Abstract
Background
The outcome of kidney transplant recipients with a history of complement-mediated thrombotic microangiopathy (cTMA) and those who develop post-transplant de novo TMA (dnTMA) is largely unknown.
Methods
We retrospectively studied all kidney transplant recipients with end-stage kidney disease secondary to cTMA and those who developed dnTMA, between Jan 2000 and Dec 2020 in our center.
Results
We identified 134 patients, 22 with cTMA and 112 had dnTMA. Patients with cTMA were younger at the time of TMA diagnosis (age at diagnosis, 28.9 ± 16.3. vs 46.5 ± 16.0 years; P < 0.001). T-cell mediated rejection, borderline rejection, and calcineurin inhibitor toxicity were more prevalent in the first kidney transplant biopsy (P < 0.05) in the dnTMA group, and antibody-mediated rejection was more prevalent in anytime-biopsy (P = 0.027). After adjusting for potential confounders, cTMA was associated with a sixfold increase in the hazard of transplant failure during the first-year post-transplant (adjusted hazard ratio (aHR): 6.37 [95%CI: 2.17 to18.68; P = 0.001]; the aHR decreased by 0.87 (95% CI: 0.76 to 0.99: P = 0.033) per year elapsed since transplantation. Long-term allograft survival was similar in both groups.
Conclusion
Post kidney transplant TMA is an important cause of poor allograft survival. More studies are needed to enhance our understanding and management of this disorder.
Keywords: Kidney, Transplant, Thrombotic Microangiopathy, Complement-mediated TMA, De novo TMA
Background
Kidney transplantation is the treatment of choice for patients with end-stage kidney disease (ESKD) secondary to thrombotic microangiopathy (TMA) [1]. TMA is a clinical syndrome characterized by microangiopathic hemolytic anemia, thrombocytopenia, organ ischemia, and dysfunction due to microvascular thrombosis [2]. Post-transplant TMA is a well-recognized complication that affects 0.8 -15% of renal transplant recipients [3] [4] [5], and is associated with poor graft and patient outcomes [6]. TMA is caused by various factors, including infection, drug toxicity, and immunological factors [7].
Post-transplant TMA can occur at any time after kidney transplantation, but most commonly manifests in the first 6 months [8], and can be either de novo (dnTMA) with no evidence of the disease before transplant or recurrence of the native kidney TMA (cTMA) [9]. dnTMA accounts for most post-transplant cases [10] [11], with incidence ranges from 1% − 15% in different reports [12]. The pathogenesis is multifactorial, and the most common causes include medications, such as calcineurin inhibitor (CNI) toxicity, ischemia–reperfusion injury, antibody-mediated rejection (ABMR), and infections, such as cytomegalovirus [13] [14–16]. The mechanism of CNI-related dnTMA includes arteriolar vasoconstriction due to the enhanced production of vasoconstrictive factors, particularly endothelin-1 and angiotensin II [17] [18]. Genetic abnormalities in complement regulation were the precipitating factors in some dnTMA cases [19].
Atypical hemolytic uremic syndrome (aHUS) is a rare cause of ESKD that manifests as TMA in the native kidneys and is caused by uninhibited activation of the complement alternative pathway [20] [21]. Genetic mutations in the regulatory complement system were identified in approximately 50–60% of the patients [22]. Recurrence rate of aHUS post kidney transplant has been reported in 50–60% of cases [23] [24] [25]. The risk of recurrence depends on the individual genetic mutation[26] [27] [28] [29].
The kidney transplant biopsy findings in acute TMA are similar to those of the native kidneys, including mucoid intimal edema, arteriolar or glomerular capillary loop fibrin thrombi, endothelial swelling with occlusion of capillary loops, and mesangiolysis [30].
Eculizumab use for post-transplant aHUS recurrence was first reported in 2009 [31], and it is effective in the treatment and prevention of recurrent cTMA [32] [33] [34] [35].
In this study, we present our center’s comprehensive long-term outcome of adult kidney transplant recipients with cTMA and those who developed dnTMA post-transplant.
Methods
Study population and data collection
We conducted a retrospective study including all adult patients ≥ 18-year-old kidney transplant recipients with cTMA and those who developed dnTMA post-transplant between January 2000 and December 2020 in our center. The study was approved by the Johns Hopkins Hospital’s Institutional Review Board. The data were collected from the electronic medical records.
TMA before transplant, or cTMA, was defined as the disorder in the native kidneys that led to ESKD. While dnTMA was defined as any TMA disorder that occurred post kidney transplant in patients for whom the native kidney disease was not caused by cTMA. All kidney biopsy obtained in the dnTMA were for clinical cause.
We sought to compare between TMA before and after a transplant to provide insights into the pathogenesis, risk factors, and outcomes associated with TMA in the context of transplantation.
We reviewed all available clinical data, including transplant-related variables, recipient and donor information, genetic testing, histological data, and treatment modalities including the use of eculizumab. We compared the baseline characteristics, clinical and histological characteristics at the time of post-transplant TMA diagnosis, and short and long-term transplant outcomes between the two groups.
All kidney biopsies were reviewed by our internal renal pathologists, employing the contemporary Banff Classification during the initial biopsy assessment.
For the purpose of our study, “highly sensitized” patients refer to those who have a pre-formed HLA antibodies define by a calculated panel reactive antibody (CPRA) level of ≥ 80%.
