in the four experimental studies exploring the effect of cTBS on the recovery of upper limb motor function, there is no quantitative evaluation of cerebral cortex or corticospinal cord excitability through instruments, so as to objectively reflect the correlation between the reorganization of brain neural network and the recovery of upper limb motor function[18–21]. An experimental protocol by van Lieshout et al. [79] made up for this deficiency by using functional magnetic resonance imaging (fMRI) to assess the brain functional status of patients. That is to say, fMRI can reflect ischemic injury, white matter integrity, functional connectivity and cortical activation, which provides a new idea for the design of objective evaluation methods for later clinical research.
LF-rTMS and cTBS are the most commonly used inhibitory non-invasive brain stimulation techniques to regulate the balance of neural network after stroke. Kuzu et al. [20] compared the effects of cTBS and LF-rTMS on upper limb spasticity and functional recovery in patients with chronic ischemic stroke. This study showed that the therapeutic effects of these two non-invasive brain stimulation techniques were similar, but the application time of cTBS was shorter, which could improve the comfort of patients during treatment and produce more clinical benefits. However, Kondo et al. [21] showed in a study that the LF-rTMS group had a more significant increase in the Fugl-Meyer assessment (FMA) score compared with the cTBS group, and LF-rTMS combined with OT was more recommended for hemiplegic patients. Whether cTBS is the best inhibitory noninvasive brain stimulation technology is still in doubt, and we still need a large number of clinical experiments to prove it.
In a word, future research needs to apply more objective equipment and technology to explore the relationship between brain network function reorganization and upper limb motor function improvement, and constantly improve the design of clinical experiments, explore the best parameters of cTBS treatment, and explore more therapeutic advantages of cTBS. To provide individualized and targeted treatment programs for stroke patients with different stages and degrees of injury.
Application of iTBS in improving upper limb motor function after stroke
So far, many studies have verified the effectiveness of iTBS in improving the upper limb motor function of stroke patients and shown that it has produced significant clinical benefits. Talelli et al. [22] first used iTBS to improve upper limb motor function in patients with chronic stroke and observed improvements in grip strength and reaction speed. Ackerley et al. [23] believed that iTBS may achieve neural network reorganization by increasing cortical excitability and other mechanisms to promote the recovery of upper limb motor function. A recent meta-analysis also showed that iTBS has a good therapeutic effect on the recovery of motor function in stroke patients [80]. Among them, the results of sham-controlled studies on the effects of iTBS on upper limb motor function in patients with chronic stroke are controversial [22–26]. Four studies have shown that iTBS can significantly improve motor function [22, 23, 25, 26], while one study showed no difference between the iTBS group and the sham stimulation group [24]. The reason for the above difference may be related to the inconsistency in the selection of evaluation indicators, the age of patients included and the course of disease. Previous studies have reported that patients in the acute phase have higher neuroplasticity than those in the chronic phase [18], and a large number of functional recovery and high levels of neuroplasticity can be observed [81]. It is suggested that rehabilitation after stroke should be carried out as early as possible, which may better improve the clinical benefits of motor recovery.
Some studies are no longer limited to the use of traditional clinical exercise scales, but through the perspective of electrophysiology and biomechanics to conduct quantitative analysis, the purpose is to deeply understand the specific impact of iTBS on upper limb spasticity after stroke, and understand the recovery process of motor ability accordingly. Chen et al. [27] conducted a study using electrophysiological indicators related to the degree of spasticity, and the results showed that compared with the sham stimulation group, iTBS combined with conventional neurological rehabilitation treatment could reduce the spasticity of patients with subacute stroke and improve the motor function of upper limbs, especially the fine motor function. Kim et al. [28] verified the effectiveness of iTBS in reducing post-stroke upper limb spasticity on the basis of clinical, electrophysiological, and biomechanical evaluations in a crossover experiment, and the results showed that both the degree of upper limb spasticity and motor function of patients were significantly improved after iTBS stimulation. In addition, the results of the above studies have shown that a single iTBS on the affected side of the brain or multiple cerebellar iTBS can produce short-term therapeutic effects [27, 28]. When using TBS to improve upper limb motor dysfunction, we can change the treatment ideas and promote the recovery of upper limb motor function by directly improving upper limb spasticity. Further studies are needed in the future to explore the potential mechanism of different target iTBS, induce longer-term ameliorative effects, and determine its benefits in the clinical setting.
As to which mode of iTBS or cTBS will produce a more significant effect on improving upper limb motor function in stroke patients, Talelli et al. [22] explored the effect of a single TBS on hand functional behavior and physiological indexes in patients with chronic stroke, and the results showed that ipsilateral iTBS temporarily improved the motor behavior and corticospinal output of the affected hand. Compared with cTBS, iTBS may be a more effective transcranial magnetic treatment. A recent meta-analysis also showed that iTBS was more effective than cTBS in treating upper limb motor deficits after stroke [82]. In addition, some studies suggest that the combination of iTBS and cTBS has a more significant effect on improving upper limb motor dysfunction in stroke patients than the intervention of iTBS or cTBS alone. The inhibitory priming stimulation through cTBS can stabilize or even enhance the stimulatory effect of the subsequent excitatory conditioned reflex stimulation through iTBS. ITBS priming (i.e., performing contralateral cTBS before the iTBS of the affected side) has a stronger amplification effect on the motor-evoked potential at the stimulation site [83, 84]. Zhang et al. [29] found that iTBS priming can promote the recovery of hemiplegic upper limbs after chronic stroke, and it is significantly better than non-priming iTBS and sham stimulation, especially for patients with higher upper limb function. In the treatment of chronic stroke, cTBS stimulation before iTBS stimulation can promote the activation of cerebral cortex, enhance motor control and learning ability, and is an effective treatment to improve the level of upper limb motor function of patients.
