Abstract
Objectives
To explore correlations between biomarker indices and urosepsis severity, and investigate the prevalence of drug-resistant Escherichia coli in a patient population at the General Hospital of Ningxia Medical University in the Ningxia region of China.
Methods
Patients with urinary tract infection-associated sepsis were categorized into three groups: a septic non-shock group (NSSPU), a septic shock group (USG), and a control group with non-sepsis cases of simple urinary tract infections (CG). The study analyzed various biomarkers, including the percentage of neutrophils (N%), neutrophil-to-lymphocyte ratio (NLR), and lactate (La), to assess their predictive value for urogenital sepsis severity.
Results
The Kruskal–Wallis test showed significant differences in all measured biomarkers between the groups. ROC curve analysis identified N%, NLR, total protein (TP), albumin (ALB), and La as meaningful predictors of urosepsis severity. The combined detection indicators hold greater value in diagnosing uroseptic shock compared to individual test indicators. In addition, the study confirmed the prevalence of drug-resistant E. coli in cases of septic shock.
Conclusion
The combined monitoring of N%, NLR, La, TP, and ALB proves beneficial in the clinical diagnosis of uroseptic shock. This study emphasizes the significance of monitoring Escherichia coli and its resistance patterns to decrease the occurrence of sepsis complications.
Keywords: Urinary tract infection, Urosepsis, Biomarkers, Drug resistance, Escherichia coli
Background
Urogenic sepsis is a severe complication of urinary tract infections that poses a significant threat to both individual and public health [1–3]. In high-risk groups, it can progress to septic shock, which has a very high mortality rate [4, 5]. Additionally, it places a significant burden on socioeconomic and family financial status [6]. As urologic diseases and surgeries become more common, the risk of urinary tract infections and resulting bloodstream infections also increases. The therapeutic challenge is exacerbated by the increasing resistance of causative organisms to conventional antibiotics. For instance, a high proportion of Escherichia coli, Enterococcus, Pseudomonas aeruginosa, and Klebsiella exhibit resistance to commonly used antibiotics [7, 8]. The European Guidelines for Urinary Tract Infections offer valuable guidance for diagnosing and treating various urinary tract infections. However, clinical management remains challenging due to regional differences in causative organisms and drug resistance. Therefore, investigating the influencing factors of UTI-associated bloodstream infections and local pathogen characteristics is crucial to enhance preventive and therapeutic strategies.
To address this issue, the aim of this study was to optimize the therapeutic strategy by analyzing the clinical data, pathogenetic composition of common strains and drug resistance in patients with urogenic sepsis admitted to the General Hospital of Ningxia Medical University.
Data and methodology
Participants
This study included individuals diagnosed with UTIs complicated by sepsis at Ningxia Medical University General Hospital between January 1, 2020, and December 1, 2023 in the Ningxia region of China. Sepsis is diagnosed according to the criteria of the American Society of Critical Care Medicine and the European Society of Intensive Care Medicine’s Campaign to Save Sepsis: International Guidelines for the Management of Sepsis and Septic Shock 2016, combined with urological practice and the diagnostic criteria for urinary septicaemia: (1) a clinically confirmed urinary tract infection (UTI); (2) a Sequential Organ Failure Assessment (SOFA) score of 2 or greater; (3) sustained hypotension post-adequate fluid resuscitation requiring vasopressors to maintain a mean arterial pressure (MAP) of ≥ 65 mmHg and serum lactate levels > 2 mmol/L are the criteria for sepsis and septic shock [9]. Exclusions included only initial diagnoses of sepsis or septic shock resulting in replicative hospital admissions, infections originating from alternate loci, individuals younger than 18, and incomplete clinical data sets.
Analytical approach
In this retrospective study, patients were organized into three cohorts for comparative analysis: a control group with non-sepsis cases of simple urinary tract infections (CG), a septic non-shock group (NSSPU), and a septic shock group (USG).
The study received ethical approval from the Institutional Review Board (IRB) of Ningxia Medical University General Hospital on January 17, 2024, under approval number KYLL-2024-066. Owing to the retrospective nature of the investigation, the necessity for informed consent was waived, with the study utilizing pre-existing clinical data that were anonymized in adherence to the ethical standards stipulated by the Declaration of Helsinki.
Statistical analysis
Data analysis was facilitated using SPSS version 21.0. Normality assessments were conducted on the dataset. Median values were used to describe non-normally distributed enumerative data. The Kruskal–Wallis H test was used to discern disparities across groups for non-normally distributed metrics. Post hoc two-by-two comparisons were made for factors with a P-value less than 0.05. Logistic regression analysis was utilized to investigate the correlation between the test indicators and sepsis, as well as to evaluate the impact of test indicators on the severity of the disease. Afterwards, a multi-factor analysis of statistically significant test indicators was performed using ROC curve analysis to assess the influence of relevant indicators on the severity of urogenital sepsis. This study evaluates the predictive value of relevant indicators for the severity of urosepsis by analyzing ROC curves and compares the diagnostic performance of various testing indicators. Categorical variables, such as gender, comorbidity profiles, and culture results, were presented as frequencies with percentages. Count data were analyzed using the χ2 test with R × C. Successive corrections were used when the theoretical frequency was less than 5. Fisher’s exact probability method was used when the number of each cell less than 1. Statistically significant differences were considered at P < 0.05.
Results
Basic characteristics of urinary sepsis
This study analyzed clinical data from 111 patients diagnosed with sepsis, comprising 46 males and 65 females. Of these, 83 had positive urine cultures, while 28 had negative urine cultures. Moreover, 61 had positive blood cultures, and 50 had negative blood cultures. The age of the patients ranged from 18 to 97 years, with a median age of 67. The statistical analysis revealed that age was significantly associated with urosepsis (P < 0.05), individuals over the age of 65 are at a higher risk of developing urogenital sepsis (Table 1). Of the patients, 25 had a normal body temperature, while 86 had a fever. The median body temperature was 38.8 °C The median systolic blood pressure was 114 mmHg, and the median diastolic blood pressure was 70 mmHg. The median pulse rate was 90 beats per minute, and the median respiratory rate was 20 breaths per minute. Urine routine WBC (+) ~ (++++). The study categorized patients with urinary sepsis into non-shock and shock groups. There were 59 cases in the non-shock group and 52 cases in the shock group. Additionally, 71 patients selected as a control group with non-sepsis cases of simple urinary tract infections (CG). Among these patients, death occurred in two cases, resulting in a mortality rate of 1.92% in the USG and 1.69% in the NSSPU.