Statistical analysis
We compared between the cTMA and dnTMA groups. The demographic and clinical characteristics were described using descriptive statistics. We used the Mann–Whitney test for continuous variables and Fisher's exact test for categorical variables. Patients were followed from the time of transplantation until graft failure or death, whichever came first. We estimated the crude survival probability using the Kaplan–Meier estimator. We estimated the adjusted hazard ratio (aHR) associated with cTMA using multiple Cox regression models. We fitted non-linear continuous variables with fractional polynomials and tested proportional hazard based on Schoenfeld residuals. We included biopsy diagnoses as time-varying cumulative sum (i.e. cumulative sum of each diagnosis that is updated every time the patient undergoes a new biopsy). Because the non-proportional hazard assumption did not hold, we allowed the HR associated with cTMA to vary over follow-up by including an interaction term with time. We performed an additional analysis in which the Cox regression model was modify so that 1) dnTMA were classified as such from the time of recurrence (i.e., it was included as a time-varying variable from the time of recurrence onward), and 2) we included a time-varying indicator variable for recurrent cTMA. Therefore, in the modified model, we had three virtual groups namely, dnTMA as a time-varying indicator variable (patients were classified as dnTMA only from the time on recurrence onward), cTMA, and cTMA that recurred (time-varying indicator variable from the time of recurrence onward). The time-varying indicator variables (i.e., patients classified as recurrent only from the time of recurrence onward) was required to avoid fallacies in the Cox-regression model. We considered two-sided P values of less than 0.05 as statistically significant.
Results
Patients’ characteristics
We identified 134 kidney transplant recipients in our center who met the inclusion criteria. All patients had the histological diagnosis of TMA before or after kidney transplant. Out of the 134 patients, 22 (16.4%) had cTMA and 112 (83.6%) developed dnTMA post-transplant. The demographic characteristics of all patients are shown in Table 1.
Table 1.
Baseline Patients Characteristic
| dnTMA | cTMA | p-value | |
|---|---|---|---|
| N | 112 (83.6%) | 22 (16.4%) | |
| † Mean (SD) Age at TMA diagnosis, years | 46.5 ± 16.0 | 28.9 ± 16.3 | < 0.001 |
| Mean (SD) Age at transplant, years | 46.1 ± 14.8 | 38.5 ± 10.6 | 0.022 |
| Ethnicity | |||
| White | 66 (58.9%) | 17 (77.3%) | 0.287 |
| Black | 42 (37.5%) | 5 (22.7%) | |
| Other | 4 (3.6%) | 0 (0.0%) | |
| Gender, Female | 56 (50.0%) | 15 (68.2%) | 0.161 |
| Donor type | |||
| Deceased donor | 69 (61.6%) | 9 (40.9%) | 0.185 |
| Living unrelated | 20 (17.9%) | 6 (27.3%) | |
| Living related | 23 (20.5%) | 7 (31.8%) | |
| Donor's age, years | 43.5 ± 14.9 | 37.0 ± 13.7 | 0.038 |
| Donor's terminal Creatinine, mg/dL | 1.1 (0.4–4.0) | 1.4 (0.5–6.5) | 0.540 |
| Highly sensitized | 47 (67.1%) | 15 (75.0%) | 0.592 |
| ‡ HLAi or ABOi transplant (required desensitization) | 46 (51.1%) | 6 (30.0%) | 0.136 |
| § Pre-transplant DSA | 50 (66.7%) | 8 (40.0%) | 0.04 |
| Re-transplantation | 63 (56.2%) | 13 (59.1%) | 1 |
| Warm ischemic time, minutes | 47.2 ± 24.8 | 42.5 ± 11.8 | 0.641 |
| Cold ischemic time, hours | 19.7 ± 17.2 | 10.3 ± 10.8 | 0.023 |
| Delayed graft function | 45 (40.9%) | 4 (18.2%) | 0.054 |
| Reason for previous graft loss | |||
| TMA | 4 (5.3%) | 15 (93.8%) | < 0.001 |
| ¶ Acute ABMR | 9 (11.8%) | 0 (0.0%) | |
| Chronic ABMR | 30 (39.5%) | 0 (0.0%) | |
| BK nephropathy | 3 (3.9%) | 0 (0.0%) | |
†SD standard deviation, TMA thrombotic microangiopathy
‡HLAi Human leukocyte antigens incompatible, ABOi ABO-incompatible
§DSA donor specific antibody
¶ABMR antibody mediated rejection
The causes of ESKD in the dnTMA patients were diabetic nephropathy in 24 (21%), glomerular diseases in 33 (29%), lupus nephritis in 8 (7%), polycystic kidney disease and other congenital kidney diseases in 22 (20%), hypertension in 11 (10%), and other causes or unknown etiology in 14 (13%) patients.
Compared with dnTMA, patients with cTMA were younger at TMA diagnosis, mean (SD) of 28.9 ± 16.3. vs 46.5 ± 16.0 years, p < 0.001, and at transplantation, mean (SD) of 38.5 ± 10.6 vs 46.1 ± 14.8, p = 0.022, respectively. There were no statistical differences in race or gender between the two groups. The majority of the dnTMA group received deceased donor kidney transplantation (DDKT), 69 (61.6%), compared to 9 (40.9%) in the cTMA group; however, the difference was not statistically significant. Thirteen patients (59.1%) had previous kidney transplants in the cTMA and 63 (56.2%) in the dnTMA. Donors were significantly younger in the cTMA group with a mean (SD) of 37.0 (13.7) years, compared to 43.5 (14.9) years in the dnTMA group, p = 0.038.
There was no statistical difference in the number of patients who were highly sensitized in the two groups, however, preformed donor specific antibody (DSA) was more prevalent in the dnTMA group compared to the cTMA group, 50 (66.7%) vs 8 (40.0%), p = 0.04.
Cold ischemia time was significantly shorter in the cTMA group with a mean (SD) of 10.3 ± 10.8 h compared with 19.7 ± 17.2 in the dnTMA group, p = 0.023; this translated into a trend toward a higher rate of delayed graft function (DGF) in the dnTMA group 45 (40.9%) vs 4 (18.2%) in the cTMA group, p = 0.054.
