Abstract
Background
The gut microbiota of venous thromboembolism (VTE) patients exhibited significant alterations. However, the causal relationship between gut microbiota and VTE has not been fully understood. This study aimed to assess the causal relationship between gut microbiota and the risk of VTE using a two-sample Mendelian Randomization (MR) study.
Methods
The gut microbiota and VTE genetic data were collected from the MiBioGen consortium and the UK biobank, respectively. The potential causal relationship between gut microbiota and VTE was investigated using a two-sample MR analysis, including inverse variance weighted (IVW), weighted median, MR-Egger, simple mode, and weighted mode methods. Cochran’s Q-test, MR-PRESSO, and MR-Egger regression intercept analysis were utilized to perform sensitivity analysis.
Results
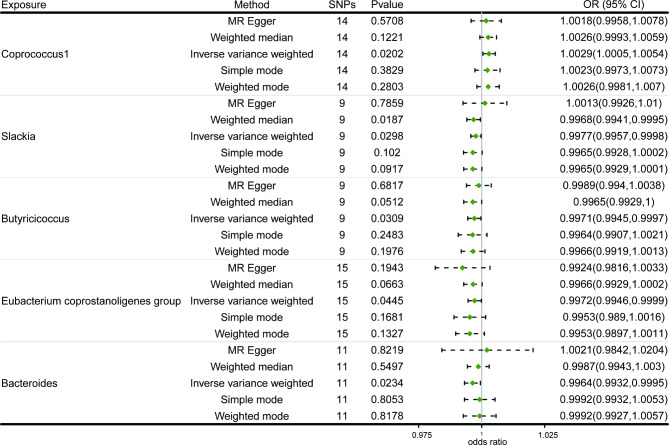
At the genus level, the results of MR analysis found that Coprococcus1 (OR: 1.0029, 95% CI: 1.0005–1.0054, p = 0.0202) was suggestively linked with an increased risk of VTE, while Slackia (odds ratio (OR): 0.9977, 95% confidence interval (CI): 0.9957–0.9998, p = 0.0298), Butyricicoccus (OR: 0.9971, 95% CI: 0.9945–0.9997, p = 0.0309), Eubacterium coprostanoligenes group (OR: 0.9972, 95% CI: 0.9946–0.9999, p = 0.0445), and Bacteroides (OR: 0.9964, 95% CI: 0.9932–0.9995, p = 0.0234) were suggestively associated with a reduced risk of VTE. No heterogeneity and horizontal pleiotropy was detected.
Conclusion
This study found that there were potential causal relationships between five gut microbiota and VTE. Our findings may provide new insights into the mechanisms of VTE.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12959-024-00676-7.
Keywords: Gut microbiota, Venous thromboembolism, Causal relationship, Mendelian randomization analysis
Introduction
Venous thromboembolism (VTE), encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major global health concern with significant morbidity and mortality [1]. Annually, approximately 10 million individuals worldwide are affected by VTE, making it the third most common vascular disease after acute myocardial infarction and stroke [2]. Oral anticoagulation therapy is the primary treatment for most patients with VTE [3]. Traditional risk factors for VTE include surgery, trauma, cancer, pregnancy, advanced age, obesity, prolonged immobility, and genetic predisposition [3]. However, these factors can not fully explain the incidence of VTE, indicating the need to investigate additional risk factors [4].
Recent research has begun to explore the potential role of the gut microbiota in cardiovascular diseases, including VTE [5, 6]. The gut microbiota, a complex ecosystem of microorganisms residing in the human gastrointestinal tract, plays a crucial role in maintaining host health [7]. Advances in high-throughput sequencing technologies have expanded our understanding of the gut microbiome’s composition and functions [8]. Environmental or genetic perturbations of the gut microbiome can trigger inflammatory cascades in vascular endothelium, platelets, and innate immune cells, leading to the release of procoagulant factors and the development of a prothrombotic state [9]. Emerging evidences suggest that alterations in the gut microbiota may be associated with VTE [9, 10]. For example, 16 S rRNA gene sequencing on feces samples revealed altered gut microbiota in VTE patients, with abnormal proliferation of Blautia, Roseburia, Coprococcus, and Ruminococcus compared to healthy controls [6]. However, the potential causal relationship of the gut microbiome on VTE remains unclear.
Mendelian randomization (MR) is a method that leverages genetic variants as instrumental variables (IVs) to infer causal relationship between exposure and outcome [11]. By exploiting the random assortment of alleles during meiosis, MR analysis reduces confounding factors, enhancing causal inference reliability [12]. This approach has been widely adopted to explore gut microbiome-disease associations, including metabolic, autoimmune, and cardiovascular disorders [13–15]. Our study employs MR analysis to investigate the potential causal link between the gut microbiome and VTE, aiming to unveil novel insights into VTE pathogenesis.
Materials and methods
Study design
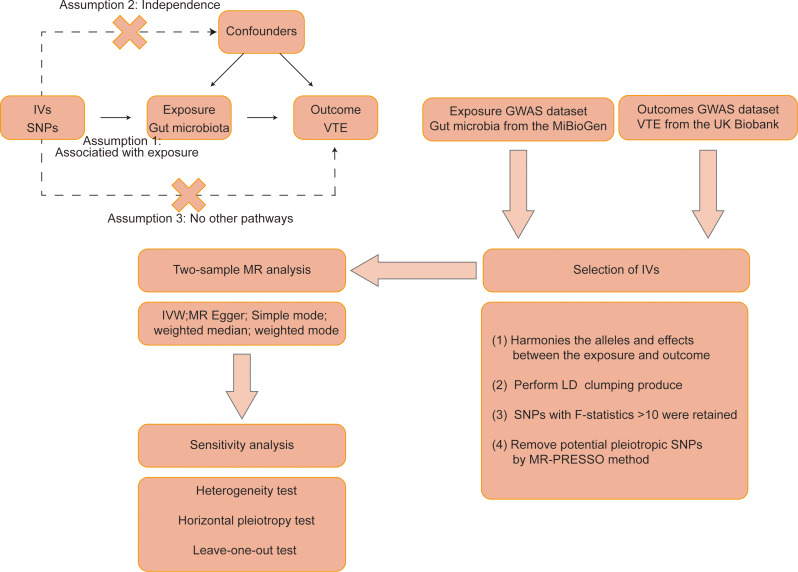
In this study, the causal effect between gut microbiota and VTE was investigated using the MR analysis. The study schematic diagram is presented in Fig. 1. The exposure was gut microbiota, and the outcome was VTE. The MR analysis needs to satisfy the following assumptions: (1) The IVs must be closely related to exposure; (2) The IVs are not associated with confounders; (3) The IVs affect outcome only via exposure. This study was a secondary analysis of data from the original study and did not require additional ethical approval.
Fig. 1.
The schematic diagram of this study. IVs: instrumental variables, SNPs: single-nucleotide polymorphisms, VTE: venous thromboembolism, MR: Mendelian randomization, GWAS: genome-wide association study, IVW: inverse-variance weighted, LD: linkage disequilibrium, MR-PRESSO: MR pleiotropy residual sum and outlier
Data sources
The gut microbiota genetic data were obtained from the largest genome-wide meta-analysis published to date for gut microbiota composition conducted by the MiBioGen consortium, which included genome-wide association studies (GWAS) data and 16 S fecal microbiome data for 18,340 individuals from 24 cohorts, approximately 78% of whom were European (14,306 individuals from 18 cohorts). Additionally, sex, age, and other covariates were adjusted in all cohorts. The GWAS summary data included a total of 211 gut microbiota taxa (131 genera, 35 families, 20 orders, 16 classes, and 9 phyla). After excluding 12 unknown genera, 119 taxa at the genera level as the exposure were included in the follow-up study. For those interested in further details, the complete information of the dataset can be accessed through the link (https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90016908/) and literature [16].
The GWAS summary data of VTE was collected from the UK biobank. The phenotypic code for VTE was “ukb-d-I9_VTE”, including 4,620 VTE cases, 356,574 controls, and 11,901,177 single nucleotide polymorphisms (SNPs) (https://gwas.mrcieu.ac.uk/datasets/ukb-d-I9_VTE/).
IVs selection
The IVs were selected according to the following criteria: (1) SNPs that significantly (p-value < 1 × 10− 5) associated with gut microbiota were selected as potential IVs; (2) Independent SNPs were further acquired to minimize the effect of linkage disequilibrium (LD), with parameters set to r2 < 0.001 and clumping distance = 10,000 kb; (3) Palindromic SNPs (SNPs with A/T or G/C alleles) and SNPs that were not present in the outcome were eliminated; (4) The strength of SNPs with F-statistic > 10 were retained to avoid weak instruments bias [17].
Statistical methods
The inverse variance weighted (IVW), weighted median, MR-Egger, simple mode, and weighted mode methods were utilized to perform a two-sample MR analysis to investigate the causal effect of gut microbiota on VTE. Among these, the IVW method served as the primary approach, integrating Wald ratio estimates from multiple SNPs and providing a consistent assessment of the causal effect between exposure and outcome when the IV assumptions are met [18]. The IVW method yields the most reliable results when the IVs are not subject to horizontal pleiotropy [19]. MR-Egger regression is based on the assumption of instrument strength independent of direct effect (InSIDE), which makes it possible to evaluate the existence of pleiotropy with the intercept term. MR-Egger regression was used to detect the presence of horizontal pleiotropy in the IVs and to provide unbiased estimates of causal effect even in the presence of horizontal pleiotropy [20]. The MR-Egger method is able to generate reliable causal estimates even in situations where all IVs are invalid. The weighted median method provides consistent causal effect estimates when at least 50% of the SNPs are valid IVs [21]. Compared to the MR-Egger method, the weighted median method improves the accuracy of the results [22]. The simple mode and weighted mode approaches were employed as supplementary methods to further enhance the robustness of the analysis [23]. The simple mode is a model-based assessment approach that offers pleiotropy robustness. For mode assessment, the weighted mode is sensitive to the hard throughput collection [24].