Application of iTBS to improve lower limb motor function after stroke
The choice of transcranial magnetic stimulation target is very important in research. Cerebellar iTBS is the most commonly used treatment to improve lower extremity motor function after stroke, but there is no consensus on the best stimulation target to improve lower extremity motor dysfunction in stroke patients, and many researchers have made different attempts. Liao et al. [30] compared the efficacy of iTBS on the stimulation of the cerebellum or M1 in stroke patients, and the results showed that both interventions could improve the balance function of patients, but only iTBS on the cerebellum could promote the recovery of lower limb movement. Therefore, iTBS targeting the cerebellum may be a valuable new therapeutic option in stroke rehabilitation programs. At present, there are four randomized controlled trials using the affected cerebellum as the target of iTBS stimulation, which found that iTBS stimulation of the affected cerebellum can improve the balance function and gait parameters (step width and step speed) of patients with subacute or acute stroke, and improve trunk stability and control ability, but there is no additional benefit for lower limb activities of daily living [31–33, 35]. Other studies have tried different combinations of targets to improve the level of patients with functional. Xia et al. [85] compared unilateral cerebellar (CB-single), cerebellar-primary motor cortex (CB-M1) and cerebellar-supplementary motor area (CB-SMA), the results showed that the effect of combined stimulation was better, and CB-SMA was more significant in improving the balance function of stroke patients. Lin et al. [34] discussed the feasibility and effectiveness of using iTBS to stimulate bilateral leg motor cortex (LE-M1) before PT to improve lower limb balance and motor function in stroke patients, but found no significant advantages. In addition, the cerebellar vermis plays an important role in integrating visual, proprioceptive, and sensory skin inputs, and may be a candidate stimulus target for modulating the motor network associated with balance [35]. There are two literatures that take the cerebellar vermis as the stimulation target, design the experimental scheme of using iTBS to stimulate the cerebellar vermis to improve the lower limb balance ability and motor function of patients after stroke, and design to use resting-state functional magnetic resonance imaging and functional near-infrared spectroscopy to clarify the mechanism of neural remodeling [35, 86]. In order to verify the actual effect of iTBS stimulation in the cerebellar vermis, more clinical studies are needed in the future.
Some studies used corticospinal cord excitability as an evaluation index to compare the efficacy of lower limb motor function before and after iTBS, and found that the choice of peripheral target muscles affected the reliability and validity of the experimental results to a certain extent. Liao et al. [33] selected the abductor pollicis brevis (APB) on the affected side as the peripheral target muscle, and used the amplitude of MEP on the affected side to evaluate the corticospinal excitability, while Xie et al. [31] recorded the surface electromyogram of the APB on the healthy side to measure the cortical excitability of the M1 on the healthy side. The results of both studies showed that there was no significant difference in corticospinal excitability between the experimental group and the control group, which speculated that the leg muscle response might be more specific without the difficulty of device testing. In a recent study, MEP was measured by taking the tibialis anterior muscle of the affected side as the peripheral target muscle, and the latency of MEP was recorded to reflect the integrity of motor nerve conduction pathway and nerve recovery. The results showed that compared with the control group, the latency of MEP in the experimental group decreased significantly [35], which verified the reliability of the conjecture, but a large number of clinical experiments are still needed to demonstrate it. Moreover, changes in corticospinal excitability can only be used to explain some of the underlying mechanisms of cerebellar iTBS, and future studies should use more non-invasive brain function testing techniques to assess changes in multiple regions of the brain. In addition, the difference in therapeutic coils will also affect the therapeutic effect. In previous literature on iTBS to improve lower extremity motor dysfunction [31–35], the fig-eight coil was mainly used, which has the characteristics of a small stimulation area, shallow stimulation depth, and good focusing. Studies have found that the double-cone coil can achieve a deeper stimulation effect compared with the eight-coil [87] and produce a stronger electric field [88]. Hardwick et al. [89] showed that the cerebellum tissue is deeper than the M1 area of the brain. Under the same parameters of magnetic stimulation, compared with the eight-coil, Cerebellar stimulation with a biconical coil can achieve better stimulation effect and better therapeutic effect, so the best therapeutic coil should be selected according to the stimulation site in the follow-up study.
Application summary
Most of the subjects included in this study are in the chronic phase, which may be due to the consideration of the safety of the subjects and the degree of cooperation in treatment. However, some studies have shown that the neuroplasticity of the brain in patients in the acute phase is higher, and early intervention can better enhance the recovery of motor function [19]. If there is no interference factor in the acute phase after comprehensive examination and evaluation, Such as cerebral edema, increased intracranial pressure and unstable vital signs, it is recommended that TBS should be intervened as early as possible in clinic, which may enhance the effect of motor function recovery [18].
The incidence of ischemic stroke is usually higher than that of hemorrhagic stroke. In the epidemiological survey of most regions, ischemic stroke accounts for about 60% −80% of all stroke cases [90]. A higher proportion of ischemic stroke was included in this literature, which is consistent with the epidemiological findings. In addition, patients with hemorrhagic stroke are more likely to have symptoms of disturbance of consciousness and increased intracranial pressure, while patients with ischemic stroke are more prone to hemiplegia, sensory disorders, language disorders, etc. [91]. Hemorrhagic stroke seems to be more likely to lead to dyskinesia than ischemic stroke [92]. Some studies have compared hemorrhagic stroke and ischemic stroke from the perspective of pathophysiology, pointing out that hemorrhagic stroke is caused by blood vessel rupture leading to blood entering the brain tissue, causing brain tissue injury and compression, while ischemic stroke is caused by insufficient blood supply to the brain due to vascular blockage, causing brain tissue hypoxia and necrosis [93, 94]. This difference in pathophysiology determines the difference in treatment methods between the two groups. For example, hemorrhagic stroke requires control of bleeding and reduction of intracranial pressure, while ischemic stroke requires thrombolysis and antiplatelet therapy [93, 94]. At present, the recommended method of TBS in the rehabilitation treatment of hemorrhagic stroke and ischemic stroke is still in the stage of research and exploration, and there is no clear and unified standard recommended method. In view of the fact that the condition of patients with hemorrhagic stroke in the acute stage is usually dangerous and complex, the application of TBS needs to be more cautious [95].
