Abstract
Objective
Skilled birth attendance and place of delivery have a significant effect on child growth. The present paper examined the mode of delivery and its impact on child health among children 0–59 months in India.
Methods
A total of 200,794 samples were used in the study. Among them, 45,784 births were delivered by C-section, and the remaining 150,010 births were delivered through normal delivery. Life table estimation of mortality, as well as bivariate and multivariate logistic regression, were used to identify the association between child health and mode of delivery using data from the fifth round of the National Family Health Survey conducted in 2019-21.
Results
The study results indicate that children born through normal delivery had significantly lower rates of stunting, wasting, and underweight compared to those born via C-section. Additionally, the likelihood of a new-born baby dying during the neonatal period was higher for those delivered by C-section compared to those delivered vaginally, which holds true for various background characteristics. Mothers with a 3rd or higher order birth who deliver via C-section face a higher risk of their baby dying during the neonatal and infant periods compared to those with a 2nd order birth.
Conclusion
The conclusion of the study found that C-section delivery may adversely affect child undernutrition as compared to normal birth. These findings would help formulate the policies and implement actions that would improve the quality of painless labor and immediate delivery in health facilities particularly public hospitals and shall reduce the C-section birth.
Keywords: Child growth, Life-table, Multivariate regression, Stunting, Neo-natal, Infant
Introduction
Child undernutrition and mortality are serious health issues in low- and middle-income countries like India. According to a global report from 2017, nearly 151 million children, or 22%, were stunted [1], and 45% of child deaths occurred in those under the age of five [2, 3]. The global report on child malnutrition in developing countries revealed that undernutrition, infectious diseases, household environments, and different modes of birth delivery (C-section and vaginal) are major causes of child malnutrition and early mortality [4]. Human births can occur through various delivery methods, including natural delivery, assisted delivery, institutional C-section births, and C-section births influenced by social factors [5]. A growing number of studies suggest that “C-section birth delivery has a negative impact on maternal and child health outcome” [6–11]. The World Health Organization (WHO) recommended that “the utilization of C-section birth should lie between 5 to 15% in any population without any negative health impact” [12–17]. A rate below 5% indicates that the women do not have access to surgical, obstetric, and unmet needs for skilled delivery services [18]. And it is a good sign for saving infants and mothers in emergency obstetric circumstances and also reduces the risk of maternal and neonatal mortality [19]. While rates above 15% inform that both mother and newborn babies have lower safety and increases the risk of mortality and further superimpose a financial burden in the family and creates the clinical risks on the healthcare system [20–24]. Polidano et al. (2017) suggested that C-section birth may be directly and indirectly linked with the negative cognitive development [25]. Indirect links with asthma, type I diabetes, allergies [26–28], and obesity [29] have also been associated with poor learning and academic performance [30, 31]. Additionally, a direct association may occur through the infant’s gut microbiota” [25]. An early study by Rowe-Murray and Fisher (2002), found that babies born through C-section were less likely to have immediate skin-to-skin contact with their mothers after birth and were more likely to experience a delay in breastfeeding within the first 24 h post-delivery” [32].
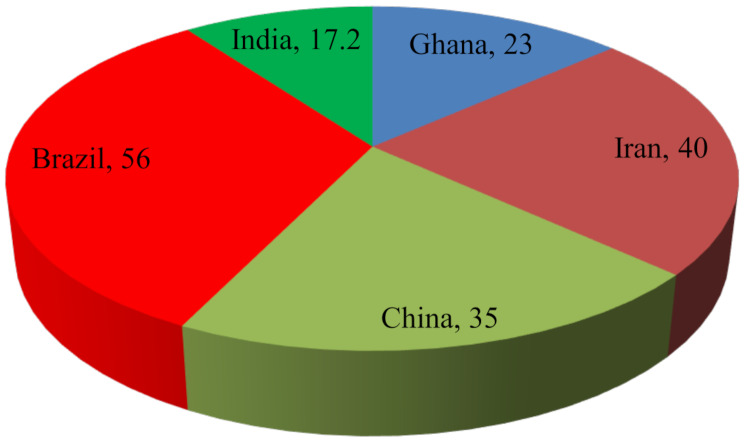
Worldwide C-section birth deliveries have increased and varied across different countries due to diverse socio-economic factors and differential health care services. The ratio is even higher in Asian countries than in others. In Ghana C-section, birth delivery increased from 3 to 23% from 2003 to 2014 [33, 34]. In Iran, the proportion of C-section birth operations is around 40.0% [35], and China is 34.9% [36], Brazil contributes the highest percentage, with C-section birth delivery accounting for 56%, and in the private sector it is close to 90% [37]. The Fig. 1 shows the global report of C-section delivery and found India has 17.2% of C-section births which is lower than the other countries like Brazil and China [38]. The low coverage of maternal healthcare utilization is one of the crucial factors that may affect child health negatively.