Table 1.
Comparison of clinical and laboratory characteristics collected at hospital discharge in each study group
| Features | CG% (n = 71) | NSSPU% (n = 59) | USG % (n = 52) | P-value |
|---|---|---|---|---|
| Underlying disease | ||||
| Hypertension | 1.41 (1) | 20.60 (13) | 37.50 (18) | < 0.001* |
| Diabetes mellitus | 4.23 (3) | 38.10 (24) | 33.33 (16) | < 0.001* |
| Hypertension with diabetes mellitus | 1.41 (1) | 19.05 (12) | 18.80 (9) | 0.001* |
| Cardiovascular and cerebrovascular diseases | 2.82 (2) | 26.98 (17) | 41.67 (20) | < 0.001* |
| Prostatic hypertrophy | 5.63 (4) | 6.35 (4) | 10.42 (5) | 0.763 |
| Chronic kidney disease | 7.04 (5) | 41.27 (26) | 62.50 (30) | < 0.001* |
| Urinary calculus | 2.82 (2) | 19.05 (12) | 33.33 (16) | < 0.001* |
| Malignant tumor | 0 (0) | 14.29 (9) | 14.58 (7) | 0.002* |
| Post-urinary tract surgery | 5.63 (4) | 7.94 (5) | 14.58 (7) | 0.346 |
| Hematologic disorders | 0 (0) | 19.05 (12) | 33.33 (16) | < 0.001* |
| Malnutrition | 0 (0) | 20.63 (13) | 39.58 (19) | < 0.001* |
| Fluid in the chest and abdomen | 0 (0) | 15.87 (10) | 12.50 (6) | 0.002* |
| Digestive tract disease | 1.41 (1) | 12.70 (8) | 22.92 (11) | 0.001* |
| Age distribution | ||||
| 18–34 | 23.94 (17) | 6.78 (4) | 0 (0) | < 0.001* |
| 35–49 | 5.63 (4) | 5.09 (3) | 7.69 (4) | |
| 50–64 | 35.21 (25) | 25.42 (15) | 42.31 (22) | |
| > 65 | 35.21 (25) | 62.71 (37) | 50.00 (26) | |
| Gender | ||||
| Male | 22.54 (16) | 40.68 (24) | 42.31 (22) | 0.031* |
| Female | 77.46 (55) | 59.32 (35) | 59.69 (30) | |
| Blood culture | ||||
| Positive | 4.23 (3) | 50.85 (30) | 59.62 (31) | < 0.001* |
| Negative | 95.77 (68) | 49.15 (29) | 40.38 (21) | |
| Urine culture | ||||
| Positive | 4.23 (3) | 77.97 (46) | 71.15 (37) | < 0.001* |
| Negative | 95.77 (68) | 22.03 (13) | 28.85 (15) | |
Values are represented as % (n) where n is the number of cases and (%) represents the percentage of the total within each column. If the superscript a or b is the same, there is no statistical difference between the two groups
*Indicates a statistically significant difference at P < 0.05
Analysis of underlying diseases in the NSSPU and USG
Patients with urinary sepsis often have underlying diseases. In the NSSPU group, the most common underlying diseases were chronic kidney disease (41.27%), diabetes mellitus (38.1%), cardiovascular and cerebrovascular diseases (26.98%), malnutrition (20.63%), hypertension (20.6%), hypertension with diabetes mellitus (19.05%), and hematologic disorders (19.05%). In the USG, common underlying diseases included chronic kidney disease (62.5%), cardiovascular and cerebrovascular diseases (41.67%), malnutrition (39.58%), hypertension (37.5%), diabetes mellitus (33.33%), hematologic disorders (33.33%), hypertension with diabetes mellitus (18.8%), and post urinary tract surgery (14.58%). The statistical analysis revealed that the incidence of urinary sepsis was significantly associated with certain underlying diseases, such as hypertension and diabetes mellitus, but not with prostatic hypertrophy or post-urologic surgery (Table 1).
Normality test
To test the normality of the three groups of test indexes, skewness and kurtosis were used. It was found that leukocyte count (WBC), neutrophils (N%), neutrophil-to-lymphocyte ratio (NLR), platelet (PLT), D-dimer, total protein (TP), albumin (ALB), urea (URE), serum creatinine (SCR), and lactate (La) did not follow a normal distribution simultaneously.
Univariate analysis
After WBC, N%, NLR, PLT, D-Dimer, TP, ALB, URE, SCR, La, and other factors were subjected to the Kruskal–Wallis test. The test results indicated statistically significant differences between the control group, the sepsis non-shock group, and shock group. For qualitative data, such as blood culture, urine culture, and gender distribution, significant differences between groups were found using the Chi-square test (Table 1). Therefore, it can be inferred that patients who test positive for blood and urine cultures and are female are more likely to develop urogenic septic shock.
A post hoc two-by-two comparison was conducted to analyze the test indicators between different groups
The comparison revealed significant differences in N%, NLR, TP, ALB, and La between the CG and NSSPU, CG and USG, and NSSPU and USG. The test indices’ median results in different groups indicate that TP and ALB values decrease with increasing sepsis severity, while N%, NLR, and La values increase (Table 2).
Table 2.