Since 2000, our center has been using mostly the same immunosuppression protocol including induction therapy with a T-cell depleting agent (mostly thymoglobulin), and maintenance therapy with calcineurin inhibitor (tacrolimus and less likely cyclosporine), steroids, and anti-metabolites mycophenolate mofetil. Most patients in our cohort received induction therapy with thymoglobulin and maintenance immunosuppression with tacrolimus, mycophenolate mofetil, and steroids. There were no statistical differences in induction and maintenance therapies between the two groups.
Treatment of post-transplant TMA, before the utilization of eculizumab for this disorder, was consistent of plasmapheresis and in some cases high doses of steroids. In our cohort, treatment with plasmapheresis was implemented in 19 patients (86.4%) of the cTMA group compared to 41 patients (36.9%) of the dnTMA group, p < 0.001.
Eculizumab was first used off-label in our center in 2010, since then it has become the treatment of choice for prevention and treatment of recurrent cTMA post-transplant. In our cohort, Eculizumab was used in 13 patients (59.1%) for recurrent or prevention of cTMA post-transplant, compared to 6 patients (5.4%) in the treatment of dnTMA group, p < 0.001.
Diagnostic findings of post-transplant TMA
The median time (interquartile range (IQR)) to biopsy-proven TMA post-transplant was 16.4 (3.6 -79.7) months. There were 543 biopsies in the cohort, median (IQR) number per patient: of 4 (2 -5) biopsies.
Twelve patients (60%) with cTMA experienced recurrence after kidney transplantation, confirmed by kidney transplant biopsy. Pathogenic mutations were identified in 10 patients (45.5%) while 12 (54.5%) patients had either no identified 7 (31.8%) mutation or testing was not done 5 (22.7%).
Laboratories results including hemoglobin, platelets, and kidney function were not statistically significant on the day of discharge post hospitalization for kidney transplant between the two groups. At the time of TMA diagnosis post-transplant, which was confirmed by kidney transplant biopsy, the laboratory parameters did not differ significantly except for serum creatinine, Table 2. Median serum creatinine (IQR) was much higher at the time of dnTMA diagnosis compared with cTMA recurrence post-transplant, 3.6 (0.4–24.0) vs 2.0 (0.7–15.6) mg/dL, p = 0.043.
Table 2.
Clinical and pathological findings at the time of post-transplant TMA diagnosis
| dnTMA (N = 112) | cTMA (N = 22) | p-value | |
|---|---|---|---|
| Number of graft biopsies | 4.0 (1.0–12.0) | 3.5 (0.0–10.0) | 0.134 |
| Time from first to last biopsy, months | 18.8 (0.0–296.2) | 45.2 (0.6–1290.0) | 0.197 |
| † Median (IQR) of SCr at time of diagnostic biopsy, mg/dL | 3.6 (0.4–24.0) | 2.0 (0.7–15.6) | 0.043 |
| ‡ Mean (SD) of Hg at time of diagnostic biopsy, g/dL | 9.4 ± 2.0 | 9.6 ± 1.5 | 0.449 |
| Median (IQR) of platelets count at time of diagnostic biopsy, × 1000/dL | 167 (21.0–459) | 176 (47.0–396) | 0.855 |
| Median (IQR) of LDH at time of diagnostic biopsy, UI/L [Ref. 100–200] | 312 (91–1547) | 286 (200–1183) | 0.753 |
| Median (IQR) of last SCr, mg/dL | 3.1 (0.3–17.8) | 2.3 (0.4–5.0) | 0.025 |
| § Mean (SD) of last eGFR, mL/min/1.73 m2 | 23.5 ± 22.1 | 42.2 ± 27.3 | 0.003 |
| Mean (SD) of last Hemoglobin, g/dL | 10.6 ± 2.2 | 9.5 ± 2.7 | 0.212 |
| Rejection in the diagnostic biopsy | 49 (43.8%) | 2 (10.0%) | 0.005 |
| ¶ TCMR in diagnostic biopsy | 26 (23.2%) | 0 (0.0%) | 0.007 |
| # CNI toxicity in diagnostic biopsy | 34 (30.4%) | 1 (5.0%) | 0.025 |
| Rejection, at least in one biopsy | 90 (80.4%) | 11 (50.0%) | 0.005 |
| ††ABMR, at least in one biopsy | 29 (25.9%) | 1 (4.5%) | 0.027 |
| ‡‡ CTG, at least in one biopsy | 11 (9.8%) | 3 (13.6%) | 0.701 |
| CNI toxicity, at least in one biopsy | 80 (71.4%) | 5 (22.7%) | < 0.001 |
†SCr serum creatinine
‡Hg Hemoglobin
§eGFR estimated glomerular filtration rate
¶TCMR T cell mediated rejection, antibody-mediated rejection
#CNI calcineurin inhibitor
††ABMR antibody mediated rejection
‡‡CTG chronic transplant glomerulopathy
Patients with dnTMA had a much higher rate of rejection confirmed by kidney transplant biopsy at the time of TMA diagnosis compared with cTMA, 49 (43.8%) vs 2 (10%), p = 0.005, Table 1. Similarly, CNI toxicity in the diagnostic biopsy was more frequent in the dnTMA group compared to the cTMA group, 80 (71.4%) vs 5 (22.7%), p < 0.001. In anytime kidney transplant biopsy, ABMR was more prevalent in the dnTMA group compared to the cTMA group, 29 (25.9%) vs 1 (4.5%) patients, p = 0.027. Otherwise, there was no difference in the other Banff scores of the first kidney biopsy, including g, I, ti, t, v, ptc, C4d, cg, ci, ct, cv, cg, mm, ah, IFTA, Table 2.
Allograft and patient outcome
The survival analysis was performed in the 129 patients with available follow-up (109 with dnTMA, and 20 with cTMA), Fig. 1. The mean follow-up was 4.5 years during which 73 (54%) had allograft failure and 22 (16%) died. Black race was associated with a higher risk of allograft failure. Pathological changes of any type of acute rejection, including borderline rejection, and tacrolimus toxicity in the kidney transplant biopsy at the time of TMA diagnosis post kidney transplant were associated with a significantly higher risk of allograft failure, Table 3.
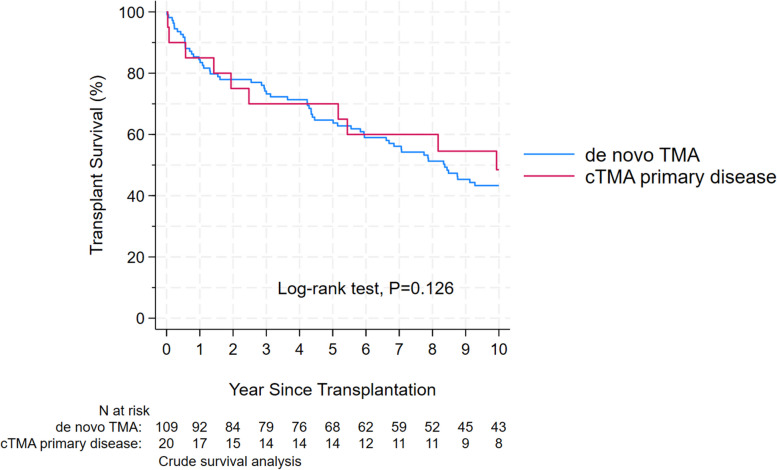
Fig. 1.
Kaplan–Meier plot of transplant survival (death or end-stage kidney disease, ESKD) of primary cTMA vs dnTMA. Time at risk starts from transplantation and includes patients with available follow-up. The population is divided into those who had known cTMA before transplantation (red) and those who had dnTMA (blue). There was a sudden drop by 10% in cTMA patients shortly after follow-up, but survival was similar between the groups in the long term; cTMA
Table 3.
Hazard ratio of transplant failure from Cox proportional hazards regression model
| HR | 95% CI | P value | |
|---|---|---|---|
| Male gender | 1.70 | (1.06 to 2.75) | 0.029 |
| Ethnicity (vs White) | |||
| Black | 1.76 | (1.06 to 2.93) | 0.029 |
| Other | 0.37 | (0.09 to 1.57) | 0.176 |
| Donor type (vs Deceased donor) | |||
| Living unrelated | 1.22 | (0.65 to 2.29) | 0.526 |
| Living related | 0.47 | (0.22 to 1.00) | 0.051 |
| Delayed graft function | 1.38 | (0.78 to 2.42) | 0.267 |
| Recurrent cTMA, first year | 6.37 | (2.17 to18.68) | 0.001 |
| † ABMR | 1.23 | (0.90 to 1.67) | 0.188 |
| ‡ TCMR grade 1 | 1.37 | (1.12 to 1.67) | 0.002 |
| TCMR grade 2 | 1.55 | (1.22 to 1.98) | < 0.001 |
| Borderline rejection | 1.47 | (1.08 to 2.00) | 0.013 |
| § CNI toxicity | 1.65 | (1.28 to 2.12) | < 0.001 |
| Yearly change of the HR associated with cTMA | 0.87 | (0.76 to 0.99) | 0.033 |
†ABMR antibody mediated rejection
‡TCMR T cell mediated rejection
§CNI calcineurin inhibitor
After adjusting for age, gender, ethnicity, donor type, lymphodepleting agent induction and DGF patients with cTMA had a significant increase in the hazard risk of allograft failure in the first-year post-transplant, aHR: 6.37 (95% CI: 2.17 to18.68, P = 0.001). However, the aHR decreased by 0.87 (95% CI: 0.76 to 0.99, P = 0.033) per year elapsed since transplantation, Table 3. By the end of the study’s time, there were no statistical differences in the allograft survival between the two groups, Fig. 2. Among the 132 patients who contributed to the survival analyses, 97 (87%) in the dnTMA and 13 (65%) in the cTMA receive a TMA diagnosis in the post-transplant biopsy report (P = 0.044).
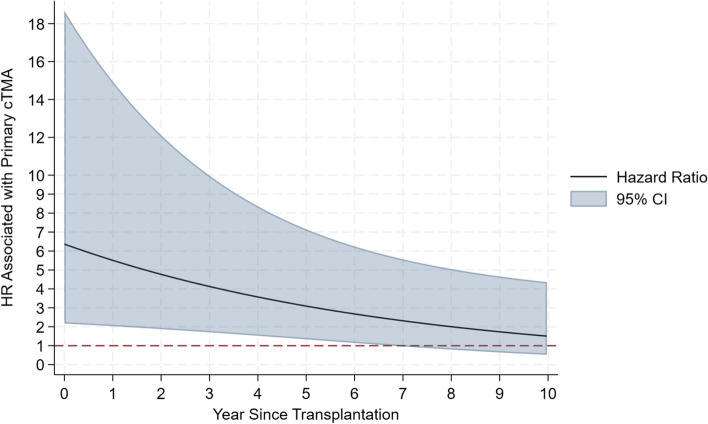
Fig. 2.
Time-varying adjusted hazard ratio (HR) of transplant failure (death or end-stage kidney disease, ESKD) of patients with cTMA compared to dnTMA. The hazard ratio is highest shortly after transplantation, and then decreases with time. The model is adjusted for age, gender, ethnicity, donor type, induction with lymphodepleting agents, DGF, eculizumab use (any time), and biopsy diagnosis (time-varying cumulative sum). The line represents the hazard ratio estimates, the blue shaded area represents the 95 percent confidence interval; cTMA, complement-mediated thrombotic microangiopathy
HR associated with dnTMA as time-varying variable biopsy diagnosis showed a relative 87% increase in rate of transplant failure which was, however, only of borderline statistical significance (aHR: 1.87 [95%CI: 0.83 to 4.23; P = 0.134). On the contrary, cTMA was associated with a striking increase in early transplant failure. The increase in the rate of early transplant failure associated with cTMA did not depend on whether or not there was a biopsy diagnosis of TMA recurrence in cTMA patients. In fact, the HR associated with biopsy-proven recurrence in the cTMA did not differ between cTMA that had biopsy-proven recurrence and those who did not have biopsy-proven recurrence: HR in cTMA with no biopsy-proven recurrence: 8.18 [95%CI: 1.69 to 39.66; P = 0.009]; HR in cTMA with biopsy-proven recurrence: 5.56 [95%CI: 1.77 to 17.52; P = 0.003]; difference between the two HRs (test for interaction): P = 0.629. The reason why biopsy-proven recurrence did not apparently affect the transplant failure rate within the cTMA group is likely related to the fact that, within the cTMA group, there were recurrences that occurred shortly post-transplantation and that we did not record as such because they were non documented by a biopsy.
In the most recent follow-up, allograft function as measured by mean (SD) eGFR (23.5 ± 22.1 vs 42.2 ± 27.3 ml/min/m2, p = 0.003), was significantly worse in the dnTMA group comparing with cTMA group. The difference in serum creatinine (SCr) at the time of diagnosis between the dnTMA and cTMA groups does not appear to be directly related to the time elapsed between the timing of biopsies for each group. The study provided data on the serum creatinine levels at the time of diagnostic biopsy, showing a significant difference between the two groups (median SCr of 3.6 mg/dL in dnTMA vs. 2.0 mg/dL in cTMA, p = 0.043). However, the time from the first to the last biopsy, which might reflect the timing of biopsy, was not significantly different between the two groups (median time of 18.8 months for dnTMA vs. 45.2 months for cTMA, p = 0.197). While not statistically significant, this may have influenced the results given larger sample size and more power.
Therefore, while there is a noted difference in serum creatinine at diagnosis, this difference does not seem to be explained by the timing of the biopsies. Instead, it could be related to other factors, such as underlying pathology or clinical management differences between the two groups.
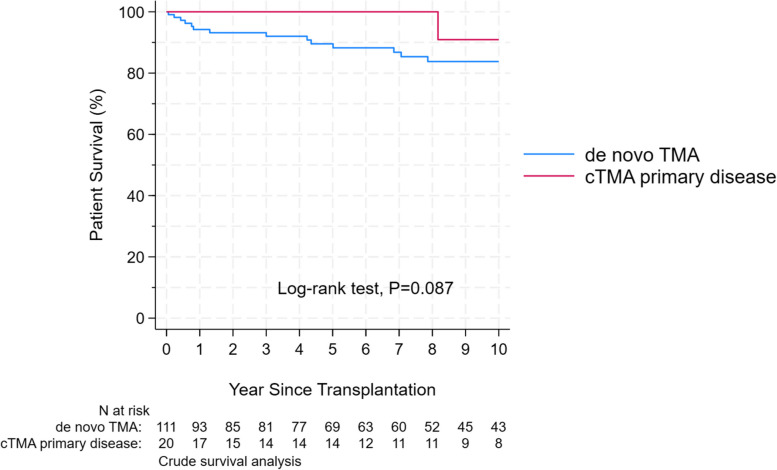
There was a trend toward worse patients’ survival in the dnTMA group, which did not reach a statistical difference, p = 0.087, Fig. 3. The Hazard Ratio (HR) for graft failure for living unrelated donors compared to deceased donors was 1.22 (95% CI: 0.65 to 2.29, p = 0.526). For living related donors compared to deceased donors, the HR was 0.47 (95% CI: 0.22 to 1.00, p = 0.051).
Fig. 3.
Kaplan–Meier patient survival estimates in cTMA and dnTMA. Time at risk started from transplantation and included patients with available follow-up. Patients starting dialysis were censored
Eculizumab effect on the allograft outcome
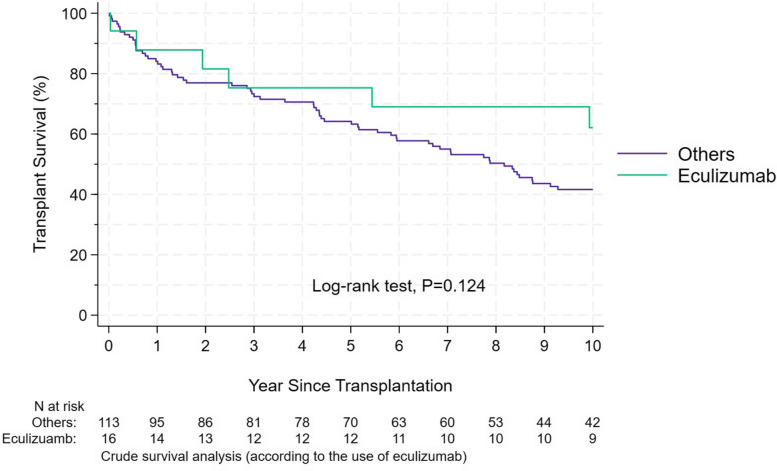
We performed a sub-group analysis of patients who received Eculizumab; mostly cTMA. There was no difference in allograft survival between those who received eculizumab and those who did not, Fig. 4. However, the treatment of eculizumab has been utilized only since 2010, and in many cases, it was used late in the course of post-transplant TMA.
Fig. 4.