Fig. 1.
Spatial variation of C-Section birth
Currently, there is a significant debate regarding the use of surgical procedure for birth delivery among women in private and public health institutions. Some studies suggested normal vaginal delivery [39–41] whereas others recommended C-section birth delivery [42]. Studies also suggested, “that vaginal delivery is commonly associated with the postpartum hemorrhage [41] whereas postpartum morbidity occurs most commonly due to C-section birth delivery” [40]. In addition, normal vaginal delivery plays the inevitable role of physiological process for human reproduction and it has positive signs for human health as compared to C-section birth delivery. For example, in normal birth or vaginal delivery, infants have early contact with the mother, and start breastfeeding within 2 hour, which is an important requirement for a child’s psychological development process [43]. While, in C-section birth is an unnatural process of delivery and it’s associated with an increased risk of ‘endometritis’, ‘pneumonia’, and other diseases, which have adverse effects on the psychological development of new-born babies [5, 41]. Therefore, the present paper aims to identify the impact of child health due to different sections of birth of delivery in India.
Methodology
Data
The present study used the data from the fifth round of the National Family Health Survey (NFHS-V) which was conducted in 2019-21 under the Ministry of Family Health and Wealth fare (MoHFW) in Govt. of India. The NFHS is one of the important large-scale demographic health surveys in India which provides sufficient information about fertility, mortality, nutritional status, family planning, and health care utilization. The survey carried out two stage sampling design in rural areas and three-stage sampling design in urban areas. In rural areas, villages were selected based on probability proportional to size (PPS) sampling scheme in first stage and second stage using systematics sampling of each household in the villages. In urban areas, first wards were selected based on PPS sampling after that census enumeration blocks (CEB) were selected at the second stage and in the final stage systematic sampling technique adopted for selected each household in urban areas. The total 200,794 samples were used from the kids file in the last five years preceding the survey with the women age group of 15–49. Among of them 45,784 birth delivered by C-section and reaming 150,010 birth delivered through normal section. During the survey, all women were provided with complete birth history including sex, date of live birth, and survival status of each live birth. Detailed information was available in the national report [38].
Outcome variables
The child’s health is the main outcome variable study. For child health, study has considered the mortally variables such as Neo-natal and Infant mortality and nutritional variables like stunting, wasting, underweight and anemia. The neo-natal mortality is defined as the death of newborn babies within 28 days of life after birth, while infant mortality is defined as the death of a newborn baby before reaching 12 months. Childhood malnutrition is measured using standard indicators such as height-for-age (HAZ), weight-for-age (WAZ), and weight-for-height (WHZ), along with anemia. Stunting is defined as a HAZ score of less than − 2 SD, wasting as a WHZ score of less than − 2 SD, and underweight as a WAZ score of less than − 2 SD. All these child malnutrition indicators, based on Z-scores, are calculated using the WHO-recommended reference population [44]. These variables were classified as dichotomous, where ‘0’ indicates no stunting, wasting and underweight (Z ≥ -2 standard deviations) and ‘1’ indicates stunting, wasting and underweight (Z < -2 standard deviations). Mortality was also coded as ‘1’ if the baby died and ‘0’ if the baby survived. And finally, children’s anemia level is defined as a hemoglobin level < 110 g/L.