For sensitivity analysis, Cochran’s Q-test for random effect IVW was used to assess heterogeneity. MR-PRESSO and MR-Egger regression intercept analysis were applied to identify horizontal pleiotropy [25]. Additionally, MR-PRESSO analysis was used to eliminate outliers and correct for horizontal pleiotropy [26]. Moreover, leave-one-out analysis was conducted to evaluate whether the causal effect was driven by single SNPs, and the results were visualized by forest plot [27]. The Bonferroni correction method was used to identify false-positive results caused by multiple tests. Associations with p < 4.2 × 10− 4 (0.05 divided by 119) were considered statistically significant, whereas associations with p > 4.2 × 10− 4 and p < 0.05 were defined as suggestive associations. The “TwoSampleMR” and “MR-PRESSO” packages in R software (version 4.4.0) were used to carry out statistical analysis [28, 29].
Results
IVs selection
A total of 1,529 SNPs with p-value < 1 × 10− 5 were selected as IVs from 119 taxa at the genus level after eliminating LD (r2 < 0.001 and clumping distance = 10,000 kb), and removing palindromic SNPs and SNPs that were not present in the outcome (Supplementary Table 1). The F-statistic values of all IVs were more than 14 in this study, indicating no evidence of weak instrument bias.
The causal effect of gut microbiota on VTE
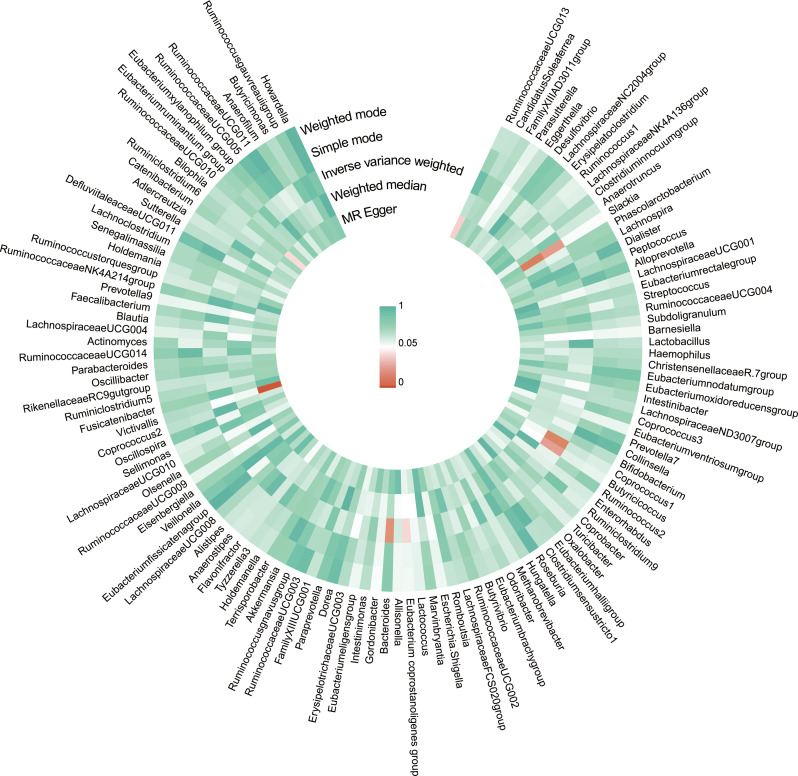
The causal effect of gut microbiota on VTE was investigated using a two-sample MR analysis (Fig. 2). We found that five microbial taxa were suggestively associated with the risk of VTE. According to the IVW method, four microbial taxa showed a potential protective effect on VTE, while one was associated with an increased risk of VTE (Figs. 3 and 4). The microbial taxa that play a protective role in VTE are as follows: Slackia (odds ratio (OR): 0.9977, 95% confidence interval (CI): 0.9957–0.9998, p = 0.0298), Butyricicoccus (OR: 0.9971, 95% CI: 0.9945–0.9997, p = 0.0309), Eubacterium coprostanoligenes group (OR: 0.9972, 95% CI: 0.9946–0.9999, p = 0.0445), and Bacteroides (OR: 0.9964. 95% CI: 0.9932–0.9995, p = 0.0234). In contrast, Coprococcus1 (OR: 1.0029, 95% CI: 1.0005–1.0054, p = 0.0202) was associated with an increased risk of VTE.
Fig. 2.
A circus plot showing the causal effects of gut microbiota on VTE. From the outer circle to the inner circle, five methods are represented: weighted mode, simple mode, inverse variance weighted, weighted median, and MR Egger. The colour shades indicated the size of the p-value
Fig. 3.
Forest plot of the causal effects of Coprococcus1, Slackia, Butyricicoccus, Eubacterium coprostanoligenes group, and Bacteroides on VTE. SNPs: single nucleotide polymorphisms, OR: odds ratio, CI: confidence interval
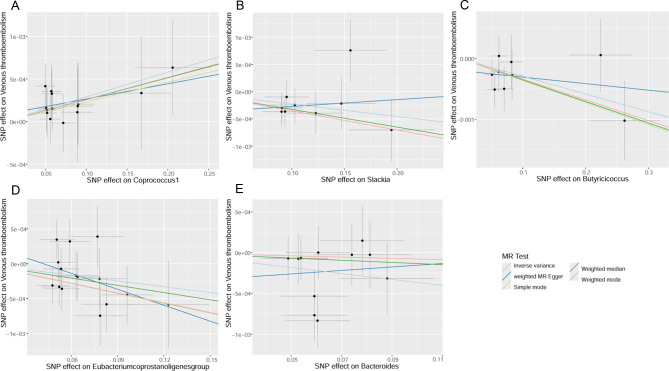
Fig. 4.
Scatterplots of the causal effects of Coprococcus1 (A), Slackia (B), Butyricicoccus (C), Eubacterium coprostanoligenes group (D), and Bacteroides (E) on VTE
For Slackia and Bacteroides, the causal effects evaluated by ME-Egger are opposite to those estimated by the other four methods, although not significant (Fig. 4B, E). These potential causal effects warrant cautious interpretation. However, given the absence of horizontal pleiotropy and heterogeneity (explained later), the IVW method has the strongest statistical power compared to the MR-Egger method. Consequently, Slackia and Bacteroides may reduce the risk of VTE.
Sensitivity analyses
The results of the sensitivity analyses are presented in Table 1. Cochran’s Q tests revealed no significant heterogeneity (p-value > 0.05, Table 1). Furthermore, the MR-Egger intercept and MR-PRESSO analyses showed no horizontal pleiotropy (p-value > 0.05, Table 1). Leave-one-out analyses indicated that the results were not influenced by any single SNP (Fig. 5). These sensitivity analyses support the robustness of our main findings.
Table 1.
Heterogeneity and pleiotropy test
| Exposure | Heterogeneity test | Pleiotropy test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MR-Egger | Inverse variance weighted | MR-Egger | |||||||
| Q | Q_df | Q_pval | Q | Q_df | Q_pval | Egger intercept | se | pval | |
| Coprococcus1 | 2.376 | 12 | 0.999 | 2.536 | 13 | 0.999 | 8.64E-05 | 0.0002 | 0.696 |
| Slackia | 4.513 | 7 | 0.719 | 5.182 | 8 | 0.738 | -0.0004 | 0.0005 | 0.441 |
| Butyricicoccus | 3.543 | 7 | 0.831 | 4.312 | 8 | 0.828 | -0.0002 | 0.0002 | 0.409 |
| Eubacterium coprostanoligenes group | 10.539 | 13 | 0.649 | 11.352 | 14 | 0.658 | 0.0003 | 0.0004 | 0.384 |
| Bacteroides | 9.633 | 9 | 0.381 | 10.071 | 10 | 0.434 | -0.0004 | 0.0006 | 0.538 |
Q: Heterogeneity static Q; df: Degree of freedom; se: Standard error
Fig. 5.
Leave-one-out plots of the causal effects of Coprococcus1 (A), Slackia (B), Butyricicoccus (C), Eubacterium coprostanoligenes group (D), and Bacteroides (E) on VTE
Discussion
The potential causal relationship of gut microbiota on VTE was explored in this study using GWAS data through two-sample MR analysis. We found that Slackia, Butyricicoccus, Eubacterium coprostanoligenes group, and Bacteroides were suggestively associated with a reduced risk of VTE, whereas Coprococcus1 was suggestively associated with an increased risk of VTE. These findings contribute to the current understanding of the potential role of gut microbiota in VTE pathogenesis and elucidate specific taxa that may either promote or inhibit VTE development.
The etiology of VTE involves complex interactions among the coagulation cascade, innate immune system, and inflammatory processes [30]. Research in vivo has demonstrated that targeting inflammatory pathways can effectively reduce the incidence of venous thrombosis [31]. Isoflavones, phenolic compounds with estrogenic and anti-inflammatory properties, have been reported to inhibit thrombus formation and platelet aggregation in animal models [32, 33]. However, isoflavones require microbial transformation into bioactive metabolites for human absorption and utilization [34]. These active compounds include dihydrodaidzein, equol, and 5-hydroxy-equol [34]. Slackia isoflavoniconvertens, a human gut bacterium, converts isoflavones into equol and 5-hydroxy-equol [35, 36]. Additionally, the activation and aggregation of platelets contributed to the venous thrombus formation [37]. It is notable that thromboxane A2 (TxA2), a primary platelet metabolite, promotes these processes [38]. Muñoz et al. reported that equol, which has the highest affinity for TxA2 receptors compared to daidzein and genistein, inhibits platelet activation and aggregation through competitive receptor antagonism [39].