The pulse number commonly used in TBS treatment is 600, but some studies have used 1200 pulses and 2400 pulses [21, 30, 32, 34]. TBS with higher pulse number may enhance the connection and remodeling of nerve synapses to a certain extent, which can achieve better motor function recovery, but also increase the risk of adverse reactions. TBS with a lower pulse number is relatively mild and may have a more limited regulatory effect on the cerebral cortex, but it may be safer. At present, the results of studies on the optimal pulse number are not consistent, which may vary with individual differences, stroke types and course of disease. In many stroke patients, it is often difficult to measure the motor threshold of the target muscle because of the interruption of the motor conduction pathway. At this time, researchers may choose the other side of the mirror muscle for motor threshold measurement, but this may lead to low stimulation intensity [96]. In addition, when determining the stimulation intensity of TBS, the selection of target muscles will also affect the efficacy. In the current study, the target muscles selected for determining the motor threshold are mostly FDI, APB, FCR and other hand muscles, but when TBS improves the motor dysfunction of lower limbs, the response of leg muscles may be more specific [35]. Taking the lower limb muscle as the target muscle to measure the motor threshold to define the stimulation intensity may improve the effect of lower limb motor function recovery.
There are many types of TBS stimulation coils, such as circular coils, figure-of-eight coils, double-cone coils, and H-shaped coils. These different types of coils have their own characteristics in clinical application, and there are differences in the effective stimulation area and depth produced by them. The circular coil has a simple structure and can usually generate a relatively uniform magnetic field, but the effective stimulation area is relatively large and scattered, and the stimulation depth is shallow [97]. All the literatures included in this study used figue-8 coil, which was composed of two circular coils, with a more focused magnetic field, a relatively small but more concentrated effective stimulation area, and a slightly deeper stimulation depth for specific brain regions than the circular coil [88]. However, when deep brain regions (such as bilateral leg motor cortex) are stimulated, the double-cone coil and H-shaped coil seem to have more advantages and can achieve deeper stimulation depth. Among them, the focusing degree of the double-cone coil is better than that of the H-shaped coil, but the focusing degree is not as good as the Fig. 8 coil [98]. When choosing a TBS stimulation coil, it is necessary to comprehensively consider the characteristics of different coils according to the specific research purpose, the patient's condition, and the desired stimulation effect, so as to achieve the best therapeutic effect.
The stimulation site is the key factor of TBS stimulation protocol, which can cause corresponding local neurophysiological changes, so as to achieve different clinical effects. The stimulating brain areas included M1, Cerebellum, hand cortical motor area and lower limb cortical motor area. In addition, Supplementary Motor Area (SMA), Sensory Motor Cortex, and Prefrontal Cortex are also key brain regions for motor function recovery [99, 100]. One study compared the difference in the efficacy of iTBS on M1 and cerebellum, and found that the recovery of motor function of patients was more significant when the cerebellum was used as the stimulation target [30]. However, there is no consensus on the best target of transcranial magnetic stimulation. Moreover, compared with single-target stimulation, the effect of combined target stimulation may be better [85].
In the clinic, TBS is often combined with PT or OT. Among them, PT can promote muscle strength recovery, improve joint range of motion, enhance balance and coordination, and also promote neural plasticity. OT can improve patients' activities of daily living, promote occupational rehabilitation, provide psychological support and increase social participation. The course of treatment and duration should be appropriate; too short can not achieve the desired effect, and too long will cause fatigue in patients. It is recommended that stroke patients should have exercise training at least 3–4 times a week, 40 min of moderate intensity aerobic exercise each time, and the duration of exercise can be adjusted according to the specific situation of patients with in stable condition and tolerance [101]. The specific frequency and duration of exercise therapy should be formulated and adjusted by professional rehabilitation doctors or therapists according to the patient's specific condition, physical condition, rehabilitation progress and other factors.
Comparison of TBS with other rehabilitation modalities
In addition to TBS, many studies have explored other advanced technologies, such as robotics, gamified and virtual reality based rehabilitation models, which all have a certain effect on improving motor dysfunction after stroke [102–105], but these rehabilitation strategies have their own characteristics in terms of the way and mechanism of action on the brain. In terms of the way of influence, the influence of TBS on the brain is relatively direct. It directly regulates nerve activity and plasticity through magnetic field stimulation, and affects blood circulation and substance metabolism in the brain [46, 63]. However, rehabilitation methods based on robotics, gamification and virtual reality are more likely to indirectly affect the brain by guiding the patient's motor, cognitive and perceptual experience [106]. In terms of mechanism of action, TBS can precisely stimulate specific brain regions, regulate their excitability and plasticity, and has a certain pertinence [46]. Robotic rehabilitation is mainly aimed at the recovery of motor function, focusing on the remodeling of motor cortex and related neural pathways [107]. Gamification rehabilitation is more focused on the comprehensive impact on the brain from the aspects of psychological motivation and cognitive function, with a wide range of comprehensiveness [106]. By providing immersive multi-sensory experience, virtual reality rehabilitation comprehensively promotes the functional integration of brain perception, cognition, movement and other aspects, and has a strong comprehensiveness, which also has a certain impact on the changes of cerebral blood flow [108, 109]. In conclusion, TBS and other advanced rehabilitation modalities have their own characteristics and advantages in terms of their effects on brain changes, and they can complement each other. According to the specific conditions and rehabilitation needs of stroke patients, the comprehensive use of a variety of rehabilitation modalities is expected to achieve better rehabilitation effects. In the future rehabilitation treatment, further study on the synergistic mechanism between them will provide new ideas and methods for improving the level of rehabilitation treatment after stroke.