Comparison of test indicators across study groups
| Test indicators | CG (M) | NSSPU (M) | USG (M) | Statistics (H) | P-value |
|---|---|---|---|---|---|
| WBC | 8.35a | 13.02b | 42.61b | 15.90 | < 0.001* |
| N% | 71.90a | 87.40b | 92.80c | 90.38 | < 0.001* |
| NLR | 4.25a | 13.34b | 23.78c | 85.14 | < 0.001* |
| La | 1.10a | 2.01b | 2.65c | 89.28 | < 0.001* |
| PLT | 244.00a | 166.00b | 126.00b | 34.28 | < 0.001* |
| D-dimer | 0.82a | 2.01b | 5.10b | 83.38 | < 0.001* |
| URE | 5.09a | 8.73b | 9.96b | 44.52 | < 0.001* |
| SCR | 58.50a | 115.40b | 123.55b | 44.19 | < 0.001* |
| TP | 71.40a | 63.60b | 56.35c | 47.04 | < 0.001* |
| ALB | 41.30a | 34.10b | 29.35c | 59.71 | < 0.001* |
Data are presented as median (M)
*P-value < 0.05 is considered statistically significant. If the superscript a, b or c is the same, there is no statistical difference between the two groups
Analysis of the indicators for the progression of urinary tract infection to septic shock using multiple logistic regression
Compared to the non-sepsis cases of simple urinary tract infections, NLR and La have significant effects on the septic non-shock and septic shock. An increase in NLR and La levels increases the probability of septic non-shock to 1.3 times and 3.95 times, respectively, compared to when these levels are not increased. The probability of septic shock increases to 1.4 times and 8.2 times, respectively, as shown in Table 3.
Table 3.
Multiple logistic regression analysis of factors related to sepsis
| Progression | Variable | B | S.E | Wald χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| NSSPU | WBC | 0.010 | 0.075 | 0.018 | 0.893 | 1.010 | 0.872–1.169 |
| N | 0.010 | 0.044 | 0.049 | 0.825 | 1.010 | 0.926–1.102 | |
| NLR | 0.229 | 0.087 | 6.919 | 0.009* | 1.257 | 1.060–1.492 | |
| PLT | − 0.010 | 0.004 | 7.322 | 0.007* | 0.990 | 0.983–0.997 | |
| La | 1.373 | 0.433 | 10.069 | 0.002* | 3.946 | 1.690–9.212 | |
| TP | 0.002 | 0.064 | 0.001 | 0.977 | 1.002 | 0.884–1.135 | |
| ALB | − 0.082 | 0.087 | 0.898 | 0.343 | 0.921 | 0.777–1.092 | |
| SCR | 0.011 | 0.006 | 3.034 | 0.082 | 1.011 | 0.999–1.023 | |
| D-dimer | 0.073 | 0.093 | 0.623 | 0.430 | 1.076 | 0.897–1.290 | |
| URE | − 0.137 | 0.090 | 2.349 | 0.125 | 0.872 | 0.731–1.039 | |
| USG | WBC | − 0.020 | 0.083 | 0.060 | 0.807 | 0.980 | 0.833–1.153 |
| N | 0.012 | 0.070 | 0.031 | 0.860 | 1.012 | 0.883–1.161 | |
| NLR | 0.318 | 0.092 | 12.054 | 0.001* | 1.375 | 1.149–1.645 | |
| PLT | − 0.006 | 0.004 | 2.162 | 0.141 | 0.994 | 0.986–1.002 | |
| La | 2.106 | 0.473 | 19.794 | < 0.001* | 8.211 | 3.248–20.761 | |
| TP | − 0.071 | 0.083 | 0.722 | 0.395 | 0.932 | 0.792–1.097 | |
| ALB | − 0.071 | 0.117 | 0.368 | 0.544 | 0.932 | 0.741–1.171 | |
| SCR | 0.011 | 0.007 | 2.864 | 0.091 | 1.011 | 0.998–1.024 | |
| D-dimer | 0.094 | 0.096 | 0.962 | 0.327 | 1.099 | 0.910–1.327 | |
| URE | − 0.112 | 0.100 | 1.265 | 0.261 | 0.894 | 0.735–1.087 |
Multiple logistic regression analysis was performed to identify factors associated with different stages of sepsis severity. B represents the regression coefficient for each independent variable
S.E standard error
Wald χ2 is the Chi-squared statistic from the Wald test. P-values show the level of statistical significance (*P < 0.05). The OR (odds ratio) value represents the degree of influence one variable has on another in the relationship between two categorical variables. Confidence intervals (CI) are provided at the 95% level
Predictive value of N%, NLR, TP, ALB and La for urosepsis
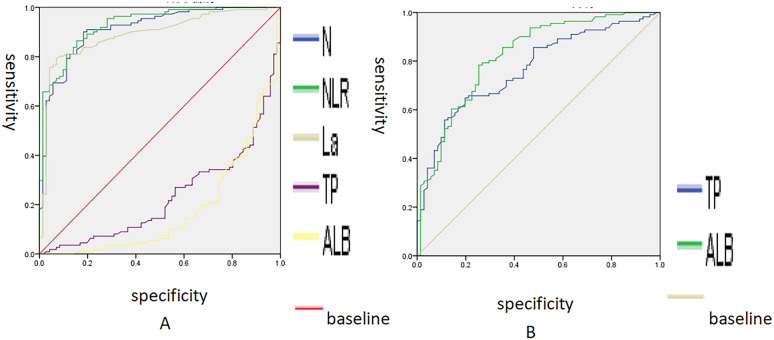
We evaluated the diagnostic value of N%, NLR, TP, ALB and La levels in diagnosing sepsis by comparing them between the non-sepsis control group (CG) and sepsis groups (NSSPU and USG). Receiver operating characteristics (ROC) were also plotted (Fig. 1). At the maximum Youden index, the corresponding critical value was determined. The results showed that an increase of N% had diagnostic value for the severity of urogenital sepsis (AUC = 0.913, 95% CI = 0.871–0.956, P = 0.000). The optimal threshold was 80.8%, at which the Youden index was 0.718, with a sensitivity of 90.1% and specificity of 81.7%. An increase in NLR% is a diagnostic indicator of the severity of urogenital sepsis (AUC = 0.929, 95% CI = 0.893–0.966, P = 0.000), with an optimal cut-off value of 9.23, and a Jordon’s index of 0.71. The sensitivity and specificity of this indicator are 86.5% and 84.5%, respectively. Similarly, elevated levels of La (AUC = 0.891, 95% CI = 0.831–0.933, P = 0.000) was found to be correlated with the severity of urogenic sepsis and showed some diagnostic value. However, severity of urinary sepsis correlated with changes in TP and ALB with some but not much specificity. Comparing the levels of N%, NLR and La between the septic non-shock group and the septic shock group, the cut-off values for diagnosing septic shock were identified as N% at 90.15%, NLR at 18.44, and La at 2.00 mmol/L. The sensitivity was 71.20%, 73.10%, and 100%, and the specificity was 72.90%, 76.30%, and 49.20%. In the diagnosis of predicting uroseptic shock, the combined indicator exhibits high levels of cut-off value, sensitivity, and specificity, which are 42.10%, 80.80%, and 83.10%, respectively (Table 4).