Kaplan–Meier allograft survival estimates according to the use of eculizumab
Discussion
In this single-center study, we identified a total of 134 patients with TMA, 22 patients with cTMA, and 112 developed dnTMA post-transplant. Patients with cTMA were younger both at transplantation and at the time of TMA diagnosis. We found that biopsy-proven acute TCMR and ABMR rejections and biopsy-proven CNI toxicity were much more common in the dnTMA group. Rejection and CNI toxicity were most likely the causes of dnTMA post kidney transplantation.
Additionally, we found that cTMA was associated with a sixfold increase in the hazard risk of allograft failure in the first year after transplant but the aHR decreased as time elapsed after transplant. This finding may be explained by the high recurrence rate of cTMA early post-transplant triggered by several factors that lead to the activation of the alternative complement pathway, e.g., ischemia–reperfusion injury, infections, and the use of immunosuppressive drugs, especially before the utilization of anti-C5 antibodies. However, the long-term allograft survival of the two groups was similar.
Before the utilization of eculizumab in the treatment of post-transplant TMA in our center, plasma exchange was the main treatment of choice for recurrent cTMA and in some cases of dnTMA. Although eculizumab improved the allograft survival in published case reports, our study did not capture this benefit. This can be explained by the underpowered sample size and the fact that the utilization of eculizumab was delayed in some cases.
cTMA is a systemic disorder caused by uncontrolled activation of the alternative complement pathway and can lead to ESKD. A variety of genetic defects in complement-related factors have been identified and recurrence rate post-transplant largely depends on the pathogenetic mutations involved [33]. Before the utilization of anti-C5 antibodies in cTMA, kidney transplant outcome of recurrent cTMA was dire [36].
Although acute rejection episodes commonly occur in the first year post-transplant, mostly in the first 6 months, successful rejection treatment and allograft functional recovery may not have a negative long-term impact on the allograft survival [37]. However, the detection of dnTMA in the early post-transplant period holds significant implications for the long-term allograft outcome. Our study demonstrates that dnTMA may serve as a pivotal early pathological marker associated with poor long-term allograft survival.
The incidence of recurrent cTMA or dnTMA is not very well defined, likely because most of the transplant centers do not do protocol biopsies. In a small retrospective study of 57 renal transplant recipients with early allograft dysfunction, post-transplant biopsy-proven TMA was detected in 10.5% of cases [38].
The significant advances in our understanding of the cTMA disorder and the approval of anti-C5 antibodies have resulted in a major improvement in the outcome of kidney transplants in patients with cTMA [39] [40].Eculizumab has been used for the treatment of recurrent cTMA and as a preventive measurement that decreases or prevents recurrent cTMA post-transplant [41]. On the other hand, limited options are available for dnTMA that mostly depend on the cause. In cases of CNI toxicity, many providers switch to mTOR inhibitors or belatacept with some success [42] [43].
Furthermore, there is limited data on the long-term outcome of renal transplantation in patients with post-transplant TMA. In a study from Brazil, 17 (1.1%) out of 1549 kidney transplant recipients developed dnTMA that occurred at a median of 25 (1–1755) days after transplantation. CNI withdrawal or reduction was the first step in the management of 10/15 (66%) patients, and 6 (35%) received fresh frozen plasma (FFP) and/or plasmapheresis. Eight (47%) patients needed dialysis after TMA diagnosis and 75% remained on dialysis. At 4 years of follow-up, death-censored graft survival was worse for the dnTMA group (43.0% versus 85.6%, log-rank = 0.001; hazard ratio = 3.74), with no difference in patient survival (53.1% versus 82.2%, log-rank = 0.24) [9].
In most published data the two types of post-transplant TMA were grouped. In a retrospective study of 89 patients with post-transplant TMA, underlying precipitating factors were infection (54%), acute rejection (34%), CNI toxicity (13%), and pregnancy (3%). The 1-year patient survival was 97% and graft survival was 66%. Allograft survival was inferior when ABMR occurred (with 41%; without 70%, p = 0.01) [44].
Despite the significant novel findings of our study, it has several limitations, primarily stemming from its retrospective nature. Additionally, the absence of protocol biopsy might have led to the oversight of numerous other patients with post-transplant TMA. Furthermore, our understanding of cTMA, primarily attributed to its association with genetic disorders within the complement alternative pathway, remains relatively new. Consequently, treatment modalities for both cTMA and dnTMA were notably limited in the first ten years of our study. Other limitation of our study is the limited available data on eculizumab use in TMA. However, despite these limitations, our study represents one of the largest cohorts of kidney transplant recipients with TMA. Moreover, the outcomes derived from our study carry substantial implications for advancing comprehension of this disorder and elucidating its ramifications on allograft outcomes. The fact that we only classified recurrence based on biopsy report is a limitation of the study that we acknowledged. Nonetheless, we contend that the study shows clear evidence that cTMA and dnTMA are, in fact different and that by no means they can be regarded the same disease. Additionally, the possibility that cTMA led to heightened awareness and potentially earlier biopsying/diagnosis is indeed a valid concern. It is possible that clinicians, being more vigilant with patients who had a history of cTMA, might have detected TMA earlier, particularly when prompted by subtler signs like a lower rise in creatinine. This could, in turn, contribute to an earlier diagnosis in the cTMA group compared to dnTMA, where the suspicion of TMA might not be as immediately high. Future studies could aim to control for this bias by standardizing biopsy protocols or using additional biomarkers to guide the timing of biopsy across all patient groups, regardless of their pre-transplant history.
Conclusion
Post-transplant recurrent cTMA is an important cause of poor allograft survival in the first-year post kidney transplant. On the other hand, dnTMA is associated strongly with poor long-term allograft survival.
Acknowledgements
None.
Abbreviations
- cTMA
Complement-mediated thrombotic microangiopathy
- dnTMA
De novo thrombotic microangiopathy
- aHR
Adjusted hazard ratio
- ESKD
End-stage kidney disease
- CNI
Calcineurin inhibitor toxicity
- ABMR
Antibody-mediated rejection
- TCMR
T cell-mediated rejection
- aHUS
Atypical hemolytic uremic syndrome
- MCP
Membrane cofactor protein
- DDKT
Deceased donor kidney transplant
- LURT
Living unrelated kidney transplant
- LRT
Living-related kidney transplant
- GN
Glomerulonephritis
- DSA
Donor-specific antibodies
- IFTA
INTERSTITIAL Fibrosis Tubular Atrophy
- eGFR
Estimated glomerular filtration rate
- rATG
Rabbit anti-thymocyte globulins
- MMF
Mycophenolate mofetil
- mTOR
Mammalian target of rapamycin
Authors’ contributions
All authors reviewed the manuscript.
Funding
None.
Data availability
Data is provided within the manuscript.
Declarations
Ethics approval and consent to participate
The ethics committee is the Johns Hopkins Hospital’s Institutional Review Board. This is a retrospective study, data were collected from the electronic medical records, consents were waived by Johns Hopkins Hospital’s Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hariharan S, Israni AK, Danovitch G. Long-Term Survival after Kidney Transplantation. N Engl J Med. 2021Aug 19;385(8):729–43. [DOI] [PubMed] [Google Scholar]
- 2.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002Aug 22;347(8):589–600. [DOI] [PubMed] [Google Scholar]
- 3.Ávila A, Gavela E, Sancho A. Thrombotic Microangiopathy After Kidney Transplantation: An Underdiagnosed and Potentially Reversible Entity. Front Med (Lausanne). 2021;8: 642864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarifian A, Meleg-Smith S, O’donovan R, Tesi RJ, Batuman V. Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int. 1999 Jun;55(6):2457–66. [DOI] [PubMed]
- 5.Reynolds JC, Agodoa LY, Yuan CM, Abbott KC. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003Nov;42(5):1058–68. [DOI] [PubMed] [Google Scholar]
- 6.Satoskar AA, Pelletier R, Adams P, Nadasdy GM, Brodsky S, Pesavento T, et al. De novo thrombotic microangiopathy in renal allograft biopsies-role of antibody-mediated rejection. Am J Transplant. 2010Aug;10(8):1804–11. [DOI] [PubMed] [Google Scholar]
- 7.Bayer G, von Tokarski F, Thoreau B, Bauvois A, Barbet C, Cloarec S, et al. Etiology and Outcomes of Thrombotic Microangiopathies. Clin J Am Soc Nephrol. 2019Apr 5;14(4):557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas F, El Kossi M, Kim JJ, Sharma A, Halawa A. Thrombotic microangiopathy after renal transplantation: Current insights in de novo and recurrent disease. World J Transplant. 2018Sep 10;8(5):122–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caires RA, Marques IDB, Repizo LP, Sato V a. H, Carmo LPF, Machado DJB, et al. De novo thrombotic microangiopathy after kidney transplantation: clinical features, treatment, and long-term patient and graft survival. Transplant Proc. 2012 Oct;44(8):2388–90. [DOI] [PubMed]
- 10.Schwimmer J, Nadasdy TA, Spitalnik PF, Kaplan KL, Zand MS. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003Feb;41(2):471–9. [DOI] [PubMed] [Google Scholar]
- 11.Ponticelli C, Banfi G. Thrombotic microangiopathy after kidney transplantation. Transpl Int. 2006Oct;19(10):789–94. [DOI] [PubMed] [Google Scholar]
- 12.Pham PT, Peng A, Wilkinson AH, Gritsch HA, Lassman C, Pham PC, et al. Cyclosporine and tacrolimus-associated thrombotic microangiopathy. Am J Kidney Dis. 2000Oct;36(4):844–50. [DOI] [PubMed] [Google Scholar]
- 13.De Keyzer K, Van Laecke S, Peeters P, Vanholder R. De novo thrombotic microangiopathy induced by cytomegalovirus infection leading to renal allograft loss. Am J Nephrol. 2010;32(5):491–6. [DOI] [PubMed] [Google Scholar]
- 14.Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001Sep;60(3):831–46. [DOI] [PubMed] [Google Scholar]
- 15.Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009Feb;4(2):481–508. [DOI] [PubMed] [Google Scholar]
- 16.Liptak P, Ivanyi B. Primer: Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006 Jul;2(7):398–404; quiz following 404. [DOI] [PubMed]
- 17.Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993May;91(5):2144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English J, Evan A, Houghton DC, Bennett WM. Cyclosporine-induced acute renal dysfunction in the rat. Evidence of arteriolar vasoconstriction with preservation of tubular function. Transplantation. 1987 Jul;44(1):135–41. [DOI] [PubMed]
- 19.Le Quintrec M, Lionet A, Kamar N, Karras A, Barbier S, Buchler M, et al. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant. 2008Aug;8(8):1694–701. [DOI] [PubMed] [Google Scholar]
- 20.Sperati CJ, Moliterno AR. Thrombotic microangiopathy: focus on atypical hemolytic uremic syndrome. Hematol Oncol Clin North Am. 2015Jun;29(3):541–59. [DOI] [PubMed] [Google Scholar]
- 21.Kant S, Bhalla A, Alasfar S, Alachkar N. Ten-year outcome of Eculizumab in kidney transplant recipients with atypical hemolytic uremic syndrome- a single center experience. BMC Nephrol. 2020May 20;21(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuber J, Le Quintrec M, Sberro-Soussan R, Loirat C, Frémeaux-Bacchi V, Legendre C. New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol. 2011Jan;7(1):23–35. [DOI] [PubMed] [Google Scholar]