Innovation
At present, similar systematic reviews and Meta-analyses have found that TBS has a certain improvement effect on motor dysfunction after stroke. Compared with traditional rehabilitation methods, TBS combined with conventional rehabilitation therapy has a statistically significant advantage in improving motor function scores. In different stages of stroke, the therapeutic effect of TBS may be different. In addition, different stimulation parameters and stimulation schemes may affect the therapeutic effect [80, 110–112]. However, most studies only explored the effect of iTBS on upper limb motor function after stroke, and few literatures explored the effect of iTBS on lower limb motor function recovery. There were few reviews on cTBS treatment of motor dysfunction after stroke, and most of the efficacy evaluation indicators focused on scales and tests related to motor function. Compared with previous studies, this study has the following new contributions: (1) It comprehensively searches the experimental studies of iTBS and cTBS on motor dysfunction after stroke, which are no longer limited to unilateral discussion, but systematically explores the findings of TBS on limb motor recovery from three aspects. (2) The neurobiological mechanism of TBS in improving motor dysfunction after stroke was further studied, which provided deeper molecular evidence. At the same time, combined with DTI technology, we discussed the effect of TBS on brain network connectivity and functional integration, as well as the relationship between this effect and motor function recovery, providing a more comprehensive mechanism. (3) In addition to finding that TBS combined with conventional rehabilitation therapy was more effective than conventional rehabilitation therapy alone, this study also compared the efficacy of TBS with rTMS, and concluded that the combination of the two TBS modes was more effective than iTBS or cTBS alone. These are parts that have not been discussed in previous reviews. (4) This study not only used the traditional clinical exercise scale, but also used neuroelectrophysiological indicators to evaluate the efficacy and narrative summary, which can provide a more comprehensive and multi-dimensional understanding of the recovery of patients after treatment.
Limitations of evidence
Among the 18 included trials, including 16 randomized controlled trials and 2 non-randomized controlled trials, the types of trials were inconsistent, and there was significant heterogeneity in the general condition of patients, the intervention protocols of TBS and adjuvant therapy, and outcome measures. Therefore, this paper does not carry out Meta-analysis, but uses the method of systematic review to sort out the same characteristics of different studies, analyze the possible reasons for the difference in treatment effect, and make a narrative summary of the factors of the included studies. Without using any statistical methods to provide more precise information, it is difficult to draw strong evidence on the effectiveness of TBS in improving motor function in stroke patients. Most of the included studies selected stroke patients in the chronic phase, with little potential for rehabilitation, which may lead to no significant difference in outcome measures within or between groups before and after TBS treatment. Moreover, some studies included small sample sizes, did not randomly assign subject groups, and did not blind the outcome index assessors, which also led to a large bias in the interpretation of the results.
Future research
Future studies should further clarify the exact mechanism of TBS in improving motor dysfunction after stroke and improve the theoretical model. Whether the IHI model is suitable for the recovery of lower limb motor function also needs a large number of clinical trials to verify. At present, there are few studies on the application of iTBS in the recovery of lower limb motor function. Moreover, there is no relevant literature on the application of cTBS in the improvement of lower limb motor function at home and abroad. It is suggested that follow-up studies should be carried out to explore the effect of TBS on the rehabilitation of lower limb motor function. In addition, the effect of cTBS in upper limb treatment is controversial. When using TBS to improve upper limb motor dysfunction, we can change the treatment thinking and promote the recovery of upper limb motor function by directly improving upper limb spasticity. Early intervention should be carried out after the onset of stroke, and transcranial magnetic stimulation should be carried out at least within four weeks after the onset of stroke to obtain greater efficacy. More objective evaluation indicators should be included in follow-up related studies, and non-invasive brain functional imaging technology should be used to detect the reorganization of neural network in different brain regions. Such as functional magnetic resonance imaging, near-infrared functional brain imaging, electroencephalogram, and so on, through some visual quantitative indicators to evaluate the improvement of patients' motor function before and after treatment.
In a word, it is suggested that the mechanism of TBS should be further clarified in the follow-up studies, the best target of brain stimulation should be explored, the appropriate stimulation coil should be selected, the treatment parameters of TBS should be improved, the improvement effect should be induced for a longer time, the individualized treatment should be formulated for stroke patients with different stages and degrees of injury, and the follow-up should be followed up in the later stage of the experiment. To determine the long-term efficacy of TBS.
Conclusion
The results of this study indicate that TBS is an effective treatment for improving limb function in stroke patients, but the optimal timing of treatment, dose of adjuvant therapy, and TBS parameters need to be further clarified according to the course of stroke and the degree of injury. Future studies need to explore the mechanism of TBS combined with other advanced rehabilitation methods, which will provide new ideas and methods for improving the level of rehabilitation treatment after stroke.
Supplementary Information
Author contributions
Y. X. Fu and C. S. Wang Fu put forward the theme and ideas of the article, and were responsible for the main writing work of the article; L. Wu, S. S. Jin and Q. Zhang are the corresponding authors, responsible for the guidance, review and revision of the articles; L. L. Zhang, D. Q. Ji and A. M. Xiang are responsible for literature screening, collecting and sorting out relevant data; J. M. Qi and R. X. Zhao are responsible for the arrangement and typesetting of the articles and participate in the writing of the articles. All authors reviewed the manuscript.
Funding
This work was supported by the Beijing Municipal Administration of Hospitals Incubating Program (No.PX2024074).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanxin Fu and Chengshuo Wang contributed equally to this work.
Contributor Information
Liang Wu, Email: 1972wuliang@sina.com.
Shasha Jin, Email: jinshasha2007@126.com.
Qin Zhang, Email: 2767348230@qq.com.
References
- 1.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet, Neurol. 2021;20:795–820 [DOI] [PMC free article] [PubMed]
- 2.Langhorne P, O’Donnell MJ, Chin SL, Zhang H, Xavier D, Avezum A, et al. Practice patterns and outcomes after stroke across countries at different economic levels (INTERSTROKE): an international observational study. Lancet. 2018;391:2019–27. [DOI] [PubMed] [Google Scholar]
- 3.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the american heart association/american stroke association. Upd Int Car. 2016;47:e98-169. [DOI] [PubMed] [Google Scholar]
- 4.Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther: Off J Am Soc Hand Ther. 2013;26:104–114;quiz 115. [DOI] [PMC free article] [PubMed]
- 5.Zhang Y, Wang C, Yang J, Qiao L, Xu Y, Yu L, et al. Comparing the effects of short-term liuzijue exercise and core stability training on balance function in patients recovering from stroke: a pilot randomized controlled trial. Front Neurol. 2022;13: 748754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2016 Lifetime Risk of Stroke Collaborators, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–37. [DOI] [PMC free article] [PubMed]
- 7.Teasell RW, Foley NC, Bhogal SK, Speechley MR. An evidence-based review of stroke rehabilitation. Top Stroke Rehabil. 2003;10:29–58. [DOI] [PubMed] [Google Scholar]
- 8.Lee T-H, Uchiyama S, Kusuma Y, Chiu HC, Navarro JC, Tan KS, et al. A systematic-search-and-review of registered pharmacological therapies investigated to improve neuro-recovery after a stroke. Front Neurol. 2024;15:1346177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roesner K, Scheffler B, Kaehler M, Schmidt-Maciejewski B, Boettger T, Saal S. Effects of physical therapy modalities for motor function, functional recovery, and post-stroke complications in patients with severe stroke: a systematic review update. Syst Rev. 2024;13:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–37. [DOI] [PubMed] [Google Scholar]
- 11.Wessel MJ, Zimerman M, Hummel FC. Non-invasive brain stimulation: an interventional tool for enhancing behavioral training after stroke. Front Hum Neurosci. 2015;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–50. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y-Z, Rothwell JC, Chen R-S, Lu C-S, Chuang W-L. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2011;122:1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane handbook for systematic reviews of interventions. [cited 2024 Nov 2]. https://training.cochrane.org/handbook/archive/v6.2
- 16.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao D, Liang G-H, Pan J-K, Zeng L-F, Luo M-H, Huang H-T, et al. Risk factors for postoperative surgical site infections after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2023;57:118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolo P, Magnin C, Pedrazzini E, Plomp G, Mottaz A, Schnider A, et al. Comparison of neuroplastic responses to cathodal transcranial direct current stimulation and continuous theta burst stimulation in subacute stroke. Arch Phys Med Rehabil. 2018;99:862-872.e1. [DOI] [PubMed] [Google Scholar]
- 19.Vink JJT, van Lieshout ECC, Otte WM, van Eijk RPA, Kouwenhoven M, Neggers SFW, et al. Continuous theta-burst stimulation of the contralesional primary motor cortex for promotion of upper limb recovery after stroke: a randomized controlled trial. Stroke. 2023;54:1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuzu Ö, Adiguzel E, Kesikburun S, Yaşar E, Yılmaz B. The effect of sham controlled continuous theta burst stimulation and low frequency repetitive transcranial magnetic stimulation on upper extremity spasticity and functional recovery in chronic ischemic stroke patients. J Stroke Cerebrovasc Dis: Off J Natl Stroke Assoc. 2021;30: 105795. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Yamada N, Momosaki R, Shimizu M, Abo M. Comparison of the effect of low-frequency repetitive transcranial magnetic stimulation with that of theta burst stimulation on upper limb motor function in poststroke patients. Biomed Res Int. 2017;2017:4269435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talelli P, Greenwood RJ, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118:333–42. [DOI] [PubMed] [Google Scholar]
- 23.Ackerley SJ, Byblow WD, Barber PA, MacDonald H, McIntyre-Robinson A, Stinear CM. Primed physical therapy enhances recovery of upper limb function in chronic stroke patients. Neurorehabil Neural Repair. 2016;30:339–48. [DOI] [PubMed] [Google Scholar]
- 24.Talelli P, Wallace A, Dileone M, Hoad D, Cheeran B, Oliver R, et al. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair. 2012;26:976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y-J, Huang Y-Z, Chen C-Y, Chen C-L, Chen H-C, Wu C-Y, et al. Intermittent theta burst stimulation enhances upper limb motor function in patients with chronic stroke: a pilot randomized controlled trial. BMC Neurol. 2019;19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sj A, Cm S, Pa B, Wd B. Combining theta burst stimulation with training after subcortical stroke. Upd Int Car [Internet]. 2010; 41. [DOI] [PubMed]
- 27.Chen Y, Wei Q-C, Zhang M-Z, Xie Y-J, Liao L-Y, Tan H-X, et al. Cerebellar intermittent theta-burst stimulation reduces upper limb spasticity after subacute stroke: a randomized controlled trial. Front Neural Circuits. 2021;15: 655502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Shin JC, Jung S, Jung T-M, Kim DY. Effects of intermittent theta burst stimulation on spasticity after stroke. NeuroReport. 2015;26:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JJ, Bai Z, Fong KNK. Priming intermittent theta burst stimulation for hemiparetic upper limb after stroke: a randomized controlled trial. Stroke. 2022;53:2171–81. [DOI] [PubMed] [Google Scholar]
- 30.Liao L-Y, Zhu Y, Peng Q-Y, Gao Q, Liu L, Wang Q-H, et al. Intermittent theta-burst stimulation for stroke: primary motor cortex versus cerebellar stimulation: a randomized sham-controlled trial. Stroke. 2024;55:156–65. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y-J, Wei Q-C, Chen Y, Liao L-Y, Li B-J, Tan H-X, et al. Cerebellar theta burst stimulation on walking function in stroke patients: a randomized clinical trial. Front Neurosci. 2021;15: 688569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch G, Bonnì S, Casula EP, Iosa M, Paolucci S, Pellicciari MC, et al. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol. 2019;76:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao L-Y, Xie Y-J, Chen Y, Gao Q. Cerebellar theta-burst stimulation combined with physiotherapy in subacute and chronic stroke patients: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2021;35:23–32. [DOI] [PubMed] [Google Scholar]
- 34.Lin L-F, Chang K-H, Huang Y-Z, Lai C-H, Liou T-H, Lin Y-N. Simultaneous stimulation in bilateral leg motor areas with intermittent theta burst stimulation to improve functional performance after stroke: a feasibility pilot study. Eur J Phys Rehabil Med. 2019;55:162–8. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Huang G, Zhang L, Yang J, Ren C, Liang C, et al. Effects of the intermittent theta burst stimulation of the cerebellar vermis on balance recovery after stroke: a study protocol for a randomized controlled trial. Front Aging Neurosci. 2022;14: 881311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlsson H, Gard G, Brogårdh C. Upper-limb sensory impairments after stroke: Self-reported experiences of daily life and rehabilitation. J Rehabil Med. 2018;50:45–51. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, You H, Zhang H, Zhao W, Han T, Liu J, et al. Effects of visual feedback balance training with the Pro-kin system on walking and self-care abilities in stroke patients. Medicine. 2020;99: e22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. [DOI] [PubMed] [Google Scholar]
- 39.Hess G, Donoghue JP. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Wars). 1996;56:397–405. [DOI] [PubMed] [Google Scholar]
- 40.Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, et al. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord. 2019;12:1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L, Liu Y, Ningbo Wu, Liu H. Effects of low-frequency repetitive transcranial magnetic stimulation on serum neurotransmitters and cytokines in patients with post-ischemic stroke depression. J Hainan Med Univ. 2017;23:1434–7. [Google Scholar]
- 42.Luo J, Zheng H, Zhang L, Zhang Q, Li L, Pei Z, et al. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int J Mol Sci. 2017;18:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang D, Xie J, Wang X. Effects of repetitive transcranial magnetic stimulation on cerebral blood flow perfusion and velocity in normal humans. Chin J Tissue Eng Res. 2005;5:50–1. [Google Scholar]
- 44.Mei Y, Liu C. Zhang X [effects of transcranial magnetic stimulation on recovery of neural functions and changes of synaptic interface and dendritic structure in the contralateral brain area after cerebral infarction: experiment with rats]. Zhonghua Yi Xue Za Zhi. 2006;86:2639–42. [PubMed] [Google Scholar]
- 45.Oosterveer DM, Mishre RR, van Oort A, Bodde K, Aerden LAM. Depression is an independent determinant of life satisfaction early after stroke. J Rehabil Med. 2017;49:223–7. [DOI] [PubMed] [Google Scholar]
- 46.Diekhoff-Krebs S, Pool E-M, Sarfeld A-S, Rehme AK, Eickhoff SB, Fink GR, et al. Interindividual differences in motor network connectivity and behavioral response to iTBS in stroke patients. NeuroImage, Clin. 2017;15:559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol. 2007;118:1028–32. [DOI] [PubMed] [Google Scholar]
- 48.Tsumoto T. Long-term potentiation and long-term depression in the neocortex. Prog Neurobiol. 1992;39:209–28. [DOI] [PubMed] [Google Scholar]
- 49.Li C-T, Huang Y-Z, Bai Y-M, Tsai S-J, Su T-P, Cheng C-M. Critical role of glutamatergic and GABAergic neurotransmission in the central mechanisms of theta-burst stimulation. Hum Brain Mapp. 2019;40:2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188:249–61. [DOI] [PubMed] [Google Scholar]
- 51.Thimm A, Funke K. Multiple blocks of intermittent and continuous theta-burst stimulation applied via transcranial magnetic stimulation differently affect sensory responses in rat barrel cortex. J Physiol. 2015;593:967–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, et al. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci: Off J Soc Neurosci. 2011;31:1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. [DOI] [PubMed] [Google Scholar]
- 54.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med. 2014;46:193–9. [DOI] [PubMed] [Google Scholar]
- 56.Chen YJ, Nabavizadeh SA, Vossough A, Kumar S, Loevner LA, Mohan S. Wallerian degeneration beyond the corticospinal tracts: conventional and advanced MRI findings. J Neuroimaging: Off J Am Soc Neuroimaging. 2017;27:272–80. [DOI] [PubMed] [Google Scholar]
- 57.Fujiki M, Matsushita W, Kawasaki Y, Fudaba H. Monophasic-quadripulse theta burst magnetic stimulation for motor palsy functional evaluation after intracerebral hemorrhage. Front Integr Neurosci. 2022;16: 827518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moura LM, Luccas R, de Paiva JPQ, Amaro E, Leemans A, Leite C da C, et al. Diffusion tensor imaging biomarkers to predict motor outcomes in stroke: a narrative review. Front Neurol. 2019;10:445. [DOI] [PMC free article] [PubMed]