Fig. 1.
ROC curve analysis of N%, NLR, La, TP and ALB. A A larger test result indicates a more positive test; B smaller test results indicate a more positive test. A Orange solid line—La; purple solid line—TP; black solid line—ALB; steel blue solid line—N; green solid line—NLR; red solid line—baseline. B Steel blue solid line—TP; green solid line—ALB; orange solid line—baseline. X-axis: specificity; Y-axis: sensitivity; scale: the range for both the x-axis (specificity) and the y-axis (sensitivity) extends from 0.0 to 1.0, representing 0% to 100%. AUC area under the receiver operating characteristic curve
Table 4.
ROC curve analysis of test Indicators in the urosepsis
| Test index | AUC | P value | 95% CI | Threshold | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| Urosepsis | N% | 0.913 | < 0.001* | 0.871–0.956 | 80.80 | 90.10 | 81.70 |
| NLR | 0.929 | < 0.001* | 0.893–0.966 | 9.23 | 86.50 | 84.50 | |
| La | 0.891 | < 0.001* | 0.831–0.933 | 1.71 | 79.30 | 93.00 | |
| TP | 0.767 | < 0.001* | 0.699–0.836 | 39.40 | 100.00 | 0 | |
| ALB | 0.821 | < 0.001* | 0.758–0.885 | 18.65 | 100.00 | 0 | |
| USG | N% | 0.773 | < 0.001* | 0.684–0.863 | 90.15 | 71.20 | 72.90 |
| NLR | 0.775 | < 0.001* | 0.686–0.863 | 18.44 | 73.10 | 76.30 | |
| La | 0.772 | < 0.001* | 0.687–0.858 | 2.00 | 100.00 | 49.20 | |
| TP | 0.696 | < 0.001* | 0.598–0.795 | 39.40 | 100.00 | 0 | |
| ALB | 0.661 | 0.002* | 0.558–0.763 | 18.20 | 100.00 | 0 | |
| PRE | 0.863 | < 0.001* | 0.795–0.931 | 42.10 | 80.80 | 83.10 | |
The PRE variable can be used to assess the likelihood of an individual having a certain disease
PRE predicted probability, AUC area under the receiver operating characteristic curve
P-values show the level of statistical significance (*P < 0.05). Confidence intervals (CI) are provided at the 95% level
Compare the ROC curves of N%, NLR, TP, ALB, La, and the combined indicator to assess the differences in diagnostic performance for predicting uroseptic shock
Comparison of the predictive value differences for N%, NLR, TP, ALB, La, and the combined indicator in predicting uroseptic shock showed that there were significant differences in predictive value among N–TP, N–ALB, N–PRE, NLR–TP, NLR–ALB, NLR–PRE, La–TP, La–ALB, La–PRE, TP–PRE, and ALB–PRE. It is evident that the diagnostic performance of the combined indicator is significantly superior to that of the individual tests. Furthermore, the diagnostic performance of N, NLR, and La individually is higher than that of TP and ALB individually (Table 5).
Table 5.
Comparative analysis of ROC curves for different diagnostic indicators in predicting uroseptic shock
| Test index | AUC difference | S.E | Statistics (Z) | P | 95% CI |
|---|---|---|---|---|---|
| N–NLR | − 0.001 | 0.297 | − 0.043 | 0.966 | − 0.119–0.058 |
| N–LA | 0.001 | 0.299 | 0.016 | 0.987 | − 0.240–0.121 |
| N–TP | 0.470 | 0.312 | 6.057 | < 0.001* | 0.318–0.622 |
| N–ALB | 0.434 | 0.315 | 5.648 | < 0.001* | 0.283–0.585 |
| N–PRE | − 0.090 | 0.280 | − 2.416 | 0.016* | − 0.145–(− 0.017) |
| NLR–LA | 0.002 | 0.299 | 0.036 | 0.971 | − 0.249–0.127 |
| NLR–TP | 0.471 | 0.312 | 6.138 | < 0.001* | 0.321–0.622 |
| NLR–ALB | 0.435 | 0.315 | 5.639 | < 0.001* | 0.284–0.587 |
| NLR–PRE | − 0.088 | 0.279 | − 2.673 | 0.008* | − 0.129–(− 0.024) |
| LA–TP | 0.469 | 0.307 | 6.842 | < 0.001* | 0.335–0.603 |
| LA–ALB | 0.433 | 0.310 | 6.271 | < 0.001* | 0.298–0.568 |
| LA–PRE | − 0.091 | 0.277 | − 2.270 | 0.023* | − 0.157–(− 0.012) |
| TP–ALB | − 0.036 | 0.314 | − 1.218 | 0.223 | − 0.116–0.022 |
| TP–PRE | − 0.559 | 0.294 | − 7.828 | < 0.001* | − 0.281–(− 0.419) |
| ALB–PRE | − 0.524 | 0.297 | − 7.265 | < 0.001* | − 0.283–(− 0.382) |
The PRE variable can be used to assess the likelihood of an individual having a certain disease
PRE predicted probability, AUC area under the receiver operating characteristic curve, S.E standard error
P-values show the level of statistical significance (*P < 0.05). Confidence intervals (CI) are provided at the 95% level
Distribution characteristics of pathogenic bacteria
In the analysis of blood and urine samples, a total of 195 strains were identified, of which 155 were Gram-negative bacteria, accounting for 79.5% of the total number of isolates. Additionally, 29 Gram-positive bacteria (14.87%) and 11 fungi (5.60%) were also identified. Among patients with urinary sepsis, the most commonly isolated Gram-negative bacteria were Escherichia coli (51.42%), followed by Klebsiella pneumoniae (7.14%), and Acinetobacter baumannii (2.86%). Among Gram-positive bacteria, Enterococcus faecalis (14.29%) and fungi (2.86%) were the most common causative agents. In cases of urinary tract infections related to urosepsis, Escherichia coli and Klebsiella pneumoniae were the most commonly isolated pathogens, with detection rates of 65.75% and 9.59% (Table 6). Among patients with urogenic septic shock, Escherichia coli showed significant antibiotic resistance, with rates exceeding 66.67% for antibiotics such as penicillin, cefazolin, ceftriaxone, and levofloxacin. The bacterial strain exhibited resistance rates of 61.9% to Aztreonam and 38.1% to cefepime. Among the cases of urinary tract infection, Escherichia coli showed the highest resistance rate to ampicillin at 88.57%, followed by ciprofloxacin and piperacillin at 68.57% and 62.86%, respectively. The resistance rate to levofloxacin was 61.90% (Table 7).