- 23.Salvadori M, Bertoni E. Update on hemolytic uremic syndrome: Diagnostic and therapeutic recommendations. World J Nephrol. 2013Aug 6;2(3):56–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahlou A, Lang P, Charpentier B, Barrou B, Glotz D, Baron C, et al. Hemolytic uremic syndrome. Recurrence after renal transplantation. Groupe Coopératif de l’Ile-de-France (GCIF). Medicine (Baltimore). 2000 Mar;79(2):90–102. [DOI] [PubMed]
- 25.Zuber J, Le Quintrec M, Morris H, Frémeaux-Bacchi V, Loirat C, Legendre C. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando). 2013Oct;27(4):117–25. [DOI] [PubMed] [Google Scholar]
- 26.Artz MA, Steenbergen EJ, Hoitsma AJ, Monnens LAH, Wetzels JFM. Renal transplantation in patients with hemolytic uremic syndrome: high rate of recurrence and increased incidence of acute rejections. Transplantation. 2003Sep 15;76(5):821–6. [DOI] [PubMed] [Google Scholar]
- 27.Loirat C, Noris M, Fremeaux-Bacchi V. Complement and the atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2008Nov;23(11):1957–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007Aug;18(8):2392–400. [DOI] [PubMed] [Google Scholar]
- 29.Bresin E, Daina E, Noris M, Castelletti F, Stefanov R, Hill P, et al. Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: prognostic significance of genetic background. Clin J Am Soc Nephrol. 2006Jan;1(1):88–99. [DOI] [PubMed] [Google Scholar]
- 30.Lusco MA, Fogo AB, Najafian B, Alpers CE. AJKD Atlas of Renal Pathology: Thrombotic Microangiopathy. Am J Kidney Dis. 2016Dec;68(6):e33–4. [DOI] [PubMed] [Google Scholar]
- 31.Chatelet V, Frémeaux-Bacchi V, Lobbedez T, Ficheux M, Hurault de Ligny B. Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant. 2009 Nov;9(11):2644–5. [DOI] [PubMed]
- 32.Siedlecki AM, Isbel N, Vande Walle J, James Eggleston J, Cohen DJ, Global aHUS Registry. Eculizumab Use for Kidney Transplantation in Patients With a Diagnosis of Atypical Hemolytic Uremic Syndrome. Kidney Int Rep. 2019 Mar;4(3):434–46. [DOI] [PMC free article] [PubMed]
- 33.Matar D, Naqvi F, Racusen LC, Carter-Monroe N, Montgomery RA, Alachkar N. Atypical hemolytic uremic syndrome recurrence after kidney transplantation. Transplantation. 2014Dec 15;98(11):1205–12. [DOI] [PubMed] [Google Scholar]
- 34.Alachkar N, Bagnasco SM, Montgomery RA. Eculizumab for the treatment of two recurrences of atypical hemolytic uremic syndrome in a kidney allograft. Transpl Int. 2012Aug;25(8):e93-95. [DOI] [PubMed] [Google Scholar]
- 35.Alasfar S, Alachkar N. Atypical hemolytic uremic syndrome post-kidney transplantation: two case reports and review of the literature. Front Med (Lausanne). 2014;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuber J, Frimat M, Caillard S, Kamar N, Gatault P, Petitprez F, et al. Use of Highly Individualized Complement Blockade Has Revolutionized Clinical Outcomes after Kidney Transplantation and Renal Epidemiology of Atypical Hemolytic Uremic Syndrome. J Am Soc Nephrol. 2019Dec;30(12):2449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opelz G, Döhler B, Collaborative Transplant Study Report. Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation. 2008 Mar 15;85(5):661–6. [DOI] [PubMed]
- 38.Ardalan MR, Shoja MM, Tubbs RS, Etemadi J, Esmaili H, Khosroshahi HT. Thrombotic microangiopathy in the early post-renal transplant period. Ren Fail. 2008;30(2):199–203. [DOI] [PubMed] [Google Scholar]
- 39.Legendre CM, Campistol JM, Feldkamp T, Remuzzi G, Kincaid JF, Lommelé Å, et al. Outcomes of patients with atypical haemolytic uraemic syndrome with native and transplanted kidneys treated with eculizumab: a pooled post hoc analysis. Transpl Int. 2017Dec;30(12):1275–83. [DOI] [PubMed] [Google Scholar]
- 40.de Andrade LGM, Contti MM, Nga HS, Bravin AM, Takase HM, Viero RM, et al. Long-term outcomes of the Atypical Hemolytic Uremic Syndrome after kidney transplantation treated with eculizumab as first choice. PLoS ONE. 2017;12(11): e0188155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levi C, Frémeaux-Bacchi V, Zuber J, Rabant M, Devriese M, Snanoudj R, et al. Midterm Outcomes of 12 Renal Transplant Recipients Treated With Eculizumab to Prevent Atypical Hemolytic Syndrome Recurrence. Transplantation. 2017Dec;101(12):2924–30. [DOI] [PubMed] [Google Scholar]
- 42.Koppula S, Yost SE, Sussman A, Bracamonte ER, Kaplan B. Successful conversion to belatacept after thrombotic microangiopathy in kidney transplant patients. Clin Transplant. 2013;27(4):591–7. [DOI] [PubMed] [Google Scholar]
- 43.Ashman N, Chapagain A, Dobbie H, Raftery MJ, Sheaff MT, Yaqoob MM. Belatacept as maintenance immunosuppression for postrenal transplant de novo drug-induced thrombotic microangiopathy. Am J Transplant. 2009Feb;9(2):424–7. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira CM, Tedesco Silva Junior H, Moura LAR de, Proença HM de S, de Marco R, Gerbase de Lima M, et al. Clinical and pathological features of thrombotic microangiopathy influencing long-term kidney transplant outcomes. PLoS One. 2020;15(1):e0227445. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript.