Abstract
Background
Pseudomonas aeruginosa is able to survive, grow, and cause severe infections at different sites throughout the human body owing to its ability to sense diverse signals and precisely modulate target gene expression using its abundant signaling systems. Release of zinc (Zn) and hydrogen peroxide (H2O2) within the phagocyte are two major host strategies to defend against bacterial infections. It was previously shown that the response regulator CzcR controls global gene expression including catalase genes during Zn excess, but regulatory mechanisms of catalase gene expression and the role of CzcR in H2O2 tolerance remain unclear.
Results
In the study, comparative transcriptome analysis comprehensively described the CzcR-dependent and -independent gene regulatory pattern in P. aeruginosa during Zn excess, which revealed the counteractive co-regulation of two key H2O2-detoxifying catalase genes katA and katB through CzcR-dependent and -independent pathways in response to Zn excess. Protein-DNA interaction assay demonstrated that CzcR negatively regulates the expression of catalase genes katA and katB by directly binding to their promoters. While interestingly, we further showed that CzcR positively regulates H2O2 tolerance by inducing the catalase activity during Zn excess.
Conclusion
This study reported the opposite functions of CzcR in negatively regulating the expression of catalase genes katA and katB but in positively regulating the activity of catalase and H2O2 tolerance during Zn excess in P. aeruginosa.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-024-03671-0.
Keywords: P. aeruginosa, Two-component system, CzcS/CzcR, Catalase, Oxidative tolerance
Background
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen that can cause a variety of acute and chronic infections in patients with compromised immune defenses [1]. It is able to survive and grow under adverse environmental or host-related conditions owing that the pathogen is equipped with a wide range of mechanisms for adaptation [2]. For example, P. aeruginosa frequently encounters oxidative stress due to the emergence of toxic reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide, and hydroxyl free radicals during infections [3]. To cope with endogenously produced or exogenous ROS, P. aeruginosa encodes antioxidant enzymes including catalases KatA and KatB, which are two critical enzymes for the adaptation of this pathogen to high levels of H2O2 [4, 5].
Adaptation to diverse environmental conditions is exquisitely regulated, which largely depends on the abundant signaling systems in P. aeruginosa. With regards to the major catalase genes katA and katB, their expression can be upregulated at both transcriptional and translational levels by a variety of regulatory systems or pathways when the pathogen is surrounded by oxidizing agents [3]. For example, OxyR is the key transcriptional activator of katA and katB, contributing significantly to H2O2 tolerance [6]. In addition, expression of catalase genes and activity of catalases are partly controlled by other regulators such as IscR, ANR and are also associated with metal availability, oxygen availability, quorum sensing activity, nitrate respiration, etc [7–10].
An important kind of regulatory system in P. aeruginosa is the two-component system (TCS) that typically employs a histidine kinase to sense specific intracellular or extracellular signals and a response regulator to control target gene expression [11, 12]. CzcS/CzcR, composed of the histidine kinase CzcS and the response regulator CzcR, is a well-studied TCS that was found for zinc (Zn) detoxification by upregulating the expression of Zn exporters during Zn excess [13]. It was also found to control global gene expression and demonstrated to regulate multiple physiological processes such as antibiotic resistance, quorum sensing, motility, copper (Cu) tolerance, iron (Fe) homeostasis [14–17].
Interestingly, when we were investigating the co-regulation of Cu tolerance and pyochelin biosynthesis by CzcR-dependent and -independent pathways during Zn excess [17], we also noticed that the expression of the major H2O2-detoxifying catalase genes katA and katB was significantly induced due to the loss of the response regulator CzcR during Zn excess, however, we observed a significant growth defect of the ΔczcR mutant in the presence of both Zn and H2O2 [17], implying the complicated and elusive regulation of P. aeruginosa susceptibility to H2O2 by CzcR and its inducing signal Zn. Zn is a traditional antimicrobial agent. Unlike Fe and Cu which toxify microbes by inducing oxidative stress through Fenton reaction [18], Zn is a non-redox transition metal and it mainly kills microbes by disrupting the homeostasis of other key trace metals or inhibiting enzymatic activity by competing with other metals due to its top position at the Irving-Williams series [19]. It is interesting that Zn was found to induce the sensitivity of Streptococcus pneumoniae to oxidative stress but also provide protection against the toxicity of H2O2 in Burkholderia pseudomallei [20, 21]. Regulatory mechanisms of oxidative tolerance in microbes including P. aeruginosa in response to Zn remain largely elusive. Therefore, this study aimed to further explore the mechanisms underlying the regulation of catalase gene expression and catalase activity mediated by CzcR and its inducing signal Zn in P. aeruginosa.
Methods
Bacterial strains, plasmids, primers, and growth conditions
Bacterial strains, plasmids, and primers used in this study are listed in Table S1. 0.5 mM ZnSO4 was supplemented in the medium which is prepared from Invitrogen LB Broth Base for Zn treatment. Antibiotics were supplemented in the medium when required: 15 µg/ml tetracycline (reporters of promoter activity) for E. coli DH5α; 50 µg/ml tetracycline (reporters of promoter activity), 50 µg/ml gentamycin (complementation of czcR) for P. aeruginosa PAO1.
Comparative transcriptome analysis
The transcriptome data was retrieved from NCBI (project accession number: PRJNA896733). Mapping of qualified reads was performed using BWA v0.7.17-r1188 [22], SAMtools v1.15 [23] and bamkeepgoodreads of Stampy v1.0.32 [24] with the genome of PAO1 (NC_002516.2) as the reference. Count matrix was generated by featureCounts of Subread v2.0.3 [25] and then used to identify differentially expressed genes (DEGs) by DEseq2 [26]. KEGG pathway enrichment analysis was performed using clusterProfile v4.0 [27].
Reverse transcription-quantitative PCR (RT-qPCR)
Overnight culture of P. aeruginosa strains was 1:50 diluted and then cultured with or without the supplementation of Zn for 6 h. 1 ml bacterial culture was harvested by centrifugation and total RNA was extracted using the Eastep® Super Total RNA Extraction Kit (Promega). cDNA was reverse transcribed using TransScript® OneStep gDNA Removal and cDNA Synthesis SuperMix (TransGen). qPCR was performed using the ChamQ Universal SYBR qPCR Master Mix (Vazyme). The house-keeping recA gene was used as the internal reference control.
Promoter activity assay
Promoter regions were amplified from the PAO1 genome and then inserted into the mini-CTX-lux plasmid to generate reporters (PkatA-lux and PkatB-lux) for evaluating promoter activities. Overnight cultures of P. aeruginosa strains containing PkatA-lux or PkatB-lux were 1:50 diluted and then cultured with or without the supplementation of 0.5 mM Zn for 6 h. Luminescence and OD600 of the bacterial culture were measured in a microplate reader (BioTek).
Electrophoretic mobility shift assay (EMSA)
His6-tagged CzcR protein was purified according to the previously reported procedures [17]. Promoters of the katA, katB, and pvdR genes were amplified from the PAO1 genome by PCR and labeled by the Biotin 3’ End DNA Labeling Kit (ThermoFisher Scientific). Biotin-labeled promoter fragments were incubated with CzcR at different concentrations and then separated by polyacrylamide gel electrophoresis. Interactions between CzcR and target promoters were examined using the LightShift® Chemiluminescent EMSA Kit (ThermoFisher Scientific). Unprocessed gel images of the EMSA results were shown in Figure S1.
H2O2 tolerance assays
Overnight culture of P. aeruginosa strains was 1:50 diluted and then cultured with or without the supplementation of Zn for 6 h. Cells were harvested by centrifugation and washed by fresh LB medium without the supplementation of Zn. Next, cells were resuspended in fresh LB medium and cell density of each sample was adjusted to OD600 of 1.0. For survival rate assay, H2O2 was added into the medium to the concentrations of 1.5 mM, 2.5 mM, or 3.5 mM. After incubation for 30 min, cell culture was serially diluted and plated. Recovered colonies were counted and the survival rate was calculated. For growth curve measurement, bacterial suspension was 1:100 diluted and 0.5 mM H2O2 was added into the medium. OD600 was measured every 1 h to plot the growth curve.
Catalase activity assay
Overnight culture of P. aeruginosa strains was 1:50 diluted and then cultured with or without the supplementation of Zn for 6 h. P. aeruginosa cells were collected from 0.5 ml cell culture by centrifugation and lysed by sonication. After centrifugation, suspension was collected for catalase activity measurement using the Catalase (CAT) Activity Assay Kit (Sangon Biotech) according to the manufacturer’s instructions.
Results
Transcriptomic profile changes of PAO1 WT and ΔczcR strains in response to Zn excess
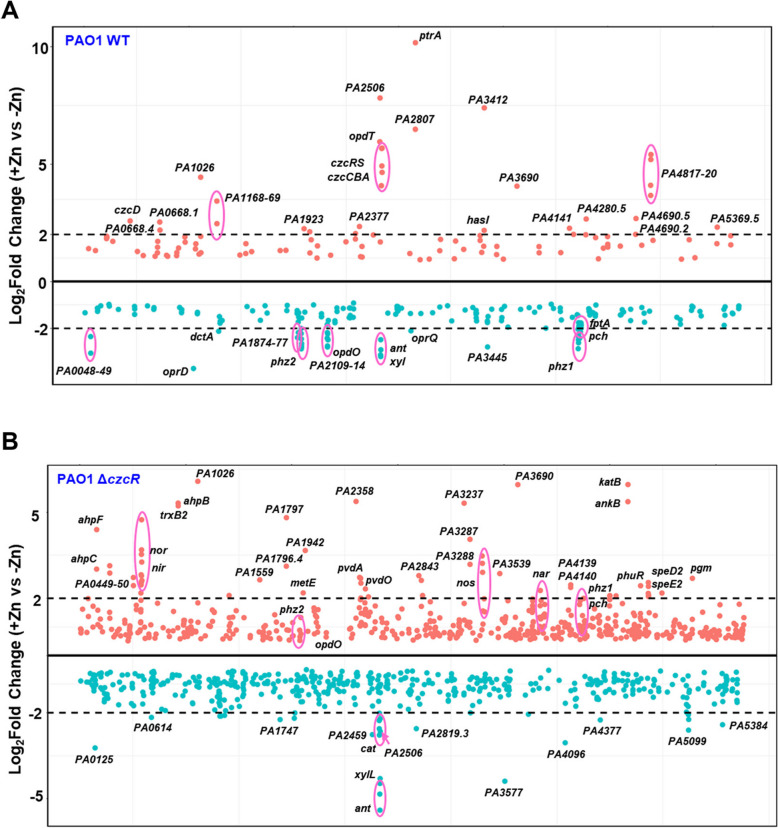
Considering the interesting co-regulation of Cu tolerance and PCH biosynthesis by CzcR-dependent and -independent pathways as we recently observed during Zn excess [17], we sought to first comprehensively explore CzcR-dependent and -independent gene regulatory patterns during Zn excess by re-analyzing the transcriptome data of the PAO1 WT and ΔczcR strains after they were cultured with or without the treatment of 0.5 mM ZnSO4. Firstly, it was shown that Zn treatment led to significant expression changes (P value < 0.05) of 279 genes in the PAO1 WT strain (Fig. 1A and Table S2). Among them, 100 genes were upregulated, and 179 genes were downregulated during Zn excess. In contrast, there were 1,055 genes differentially expressed with statistical significance (P value < 0.05) in the ΔczcR mutant after Zn treatment (Fig. 1B and Table S3), which contained 776 more genes than that was displayed in the WT strain. This result further indicated the importance of the TCS CzcS/CzcR in maintaining the normal expression levels of abundant genes and the physiological balance in P. aeruginosa during Zn excess.
Fig. 1.
Transcriptomic profile changes in PAO1 WT and ΔczcR strains during Zn excess. A Genome-wide transcriptomic profiles of the PAO1 WT strain when it was cultured with or without Zn treatment. B Genome-wide transcriptomic profiles of the ΔczcR mutant when it was cultured with or without Zn treatment. Orange dots were the genes upregulated during Zn excess and green dots were the genes downregulated during Zn excess. The black dot lines represented 2-Log2Fold expression changes
CzcR-dependent and -independent target gene regulation during Zn excess
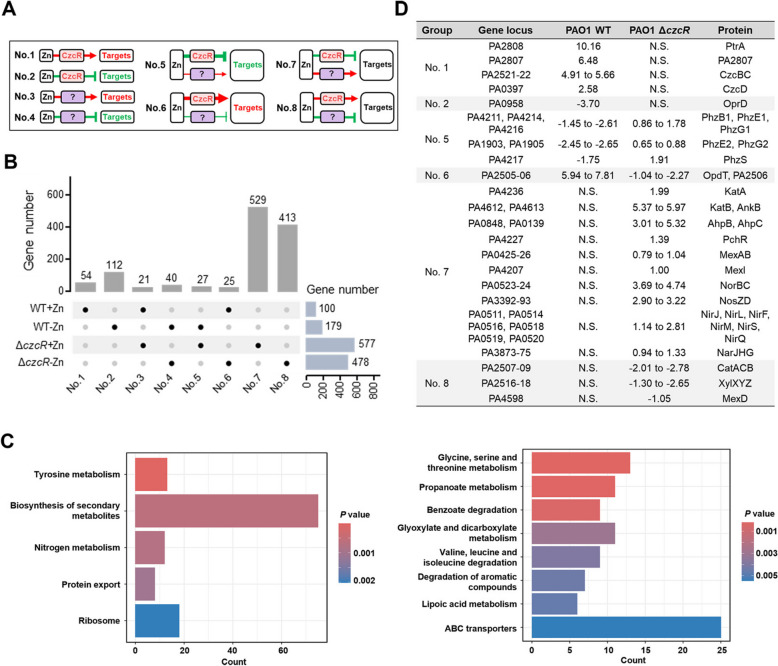
We next moved to classify the genes with different regulatory patterns involving Zn and/or CzcR by combining two datasets from the WT strain and the ΔczcR mutant. According to the gene expression changes in both strains, all the differentially expressed genes (DEGs) listed in TablesS2 and S3 could be generally classified into 8 groups (Fig. 2A and Table S4), displaying the complicated CzcR-dependent and -independent gene regulatory patterns in P. aeruginosa during Zn excess. Group No.1 included 54 genes such as ptrA, PA2807, czcC, czcB, and czcD that were exclusively found as up-regulated genes in the WT strain but not the ΔczcR mutant during Zn excess, meaning that their expression is induced by Zn through CzcR exclusively. Likewise, group No.2 included 112 genes such as oprD that were repressed by Zn through CzcR exclusively. Groups No.3 and No.4 contained 21 and 40 genes that are positively and negatively regulated by Zn, respectively, possibly through CzcR-independent pathways. Groups No.5 to 8 included genes that are regulated by Zn through a combination of CzcR-dependent and -independent pathways. For example, the pchR gene was found in the group No. 7 which indicated that its expression is unchanged in the WT strain during Zn excess. This result was consistent with the unchanged expression of pchR in the WT strain but not the ΔczcR mutant as we recently reported [17], suggesting the accuracy of the classification.
Fig. 2.
Global gene regulation by CzcR-dependent and -independent pathways during Zn excess. A A diagram showing eight groups of target genes that were regulated by Zn in CzcR-dependent or/and CzcR-independent pathways. Question marks indicate unclear factors involved in the pathways. Red arrows and green T-shaped symbols indicate upregulation and repression, respectively. Targets highlighted in red indicate genes that are upregulated by Zn in the WT strain, targets highlighted in green indicate genes that are downregulated by Zn in the WT strain, targets in black indicate genes whose expression is not changed by Zn in the WT strain. B An UpSet plot showing the distribution of DEGs within eight groups as shown in (A). C KEGG pathway enrichment of genes that were present in the groups No.7 (left) and No.8 (right). D Representative target genes in groups No.1, 2, 5, 6, 7 and 8 were presented
Interestingly, it was shown that the majority of DEGs (942 out of 1,221) were listed in groups No.7 and 8 (Fig. 2B). Genes found in these two groups are inversely regulated by CzcR-dependent and -independent pathways at comparable levels and, consequently, their expression did not show any responses to Zn excess. This result confirmed that CzcR plays a critical role in balancing the expression of about a thousand of genes that could be disturbed by Zn excess. Enrichment analysis based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways showed that genes in the group No.7 were mainly enriched in pathways of tyrosine metabolism, biosynthesis of secondary metabolites, nitrogen metabolism, protein export, and ribosome (Fig. 2C), while genes in the group No.8 were enriched in pathways of glycine, serine and threonine metabolism, propanoate metabolism, benzoate degradation, glyoxylate and dicarboxylate metabolism, valine, leucine and isoleucine degradation, degradation of aromatic compounds, lipoic acid metabolism, ABC transporters (Fig. 2C).
Counteractive co-regulation of catalase genes katA and katB through CzcR-dependent and -independent pathways during Zn excess
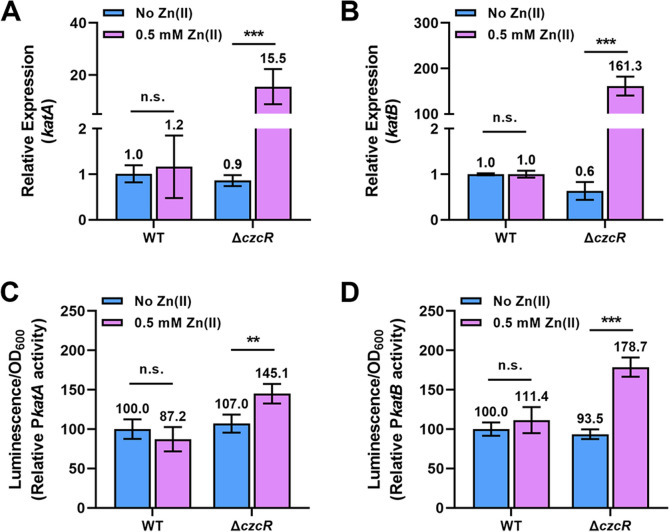
Comparative transcriptome analysis between the WT and ΔczcR strains during Zn excess revealed the negative regulation of two key catalase genes katA and katB by CzcR [17]. Here, we showed that both genes were listed in group No.7 (Fig. 2D), meaning that these genes are co-regulated by CzcR-dependent and -independent pathways in a balanced manner during Zn excess. RT-qPCR verified that the expression of katA and katB genes was not influenced in the WT strain during Zn excess but significantly upregulated by Zn in the ΔczcR mutant (Fig. 3A and B). To further confirm the regulatory patterns of katA and katB genes, we constructed two promoter activity reporters, PkatA-lux and PkatB-lux, and then used these two reporters to examine the transcriptional activities of both genes. As shown in Fig. 3C and D, the transcriptional activities of two genes were also not changed in the WT strain during Zn excess. However, the transcriptional activities of both genes were significantly induced by Zn in the ΔczcR mutant. This result not only confirmed that CzcR negatively regulates the expression of katA and katB during Zn excess but also demonstrated the simultaneous presence of CzcR-independent pathways that are induced by Zn to upregulate the expression of two genes.
Fig. 3.
CzcR represses the expression of katA and katB during Zn excess. A and B Relative expression of the katA (A) and katB (B) genes in the WT and ΔczcR strains when they were cultured with or without Zn treatment. C and D Activities of PkatA-lux (C) and PkatB-lux (D) were measured in the WT and ΔczcR strains when they were cultured with or without Zn treatment. Statistical significance was calculated based on two-way analysis of variance (ANOVA) (n.s., not significant; **, P < 0.01; ***, P < 0.001)
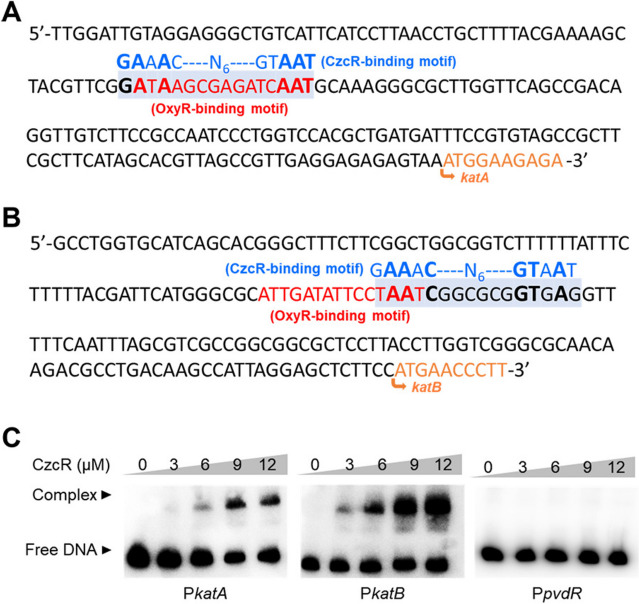
CzcR is a transcription factor that was demonstrated to regulate the expression of genes involved in quorum sensing, flagellar biosynthesis, antibiotic resistance, and metal homeostasis by directly binding to their promoters [14–17]. To understand how CzcR regulates the expression of katA and katB, we then searched for potential CzcR-binding sites in their promoters by aligning the promoter sequences with the 16-bp CzcR-binding motif (GAAACN6GTAAT) [28]. Potential CzcR-binding sites were found in both promoters (Fig. 4A and B). This analysis suggested the possible interaction between CzcR and two promoters. Therefore, EMSA was performed, which showed that CzcR could bind to the promoters of both katA and katB genes (Fig. 4C). In contrast, CzcR did not bind to the promoter of pvdR which was selected as a negative control for EMSA (Fig. 4C) [17]. These results revealed that the expression of katA and katB is repressed by CzcR through its direct binding at their promoters.
Fig. 4.
CzcR directly targets the promoters of katA and katB. A and B Prediction of the CzcR-binding site in the promoters of katA (A) and katB (B). C EMSAs showed the binding ability of CzcR at the promoters of katA (PkatA) and katB (PkatB) but not the negative control promoter PpvdR
CzcR-mediated induction of catalase activity and H2O2 tolerance during Zn excess
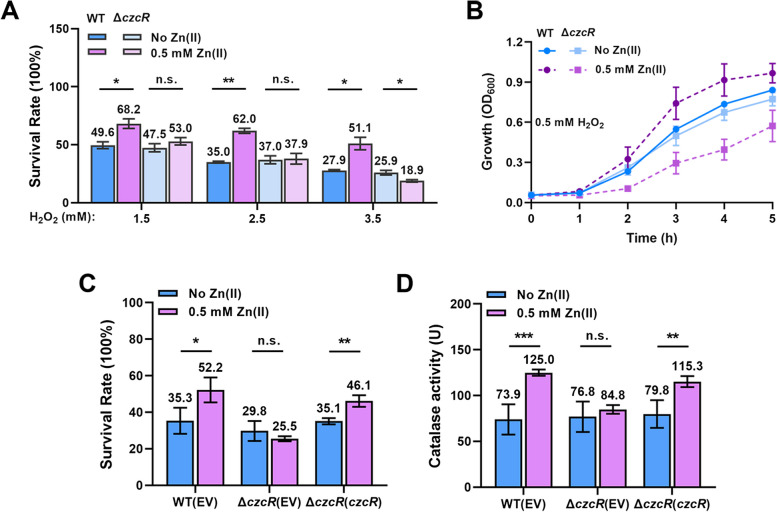
KatA and KatB are two key catalases responsible for H2O2 detoxification [4, 5]. Considering their upregulation in the ΔczcR mutant during Zn excess, we speculated that the tolerance of the mutant to H2O2 might be enhanced. We pre-treated WT and ΔczcR strains with or without Zn and then incubated them in the presence of 1.5 mM, 2.5 mM, or 3.5 mM H2O2 to compare the survival rates. Unexpectedly, we found that the survival rate of the WT strain but not the ΔczcR mutant was significantly elevated after Zn treatment (Fig. 5A). Growth of WT and ΔczcR strains in the 0.5 mM H2O2 also showed that Zn could promote the growth of the WT strain and deletion of czcR led to an obvious growth defect (Fig. 5B), meaning that CzcR was essential for the increased H2O2 tolerance during Zn excess. We further examined the survival rate of the ΔczcR mutant with the complementation of the czcR gene after the treatment of 3.5 mM H2O2. The result confirmed that the H2O2 tolerance induced by Zn is dependent on CzcR (Fig. 5C). These results displayed that Zn induced H2O2 tolerance in the WT strain instead of the ΔczcR mutant, which is not consistent with the induced expression of two catalase genes in the ΔczcR mutant but not the WT strain during Zn excess.
Fig. 5.
CzcR-mediated induction of H2O2tolerance and catalase activity during Zn excess. A Survival rate of the WT and ΔczcR strains when they were treated with 1.5 mM, 2.5 mM, and 3.5 mM H2O2 with the pre-treatment of Zn or not. B Growth curves of the WT and ΔczcR strains when they were cultured in the presence of 0.5 mM H2O2 with the pre-treatment of Zn or not. C Survival rate of the WT, ΔczcR, and ΔczcR(czcR) strains when they were cultured in the presence of 3.5 mM H2O2 with the pre-treatment of Zn or not. D Catalase activity was measured in the WT, ΔczcR, and ΔczcR(czcR) strains when they were cultured with or without Zn treatment. Statistical significance was calculated based on two-way ANOVA (n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
To explain the inconsistency, we next measured the catalase activity of the WT strain, ΔczcR mutant and the ΔczcR mutant with the complementation of czcR. It was found that the catalase activity was significantly increased by Zn treatment in the WT strain and the ΔczcR mutant with the complementation of czcR, while there was no change of the catalase activity in the ΔczcR mutant during Zn excess (Fig. 5D), supporting the enhanced H2O2 tolerance as observed in the WT strain instead of the ΔczcR mutant during Zn excess. Together, these results demonstrated that Zn induces CzcR-mediated catalase activity and H2O2 tolerance in P. aeruginosa.
Discussion
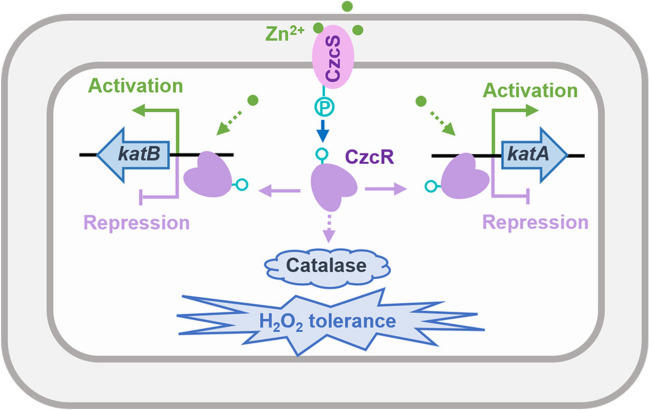
P. aeruginosa is equipped with abundant signal detection and transduction systems, which enable the pathogen to quickly adapt to the changing environment. TCS, typically composed of a histidine kinase to sense environmental signals and a response regulator to control target genes expression, is one of the important signaling systems that connect bacterial behaviors including survival, metabolism, virulence, antibiotic resistance, interbacterial competition to the diverse environmental signals [29–31]. CzcS/CzcR is a versatile TCS that regulates global genes expression in response to the elevated Zn concentration in P. aeruginosa. By re-analyzing the effects of Zn on the transcriptomic profiles of the PAO1 WT strain and the ΔczcR mutant, in this study, we discovered a delicate and complex regulatory pattern of gene expression by Zn through CzcR-dependent and -independent pathways. Focusing on the key catalase genes katA and katB, we showed an interesting function of CzcR in negatively regulating the expression of both catalase genes but in positively regulating the activity of catalase and H2O2 tolerance during Zn excess (Fig. 6).
Fig. 6.
A schematic diagram showing the regulation of catalase gene expression and catalase activity during Zn excess in P. aeruginosa. Zn positively regulates the expression of katA and katB with unclear mechanisms, and simultaneously, Zn activates CzcS/CzcR to negatively regulate the expression of katA and katB through the binding of CzcR at their promoters. As a result, the expression of the key catalase genes katA and katB is not influenced by Zn excess. In spite of the unchanged expression of katA and katB, Zn excess induces catalase activity and H2O2 tolerance of P. aeruginosa in a CzcR-dependent manner
Bacterial gene expression and phenotypes are frequently co-regulated by multiple factors or pathways [32]. With the combined transcriptome analysis between WT and ΔczcR strains, this study comprehensively revealed the co-regulatory effects exerted by CzcR and its inducing signal Zn. Owing that most (~ 1,000) genes were inversely regulated by CzcR-dependent and -independent pathways during Zn excess, this study displayed the contribution of CzcR to maintain physiological balance by sustaining the expression of target genes that are disturbed by Zn excess, further highlighting the importance of TCS in stress adaptation. Possibly for this reason, a large number of physiological processes targeted by Zn are previously neglected owing that changes of their gene expression are neutralized by CzcR. For example, DNA microarray only identified 34 DEGs with significance in P. aeruginosa after the treatment of 1 mM ZnO nanoparticles and most of them are from groups No. 1 to 6 such as the czc genes, porin genes, ptrA and phz genes [33].
Although it has been notified that CzcR negatively regulates the expression of catalase genes katA and katB during Zn excess [17], molecular mechanisms underlying the regulation was not explored. By examining the promoter activities of two genes and interactions between CzcR and two promoters, we demonstrated that CzcR regulates the expression of katA and katB by directly and specifically recognizing and binding to their promoters. Moreover, by comparing the expression of katA and katB in the WT strain when the strain was treated with Zn or not, we found that Zn did not influence their expression levels. Zn only induced their expression in the absence of CzcR, meaning that Zn simultaneously activates the expression of katA and katB independently of CzcR. However, the mechanism of activation is still unclear. Regulators such as OxyR, DksA that positively control catalase genes expression might act antagonistically with CzcR [34, 35]. Particularly, OxyR is a critical activator of both katA and katB and sequence analysis showed the potentially overlapped DNA targeting sites of OxyR and CzcR at the promoters of katA and katB (Fig. 4A and B) [35–37]. Thus, it is possible that the competitive binding of CzcR at the OxyR-binding site prevented the upregulation of katA and katB. When czcR was deleted, it could be OxyR that induced the expression of both genes in response to the elevated Zn concentration. However, expression changes of oxyR were not detected in both WT and ΔczcR strains after treatment of Zn from the transcriptome data. Whether Zn induces the activity of OxyR and how Zn directly or indirectly induces catalase genes expression independently of CzcR require further investigations. Given that abundant DEGs including katA and katB were listed in groups No.7 and 8, another two possibilities may contribute to the unchanged expression of katA and katB in the WT strain during Zn treatment. One is the presence of additional Zn-responsive regulators that control global gene expression antagonistically with CzcR. The other is one or some DEGs such as genes involved in nitrate respiration are involved in the co-regulation of katA and katB with CzcR.
RNA-seq, RT-qPCR, and promoter activity assays showed that katA and katB were dramatically upregulated during Zn excess in the ΔczcR mutant. While interestingly, when evaluating the H2O2 tolerance of WT and ΔczcR strains, we did not observe increased catalase activity and H2O2 tolerance in the ΔczcR mutant during Zn excess in spite of the upregulation of two genes in this mutant. Instead, we found that catalase activity and H2O2 tolerance were significantly induced in the WT strain during Zn excess. These results not only answered why the ΔczcR mutant showed an obvious growth defect compared to the WT strain during Zn excess as reported in the previous study [17], but further demonstrated that Zn induces the catalase activity in the CzcR-dependent manner without changing the expression of catalase genes. However, mechanisms underlying the CzcR-dependent elevation of catalase activity during Zn excess are still unclear. Owing that expression of katA and katB genes were measured at the transcriptional level in this work, whether their translation is activated by CzcR or members from the CzcR regulon is unknown. Because catalase activity and H2O2 tolerance are associated with the translational levels of katA and katB as well. For example, decreased protein levels of KatA and KatB were observed in mutants lacking the tRNA-modifying enzymes GidA or TrmB, which showed a decreased catalase activity and a H2O2-sensitive phenotype [38, 39]. Nonetheless, this finding provided new insights into the host adaptation of P. aeruginosa. Because release of both Zn and H2O2 in the phagocyte is a main strategy to eliminate engulfed pathogens [40, 41]. In addition to the activation of Zn export systems and repression of Zn import systems following the switch from Zn-deficient environment to Zn-rich environment [42], induced H2O2 tolerance in response to the elevation of Zn will further promote the survival of P. aeruginosa during infection. Therefore, the development of antimicrobial agents targeting the CzcS/CzcR signaling pathway may help to alleviate or prevent P. aeruginosa infections.
Supplementary Information
Authors’ contributions
T.L. and Z.X. designed the research; T.L., Z.M., and Y.Z. performed experiments; T.L., H.C., and Z.X. analyzed the data; T.L. drafted the manuscript; S.S. and Z.X. acquired fundings; H.C., S.S., and Z.X. revised the manuscript.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation [No. 2023A1515012775], National Natural Science Foundation of China [No. 32100020 and 32370188], Science and Technology Project of Guizhou Province [Qian Ke He Ji Chu - ZK (2022) No. 604 and ZK (2024) Zhong Dian No. 065] and Zunyi City [Zun Shi Ke He HZ Zi (2023) No. 171].
Data availability
The transcriptome data was retrieved from NCBI (project accession number: PRJNA896733).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ting Li and Zhifeng Mo contributed equally to this work.
Contributor Information
Shuo Sheng, Email: shengshuo21@foxmail.com.
Zeling Xu, Email: zelingxu@scau.edu.cn.
References
- 1.Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, Liang H, Song X, Wu M. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Therapy. 2022;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front Cell Infect Microbiol. 2017;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Cruz Nizer WS, Inkovskiy V, Versey Z, Strempel N, Cassol E, Overhage J. Oxidative stress response in Pseudomonas aeruginosa. Pathogens. 2021;10(9):1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J-S, Heo Y-J, Lee Jeong K, Cho Y-H. KatA, the Major Catalase, is critical for Osmoprotection and Virulence in Pseudomonas aeruginosa PA14. Infect Immun. 2005;73:4399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SM, Howell ML, Vasil ML, Anderson AJ, Hassett DJ. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J Bacteriol. 1995;177:6536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae H-W, Cho Y-H. Mutational analysis of Pseudomonas aeruginosa OxyR to define the regions required for peroxide resistance and acute virulence. Res Microbiol. 2012;163(1):55–63. [DOI] [PubMed] [Google Scholar]
- 7.Frederick Jesse R, Elkins James G, Bollinger N, Hassett Daniel J, McDermott Timothy R. Factors affecting catalase expression in Pseudomonas aeruginosa Biofilms and Planktonic cells. Appl Environ Microbiol. 2001;67(3):1375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seol-Hee K, Bo-Young L, Gee WL. IscR modulates catalase A (KatA) activity, peroxide resistance and full virulence of Pseudomonas aeruginosa PA14. J Microbiol Biotechnol. 2009;19(12):1520–6. [DOI] [PubMed] [Google Scholar]
- 9.Platt Mark D, Schurr Michael J, Sauer K, Vazquez G, Kukavica-Ibrulj I, Potvin E, et al. Proteomic, microarray, and signature-tagged mutagenesis analyses of anaerobic Pseudomonas aeruginosa at pH 6.5, likely representing chronic, late-stage cystic fibrosis Airway conditions. J Bacteriol. 2008;190(8):2739–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassett DJ, Ma J-F, Elkins JG, McDermott TR, Ochsner UA, West SEH, et al. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol Microbiol. 1999;34(5):1082–93. [DOI] [PubMed] [Google Scholar]
- 11.Wang Benjamin X, Cady Kyle C, Oyarce Gerardo C, Ribbeck K, Laub Michael T. Two-Component Signaling systems regulate diverse virulence-Associated traits in Pseudomonas aeruginosa. Appl Environ Microbiol. 2021;87:e03089–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sultan M, Arya R, Kim KK. Roles of two-Component systems in Pseudomonas aeruginosa Virulence. Int J Mol Sci. 2021;22(22):12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perron K, Caille O, Rossier C, van Delden C, Dumas J-L, Köhler T. CzcR-CzcS, a two-component system involved in Heavy Metal and Carbapenem Resistance in Pseudomonas aeruginosa. J Biol Chem. 2004;279:8761–8. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Xu Z, Chen S, Huang J, Li T, Duan C, Zhang L-H, Xu Z. CzcR is essential for swimming motility in Pseudomonas aeruginosa during zinc stress. Microbiol Spectr. 2022;10:e02846–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Cao H, Xu Z, Huang J, Liu Z, Li T, Duan C, Wu W, Wen Y, Zhang L-H, Xu Z. A type I-F CRISPRi system unveils the novel role of CzcR in modulating multidrug resistance of Pseudomonas aeruginosa. Microbiol Spectr. 2023;11:e01123–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieppois G, Ducret V, Caille O, Perron K. The Transcriptional Regulator CzcR modulates Antibiotic Resistance and Quorum sensing in Pseudomonas aeruginosa. PLoS ONE. 2012;7:e38148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T, Cao H, Duan C, Chen S, Xu Z. Activation of CzcS/CzcR during zinc excess regulates copper tolerance and pyochelin biosynthesis of Pseudomonas aeruginosa. Appl Environ Microbiol. 2024;90:e02327–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol. 2012;10(8):525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djoko KY, Ong C-lY, Walker MJ, McEwan AG. The role of copper and zinc toxicity in Innate Immune Defense against Bacterial pathogens. J Biol Chem. 2015;290(31):18954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeShazer D. A novel contact-independent T6SS that maintains redox homeostasis via Zn2+ and Mn2+ acquisition is conserved in the Burkholderia pseudomallei complex. Microbiol Res. 2019;226:48–54. [DOI] [PubMed] [Google Scholar]
- 21.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, et al. A molecular mechanism for bacterial susceptibility to Zinc. PLoS Pathog. 2011;7(11):e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunter G, Goodson M. Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 2011;21(6):936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–30. [DOI] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innov. 2021;2(3):100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan K, Cao Q, Lan L. Genome-wide mapping reveals Complex Regulatory activities of BfmR in Pseudomonas aeruginosa. Microorganisms. 2021;9(3):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou T, Huang J, Liu Z, Lin Q, Xu Z, Zhang L-H. The two-component system FleS/FleR represses H1-T6SS via cyclic di-GMP Signaling in Pseudomonas aeruginosa. Appl Environ Microbiol. 2022;88:e01655–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou T, Huang J, Liu Z, Xu Z, Zhang L-h. Molecular mechanisms underlying the regulation of Biofilm formation and swimming motility by FleS/FleR in Pseudomonas aeruginosa. Front Microbiol. 2021;12:707711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol. 2006;9:143–52. [DOI] [PubMed] [Google Scholar]
- 32.Shao X, Yao C, Ding Y, Hu H, Qian G, He M, Deng X. The transcriptional regulators of virulence for Pseudomonas aeruginosa: therapeutic opportunity and preventive potential of its clinical infections. Genes Dis. 2023;10:2049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J-H, Kim Y-G, Cho MH, Lee J. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol Res. 2014;169:888–96. [DOI] [PubMed] [Google Scholar]
- 34.Fortuna A, Collalto D, Schiaffi V, Pastore V, Visca P, Ascenzioni F, et al. The Pseudomonas aeruginosa DksA1 protein is involved in H2O2 tolerance and within-macrophages survival and can be replaced by DksA2. Sci Rep. 2022;12(1):10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Q, Le Minh PN, Dötsch A, Hildebrand F, Panmanee W, Elfarash A, et al. Global regulation of gene expression by OxyR in an important human opportunistic pathogen. Nucleic Acids Res. 2012;40(10):4320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heo Y-J, Chung I-Y, Cho W-J, Lee B-Y, Kim J-H, Choi K-H, Lee J-W, Hassett Daniel J, Cho Y-H. The Major Catalase Gene (katA) of Pseudomonas aeruginosa PA14 is under both positive and negative control of the global transactivator OxyR in response to Hydrogen Peroxide. J Bacteriol. 2010;192:381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochsner Urs A, Vasil Michael L, Alsabbagh E, Parvatiyar K, Hassett Daniel J. Role of the Pseudomonas aeruginosa Oxyr-Recg Operon in oxidative stress defense and DNA repair: OxyR-Dependent regulation of katB-ankB, ahpB, and ahpc-ahpf. J Bacteriol. 2000;182:4533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srimahaeak T, Thongdee N, Chittrakanwong J, Atichartpongkul S, Jaroensuk J, Phatinuwat K, et al. Pseudomonas aeruginosa GidA modulates the expression of catalases at the posttranscriptional level and plays a role in virulence. Front Microbiol. 2023;13:1079710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thongdee N, Jaroensuk J, Atichartpongkul S, Chittrakanwong J, Chooyoung K, Srimahaeak T, et al. TrmB, a tRNA m7G46 methyltransferase, plays a role in hydrogen peroxide resistance and positively modulates the translation of katA and katB mRNAs in Pseudomonas aeruginosa. Nucleic Acids Res. 2019;47(17):9271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adolfsen KJ, Brynildsen MP. A kinetic platform to Determine the Fate of Hydrogen Peroxide in Escherichia coli. PLoS Comput Biol. 2015;11:e1004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stafford Sian L, Bokil Nilesh J, Achard Maud ES, Kapetanovic R, Schembri Mark A, McEwan Alastair G, Sweet Matthew J. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci Rep. 2013;33:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ducret V, Abdou M, Goncalves Milho C, Leoni S, Martin–Pelaud O, Sandoz A, Segovia Campos I, Tercier-Waeber M-L, Valentini M, Perron K. Global Analysis of the Zinc Homeostasis Network in Pseudomonas aeruginosa and its gene expression Dynamics. Front Microbiol. 2021;12:739988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptome data was retrieved from NCBI (project accession number: PRJNA896733).