- 59.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–80. [DOI] [PubMed] [Google Scholar]
- 60.Wadden KP, Peters S, Borich MR, Neva JL, Hayward KS, Mang CS, et al. White matter biomarkers associated with motor change in individuals with stroke: a continuous theta burst stimulation study. Neural Plast. 2019;2019:7092496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zong X, Gu J, Zhou S, Ding D, Hu Y, Tucker L, et al. Continuous theta-burst stimulation enhances and sustains neurogenesis following ischemic stroke. Theranostics. 2022;12:5710–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Torbey MT. Angiogenesis and blood-brain barrier permeability in vascular remodeling after stroke. Curr Neuropharmacol. 2020;18:1250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79:S52-57. [DOI] [PubMed] [Google Scholar]
- 64.Zong X, Li Y, Liu C, Qi W, Han D, Tucker L, et al. Theta-burst transcranial magnetic stimulation promotes stroke recovery by vascular protection and neovascularization. Theranostics. 2020;10:12090–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He W, Au-Yeung S-YS, Mak M, Leung TWH, Leung H, Wong LKS. The potential synergism by combining external counterpulsation with intermittent theta burst stimulation in post-stroke motor function recovery. Med Hypotheses. 2016; 93: 140–2. [DOI] [PubMed]
- 66.Pichiorri F, Vicenzini E, Gilio F, Giacomelli E, Frasca V, Cambieri C, et al. Effects of intermittent theta burst stimulation on cerebral blood flow and cerebral vasomotor reactivity. J Ultrasound Med: Off J Am Inst Ultrasound Med. 2012;31:1159–67. [DOI] [PubMed] [Google Scholar]
- 67.Cho SS, Pellecchia G, Ko JH, Ray N, Obeso I, Houle S, et al. Effect of continuous theta burst stimulation of the right dorsolateral prefrontal cortex on cerebral blood flow changes during decision making. Brain Stimul. 2012;5:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chieffo R, Comi G, Leocani L. Noninvasive neuromodulation in poststroke gait disorders: rationale, feasibility, and state of the art. Neurorehabil Neural Repair. 2016;30:71–82. [DOI] [PubMed] [Google Scholar]
- 69.Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev, Neurol. 2014;10:597–608. [DOI] [PubMed] [Google Scholar]
- 70.Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol. 2010;121:1922–9. [DOI] [PubMed] [Google Scholar]
- 71.Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the “affected” and “unaffected” hemispheres in human stroke. Brain Res. 1998;803:1–8. [DOI] [PubMed] [Google Scholar]
- 72.Chang WH, Kim Y-H, Bang OY, Kim ST, Park YH, Lee PKW. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. 2010;42:758–64. [DOI] [PubMed] [Google Scholar]
- 73.Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128:1122–38. [DOI] [PubMed] [Google Scholar]
- 74.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–72. [DOI] [PubMed] [Google Scholar]
- 75.Huang Y-Z, Lin L-F, Chang K-H, Hu C-J, Liou T-H, Lin Y-N. Priming with 1-Hz repetitive transcranial magnetic stimulation over contralesional leg motor cortex does not increase the rate of regaining ambulation within 3 months of stroke: a randomized controlled trial. Am J Phys Med Rehabil. 2018;97:339–45. [DOI] [PubMed] [Google Scholar]
- 76.Belyk M, Banks R, Tendera A, Chen R, Beal DS. Paradoxical facilitation alongside interhemispheric inhibition. Exp Brain Res. 2021;239:3303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Pino G, Di Lazzaro V. The balance recovery bimodal model in stroke patients between evidence and speculation: do recent studies support it? Clin Neurophysiol. 2020;131:2488–90. [DOI] [PubMed] [Google Scholar]
- 78.van Lieshout ECC, van der Worp HB, Visser-Meily JMA, Dijkhuizen RM. Timing of repetitive transcranial magnetic stimulation onset for upper limb function after stroke: a systematic review and meta-analysis. Front Neurol. 2019;10:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Lieshout ECC, Visser-Meily JMA, Neggers SFW, van der Worp HB, Dijkhuizen RM. Brain stimulation for arm recovery after stroke (B-STARS): protocol for a randomised controlled trial in subacute stroke patients. BMJ Open. 2017;7: e016566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao B, Wang Y, Zhang D, Wang Z, Wang Z. Intermittent theta-burst stimulation with physical exercise improves poststroke motor function: a systemic review and meta-analysis. Front Neurol. 2022;13: 964627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buma F, Kwakkel G, Ramsey N. Understanding upper limb recovery after stroke. Restor Neurol Neurosci. 2013;31:707–22. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Xing G, Fan Y, Guo Z, Chen H, Mu Q. Short- and long-term effects of repetitive transcranial magnetic stimulation on upper limb motor function after stroke: a systematic review and meta-analysis. Clin Rehabil. 2017;31:1137–53. [DOI] [PubMed] [Google Scholar]
- 83.Opie GM, Vosnakis E, Ridding MC, Ziemann U, Semmler JG. Priming theta burst stimulation enhances motor cortex plasticity in young but not old adults. Brain Stimul. 2017;10:298–304. [DOI] [PubMed] [Google Scholar]
- 84.T M, F M-D, Mk L, U Z. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J Physiol. 2012; 590. [DOI] [PMC free article] [PubMed]
- 85.Xia Y, Tang X, Hu R, Liu J, Zhang Q, Tian S, et al. Cerebellum-cerebrum paired target magnetic stimulation on balance function and brain network of patients with stroke: a functional near-infrared spectroscopy pilot study. Front Neurol. 2022;13:1071328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Su W, Gui C-F, Guo Q-F, Tan H-X, He L, et al. Effectiveness of cerebellar vermis intermittent theta-burst stimulation in improving trunk control and balance function for patients with subacute stroke: a randomised controlled trial protocol. BMJ Open. 2023;13: e066356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deng Z-D, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mai L, Shoogo U. Comparison of the induced fields using different coil configurations during deep transcranial magnetic stimulation. PLoS One. 2017. 10.1371/journal.pone.0178422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hardwick RM, Lesage E, Miall RC. Cerebellar transcranial magnetic stimulation: the role of coil geometry and tissue depth. Brain Stimul. 2014;7:643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet. 2014;383:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pangprasertkul S, Borisoot W, Buawangpong N, Sirikul W, Wiwattanadittakul N, Katanyuwong K, et al. Comparison of arterial ischemic and hemorrhagic pediatric stroke in etiology, risk factors, clinical manifestations, and prognosis. Pediatr Emerg Care. 2022;38:e1569–73. [DOI] [PubMed] [Google Scholar]
- 92.Kim JS. Delayed onset mixed involuntary movements after thalamic stroke: clinical, radiological and pathophysiological findings. Brain. 2001;124:299–309. [DOI] [PubMed] [Google Scholar]
- 93.Magid-Bernstein J, Girard R, Polster S, Srinath A, Romanos S, Awad IA, et al. Cerebral hemorrhage: pathophysiology, treatment, and future directions. Circ Res. 2022;130:1204–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Majumder D. Ischemic stroke: pathophysiology and evolving treatment approaches. Neurosci Insights. 2024;19:26331055241292600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Keser Z, Ikramuddin S, Shekhar S, Feng W. Neuromodulation for post-stroke motor recovery: a narrative review of invasive and non-invasive tools. Curr Neurol Neurosci Rep. 2023;23:893–906. [DOI] [PubMed] [Google Scholar]
- 96.Wilson MT, St GL. Repetitive transcranial magnetic stimulation: a call for better data. Front Neural Circuits. 2016;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol. 2012;123:858–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deng Z-D, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol. 2014;125:1202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. [DOI] [PubMed] [Google Scholar]
- 100.Finis J, Enticott PG, Pollok B, Münchau A, Schnitzler A, Fitzgerald PB. Repetitive transcranial magnetic stimulation of the supplementary motor area induces echophenomena. Cortex J Devoted Study Nerv Syst Behav. 2013;49:1978–82. [DOI] [PubMed] [Google Scholar]
- 101.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2014;45:3754–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y, Cui L, Wang J, Xiao Z, Chen Z, Yan J, et al. Robot-assisted therapy in stratified intervention: a randomized controlled trial on poststroke motor recovery. Front Neurol. 2024;15:1453508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang H, Su X, Zheng B, Cao M, Zhang Y, Chen J. Effect and optimal exercise prescription of robot-assisted gait training on lower extremity motor function in stroke patients: a network meta-analysis. Neurol Sci. 2024. 10.1007/s10072-024-07780-6. [DOI] [PubMed] [Google Scholar]
- 104.Rubino C, Lakhani B, Larssen BC, Kraeutner SN, Andrushko JW, Borich MR, et al. Gamified Practice improves paretic arm motor behavior in individuals with stroke. Neurorehabil Neural Repair. 2024;38:15459683241286448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khan A, Imam YZ, Muneer M, Al Jerdi S, Gill SK. Virtual reality in stroke recovery: a meta-review of systematic reviews. Bioelectron Med. 2024;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tosto-Mancuso J, Tabacof L, Herrera JE, Breyman E, Dewil S, Cortes M, et al. Gamified neurorehabilitation strategies for post-stroke motor recovery: challenges and advantages. Curr Neurol Neurosci Rep. 2022;22:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang Z, Zhao Y, Sun X, Liu Y, Su W, Liu T, et al. Evidence that robot-assisted gait training modulates neuroplasticity after stroke: an fMRI pilot study based on graph theory analysis. Brain Res. 2024;1842: 149113. [DOI] [PubMed] [Google Scholar]
- 108.Xiao Y, Bai H, Gao Y, Hu B, Zheng J, Cai X, et al. Interactive virtual ankle movement controlled by wrist sEMG improves motor imagery: an exploratory study. IEEE Trans Visual Comput Graphics. 2024;30:5507–24. [DOI] [PubMed] [Google Scholar]
- 109.Firwana YMS, Zolkefley MKI, Mohamed Hatta HZ, Rowbin C, Che Mohd Nassir CMN, Hanafi MH, et al. Regional cerebral blood perfusion changes in chronic stroke survivors as potential brain correlates of the functional outcome following gamified home-based rehabilitation (IntelliRehab)-a pilot study. J Neuroeng Rehabil. 2022;19:94. [DOI] [PMC free article] [PubMed]
- 110.Chen S, Zhang S, Yang W, Chen Y, Wang B, Chen J, et al. The effectiveness of intermittent theta burst stimulation for upper limb motor recovery after stroke: a systematic review and meta-analysis of randomized controlled trials. Front Neurosci. 2023;17:1272003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang JJ, Sui Y, Sack AT, Bai Z, Kwong PWH, Sanchez Vidana DI, et al. Theta burst stimulation for enhancing upper extremity motor functions after stroke: a systematic review of clinical and mechanistic evidence. Rev Neurosci. 2024;35:679–95. [DOI] [PubMed] [Google Scholar]
- 112.Huang W, Chen J, Zheng Y, Zhang J, Li X, Su L, et al. The effectiveness of intermittent theta burst stimulation for stroke patients with upper limb impairments: a systematic review and meta-analysis. Front Neurol. 2022;13: 896651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.