Table 6.
Distribution of strains and their proportions across different groups
| Features | Total (n = 182) | CG (n = 71) | NSSPU (n = 59) | USG (n = 52) | ||||
|---|---|---|---|---|---|---|---|---|
| Strain (n) | Proportion (%) | Strain (n) | Proportion (%) | Strain (n) | Proportion (%) | Strain (n) | Proportion (%) | |
| Gram-negative bacilli | 155 | 85.17 | 63 | 88.74 | 56 | 94.91 | 37 | 71.16 |
| E. coli | 105 | 57.69 | 48 | 67.61 | 36 | 61.02 | 21 | 40.39 |
| Klebsiella pneumoniae | 15 | 8.24 | 7 | 9.86 | 5 | 8.47 | 3 | 5.77 |
| Proteus mirabilis | 7 | 3.85 | 1 | 1.41 | 2 | 3.39 | 4 | 7.69 |
| Acinetobacter baumannii | 4 | 2.20 | 0 | 0 | 2 | 3.39 | 2 | 3.85 |
| Other | 24 | 13.19 | 7 | 9.86 | 11 | 18.64 | 7 | 13.46 |
| Gram-positive bacilli | 29 | 15.94 | 8 | 11.28 | 12 | 20.34 | 9 | 17.3 |
| Enterococcus faecium | 21 | 11.54 | 3 | 4.23 | 10 | 16.95 | 8 | 15.38 |
| Enterococcus faecalis | 5 | 2.75 | 3 | 4.23 | 2 | 3.39 | 0 | 0 |
| Other | 3 | 1.65 | 2 | 2.82 | 0 | 0 | 1 | 1.92 |
| Fungus | 11 | 6.04 | 2 | 2.82 | 2 | 3.39 | 7 | 13.46 |
The total counts for each bacterial and fungal strain were calculated from the data collected across all patient groups within the study
Table 7.
Analysis of drug resistance in Escherichia coli isolated from different groups [strains (%)]
| Antibiotic | CG (n = 48) | NSSPU (n = 36) | USG (n = 21) | Total (n = 105) | ||||
|---|---|---|---|---|---|---|---|---|
| Drug resistance rate% (n) | Sensitivity % (n) | Drug resistance rate% (n) | Sensitivity % (n) | Drug resistance rate% (n) | Sensitivity % (n) | Drug resistance rate% (n) | Sensitivity % (n) | |
| Ampicillin | 87.50 (42) | 10.42 (5) | 81.08 (30) | 16.67 (6) | 100.00 (21) | 0 (0) | 88.57 (93) | 10.48 (11) |
| Piperacillin | 54.17 (26) | 12.50 (6) | 69.44 (25) | 25.00 (9) | 71.43 (15) | 14.29 (3) | 62.86 (66) | 17.14 (18) |
| Ampicillin/sulbactam | 43.75 (21) | 29.17 (14) | 41.67 (15) | 25.00 (9) | 38.10 (8) | 19.05 (4) | 41.90 (44) | 25.71 (27) |
| Cefazolin | 45.83 (22) | 54.17 (26) | 55.56 (20) | 44.44 (16) | 71.43 (15) | 23.81 (5) | 54.29 (57) | 44.76 (47) |
| Cefuroxime | 43.75 (21) | 43.75 (21) | 55.56 (20) | 38.89 (14) | 66.67 (14) | 28.57 (6) | 52.38 (55) | 39.05 (41) |
| Cefoperazone/sulbactam | 6.25 (3) | 89.58 (43) | 0 (0) | 100.00 (36) | 9.52 (2) | 80.95 (17) | 4.76 (5) | 91.43 (96) |
| Ceftazidime | 31.25 (15) | 56.25 (27) | 33.33 (12) | 58.33 (21) | 42.86 (9) | 38.10 (8) | 34.29 (36) | 53.33 (56) |
| Cefepime | 27.08 (13) | 68.75 (33) | 27.78 (10) | 52.78 (19) | 38.10 (8) | 42.86 (9) | 29.52 (31) | 58.10 (61) |
| Ceftriaxone | 47.92 (23) | 52.08 (25) | 55.56 (20) | 44.44 (16) | 66.67 (14) | 80.95 (7) | 54.29 (57) | 45.71 (48) |
| Ciprofloxacin | 66.67 (32) | 22.92 (11) | 63.89 (23) | 25.00 (9) | 80.95 (17) | 9.52 (2) | 68.57 (72) | 20.95 (22) |
| Levofloxacin | 58.33 (28) | 8.33 (4) | 58.33 (21) | 30.56 (11) | 76.19 (16) | 19.05 (4) | 61.90 (65) | 18.10 (19) |
| Amikacin | 4.17 (2) | 95.83 (46) | 2.78 (1) | 97.22 (35) | 4.76 (1) | 95.24 (20) | 3.81 (4) | 96.19 (101) |
| Gentamicin | 43.75 (21) | 56.25 (27) | 30.56 (11) | 69.44 (25) | 57.14 (12) | 42.86 (9) | 41.90 (44) | 58.10 (61) |
| Imipenem | 0 (0) | 100 (48) | 0 (0) | 100 (36) | 0 (0) | 100.00 (21) | 0 (0) | 100.00 (105) |
| Meropenem | 0 (0) | 100 (48) | 0 (0) | 100 (36) | 0 (0) | 100.00 (21) | 0 (0) | 100.00 (105) |
| Cotrimoxazole | 47.92 (23) | 52.08 (25) | 58.33 (21) | 41.67 (15) | 76.19 (16) | 23.81 (5) | 57.14 (60) | 42.86 (45) |
| Piperacillin–tazobactam | 4.17 (2) | 89.58 (43) | 2.78 (1) | 97.22 (35) | 14.29 (3) | 80.95 (17) | 5.71 (6) | 90.48 (95) |
| Aztreonam | 33.33 (16) | 66.67 (32) | 44.44 (16) | 55.56 (20) | 61.90 (13) | 38.10 (8) | 42.86 (45) | 57.14 (60) |
| Tobramycin | 12.50 (6) | 58.33 (28) | 8.33 (3) | 69.44 (25) | 23.81 (5) | 80.95 (7) | 13.33 (14) | 57.14 (60) |
| Furantoin | 4.17 (2) | 87.50 (42) | 2.78 (1) | 86.11 (31) | 19.05 (4) | 76.19 (16) | 6.67 (7) | 84.76 (89) |
| Ticarcillin/clavulanic acid | 8.33 (4) | 81.25 (39) | 5.56 (2) | 66.67 (24) | 9.52 (2) | 85.71 (18) | 7.62 (8) | 77.14 (81) |
| Tigecycline | 0 (0) | 100.00 (48) | 0 (0) | 100.00 (36) | 0 (0) | 100.00 (21) | 0 (0) | 100.00 (105) |
| Minocycline | 10.42 (5) | 75.00 (36) | 8.33 (3) | 80.56 (29) | 28.57 (6) | 57.14 (12) | 13.33 (14) | 73.33 (77) |
| Doxycycline | 20.83 (10) | 50.00 (24) | 16.67 (6) | 66.67 (24) | 38.10 (8) | 38.10 (8) | 22.86 (24) | 53.33 (56) |
| Colistin | 4.17 (2) | 93.75 (45) | 0 (0) | 83.33 (30) | 0 (0) | 90.48 (19) | 1.90 (2) | 89.52 (94) |
Both the drug resistance rate and sensitivity rate are expressed as percentages, with the number in parentheses indicating the corresponding number of bacterial strains. All data have been analyzed according to standardized statistical procedures
n number of bacterial strains analyzed
Discussion
Urogenic sepsis is a type of sepsis caused by a bacterial infection in the urinary tract that spreads to other organs or tissues through the bloodstream, resulting in new lesions. According to Nagao et al., urogenic sepsis is more common in male patients [10, 11], particularly in the elderly population, and is typically caused by Gram-negative bacteria. The study indicates that patients aged over 65 are more prone to developing sepsis following a urinary tract infection. Bou-Anton et al. studied the prevalence and risk factors of Escherichia coli bacteremia in the United Kingdom over a 2-year period. The study found that both age and female gender were associated with an increased likelihood of E. coli bacteremia [12, 13]. Furthermore, we found that the prevalence of sepsis was lower in male patients (25.27%) than in female patients (35.71%). This gender difference is likely to be related to region-specific health conditions and management [14]. When analyzing the underlying diseases of sepsis, it was found that patients in the non-shock group had mainly chronic kidney disease (41.27%), diabetes mellitus (38.1%), and cardiovascular disease (26.98%). In contrast, patients in the shock group had chronic kidney disease (62.5%), cardiovascular disease (41.67%), and malnutrition (39.58%) more commonly. In addition to prostatic hypertrophy and urological surgery, other underlying diseases were found to be statistically significant in the development of urogenic sepsis and may increase the risk of sepsis [15, 16]. Positive urine and blood cultures were significantly associated with the development of urogenital sepsis. It can be inferred that positive urine and blood culture results may increase the risk of sepsis.
The field of critical care medicine has made considerable advancements in the diagnosis and management of septic shock. The “Campaign to Save Sepsis: International Guidelines for the Management of Sepsis and Septic Shock 2016”, endorsed by the American College of Critical Care Medicine and the European Society of Intensive Care Medicine, identifies a serum lactate level above 2 mmol/L as a critical marker for septic shock diagnosis. Studies demonstrate that this threshold boasts a sensitivity of 82.5% and a specificity of 22.4%, with a 95% confidence interval (CI) ranging from 0.816 to 0.834 [17]. Additionally, the NLR has emerged as an important biomarker in detecting systemic inflammation [18–20]. In research conducted by Wu et al., it was found that an NLR value of 11.57 could serve as a diagnostic threshold for septic shock, exhibiting a sensitivity and specificity of 100%. This study explored the differences in N% across control groups, the septic shock group and non-shock group, finding that N% is statistically significant with an area under the curve (AUC) of 0.885. A cut-off value of 77% for N% in urinary sepsis diagnosis was established, yielding a sensitivity of 93.8% and specificity of 69.6% [21]. While in the present study, the receiver operating characteristic (ROC) curve analysis of patients diagnosed with septic shock showed that the AUC for La, NLR and N% were an impressive 0.772, 0.775 and 0.773, with corresponding sensitivities of 100%, 73.10% and 71.20%, and specificities of 49.20%, 76.30% and 72.90%. The 95% CIs, when setting diagnostic thresholds at 2.00 mmol/L for La, 18.44 for NLR and 90.15% for N%, ranged between 0.687–0.858, 0.686–0.863 and 0.684–0.863, respectively. The diagnostic threshold for La reported in this study aligns with prior research. Nonetheless, the broad confidence interval, possibly due to a small sample size for septic shock incidents, limits the precision, highlighting the need for larger sample sizes to enhance the accuracy. The slightly elevated diagnostic threshold for the NLR may be attributed to a swift rise in neutrophil counts, a response often observed during episodes of acute stress, pain, or trauma [22]. Furthermore, the surge in endogenous cortisol and catecholamine levels can exacerbate this effect, leading to increased neutrophil and decreased lymphocyte counts. The influence of other hormones and cytokines on these parameters cannot be discounted. Utilizing logistic regression analysis, a significant positive correlation was established between the levels of blood La, and NLR in the context of urogenic sepsis, with respective regression coefficients of 1.373 and 0.229, all bearing statistical significance with P-values less than 0.05. These findings underscore the value of lactate and NLR not simply as diagnostic tools for septic shock but also as indicators to gauge the condition’s severity and monitor its trajectory [23].
The total protein in the serum is made up of two main categories: ALB and globulin. These components are important for assessing nutritional status and diagnosing various diseases. ALB maintains osmotic pressure balance in plasma, which is essential for normal bodily functions. Hypoproteinemia is frequently observed in patients with sepsis at the onset of the condition. Several studies have confirmed a strong correlation between hypoproteinemia and poor prognosis in critically ill patients, indicating its significance as a prognostic indicator [16, 24]. In cases of sepsis where the initial concentration of ALB in the plasma drops below 20 g/L, failure to replenish ALB immediately can result in life-threatening complications. The study analyzed predictive curves to determine the area under the curve (AUC) for TP in relation to the severity of sepsis. The results showed that a TP threshold of 39.4 g/L had a sensitivity of 100% for diagnosing sepsis, but a specificity of 0%. The AUC for ALB was also analyzed and found to be 0.179. The diagnostic cut-off for ALB was set at 18.65 g/L, achieving a sensitivity of 100%. However, its specificity was as low as 1.4%. Therefore, while TP and ALB levels can predict sepsis to some extent, their effectiveness as prognostic tools is notably limited. This study only included two fatal cases, thus could not provide a more comprehensive prognosis of patients. Multiple studies have shown that nutritional assessment can predict mortality rates [25].
Kiiru et al. conducted research that identified Escherichia coli as the predominant pathogen in UTIs [26]. Literature reviews indicate that the primary pathogens causing UTIs are Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii [27]. The hospital’s surveillance data show that Escherichia coli has the highest detection rate in UTIs, standing at 53.85%. This is consistent with the range of 42% to 69.3% reported by Nicolle et al. [28]. The most prevalent Gram-negative bacteria isolated from patients with urosepsis in our hospital are Escherichia coli (51.42%), Klebsiella pneumoniae (7.14%), and Acinetobacter baumannii (2.86%). The Gram-positive bacteria most commonly isolated from these patients are Enterococcus faecalis (14.29%) and Enterococcus faecium (2.86%), along with fungi (2.86%). In septic shock patients of urologic origin, Escherichia coli shows high resistance rates to a majority of antibiotics, including penicillin, cefazolin, ceftriaxone, and levofloxacin, all exceeding a resistance rate of 66.67% [2]. Additionally, this bacterial strain exhibits a 61.9% resistance rate to aztreonam and a 38.1% resistance rate to cefepime. In the case of urinary tract infections, Escherichia coli displays the highest resistance rate to ampicillin, at 88.57%, which is slightly higher than the 80% rate found among E. coli isolates studied by Lau, Peng, and others [29, 30].
Conclusion
Blood markers are essential for assessing the severity of urogenital sepsis; elevated levels of WBC, N%, NLR, D-dimer, URE, SCR, and La suggest worsening conditions, whereas decreased PLT, TP, and ALB levels indicate disease progression. Especially in diagnosing uroseptic shock, combined testing of multiple indicators has greater diagnostic value compared to single indicators. E. coli plays a critical pathogenic role in sepsis, particularly its drug-resistant strains. Considering risk factors such as age, chronic diseases, and low socioeconomic status, the necessity of resistance monitoring is highlighted to guide therapy and mitigate antibiotic resistance issues.
Strengths and limitations
This study reports the diagnostic data, pathogenetic distribution, and drug resistance of urogenic sepsis in the General Hospital of Ningxia Medical University in the Ningxia region of China. However, there are some limitations to this study, including a low number of deaths (only two) and a lack of prognostic causes for analysis.
Acknowledgements
I would like to thank my advisor, Prof. Jia Wei, for his guidance and support in successfully completing this thesis. Mr. Jia Wei’s erudition and quick thinking played an immeasurable role in my academic exploration. Associate Director Gang Li’s professional guidance in research methodology and experimental design also deeply influenced me with his spirit of research and professional rigor. I would like to thank all those who have supported me on my academic path, as your contributions are indispensable to the success of this thesis.
Author contributions
Jia Wei—participated in the revision, responsible for the overall study planning, design and implementation, approved the final release version Gang Li—verify the feasibility of collecting cases, participate in discussions and revisions, provide experimental equipment, technical and financial support Yanxia Shao—writing thesis manuscript, case collection, processing data and analyzing data, iconography, reviewing literature.
Funding
This research was supported by the Ningxia Hui Autonomous Region Key Research and Development Plan Project [2023BEG02003].
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This survey was conducted in accordance with the Declaration of Helsinki and the ethical standards of the national and institutional guidelines. The ethical approval for the study protocol was granted by the Medical Research Ethics Committee of the General Hospital of Ningxia Medical University [Approval No. KYLL-2024-066].
Consent to publication
The author confirms and consents that all materials included in the submitted manuscript, such as photographs and tables, have received the proper ethical clearance and informed consent from the relevant participants. The author has presented the content of the manuscript to the participants and has obtained their agreement. The author is committed to cooperating and will promptly provide all necessary materials to ensure the seamless publication of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jia Wei and Li Gang contributed equally to this work.
References
- 1.Tan L, Yang C, Yang X, et al. Analysis of the correlative factors of the severity of urogenic sepsis. J South Med Univ. 2019;39(01):93–9 (in Chinese). [Google Scholar]
- 2.Jiang Y, Li J, Zhang Y, et al. Clinical situations of bacteriology and prognosis in patients with urosepsis. Biomed Res Int. 2019;2019:3080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auriti C, Fiscarelli E, Ronchetti MP, et al. Procalcitonin in detecting neonatal nosocomial sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97(5):F368–70. [DOI] [PubMed] [Google Scholar]
- 4.Tuzel E, Aktepe OC, Akdogan B. Prospective comparative study of two protocols of antibiotic prophylaxis in percutaneous nephrolithotomy. J Endourol. 2013;27(2):172–6. [DOI] [PubMed] [Google Scholar]
- 5.Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria and their differential association with mortality. J Crit Care. 2018;46:29–36. [DOI] [PubMed] [Google Scholar]
- 6.Greene MT, Saint S, Ratz D, et al. Role of transfusions in the development of hospital-acquired urinary tract-related bloodstream infection among United States Veterans. Am J Infect Control. 2019;47(4):381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saltoglu N, Karali R, Yemisen M, et al. Comparison of community-onset healthcare-associated and hospital-acquired urinary infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and antimicrobial activities. Int J Clin Pract. 2015;69(7):766–70. [DOI] [PubMed] [Google Scholar]
- 8.Cho YH, Jung SI, Chung HS, et al. Antimicrobial susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in health care-associated urinary tract infection: focus on susceptibility to fosfomycin. Int Urol Nephrol. 2015;47(7):1059–66. [DOI] [PubMed] [Google Scholar]
- 9.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagao M. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin Microbiol Infect. 2013;19(9):852–8. [DOI] [PubMed] [Google Scholar]
- 11.Saint S, Kaufman SR, Rogers MA, Baker PD, Boyko EJ, Lipsky BA. Risk factors for nosocomial urinary tract-related bacteremia: a case–control study. Am J Infect Control. 2006;34:401–7. [DOI] [PubMed] [Google Scholar]
- 12.Brolund A, Sundqvist M, Kahlmeter G, Grape M. Molecular characterisation of trimethoprim resistance in Escherichia coli and Klebsiella pneumoniae during a two-year intervention on trimethoprim use. PLoS ONE. 2010;5(2): e9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonten M, Johnson JR, van den Biggelaar AHJ, et al. Epidemiology of Escherichia coli bacteremia: a systematic literature review. Clin Infect Dis. 2021;72(7):1211–9. [DOI] [PubMed] [Google Scholar]
- 14.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway LJ, Liu J, Harris AD, Larson EL. Risk factors for bacteremia in patients with urinary catheter-associated bacteriuria. Am J Crit Care. 2016;26:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leibovici L, Greenshtain S, Cohen O, Wysenbeek AJ. Toward improved empiric management of moderate to severe urinary tract infections. Arch Intern Med. 1992;152:2481–6. [PubMed] [Google Scholar]
- 17.Schlapbach LJ, Watson RS, Sorce LR, et al. International consensus criteria for pediatric sepsis and septic shock. JAMA. 2024;331(8):665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Y, Su Y, Tu GW, et al. Neutrophil-to-lymphocyte ratio predicts mortality in adult renal transplant recipients with severe community-acquired pneumonia. Pathogens. 2020;9(11):913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cataudella E, Giraffa CM, Di Marca S, Pulvirenti A, Alaimo S, Pisano M, Terranova V, Corriere T, Ronsisvalle ML, Di Quattro R, et al. Neutrophil-to-lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J Am Geriatr Soc. 2017;65:1796–801. [DOI] [PubMed] [Google Scholar]
- 20.De Jager CP, Wever PC, Gemen EF, Kusters R, van Gageldonk-Lafeber AB, van der Poll T, Laheij RJ. The neutrophil–lymphocyte count ratio in patients with community-acquired pneumonia. PLoS ONE. 2012;7: e46561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Zhang JW, Pang D, et al. Value of neutrophil to lymphocyte ratio in early diagnosis of ureteral calculi complicated by urogenic sepsis. J Modern Urol. 2019;25(10):906–9. [Google Scholar]
- 22.Cui QP, Luo LH, Li TH, et al. Comparison of clinical features between non-shock group and shock group with urinary sepsis. J Modern Urol. 2019;24(1):24–6. [Google Scholar]
- 23.Marik PE, Stephenson E. The ability of procalcitonin, lactate, white blood cell count and neutrophil–lymphocyte count ratio to predict blood stream infection. Analysis of a large database. J Crit Care. 2020;60:135–9. [DOI] [PubMed] [Google Scholar]
- 24.Zhou JH. Impact of hypoproteinemia on the prognosis of patients with systemic inflammatory response syndrome. Shandong Med. 2008;34:98–9. [Google Scholar]
- 25.Persson MD, Brismar KE, Katzarski KS, et al. Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc. 2002;50(12):1996–2002. [DOI] [PubMed] [Google Scholar]
- 26.Kiiru S, Maina J, Katana J, et al. Bacterial etiology of urinary tract infections in patients treated at Kenyan health facilities and their resistance towards commonly used antibiotics. PLoS ONE. 2023;18(5): e0277279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez-Guerrero E, Cabello HR, Expósito-Ruiz M, et al. Antibiotic resistances of Enterobacteriaceae with chromosomal AmpC in urine cultures: review and experience of a Spanish hospital. Antibiotics. 2023;12(4):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau SM, Peng MY, Chang FY. Resistance rates to commonly used antimicrobials among pathogens of both bacteremic and non-bacteremic community-acquired urinary tract infection. J Microbiol Immunol Infect. 2004;37(3):185–91. [PubMed] [Google Scholar]
- 30.Yang Q, Zhang H, Wang Y, et al. Antimicrobial susceptibilities of aerobic and facultative Gram-negative bacilli isolated from Chinese patients with urinary tract infections between 2010 and 2014. BMC Infect Dis. 2017;17(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.