Short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate, are produced by the gut microbiota through dietary fermentation [40]. SCFAs play crucial roles in maintaining intestinal homeostasis and modulating immune and inflammatory responses [41]. These SCFAs are mainly produced by the phyla Firmicutes and Bacteroidetes [42]. Bacterial lipopolysaccharide (LPS) is a key mediator linking the microbiome to a hypercoagulable state [43]. By binding to Toll-like receptors on immune cells, LPS activates endothelial cells and platelets, triggering the coagulation cascade and promoting thrombosis [43]. Butyrate and propionate inhibit LPS-induced IL-6 and IL-12 expression in monocyte-derived mature dendritic cells [44]. Moreover, the NF-κB signaling pathway is closely linked to thrombosis [45]. Its activation elevates pro-inflammatory factors and fibrinolytic inhibitors while reducing anti-thrombotic factors, exacerbating inflammation and thrombosis [45]. Many studies have demonstrated that butyrate exerts anti-inflammatory effects by inhibiting NF-κB activation in macrophages [46–48].
Butyricicoccus and Bacteroides genera belong to the phylum Bacteroidetes [49]. Butyricicoccus pullicaecorum and Butyricicoccus desmolans comb. nov were representative of butyrate-producing bacteria [50, 51]. Bacteroides humanifaecis sp. is a strain isolated from human feces that produces acetate and isobutyrate [52]. Furthermore, using certain oligosaccharides to cultivate healthy human fecal bacteria led to an enrichment of Bacteroides and increased SCFA production [53]. Additionally, Bacteroides has been reported to alleviate LPS-induced inflammation [54]. In mice with liver cirrhosis, treatment with Bacteroides alleviated the progression of portal vein thrombosis [55]. Besides, the Eubacterium coprostanoligenes group belongs to the phylum Firmicutes [56]. The Eubacterium coprostanoligenes group has been reported to enhance the intestinal mucus barrier and relieve chemotherapy-induced intestinal mucositis [57]. The correlation between Slackia, Butyricicoccus, Eubacterium coprostanoligenes group, and Bacteroides and VTE identified in our results are consistent with their known biological functions and previous research findings.
The genus Coprococcus1 is the major butyrate producer and is often considered a probiotic [58, 59]. MR analyses have revealed a negative association between Coprococcus1 and sepsis incidence and mortality [60, 61]. Conversely, Coprococcus1 was identified as a risk factor for interstitial cystitis in MR studies [62, 63]. Our current investigation suggests that Coprococcus1 may increase the risk of VTE. However, these findings warrant further investigation for validation.
In brief, this study has several strengths and limitations. For strengths, the causal relationship of gut microbiota on VTE was analyzed, avoiding the interference of reverse causal links and reducing confounder factors. Secondly, this study leveraged genetic data from a large population cohort, enhancing the reliability of our findings. Thirdly, compared to traditional observational studies, this approach proved more time-efficient and effectively mitigated the impact of confounding factors. For limitations, the data on gut microbiome and VTE primarily derive from European populations, potentially limiting generalizability to other ethnicities. Secondly, although our findings suggest causal relationships between gut microbiota and VTE, these findings are based on predictions of genetic associations and should be considered preliminary, and the underlying mechanisms require further investigation. Moreover, our use of summary-level data precluded subgroup analyses, such as distinguishing between DVT and PE. Lastly, our study focused on genetic-level causality; applying these conclusions to VTE risk assessment necessitates careful consideration of the disease’s multifactorial nature.
Conclusion
This study found five genera of gut microbiota were suggestively associated with the risk of VTE. Slackia, Butyricicoccus, Eubacterium coprostanoligenes group, and Bacteroides were associated with a reduced risk of VTE, while Coprococcus1 was linked to an increased risk of VTE. These findings may offer new directions for clinical intervention in VTE management.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- VTE
Venous thromboembolism
- MR
Mendelian randomization
- IVW
Inverse variance weighted
- OR
Odds ratio
- CI
Confidence interval
- DVT
Deep vein thrombosis
- PE
Pulmonary embolism
- GWAS
Genome-wide association studies
- SNPs
Single nucleotide polymorphisms
- LD
Linkage disequilibrium
- TxA2
Thromboxane A2
- SCFAs
Short-chain fatty acids
- LPS
Lipopolysaccharide
Author contributions
M. L and C. W designed the study. L. X and H. W wrote the manuscript. L.X, H. W, J D, S. Z and M. L collected, analyzed, and interpreted the data. M.L, W.X and C. W critically reviewed, edited the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (82272081).
Data availability
The gut microbiota genetic data were obtained from the MiBioGen consortium. The GWAS summary data of VTE was collected from the UK biobank.
Declarations
Ethics approval and consent to participate
The requirement of ethical approval for this was waived by the Institutional Review Board of our Hospital, because this study was a secondary analysis of summary-level data. The need for informed consent was waived. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Linfeng Xi and Hongyi Wang contributed equally to this work.
Contributor Information
Min Liu, Email: mikie0763@126.com.
Chen Wang, Email: cyh-birm@263.net.
References
- 1.Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398:64–77. 10.1016/s0140-6736(20)32658-1. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–73. 10.1016/s0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 3.Renner E, Barnes GD. Antithrombotic management of venous thromboembolism: JACC Focus Seminar. J Am Coll Cardiol. 2020;76:2142–54. 10.1016/j.jacc.2020.07.070. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Zhang X, Zhang J, Wang B, Tian Q, Meng X, et al. Vascular endothelial growth factor and the risk of venous thromboembolism: a genetic correlation and two-sample mendelian randomization study. Thromb J. 2022;20:67. 10.1186/s12959-022-00427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin M, Qian Z, Yin J, Xu W, Zhou X. The role of intestinal microbiota in cardiovascular disease. 2019;23:2343–50. 10.1111/jcmm.14195 [DOI] [PMC free article] [PubMed]
- 6.Fan Z, Xu S, Deng Y, Wei L, Yang J, Xing X. Disordered gut microbiota and alterations in the serum metabolome are associated with venous thromboembolism. Thromb Res. 2024;235:68–74. 10.1016/j.thromres.2024.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Th Adv Gastroenterol. 2013;6:295–308. 10.1177/1756283x13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold JW, Roach J, Azcarate-Peril MA. Emerging technologies for Gut Microbiome Research. Trends Microbiol. 2016;24:887–901. 10.1016/j.tim.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Chen H, Huang J, Zhang S, Li Z, Kong C, et al. Early administration of vancomycin inhibits pulmonary embolism by remodeling gut microbiota. 2023;13:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Luo P, Zhang F, Xu K, Feng R, Xu P. Large-scale correlation analysis of deep venous thrombosis and gut microbiota. 2022;9. 10.3389/fcvm.2022.1025918. [DOI] [PMC free article] [PubMed]
- 11.Burgess S, Timpson NJ, Ebrahim S, Davey Smith G. Mendelian randomization: where are we now and where are we going? Int J Epidemiol. 2015;44:379–88. 10.1093/ije/dyv108. [DOI] [PubMed] [Google Scholar]
- 12.Qiu B, Shen Z, Yang D, Qin X, Ren W, Wang Q. Gut microbiota and common gastrointestinal diseases: a bidirectional two-sample mendelian randomized study. 2023;14:10.3389/fmicb.2023.1273269 [DOI] [PMC free article] [PubMed]
- 13.Yan W, Ge Y, Wang L, Wang Y, He D. Causal relationship of gut microbiota with diabetic nephropathy: a mendelian randomization analysis. 2024;14:10.3389/fmicb.2023.1281361 [DOI] [PMC free article] [PubMed]
- 14.Li H, Li C. Causal relationship between gut microbiota and type 2 diabetes: a two-sample mendelian. Randomization Study. 2023;14. 10.3389/fmicb.2023.1184734. [DOI] [PMC free article] [PubMed]
- 15.Li Y, Fu R, Li R, Zeng J, Liu T, Li X et al. Causality of gut microbiome and hypertension: a bidirectional mendelian randomization study. 2023;10:10.3389/fcvm.2023.1167346 [DOI] [PMC free article] [PubMed]
- 16.Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53:156–65. 10.1038/s41588-020-00763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Huang H, Liu Z, Li Y, Yu C, Xu L, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive mendelian randomization study. Hepatology. 2023;77:949–64. 10.1002/hep.32728. [DOI] [PubMed] [Google Scholar]
- 18.Bae SC, Lee YH. Vitamin D level and risk of systemic lupus erythematosus and rheumatoid arthritis: a mendelian randomization. Clin Rheumatol. 2018;37:2415–21. 10.1007/s10067-018-4152-9. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Tian F, Yang X, Fang S, Fan Y, Bao J. Physical activity and systemic lupus erythematosus among European populations: a two-sample mendelian randomization study. Front Genet. 2021;12:784922. 10.3389/fgene.2021.784922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89. 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang K, Wang P, Xu Z, Hu YQ, He YS, Chen Y, et al. Causal effects of Gut Microbiome on systemic lupus erythematosus: a two-sample mendelian randomization study. Front Immunol. 2021;12:667097. 10.3389/fimmu.2021.667097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun W, Zhang L, Liu W, Tian M, Wang X, Liang J, et al. Stroke and myocardial infarction: a bidirectional mendelian randomization study. Int J Gen Med. 2021;14:9537–45. 10.2147/ijgm.S337681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98. 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M, Wan X, Su Y, Xu K, Wen P, Zhang B, et al. The genetic causal relationship between type 2 diabetes, glycemic traits and venous thromboembolism, deep vein thrombosis, pulmonary embolism: a two-sample mendelian randomization study. Thromb J. 2024;22:33. 10.1186/s12959-024-00600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sang N, Gao RC, Zhang MY, Wu ZZ, Wu ZG, Wu GC. Causal relationship between sleep traits and risk of systemic lupus erythematosus: a two-sample mendelian randomization study. Front Immunol. 2022;13:918749. 10.3389/fimmu.2022.918749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo S, Li W, Li Q, Zhang M, Wang X, Wu S et al. Causal effects of gut microbiota on the risk of periodontitis: a two-sample mendelian randomization study. 2023;13. 10.3389/fcimb.2023.1160993 [DOI] [PMC free article] [PubMed]
- 28.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed]
- 29.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasan RA, Koh AY, Zia A. The gut microbiome and thromboembolism. Thromb Res. 2020;189:77–87. 10.1016/j.thromres.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potere N, Abbate A, Kanthi Y, Carrier M, Toldo S, Porreca E et al. Inflammasome Signaling, Thromboinflammation, and Venous Thromboembolism. JACC: Basic Transl Sci. 2023;8:1245-61. 10.1016/j.jacbts.2023.03.017 [DOI] [PMC free article] [PubMed]
- 32.Kondo K, Suzuki Y, Ikeda Y, Umemura K. Genistein, an isoflavone included in soy, inhibits thrombotic vessel occlusion in the mouse femoral artery and in vitro platelet aggregation. Eur J Pharmacol. 2002;455:53–7. 10.1016/S0014-2999(02)02449-4. [DOI] [PubMed] [Google Scholar]
- 33.Applová L, Karlíčková J, Říha M, Filipský T, Macáková K, Spilková J, et al. The isoflavonoid tectorigenin has better antiplatelet potential than acetylsalicylic acid. Phytomedicine. 2017;35:11–7. 10.1016/j.phymed.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Langa S, Peirotén Á, Curiel JA, de la Bastida AR, Landete JM. Isoflavone metabolism by lactic acid Bacteria and its application in the development of fermented soy food with beneficial effects on human health. Foods. 2023;12:1293. [DOI] [PMC free article] [PubMed]
- 35.Schröder C, Matthies A, Engst W, Blaut M, Braune A. Identification and expression of genes involved in the conversion of Daidzein and Genistein by the Equol-Forming bacterium Slackia isoflavoniconvertens. 2013;79:3494–502. 10.1128/AEM.03693-12 [DOI] [PMC free article] [PubMed]
- 36.Matthies A, Blaut M, Braune A. Isolation of a human intestinal bacterium capable of Daidzein and Genistein conversion. 2009;75:1740–4. 10.1128/AEM.01795-08 [DOI] [PMC free article] [PubMed]
- 37.Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. 2017;38:785–91. 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin J-H, Kwon H-W, Rhee MH, Park H-J. Inhibitory effects of thromboxane A2 generation by ginsenoside ro due to attenuation of cytosolic phospholipase A2 phosphorylation and arachidonic acid release. J Ginseng Res. 2019;43:236–41. 10.1016/j.jgr.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muñoz Y, Garrido A, Valladares L. Equol is more active than soy isoflavone itself to compete for binding to thromboxane A(2) receptor in human platelets. Thromb Res. 2009;123:740–4. 10.1016/j.thromres.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Ma L, Fu P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des Devel Ther. 2017;11:3531–42. 10.2147/dddt.S150825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sam QH, Ling H, Yew WS, Tan Z, Ravikumar S, Chang MW et al. The Divergent Immunomodulatory effects of short chain fatty acids and medium chain fatty acids. Int J Mol Sci. 2021;22:6453. [DOI] [PMC free article] [PubMed]
- 43.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–44. 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 44.Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:16148. 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Fang C, Yao M, Wu D, Chen M, Guo T et al. Research progress of NF-κB signaling pathway and thrombosis. 2023;14:10.3389/fimmu.2023.1257988 [DOI] [PMC free article] [PubMed]
- 46.Pedersen SS, Prause M, Williams K, Barrès R, Billestrup N. Butyrate inhibits IL-1β-induced inflammatory gene expression by suppression of NF-κB activity in pancreatic beta cells. J Biol Chem. 2022;298:102312. 10.1016/j.jbc.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu H, Xu X, Fu D, Gu Y, Fan R, Yi H, et al. Butyrate-producing Eubacterium rectale suppresses lymphomagenesis by alleviating the TNF-induced TLR4/MyD88/NF-κB axis. Cell Host Microbe. 2022;30:1139–50.e7. 10.1016/j.chom.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Jia J, Nie L, Liu Y. Butyrate alleviates inflammatory response and NF-κB activation in human degenerated intervertebral disc tissues. Int Immunopharmacol. 2020;78:106004. 10.1016/j.intimp.2019.106004. [DOI] [PubMed] [Google Scholar]
- 49.Liu H, Sun J, Jiang S, Jiao N, Huang L, Yuan X, et al. Effects of dietary isoleucine supplementation on the production performance, health status and cecal microbiota of arbor acre broiler chickens. Microorganisms. 2023;11. 10.3390/microorganisms11020236. [DOI] [PMC free article] [PubMed]
- 50.Takada T, Watanabe K, Makino H, Kushiro A. Reclassification of Eubacterium desmolans as Butyricicoccus desmolans comb. nov., and description of Butyricicoccus faecihominis sp. nov., a butyrate-producing bacterium from human faeces. 2016;66:4125–31. 10.1099/ijsem.0.001323 [DOI] [PubMed]
- 51.Boesmans L, Valles-Colomer M, Wang J, Eeckhaut V, Falony G, Ducatelle R, et al. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of < i > Butyricicoccus pullicaecorum to. Healthy Volunteers. 2018;3. 10.1128/msystems.00094-18. [DOI] [PMC free article] [PubMed]
- 52.Kim HS, Kim JS, Suh MK, Eom MK, Lee JH, Park SH, et al. Bacteroides humanifaecis sp. nov., isolated from faeces of healthy Korean. Arch Microbiol. 2022;204:357. 10.1007/s00203-022-02967-x. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Wang R, Zhang H, Wu J, Zhu L, Zhan X. In vitro assessment of prebiotic properties of oligosaccharides derived from four microbial polysaccharides. LWT. 2021;147:111544. 10.1016/j.lwt.2021.111544. [Google Scholar]
- 54.Tan H, Zhao J, Zhang H, Zhai Q, Chen W. Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Appl Microbiol Biotechnol. 2019;103:2353–65. 10.1007/s00253-019-09617-1. [DOI] [PubMed] [Google Scholar]
- 55.Huang X-y, Zhang Y-h, Yi S-y, Lei L, Ma T, Huang R et al. Potential contribution of the gut microbiota to the development of portal vein thrombosis in liver cirrhosis. 2023;14:10.3389/fmicb.2023.1217338 [DOI] [PMC free article] [PubMed]
- 56.Xiong LL, Mao ML, Shu QL. A preliminary study on the diversity of butyrate-producing bacteria in response to the treatment of depression with Xiaoyaosan. 2022;75:844–56. 10.1111/lam.13737 [DOI] [PubMed]
- 57.Bai D, Zhao J, Wang R, Du J, Zhou C, Gu C, et al. Eubacterium coprostanoligenes alleviates chemotherapy-induced intestinal mucositis by enhancing intestinal mucus barrier. Acta Pharm Sinica B. 2024;14:1677–92. 10.1016/j.apsb.2023.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altemani F, Barrett HL, Gomez-Arango L, Josh P, David McIntyre H, Callaway LK, et al. Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer coprococcus in their gut microbiota. Pregnancy Hypertens. 2021;23:211–9. 10.1016/j.preghy.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Yang R, Shan S, Shi J, Li H, An N, Li S, et al. Coprococcus eutactus, a potent probiotic, alleviates colitis via acetate-mediated IgA response and microbiota restoration. J Agric Food Chem. 2023;71:3273–84. 10.1021/acs.jafc.2c06697. [DOI] [PubMed] [Google Scholar]
- 60.Gao Y, Liu L, Cui Y, Zhang J, Wu X. The causality of gut microbiota on onset and progression of sepsis: a bi-directional mendelian. Randomization Anal. 2024;15. 10.3389/fimmu.2024.1266579. [DOI] [PMC free article] [PubMed]
- 61.Shang W, Zhang S, Qian H, Huang S, Li H, Liu J et al. Gut microbiota and sepsis and sepsis-related death: a mendelian randomization investigation. 2024;15:10.3389/fimmu.2024.1266230 [DOI] [PMC free article] [PubMed]
- 62.Gao J, Shao S, Shen Y. Causal effects of gut microbiota on risk of interstitial cystitis: a two-sample mendelian randomization study. 2024;15. 10.3389/fmicb.2024.1434117. [DOI] [PMC free article] [PubMed]
- 63.Jiang P, Li C, Su Z, Chen D, Li H, Chen J, et al. Mendelian randomization study reveals causal effects of specific gut microbiota on the risk of interstitial cystitis/bladder pain syndrome (IC/BPS). Sci Rep. 2024;14:18405. 10.1038/s41598-024-69543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The gut microbiota genetic data were obtained from the MiBioGen consortium. The GWAS summary data of VTE was collected from the UK biobank.