Explanatory variables
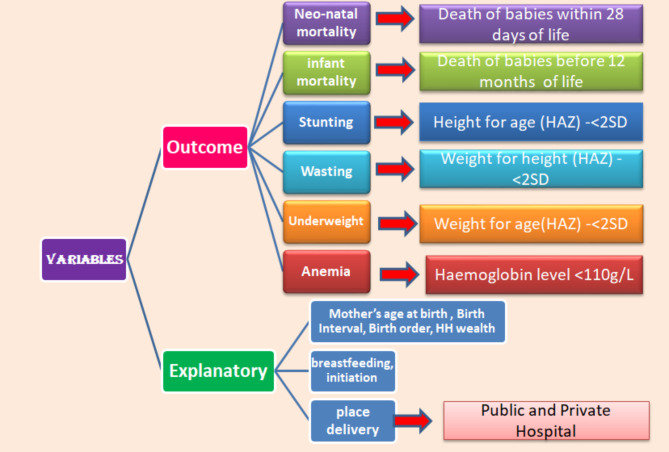
The place delivery used primary independent variables in the study. The present study only considered institutional birth delivery in either public or private hospitals. Birth deliveries at home are excluded from study. In the NFHS-V survey questionnaire, place of delivery have been identified by following categories: “Public Hospital (government hospital, government health center, government health post or other public sectors); or Private Medical Sector (private hospital or clinic, other private medical facilities)” [38]. And birth delivery is categorized by c- section or a normal vaginal section. The growing number of literature suggested that the bio-demographic and socio-economic factors had a significant impact on child health by C-section birth and normal birth delivery [45–48]. The following variables were included in the study as independent variables: breastfeeding initiation, considered a continuous variable, defined as the time in hours within the first 24 h after birth when breastfeeding begins; duration of breastfeeding, measured as 12 to 16 weeks. And other variables are mother’s age at birth (15–19 years; 20–29 years, 30–39 years and 40–49 years), preceding birth interval (< 24 months and > 24 months), birth order (1; 2; and 3), place of residence (urban; rural), household wealth (poorest; poorer; middle; richer and richest) and birth delivered by doctors or nurses. The Fig. 2 shows the variable description in the study.
Fig. 2.
Variables description
Statistical analysis
The present study carried out a comparative analysis of the impact of mode of delivery on child nutritional status and mortality. The bivariate analysis such as cross tabulation, chi-square test were carried out between total number of birth and child nutrition (stunting, wasting, under-weight, anemia and breast-feeding) with different section of birth delivery. Further, the life-table technique was developed for estimated neonatal and infant mortality rates on CMC birth history of the variables by different sections birth delivery. Binary logistic regression models were used to identify the odds of normal and cesarean birth on facility-based delivery. Besides those women who delivered their childbirth by cesarean section or normal section were coded as ‘1’ and ‘0’ if otherwise. For multi- variate analysis, multivariate logistic regression models were constructed separately for each mortality variable. The results of the regression analysis were presented by Odds ratios (OR) with 95% confidence intervals (CI). All statistical analysis was performed by statistical software in STATA ® (version 17.0).
Results
Table 1 shows the total number of births delivered by different sections of the place of delivery along with their background characteristics. It was found that the mothers aged 15–19 years had the highest number of birth delivered by normal delivery for both public and private hospitals, where public hospitals birth delivered in normal section was 88%, and C-section was 12%, while in private hospital birth delivered in same sections were 58% and 42% respectively. On the contrary, the highest percentage of C-section births were delivered in both private (51%) and public hospitals (17%) in mother’s aged above 30 years. Because the mothers aged above 25 years are more capable of giving birth through C-section delivery as compared to the immature mother of age group below 19 years. Those mothers, who were given birth more than 24 months of preceding birth interval, had a higher percentage of birth delivered in C-section as compared to those who have given birth in less than 24 months of birth interval. Another interesting finding is that as birth orders increase, the rate of C-section deliveries declines while the rate of vaginal deliveries rises. This trend may be attributed to families, particularly those from Muslim backgrounds who desire larger families, showing a preference for vaginal births over C-sections. The C-section births delivered were higher in urban residents as compared to rural residents (50.3% vs. 46%) because of the availability of medical facilities and accessibility in transport facilities. Household wealth plays a dominant role for choice of birth delivery method. Families from the wealthiest backgrounds prefer C-sections more than those from the poorest backgrounds (43.85% vs. 24.34%). C-sections were most commonly performed by doctors in both private and public hospitals, with rates of 42.35% and 16.32%, respectively, compared to births delivered by nurses.
Table 1.
Total number of births (%) delivered by different sections in place of delivery along with demographic and socio-economic background characteristics in India, 2019-21
| Background characteristic’s | Private Hospital | Public Hospital | ||
|---|---|---|---|---|
| C-section birth N (%) |
Normal birth | C-section birth N (%) | Normal birth | |
| N (%) | N (%) | |||
| Mother’s age at birth | ||||
| 15–19 | 367 (41.85) | 510 (58.15) | 450 (11.58) | 3,435 (88.42) |
| 20–24 | 5,802 (44.04) | 7,371 (55.96) | 5,762 (12.61) | 39,927 (87.39) |
| 25–29 | 9,707 (47.29) | 10,819 (52.71) | 8,696 (14.52) | 51,198 (85.48) |
| 30+ | 8,113 (50.96) | 7,806 (49.04) | 6,887 (16.87) | 33, 944 (83.13) |
| Preceding birth interval | ||||
| < 24 months | 2,435 (39.55) | 3,722 (60.45) | 2,301 (9.65) | 21,554 (90.35) |
| > 24 months | 9,040 (45.25) | 10,937 (54.75) | 8,794 (13.00) | 58,853 (87.00) |
| Birth order | ||||
| 1 | 12,232 (50.99) | 11,755 (49.01) | 10,555 (18.06) | 47,884 (81.94) |
| 2 | 8,693 (50.08) | 8,665 (49.92) | 8,135 (16.20) | 42,076 (83.80) |
| 3+ | 3,064 (33.49) | 6,086 (66.51) | 3,105 (7.46) | 38,544 (92.54) |
| Place of residences | ||||
| Rural | 15,247 (46.05) | 17,861 (53.95) | 15,690 (12.69) | 107,919 (87.31) |
| Urban | 8,742 (50.28) | 8,645 (49.72) | 6,105 (22.87) | 20,5858 (77.13) |
| Household wealth | ||||
| Poorest | 1,508 (35.75) | 2,710 (64.25) | 2,661 (6.36) | 39,164 (93.64) |
| Poorer | 3,023 (41.50) | 4,261 (58.50) | 4,593 (11.93) | 34,413 (88.22) |
| Middle | 4,718 (47.93) | 5,125 (52.07) | 5,616 (17.88) | 25,876 (82.12) |
| Richer | 6,548 (50.07) | 6,529 (49.93) | 5,317 (22.07) | 18,774 (77.93) |
| Richest | 8,192 (50.97) | 7,881 (49.03) | 3,608 (25.82) | 10,367 (74.18) |
| Delivered by | ||||
| Doctor | 20,880 (50.61) | 20,380 (49.39) | 18,161 (19.86) | 73,269 (80.14) |
| Nurse | 14,087 (45.67) | 16,757 (54.33) | 13,472 (12.12) | 97,686 (87.88) |
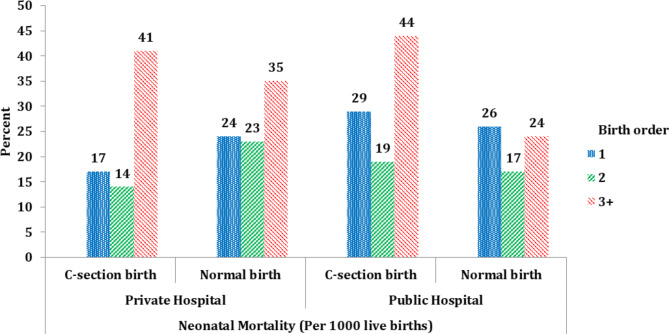
Table 2 presents the neonatal and infant mortality rates across different birth delivery methods in public and private hospitals, along with their bio-demographic and socio-economic characteristics. The results show that neonatal and infant mortality rates varied across the socio-economic and bio-demographic characteristics and the place of delivery. The overall result indicates that neonatal and infant mortality rates both were lower for C-section deliveries compared to normal deliveries at both private and public hospitals. Those mothers who had given birth in the age group 15–19 years had higher infant and neonatal mortality rates as compared to those who had given birth in the age group of 25–29 years. The higher number of birth order has increased the neonatal and infant mortality rate in C-section birth as compared to normal section birth at both places of delivery (Fig. 3). This may be explained by better utilization of full antenatal, safe delivery, and postnatal care among women with their first childbirth compared to those with previous childbirth experiences. The infant mortality rate in rural residences was higher as compared to the urban residence. The accessibility and affordability of the health care services also are the major factors that create the urban-rural divide in health care utilization. The lower use of health care services in the rural areas may be due to a number of obstacles such as the cost of care, low awareness of health-promoting behaviour and the transportation cost that contribute to higher rates infant and neo-natal mortality. The lack of motivation among health providers and poor communication between healthcare providers and patients are also among the important hurdles in the utilization of maternal health care services in the rural areas of India. Families from the wealthiest backgrounds had lower neonatal and infant mortality rates compared to those from the poorest backgrounds.
Table 2.
Neonatal and infant mortality rate (per thousand live births) by different sections in place of delivery of demographic and socio-economic background characteristics in India, 2019-21
| Neonatal Mortality (Per 1000 live births) | Infant Mortality (Per 1000 live births) | |||||||
|---|---|---|---|---|---|---|---|---|
| Background Characteristics | Private Hospital | Public Hospital | Private Hospital | Public Hospital | ||||
| C-section birth | Normal birth | C-section birth | Normal birth | C-section birth | Normal birth | C-section birth | Normal birth | |
| Mother’s age at birth | ||||||||
| 15–19 | 42 | 66 | 36 | 33 | 64 | 75 | 47 | 50 |
| 20–24 | 22 | 30 | 35 | 24 | 31 | 43 | 43 | 35 |
| 25–29 | 17 | 24 | 21 | 20 | 24 | 33 | 30 | 30 |
| 30+ | 18 | 22 | 27 | 22 | 25 | 28 | 34 | 32 |
| Preceding birth interval | ||||||||
| < 24 months | 27 | 41 | 32 | 29 | 40 | 59 | 45 | 44 |
| > 24 months | 18 | 21 | 21 | 16 | 25 | 29 | 30 | 25 |
| Birth order | ||||||||
| 1 | 17 | 24 | 29 | 26 | 25 | 31 | 37 | 35 |
| 2 | 14 | 23 | 19 | 17 | 22 | 29 | 26 | 26 |
| 3+ | 41 | 35 | 44 | 24 | 51 | 52 | 58 | 37 |
| Place of residence | ||||||||
| Rural | 23 | 33 | 31 | 24 | 32 | 41 | 40 | 35 |
| Urban | 14 | 16 | 19 | 17 | 21 | 26 | 29 | 25 |
| Household wealth | ||||||||
| Poorest | 47 | 56 | 49 | 27 | 58 | 71 | 60 | 40 |
| Poorer | 35 | 46 | 34 | 25 | 44 | 59 | 41 | 36 |
| Middle | 20 | 30 | 26 | 20 | 33 | 38 | 36 | 30 |
| Richer | 17 | 20 | 22 | 18 | 27 | 31 | 32 | 25 |
| Richest | 9 | 11 | 13 | 13 | 13 | 15 | 18 | 20 |
| Delivered by | ||||||||
| Doctor | 17 | 24 | 24 | 20 | 26 | 33 | 32 | 30 |
| Nurse | 18 | 27 | 27 | 22 | 25 | 36 | 36 | 32 |
Fig. 3.
Neonatal morality rate by mother birth order at place of delivery
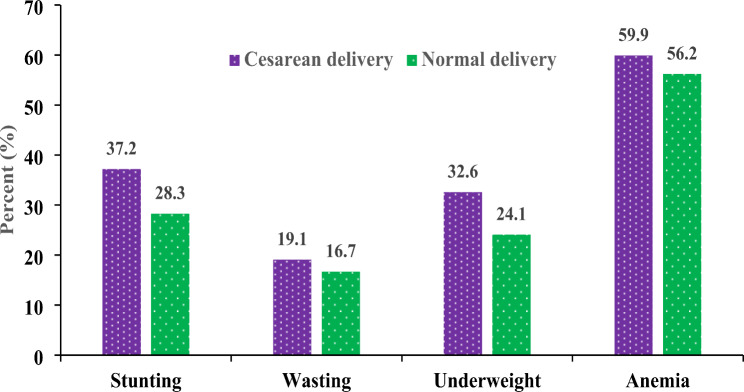
Table 3 presents overall child growth outcomes based on birth delivery methods. It was found that child undernutrition like stunting, wasting, underweight, and anemia were lower for birth delivered in normal section as compared to C-section. For example, the percentage of stunting at C-section birth delivery was 37.2% and normal delivery 28.3% (Fig. 4). Similarly the percentage of wasting and underweight at C-section and normal delivery were 19.1%, 16.7%, 32.6%, and 24.1% respectively. Furthermore 17% of normal birth delivery children have continued their breast-feeding more than 16 week. Again the initiation of breast-feeding after the birth within the first 24 h was higher in normal section delivery than the C-section delivery (48.6% vs. 30.4%) respectively.
Table 3.
Child growth indicators according to the mode of delivery
| Mode of Delivery | Child Growth | Percentage | Std. Deviation | 95% CI for mean | Test Statistics |
|---|---|---|---|---|---|
| C-section Delivery | Stunting | 37.2 | 0.5 | (0.40 - 0.40) | χ2 = 2.0, p = 0.000 |
| Normal Delivery | 28.3 | 0.4 | (0.26–0.27) | ||
| C-section delivery | Wasting | 19.1 | 0.4 | (0.20 − 0.21) | χ2 = 121.1, p = 0.000 |
| Normal delivery | 16.7 | 0.4 | (0.16 - 0.17) | ||
| C-section delivery | Underweight | 32.6 | 0.5 | (0.36 - 0.36) | χ2 = 2.1, p = 0.000 |
| Normal delivery | 24.1 | 0.4 | (0.22 - 0.23) | ||
| C-section delivery | Anemia | 59.9 | 0.5 | (0.52–0.58) | χ2 = 201.8, p = 0.000 |
| Normal delivery | 56.2 | 0.5 | (0.49–0.51) | ||
| C-section delivery | Duration of breast-feeding | 16.3 | 0.3 | (0.16–0.18) | χ2 = 6.40, p = 0.011 |
| Normal delivery | 16.9 | 0.3 | (0.14–0.18) | ||
| C-section delivery | Put the | 30.4 | 0.7 | (0.41–0.43) | χ2 = 162.0, p = 0.000 |
| Normal delivery | breast-feeding | 48.6 | 0.6 | (0.49–0.51) |
Fig. 4.
Impact of Child Growth on Mode of Delivery
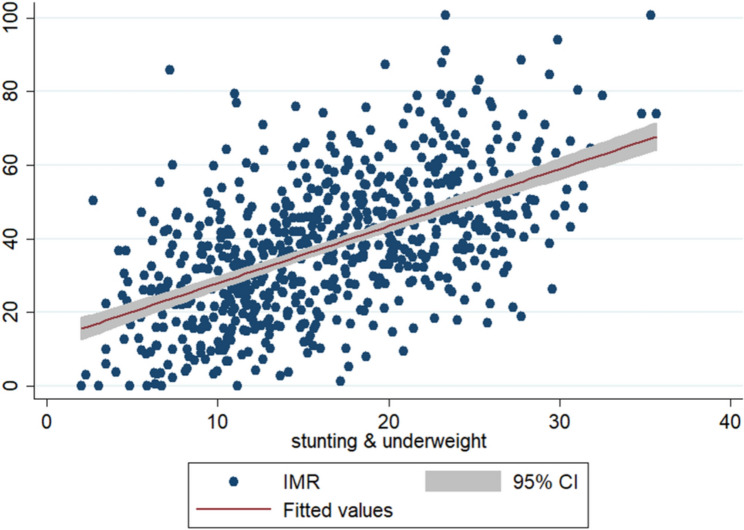
Table 4 presents the odds ratio for neonatal and infant mortality by C-section and normal birth at different places of delivery along with their background characteristics. The results suggested that the chances of a baby dying respectively in the neonatal and infant periods in case of C-section births were 0.60 times and 0.51 times lower than the normal birth with place of delivery at home. The differences between private and public hospitals in risk of neonatal and infant mortality due to C-section birth was negligible, where in normal birth, risk of a baby dying in neonatal period was 0.97 times lower in a public hospital as compared to private hospitals. Mothers aged 25–29 years had a lower risk of neonatal mortality with normal births compared to C-section births. