Abstract
Background
The association between fall risk-increasing drugs (FRIDs, medications known to be associated with falls) and the number of falls among generally healthy and active community-dwelling older adults is understudied. Prior studies have focused on individual medication classes or have predominantly relied on retrospective assessments of falls. The aim of this study was to investigate the association between FRID use at baseline and the prospective incidence rates of total, injurious and recurrent falls in community-dwelling older adults.
Methods
This is a 3-year observational analysis of DO-HEALTH, a randomized controlled trial, among community-dwelling adults aged ≥ 70 years without major diseases at baseline. The main exposures were use of at least one FRID and multiple FRIDs (≥ 2 FRIDs) at baseline. The number of total falls (including high- and low-trauma falls, as well as injurious falls) over 3 years of follow-up was defined as the primary outcome, and the number of injurious and the number of recurrent total falls (≥ 2 falls), as the two separate secondary outcomes. To examine these associations, separate negative binomial regression models controlled for the fixed effects of treatment allocation in the DO-HEALTH trial, study site, fall in the last year, age, sex, BMI, and walking aid were used. Additionally, an offset of the logarithm of each participant’s time in the study was included in the models.
Results
A total of 2157 participants were included, with a baseline median age of 74.0 years, 61.7% of whom were women, and 41.9% having experienced a prior fall in the year preceding enrolment. At baseline, 908 (42.1%) participants used at least one FRID, and 351 (16.3%) reported multiple FRIDs use. Prospectively, over 3 years of follow-up, 3333 falls were reported by 1311 (60.8%) out of the 2157 participants. Baseline use of at least one FRID was significantly associated with increased incidence rates of total falls (incidence rate ratio (IRR) [95% Confidence Interval (CI)] = 1.13 [1.01–1.27]), injurious falls (IRR = 1.15 [1.02–1.29]), and recurrent falls (IRR = 1.12 [1.01–1.23]) over 3 years. These associations were most pronounced among users of multiple FRIDs, with increased incidence rates of total falls (IRR = 1.22 [1.05–1.42]), injurious falls (IRR = 1.33 [1.14–1.54]) and recurrent falls (IRR = 1.14 [1.02–1.29]).
Conclusion
Our results suggest that FRID use is associated with increased prospective incidence rates of total, injurious, and recurrent falls even among generally healthy older adults.
Trial registration
DO-HEALTH is registered as NCT01745263 on clinicaltrials.gov, with a registration date of 2012-12-06.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-024-05557-2.
Keywords: Falls, Fall risk-increasing drugs, Prospective study, Longitudinal study, Community-dwelling older adults, DO-HEALTH, Medications, Adverse events
Introduction
Falls occur frequently among older adults and are a driver for loss of autonomy among older adults, making the prevention of falls a public health priority [1, 2]. Notably, one of three community-dwelling adults aged ≥ 65 years, and every second adult ≥ 80 years, sustains at least one fall each year [3–5]. Globally, the rate of falls increases with the rapidly growing older adult population and may cause significant morbidity and mortality among older adults [6–8].
Falls are multifactorial, with various risk factors known, including unsteady gait, impaired vision and hearing, and certain medications [2, 4, 5, 9]. Medications can contribute to falls through sedation, muscle weakness, hypotension, orthostatic reactions, and cognitive impairment [6, 10, 11]. Additionally, pharmacokinetic and pharmacodynamic changes associated with older age can lead to longer half-lives of medications (pharmacokinetic changes), altered drug sensitivities (pharmacodynamic changes) and a higher risk for adverse events [12]. Medications associated with increased risk of falls are classified as fall risk-increasing drugs (FRIDs) [3, 11]. Despite the high occurrence of falls in older adults and the potential negative impact of FRIDs, the link between FRIDs and falls in generally healthy and active community-dwelling older adults remains understudied. Prior long-term studies (≥ 3 years) focused on individual drugs or medication classes [13–18]. Further, prior evidence on FRIDs and falls is predominantly based on retrospective assessments of falls, primarily using hospital data, pharmacy claims or prescribed medications, potentially underestimating the number falls experienced and actual medication use [4, 13, 19, 20].
Therefore, the primary aim of the present study was to investigate the association between the use of at least one FRID at baseline and the prospective incidence of total falls (including high- and low-trauma falls, as well as injurious falls) in community-dwelling older adults with good cognitive function and mobility, and without major health events (e.g. cancer or major cardiovascular diseases) in the last 5 years prior enrolment [21]. Our secondary aims included multiple FRIDs use (≥ 2 FRIDs) as the exposure, along with two additional outcomes: the prospective incidence of injurious falls and the prospective incidence of recurrent falls.
Methods
Study design
This is an observational, prospective analysis of the DO-HEALTH randomized controlled trial which aims to support healthy aging in Europe (NCT01745263). In DO-HEALTH, the effects of vitamin D3, omega-3, and a home exercise program on change in systolic and diastolic blood pressure, Short Physical Performance Battery, Montreal Cognitive Assessment, and incidence rates of nonvertebral fractures and infections were tested over a 3-year follow-up [22]. DO-HEALTH enrolled 2157 generally healthy community-dwelling adults aged ≥ 70 years in seven study centers located in five European countries: Switzerland (Zurich, Basel, and Geneva), Austria (Innsbruck), Germany (Berlin), France (Toulouse), and Portugal (Coimbra) [21, 22]. Additionally, DO-HEALTH also tested the effects of vitamin D3, omega-3, and a home exercise program on fall prevention, which was a pre-defined secondary endpoint of the trial [23]. Findings from these studies are published elsewhere [22, 23].
Inclusion criteria were sufficient mobility to come to the study center and good cognitive function (Mini-Mental State Examination [MMSE] score of at least 24 points) [21]. Additionally, DO-HEALTH was designed to recruit 40% of participants with at least one fall in the year prior to enrollment [21].
Exclusion criteria included a history of cancer (except non-melanoma skin cancer), myocardial infarction, stroke, transient ischemic attack, angina pectoris, or coronary artery intervention in the past 5 years. Participants with severe renal impairment (creatinine clearance ≤ 15 ml/min), dialysis, or severe liver disease (life-threatening or affecting participation, such as chronic hepatitis B, cirrhosis, sclerosing cholangitis, liver cancer, or metastases) were also excluded. Other exclusion criteria included hypercalcaemia (> 2.6 mmol/l), hemiplegia, severe gait impairment, hypo- or primary hyperparathyroidism, granulomatous diseases (e.g. tubercolosis, sarcoidosis), osteodystrophia deformans (Paget’s disease), major visual or hearing impairment, and serious illness precluding participation. Living in assisted living or nursing homes, acute fracture (within 6 weeks), epilepsy or use of anti-epileptic drugs, and more than three falls in the past month also led to exclusion [21].
The present study was approved by the Cantonal Ethical Committee of the Canton of Zurich (BASEC N° 2021–02127). All participants provided written informed consent.
Outcomes
The primary outcome was the number of total falls (including high- and low-trauma falls, as well as injurious falls) experienced over each participant’s time in the study over 3 years. Secondary outcomes included the number of injurious and the number of recurrent falls (regarded as separate conditions) over each participant’s time in the study over 3 years. Incident falls were assessed prospectively using a fall diary and detailed questionnaires for each fall event, captured through 3-monthly phone calls and yearly clinical visits (baseline, year 1, year 2, and year 3) [23]. A fall was defined as unintentionally coming to rest at a lower level or on the ground (excluding falling against furniture or walls) [21, 23]. Injurious falls encompassed falls resulting in wounds, significant bruising, or fractures [23]. The number of recurrent falls was the number of total falls among participants who experienced two or more falls over the 3-year follow-up. Participants with one or no falls were not included in analysis for recurrent fall. For example, if a participant fell twice and another fell five times during the 3-year follow-up, the number of recurrent falls were counted as two and five, respectively.
Exposures
Participants were asked to bring their medications to the baseline visit to minimize recall bias. Details for each medication, including brand and generic names, dosage, unit, interval (as needed or regularly), duration, and indication, were recorded. Regularly taken FRIDs (both prescribed and over-the-counter) at baseline, defined as medications used daily or at regular intervals, were considered in this study. All medications were coded using the Anatomical Therapeutic Chemical (ATC) classification system [24]. The classification of FRIDs was based on a recent European consensus, including the following medications: benzodiazepines and benzodiazepines-related drugs, antipsychotics, opioids, antidepressants, anticholinergics, antiepileptics, diuretics, alpha-blockers used as antihypertensives and used for prostate hyperplasia, centrally-acting antihypertensives, antihistamines, vasodilators used in cardiac diseases and drugs used against urinary incontinence and overactive bladder [11].
Use of at least one FRID at baseline was binary and defined as the main exposure. In a secondary analysis, multiple FRIDs use, i.e. ≥2 FRIDs at baseline (binary, versus no use of multiple FRIDs [use of 0–1 FRID]) was defined as the exposure.
Demographics and clinical assessment at baseline
Baseline characteristics included sex, age, body mass index (BMI), history of a fall in the prior year to enrollment, physical activity level per week (none, 1–2 times, or ≥ 3 times; based on the Nurses’ Health Study questionnaire [25]), use of a walking aid (yes/no), number of comorbidities (based on the Self-Administered Comorbidity Questionnaire, maximum of 12 chronic diseases [26], smoking status (current smoker vs. no current smoker), living condition (alone vs. with others), and study center (Basel, Geneva, Zurich, Berlin, Coimbra, Innsbruck, Toulouse).
Statistical analyses
Baseline characteristics are described overall and by FRID use (use of at least one FRID versus no FRID use). Categorical variables are presented as frequencies and percentages. Normally and non-normally distributed continuous variables are presented as means and standard deviations, and as medians and interquartile ranges, respectively. Differences in baseline characteristics between groups were assessed by using the Chi-square test for categorical variables, t-test for continuous and normally distributed variables, and Wilcoxon rank sum test for continuous and non-normally distributed variables.
The number of incident total falls, injurious falls and recurrent falls over the 3-year follow-up were modeled using separate negative binomial regression models to test the association between at least one FRID use at baseline and the number of total, injurious, and recurrent falls experienced over 3 years. An offset of the logarithm of each participant’s time in the study was included in the models to account for early study withdrawals. Incidence rates (IR) and 95% confidence intervals (CI) for total, injurious, and recurrent falls are presented for each group: at least one FRID use and no FRID use. Incidence rate ratios (IRR) and 95% CI are presented comparing the two groups. We used the same approach to examine the association of multiple FRIDs use with the number of total, injurious, and recurrent falls.
Based on prior studies and on the DO-HEALTH design variables, all models were adjusted for the following relevant confounders at baseline: fixed effects of treatment allocation in the DO-HEALTH trial (vitamin D3, omega-3, and a home exercise program), study site, fall in the last year [4, 13], age [4, 9, 13], sex [4, 13], BMI [27], and walking aid [13]. In a sensitivity analysis, we further adjusted for the baseline number of comorbidities [4, 28].
Interactions between the use of FRIDs at baseline and subgroups of sex, age (70–74 and ≥ 75 years old), and fall in the year prior to enrolment were investigated in the models by adding the respective interaction terms. Subgroup analyses by sex, age group, and prior fall were performed when the interaction terms suggested significant effect modification (i.e. P value for the interaction term < 0.05).
All analyses were carried out using SAS version 9.4 (SAS Institute, Cary). Statistical significance was set at P values of < 0.05, and reported P values are 2-sided.
Results
Participants’ characteristics at baseline
This study included all 2157 DO-HEALTH participants, who were generally healthy, had good cognitive function and were community-dwelling to qualify for DO-HEALTH. In fact, at baseline, the mean MMSE score was 28.5, 82.6% of participants reported regular physical activity, with 52.3% engaging in physical activity ≥ 3 times per week, and the median number of medications was 3.0 (findings published elsewhere [22, 29]). Of the 2157 participants, 908 (42.1%) reported at least one FRID use, and 351 (16.3%) reported multiple FRIDs use at baseline. Diuretics (23.4%) and antidepressants (9.8%) were the two most common FRIDs (Supplementary Table 1).
Overall, the median age was 74.0 years (IQR 72.0–77.0), 61.7% were female, 1.9% used a walking aid, 41.9% reported a fall in the year prior to enrollment, the median number of comorbidities was 2.0 (IQR 1.0–3.0), 5.8% were current smokers, and 41.7% lived alone (Table 1).
Table 1.
Baseline characteristics by the use of at least one FRIDa at baseline
| No FRID use (n = 1249) |
Use of at least one FRID (n = 908) |
P valueb | Overall (n = 2157) |
|
|---|---|---|---|---|
| Age, years, median (IQR) | 73.0 (71.0, 77.0) | 74.0 (72.0, 78.0) | < 0.0001 | 74.0 (72.0, 77.0) |
| Sex, N (%) | ||||
| Female | 773 (61.9) | 558 (61.5) | 0.84 | 1331 (61.7) |
| Male | 476 (38.1) | 350 (38.5) | 826 (38.3) | |
| Prior fallc, N (%) | ||||
| Yes | 509 (40.8) | 394 (43.4) | 0.22 | 903 (41.9) |
| No | 740 (59.2) | 514 (56.6) | 1254 (58.1) | |
| Use of a walking aid, N (%) | ||||
| Yes | 13 (1.1) | 28 (3.1) | 0.0006 | 41 (1.9) |
| No | 1223 (99.0) | 872 (96.9) | 2095 (98.1) | |
| BMI, kg/m2, mean (SD) | 25.6 (4.0) | 27.4 (4.5) | < 0.0001 | 26.3 (4.3) |
| Current smokers, N (%) | ||||
| Yes | 67 (5.4) | 59 (6.5) | 0.27 | 126 (5.8) |
| No | 1182 (94.6) | 849 (93.5) | 2031 (94.2) | |
| Living alone, N (%) | ||||
| Yes | 513 (41.1) | 387 (42.6) | 0.47 | 900 (41.7) |
| No | 736 (59.9) | 521 (57.4) | 1257 (58.3) | |
| Number of comorbiditiesd, median (IQR) | 1.0 (0.0, 2.0) | 2.0 (1.0, 3.0) | < 0.0001 | 2.0 (1.0, 3.0) |
| Study site | ||||
| Basel | 174 (13.9) | 79 (8.7) | < 0.0001 | 253 (11.7) |
| Zurich | 376 (30.1) | 176 (19.4) | 552 (25.6) | |
| Geneva | 128 (10.2) | 73 (8.0) | 201 (9.3) | |
| Coimbra | 77 (6.2) | 224 (24.7) | 301 (13.9) | |
| Innsbruck | 105 (8.4) | 95 (10.5) | 200 (9.3) | |
| Berlin | 222 (17.8) | 128 (14.1) | 350 (16.2) | |
| Toulouse | 167 (13.4) | 133 (14.6) | ||
Abbreviations: BMI body mass index, FRID Fall risk-increasing drug, IQR interquartile range, N absolute number, SD standard deviation
aFRID use was defined as any FRID use at baseline, based on the European consensus by Seppala et. al. [11]
bDifferences between users and non-users of FRIDs were assessed by using the χ2 test for categorical variables, t-test for normally distributed continuous variables, and Wilcoxon rank sum test for non-normally distributed continuous variables. Significant at the 0.05 probability level
cPrior fall in the last 12 months before study start
dComorbidities was measured by the Self-Administered Comorbidity Questionnaire, which assesses the presence of current 12 comorbidities [26]
At baseline, participants in the at least one FRID use group were older (median age 74.0 vs. 73.0 years, P < 0.0001), had greater BMI (mean BMI 27.4 vs. 25.6 kg/m2, P < 0.0001), more comorbidities (median number of comorbidities 2.0 vs. 1.0, P < 0.0001), and more likely to use a walking aid (3.1% vs. 1.1%, P = 0.0006) compared to non-FRID-users. Regarding the study centers, participants from Coimbra were most likely to report the use of at least one FRID (24.7%), followed by participants from Zurich (19.4%), Toulouse (14.6%), Berlin (14.1%), Innsbruck (10.5%), Basel (8.7%), and Geneva (8.0%) (Table 1). Notably, the proportion of the intake of diuretics (43.9%), antidepressants (22.9%), benzodiazepines (32.9%) and anticholinergics (9.3%) was the highest in Coimbra compared to the other study sites (Supplementary Table 1).
FRID use and falls over the 3-year follow-up
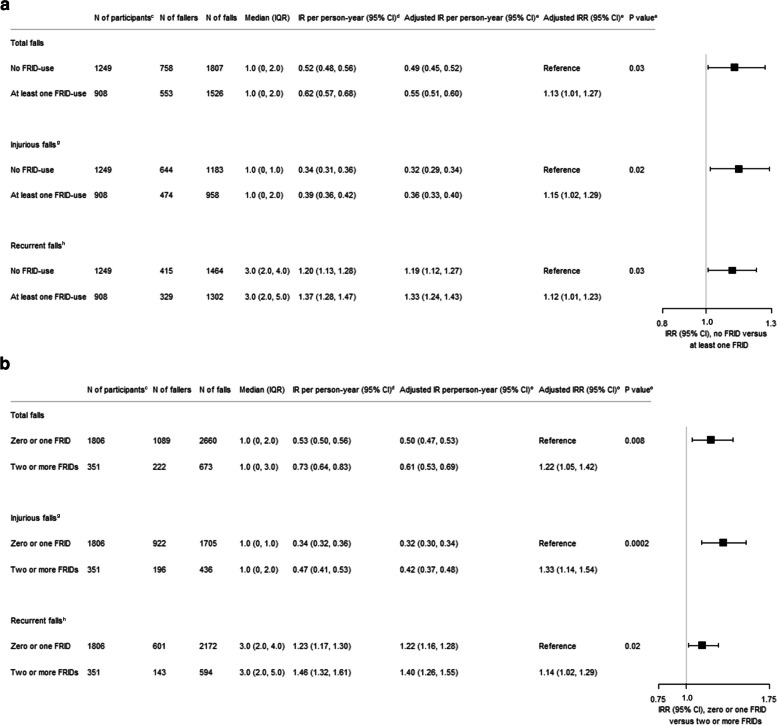
Over the 3-year follow-up, 3333 falls were reported by 1311 (60.8%) participants, of which 553 (42.2%) used at least one FRID at baseline. Over the 3-year follow-up, 1526 and 1807 total falls were observed in the at least one FRID use and no FRID use groups, respectively. After adjustment for age, sex, prior fall, BMI, study center, walking aid, participants’ follow-up times, and fixed effects of treatment allocation in the DO-HEALTH trial, at least one FRID use at baseline was associated with a 13% increased IR of total falls over the 3-year follow-up (IRR 1.13, 95% Confidence Interval (CI) [1.01–1.27]), P = 0.03) (Fig. 1A). Similarly, multiple FRIDs use was associated with a 22% increased IR of total falls (IRR 1.22, 95% CI [1.05–1.42], P = 0.008) (Fig. 1B).
Fig. 1.
Incidence rates of falls over 3 years by FRIDa use and multipleb FRID use at baseline. A Model comparing participants with no FRID to participants with at least one FRID. B Model comparing participants with zero or one FRID to participants with two or more FRIDs. Abbreviations: CI, confidence intervals; FRID, Fall risk increasing drug; IQR, interquartile range; IR, incidence rate; IRR, incidence rate ratio; N, absolute number. aFRID use was defined as any FRID use at baseline, based on the European consensus by Seppala et. al. [11]. bMultiple FRID use was defined as the use of 2 or more FRIDs at baseline, also based on the European consensus by Seppala et. al.[11]. cNumber of participants at baseline. dEstimates are from negative binomial regression models including an offset of the logarithm of participant’s time in the study, but not covariates. eEstimates are from negative binomial regression models adjusted for age, sex, prior fall, BMI, study center (Berlin, Coimbra, Innsbruck, Toulouse, Basel, Geneva, and Zurich), walking aid, participant’s follow-up times, and fixed effects of treatment allocation in the DO-HEALTH trial (vitamin D3, omega-3, and home exercise program). fThe number of total falls included high- and low-trauma falls, as well as injurious falls experienced over each participant’s time in the study over 3 years. A fall was defined as unintentionally coming to rest at a lower level or on the ground (excluding falling against furniture or walls). gInjurious falls encompassed falls resulting in wounds, significant bruising, or fractures. hThe number of recurrent falls was the number of total falls among participants who experienced two or more falls over the 3-year follow-up. Participants with one or no falls were not included in analysis for recurrent fall. For example, if a participant fell twice and another fell five times during the 3-ear follow-up, the number of recurrent falls were counted as two and five, respectively
Regarding injurious falls, 1118 (51.8%) participants reported at least one injurious fall, of which 474 (42.4%) used at least one FRID at baseline. Over the 3-year follow-up, 958 injurious falls were reported in the at least one FRID use group and 1183 injurious falls were reported in the no FRID group. Similar to total falls, the group using at least one FRID had a 15% increased IR of injurious falls, compared to the no FRID user group (IRR 1.15, 95% CI [1.02–1.29], P = 0.02) (Fig. 1A), after adjustments for age, sex, prior fall, BMI, study center, walking aid, participants’ follow-up times, and fixed effects of treatment allocation in the DO-HEALTH trial. Furthermore, multiple FRIDs use was also associated with a 33% increased IR of injurious falls (IRR 1.33, 95% CI [1.14–1.54], P = 0.0002) (Fig. 1B).
For recurrent falls, participants in the least one FRID use group reported 1302 recurrent falls, and no FRID users reported 1464 recurrent falls over the 3-year follow-up. Consistent with total falls and injurious falls, at least one FRID use at baseline was associated with 12% increased adjusted IR of recurrent falls compared to no FRID use at baseline (IRR 1.12, 95% CI [1.01–1.23], P = 0.03) (Fig. 1A). Moreover, multiple FRIDs use was also associated with a 14% increased adjusted IR of recurrent falls (IRR 1.14, 95% CI [1.02–1.29], P = 0.02) (Fig. 1B).
Subgroup analysis
No significant effect modifications were found by sex or prior falls for total, injurious, and recurrent falls. However, age group (70–74 or ≥ 75 years) showed significant effect modifications with P < 0.05 for interactions between age group and both at least one FRID use and multiple FRIDs use for total falls and recurrent falls (Supplementary Table 2). Among participants aged ≥ 75 years, at least one FRID use was associated with an increased incidence rate of total falls (IRR 1.35, 95% CI [1.15–1.58]), P = 0.0003) and recurrent falls (IRR 1.24, 95% CI [1.08–1.41]), P = 0.002) (Table 2). However, no significant association was found between at least one FRID use and the incidence rates of total and recurrent falls among participants aged 70–74 years (Table 2).
Table 2.
Association between FRIDa use and multipleb FRIDs use and falls by age group
| Model comparing participants with no FRID to participants with at least one FRID | Model comparing participants with zero or one FRID to participants with two or more FRIDs | |||
|---|---|---|---|---|
| No FRID use | At least one FRID use | Zero or one FRID | Two or more FRIDs use | |
| Total fallsc | ||||
| Age 70–74 years | ||||
| No. of participants at baseline | 767 | 470 | 1074 | 163 |
| Adjusted IRd per person-year (95% CI) | 0.51 (0.46, 0.57) | 0.50 (0.43, 0.57) | 0.51 (0.46, 0.56) | 0.52 (0.42, 0.64) |
| Adjusted IRRd (95% CI) | 0.98 (0.84, 1.14) | 1.02 (0.82, 1.27) | ||
| P valued | 0.76 | 0.87 | ||
| Age ≥ 75 years | ||||
| No. of participants at baseline | 482 | 438 | 732 | 188 |
| Adjusted IRd per person-year (95% CI) | 0.45 (0.39, 0.52) | 0.60 (0.52, 0.70) | 0.48 (0.42, 0.55) | 0.68 (0.55, 0.83) |
| Adjusted IRRd (95% CI) | 1.35 (1.15, 1.58) | 1.40 (1.16, 1.70) | ||
| P valued | 0.0003 | 0.0005 | ||
| Recurrent fallse | ||||
| Age 70–74 years | ||||
| No. of participants at baseline | 767 | 470 | 1074 | 163 |
| Adjusted IRd per person-year (95% CI) | 1.29 (1.16, 1.43) | 1.29 (1.14, 1.47) | 1.30 (1.18, 1.43) | 1.25 (1.04, 1.50) |
| Adjusted IRRd (95% CI) | 1.01 (0.88, 1.15) | 0.96 (0.80, 1.15) | ||
| P valued | 0.92 | 0.65 | ||
| Age ≥ 75 years | ||||
| No. of participants at baseline | 482 | 438 | 732 | 188 |
| Adjusted IRd per person-year (95% CI) | 1.08 (0.96, 1.22) | 1.34 (1.19, 1.51) | 1.13 (1.02, 1.26) | 1.46 (1.25, 1.69) |
| Adjusted IRRd (95% CI) | 1.24 (1.08, 1.41) | 1.28 (1.11, 1.49) | ||
| P valued | 0.002 | 0.001 | ||
Abbreviations: CI confidence intervals, FRID Fall-risk increasing drug, IR incidence rate, IRR incidence rate ratio, No absolute number
aFRID use was defined as any FRID use at baseline, based on the European consensus by Seppala et. al. [11]
bMultiple FRIDs use was defined as the use of 2 or more FRIDs at baseline, also based on the European consensus by Seppala et. al. [11]
cThe number of total falls included high- and low-trauma falls, as well as injurious falls experienced over each participant’s time in the study over 3 years. A fall was defined as unintentionally coming to rest at a lower level or on the ground (excluding falling against furniture or walls)
dEstimates are from negative binomial regression models adjusted for age, sex, prior fall, BMI, study center (Berlin, Coimbra, Innsbruck, Toulouse, Basel, Geneva, and Zurich), walking aid, participant’s follow-up times, and fixed effects of treatment allocation in the DO-HEALTH trial (vitamin D3, omega-3, and home exercise program)
eThe number of recurrent falls was the number of total falls among participants who experienced two or more falls over the 3-year follow-up. Participants with one or no falls were not included in analysis for recurrent fall. For example, if a participant fell twice and another fell five times during the 3-year follow-up, the number of recurrent falls were counted as two and five, respectively
Consistently, multiple FRIDs use was associated with increased incidence rates of total falls (IRR 1.40, 95% CI [1.16–1.70], P = 0.0005) and recurrent falls (IRR 1.28, 95% CI [1.11–1.49]), P = 0.001) among participants aged ≥ 75 years. Moreover, there was no significant association between multiple FRIDs use and the incidence rates of total and recurrent falls among participants aged 70–74 years (Table 2).
For injurious falls, no significant effect modification by age group was observed for any of the exposures (at least one FRID use and multiple FRIDs use) (Supplementary Table 2).
Sensitivity analysis
In a sensitivity analysis, we further adjusted the models for the baseline number of comorbidities. With this adjustment, the significant association between at least one FRID use at baseline and the number of total, recurrent, and injurious falls over the 3-year follow-up was attenuated and lost statistical significance. For multiple FRIDs use, however, the increased incidence rate of total and injurious falls remained significant ((IRR 1.17, 95% CI [1.01–1.36], P = 0.04) and (IRR 1.29, 95% CI [1.11–1.50], P = 0.001)) or close to statistical significance for recurrent falls (IRR 1.13, 95% CI [1.00–1.28], P = 0.05, Supplementary Table 3).
Discussion
In this 3-year observational study of 2157 generally healthy and active community-dwelling adults aged ≥ 70 years, four out of ten participants used at least one FRID and six out of ten participants sustained at least one fall. Use of at least one FRID was associated with higher incidence rates of total, injurious, and recurrent falls, independent of treatment allocation (vitamin D3, omega-3, and home exercise program), study site, fall in the last year, age, sex, BMI, walking aid, and participant’s time in the study. This association was most pronounced for multiple FRIDs use, even after adjusting for comorbidities in a sensitivity analysis. Moreover, older adults age ≥ 75 years may be most affected by FRIDs with regard to the number of total and recurrent falls experienced.
Our findings align with previous studies on increased fall risk linked to FRIDs. Short-term studies (≤ 12 months) in community-dwelling older adults have shown higher odds of falling with FRID use [4, 9, 19]. For example, a 12-month population-based cohort study among 156,488 adults aged ≥ 64 years found an increased odds of falling among new and continuing FRIDs users, with the highest odds ratio observed among those taking four or more FRIDs compared to two [4]. Another 2-month cohort study among 204 patients with admission to the emergency department after a fall event found that the number of FRIDs on discharge was associated with 1.7 higher odds of recurrent falls [19]. Prior long-term studies (≥ 3 years) which assessed the association between medications and falls in generally healthy community-dwelling older adults focused on individual drugs or medication classes [13–18]. Our study contributes by showing that both at least one FRID use and multiple FRIDs use at baseline were associated with increased incidence rates of total, injurious, and recurrent falls among generally healthy and active community-dwelling older adults over 3 years. Therefore, it is important to include medication reviews to identify and deprescribe FRID (whenever possible and according to evidence-based guidelines and algorithms) as part of the fall risk assessment in older adults. In fact, findings from prior studies suggest that deprescribing FRIDs may be effective as a single intervention [30–33] or as part of multifactorial interventions [34–36] for fall prevention.
Regarding the association of baseline FRID intake and prospective fall rate, we found effect modification by age for total and recurrent falls. Among adults aged ≥ 75 years, at least one FRID use at baseline and multiple FRIDs use at baseline were associated with a 24% or greater increase in the incidence rates of total and recurrent falls. In contrast, there was no significant association between at least one FRID use or multiple FRIDs use and total and recurrent falls among participants aged 70–74 years. These findings suggest that FRIDs at older age are potentially more harmful and may be best explained by physiologic changes that occur with older age leading to altered pharmacokinetics and pharmacodynamics [12]. These changes include altered renal and hepatic functions such as reduced glomerular filtration rate and a progressive reduction in liver volume and liver blood flow that influence drug absorption, distribution, metabolism, and clearance [12, 37–39]. Additionally, body fat increases and therefore the volume of distribution of lipophilic drugs increases leading to prolonged half-life times [40, 41]. Finally, aging is associated with an increased sensitivity to several medication classes such as psychotropic drugs [12].
In our sensitivity analyses, which also adjusted for number of comorbidities at baseline, we still found an increased rate of total and injurious falls for multiple FRIDs use at baseline which suggest that the use of ≥ 2 FRIDs may increase the rate of total and injurious falls independent of the underlying disease risk [42–44]. Consistently, another cohort study among 204 participants aged ≥ 60 years showed that the number of FRIDs remains significantly associated with an increased odds ratio of falling after adjustment for comorbidities [9]. Additionally, a threshold for the number of FRIDs that predicted falls of 2.5 and 1.5 FRIDs were suggested for robust and frail patients, respectively, based on a 2-month study among 204 patients aged 60 years and older admitted to hospital after a fall event [19].
Mechanistically, the link between FRID use and fall risk may be explained by the well-documented adverse events related to FRIDs [11, 45–47]. Prior meta-analyses showed that FRIDs can lead to dizziness, orthostatic hypotension, anticholinergic activity and hypoglycemia [11, 45–47]. Moreover, sedation and cognitive impairment are well-known adverse events associated with FRIDs [11, 46]. These adverse events individually and in combination can lead to increased risk of falling [11, 45–47].
In DO-HEALTH, despite targeting generally healthy adults aged 70 and older, 60.8% of participants fell over 3 years and 42.1% had at least one FRID at baseline. This is in-line with a Swedish population-based study where approximately 60% of community-dwellers aged 65 years and older who sustained a fall reported the use of at least one FRID [48]. Moreover, a high prevalence of FRID use (65 to 93%) at the time of a fall-related injury was reported by a recent systematic review of 14 articles including 8300 adults aged 60 years and older [3].
This study has several strengths. First, in the 5-country DO-HEALTH study, falls were assessed prospectively every 3 months with a high-quality fall assessment, with varying follow-up durations for participants up to 3 years. Second, the detailed medication ascertainment was a pre-defined work package in DO-HEALTH, including all regular medications and not only pharmacy claims or prescribed medications [4, 13, 19]. Third, the definition of FRID was based on an evidence-based European consensus published recently [11]. Fourth, our main findings show consistency between total, injurious, and recurrent falls.
Our study has limitations. First, while DO-HEALTH defined falls as a pre-defined secondary outcome and aimed to collect medication use in detail, the investigation of FRID use on falls was not part of the original protocol [21]. Second, we did not aim to assess FRID use at the moment of a fall event and therefore other causes of the fall event cannot be excluded. Third, we did not assess the indication of FRID intake and therefore the results may be biased by conditions that the medications are used for. Fourth, due to the observational design of the study, we cannot establish a causal relationship between FRID use and the different outcomes. Despite this inherent limitation, we have nonetheless endeavored to minimize potential biases by carefully adjusting for several known confounders, including a history of falls in the 12 months prior to enrolment in the DO-HEALTH trial, which is the most comprehensive risk factor for subsequent falls. These methodological adjustments align with best practices in observational research, ensuring the robustness of our findings. Fifth, the definition of FRIDs varies and the European consensus did not include medications such as hypoglycaemics. Sixth, given the select sample with low comorbidities but history of falls, our results may not be representative of the general older adult population.
Conclusion
This 3-year observational analysis of the DO-HEALTH trial, including 2157 generally health community-dwelling older adults, suggests that FRID use is associated with an increased incidence rate of total, injurious, and recurrent falls. The associations were most pronounced among adults aged 75 and older and among those taking multiple FRIDs. Therefore, medication reviews to identify and deprescribe FRIDs whenever possible are crucial to support fall prevention efforts in older adults.
Supplementary Information
Acknowledgements
We thank all the members of the DO-HEALTH Research Group, and we sincerely appreciate the dedication and participation of the study participants.
Abbreviations
- ATC
Anatomical Therapeutic Chemical classification system
- BMI
Body mass index
- CI
Confidence interval
- FRID
Fall risk increasing drug
- IQR
Interquartile range
- IR
Incidence rate
- IRR
Incidence rate ratio
- METs
Mean of metabolic equivalent tasks
- MMSE
Mini-Mental State Examination
- No
Absolute number
- SD
Standard deviation
Authors’ contributions
Concept and design: CdGRCM, HABF. Acquisition of data: AE, HABF, RWK, BV. Analysis and interpretation of data: CKF, CdGRCM, MW, EJO, GF, HABF. Drafting the article: CKF, CdGRCM, GF. Critical revision of the manuscript for important intellectual content: MW, EJO, RWK, BV, AE, HABF. Final approval: All authors.
Funding
The DO-HEALTH study was funded by the Seventh Framework Program of the European Commission (grant agreement 278588), the University of Zurich (Chair for Geriatric Medicine and Aging Research), DSM Nutritional Products, Roche, NESTEC, Pfizer, and Streuli.
The funding/supporting organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Data availability
Data described in the manuscript, code book, and analytic code will not be made available to allow primary researchers of the DO-HEALTH Research Group to fully exploit the dataset. The data will be made available to external researchers in a second step according to a controlled access system.
Declarations
Ethics approval and consent to participate
The present study was approved by the Cantonal Ethical Committee of the Canton of Zurich (BASEC N° 2021–02127). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Caroline de Godoi Rezende Costa Molino and Catherine K. Forster shared first authorship.
Gregor Freystaetter and Heike A. Bischoff-Ferrari shared last authorship.
References
- 1.Moreland B, Kakara R, Henry A. Trends in Nonfatal Falls and fall-related injuries among adults aged ≥ 65 years - United States, 2012–2018. MMWR Morb Mortal Wkly Rep. 2020;69(27):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention CfDCa. STEADI – Older Adult Fall Prevention. 2019. https://www.cdcgov/steadi/indexhtml.
- 3.Hart LA, Phelan EA, Yi JY, Marcum ZA, Gray SL. Use of fall risk-increasing drugs around a fall-related Injury in older adults: a systematic review. J Am Geriatr Soc. 2020;68(6):1334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musich S, Wang SS, Ruiz J, Hawkins K, Wicker E. Falls-Related Drug Use and Risk of Falls among older adults: a study in a US Medicare Population. Drugs Aging. 2017;34(7):555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seppala LJ, van der Velde N, Masud T, Blain H, Petrovic M, van der Cammen TJ, et al. EuGMS Task and Finish group on fall-risk-increasing drugs (FRIDs): position on knowledge dissemination, management, and Future Research. Drugs Aging. 2019;36(4):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health OrganizatioH. WHO global report on falls prevention in older age. Geneva: World Health Organization; 2008. https://iris.who.int/bitstream/handle/10665/43811/9789241563536_eng.pdf?sequence=1.
- 7.Turner S, Kisser R, Rogmans W. Falls among older adults in the EU-28: Key facts from the available statistics. Amsterdam: EuroSafe; 2015. https://eupha.org/repository/sections/ipsp/Factsheet_falls_in_older_adults_in_EU.pdf.
- 8.Crane MA, Lam A, Christmas C, Gemmill A, Romley JA. Epidemiology of mortality attributed to falls in older adults in the US, 1999–2020. J Am Geriatr Soc. 2024;72(1):303–7. [DOI] [PubMed]
- 9.Zia A, Kamaruzzaman SB, Tan MP. The consumption of two or more fall risk-increasing drugs rather than polypharmacy is associated with falls. Geriatr Gerontol Int. 2017;17(3):463–70. [DOI] [PubMed] [Google Scholar]
- 10.Kragh A, Elmståhl S, Atroshi I. Older adults’ medication use 6 months before and after hip fracture: a population-based cohort study. J Am Geriatr Soc. 2011;59(5):863–8. [DOI] [PubMed] [Google Scholar]
- 11.Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbińska K, et al. STOPPFall (Screening Tool of older persons prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on fall-risk-increasing drugs. Age Ageing. 2021;50(4):1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ham AC, Swart KM, Enneman AW, van Dijk SC, Oliai Araghi S, van Wijngaarden JP, et al. Medication-related fall incidents in an older, ambulant population: the B-PROOF study. Drugs Aging. 2014;31(12):917–27. [DOI] [PubMed] [Google Scholar]
- 14.Hill-Taylor B, Sketris IS, Gardner DM, Thompson K. Concordance with a STOPP (Screening Tool of older persons’ potentially inappropriate prescriptions) Criterion in Nova Scotia, Canada: benzodiazepine and zoplicone prescription claims by older adults with fall-related Hospitalizaions. J Popul Ther Clin Pharmacol. 2016;23(1):e1–12. [PubMed] [Google Scholar]
- 15.Trenaman SC, Hill-Taylor BJ, Matheson KJ, Gardner DM, Sketris IS. Antipsychotic drug dispensations in older adults, including continuation after a fall-related hospitalization: identifying adherence to Screening Tool of older persons’ potentially inappropriate prescriptions Criteria using the Nova Scotia seniors’ Pharmacare Program and Canadian Institute for Health’s discharge databases. Curr Ther Res Clin Exp. 2018;89:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon CG, Cahir CA, Kenny RA, Bennett K. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age Ageing. 2014;43(1):44–50. [DOI] [PubMed] [Google Scholar]
- 17.Walsh ME, Boland F, Moriarty F, Fahey T. Modification of potentially inappropriate prescribing following fall-related hospitalizations in older adults. Drugs Aging. 2019;36(5):461–70. [DOI] [PubMed] [Google Scholar]
- 18.Hanlon JT, Boudreau RM, Roumani YF, Newman AB, Ruby CM, Wright RM, et al. Number and dosage of central nervous system medications on recurrent falls in community elders: the Health, Aging and Body Composition study. J Gerontol Biol Sci Med Sci. 2009;64(4):492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett A, Gnjidic D, Gillett M, Carroll P, Matthews S, Johnell K, et al. Prevalence and impact of fall-risk-increasing drugs, polypharmacy, and drug-drug interactions in robust versus frail hospitalised falls patients: a prospective cohort study. Drugs Aging. 2014;31(3):225–32. [DOI] [PubMed] [Google Scholar]
- 20.Boyé ND, van der Velde N, de Vries OJ, van Lieshout EM, Hartholt KA, Mattace-Raso FU, et al. Effectiveness of medication withdrawal in older fallers: results from the improving medication prescribing to reduce risk of FALLs (IMPROveFALL) trial. Age Ageing. 2017;46(1):142–6. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff-Ferrari HA, de Molino GRC, Rival C, Vellas S, Rizzoli B, Kressig R. DO-HEALTH: vitamin D3 - Omega-3 - home exercise - healthy aging and longevity trial - design of a multinational clinical trial on healthy aging among European seniors. Contemp Clin Trials. 2020;100:106124. [DOI] [PubMed] [Google Scholar]
- 22.Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva JAP, Blauth M, et al. Effect of vitamin D supplementation, Omega-3 fatty acid supplementation, or a strength-training Exercise Program on Clinical outcomes in older adults: the DO-HEALTH Randomized Clinical Trial. JAMA. 2020;324(18):1855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischoff-Ferrari HA, Freystätter G, Vellas B, Dawson-Hughes B, Kressig RW, Kanis JA, et al. Effects of vitamin D, omega-3 fatty acids, and a simple home strength exercise program on fall prevention: the DO-HEALTH randomized clinical trial. Am J Clin Nutr. 2022;115(5):1311–21. [DOI] [PubMed] [Google Scholar]
- 24.Methodology WCCfDS. ATC/DDD Index. https://www.whocc.no/atc_ddd_index/.
- 25.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 26.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–63. [DOI] [PubMed] [Google Scholar]
- 27.Naharci MI, Tasci I. Frailty status and increased risk for falls: the role of anticholinergic burden. Arch Gerontol Geriatr. 2020;90:104136. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Ros P, Martínez-Arnau FM, Orti-Lucas RM, Tarazona-Santabalbina FJ. A predictive model of isolated and recurrent falls in functionally independent community-dwelling older adults. Braz J Phys Ther. 2019;23(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Molino GRC, Chocano-Bedoya C, Sadlon PO, Theiler A, Orav R, Vellas JE. Prevalence of polypharmacy in community-dwelling older adults from seven centres in five European countries: a cross-sectional study of DO-HEALTH. BMJ Open. 2022;12(4):e051881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47(7):850–3. [DOI] [PubMed] [Google Scholar]
- 31.Pit SW, Byles JE, Henry DA, Holt L, Hansen V, Bowman DA. A quality use of Medicines program for general practitioners and older people: a cluster randomised controlled trial. Med J Aust. 2007;187(1):23–30. [DOI] [PubMed] [Google Scholar]
- 32.Weber V, White A, McIlvried R. An electronic medical record (EMR)-based intervention to reduce polypharmacy and falls in an ambulatory rural elderly population. J Gen Intern Med. 2008;23(4):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20(10):1255–63. [DOI] [PubMed] [Google Scholar]
- 34.Ganz DA, Latham NK. Prevention of Falls in Community-Dwelling older adults. N Engl J Med. 2020;382(8):734–43. [DOI] [PubMed] [Google Scholar]
- 35.Ganz DA, Yuan AH, Greene EJ, Latham NK, Araujo K, Siu AL, et al. Effect of the STRIDE fall injury prevention intervention on falls, fall injuries, and health-related quality of life. J Am Geriatr Soc. 2022;70(11):3221–9. [DOI] [PMC free article] [PubMed]
- 36.Dautzenberg L, Beglinger S, Tsokani S, Zevgiti S, Raijmann R, Rodondi N, et al. Interventions for preventing falls and fall-related fractures in community-dwelling older adults: a systematic review and network meta-analysis. J Am Geriatr Soc. 2021;69(10):2973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76. [DOI] [PubMed] [Google Scholar]
- 38.Kampmann JP, Sinding J, Moller-Jorgensen I. Effect of age on liver function. Geriatrics. 1975;30(8):91–5. [PubMed] [Google Scholar]
- 39.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278–85. [DOI] [PubMed] [Google Scholar]
- 40.Fülöp T Jr., Wórum I, Csongor J, Fóris G, Leövey A. Body composition in elderly people. I. determination of body composition by multiisotope method and the elimination kinetics of these isotopes in healthy elderly subjects. Gerontology. 1985;31(1):6–14. [DOI] [PubMed] [Google Scholar]
- 41.Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975;55(2):347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibley KM, Voth J, Munce SE, Straus SE, Jaglal SB. Chronic disease and falls in community-dwelling canadians over 65 years old: a population-based study exploring associations with number and pattern of chronic conditions. BMC Geriatr. 2014;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huberty S, Freystätter G, Wieczorek M, Dawson-Hughes B, Kanis JA, Rizzoli R, et al. Association between Multimorbidity and Rate of falls: a 3-Year 5-Country prospective study in generally healthy and active Community-Dwelling adults aged ≥ 70 years. J Am Med Dir Assoc. 2023;24(6):804–810.e4. [DOI] [PubMed]
- 44.Ie K, Chou E, Boyce RD, Albert SM. Fall risk-increasing drugs, polypharmacy, and Falls among Low-Income Community-Dwelling older adults. Innov Aging. 2021;5(1):igab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries M, Seppala LJ, Daams JG, van de Glind EMM, Masud T, van der Velde N. Fall-risk-increasing drugs: a systematic review and Meta-analysis: I. Cardiovascular drugs. J Am Med Dir Assoc. 2018;19(4):371.e1-.e9. [DOI] [PubMed] [Google Scholar]
- 46.Seppala LJ, Wermelink A, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al. Fall-risk-increasing drugs: a systematic review and Meta-analysis: II. Psychotropics. J Am Med Dir Assoc. 2018;19(4):371.e11-.e17. [DOI] [PubMed] [Google Scholar]
- 47.Seppala LJ, van de Glind EMM, Daams JG, Ploegmakers KJ, de Vries M, Wermelink A, et al. Fall-risk-increasing drugs: a systematic review and Meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19(4):372.e1-.e8. [DOI] [PubMed] [Google Scholar]
- 48.Laflamme L, Monárrez-Espino J, Johnell K, Elling B, Möller J. Type, number or both? A population-based matched case-control study on the risk of fall injuries among older people and number of medications beyond fall-inducing drugs. PLoS ONE. 2015;10(3):e0123390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21(5):658–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available to allow primary researchers of the DO-HEALTH Research Group to fully exploit the dataset. The data will be made available to external researchers in a second step according to a controlled access system.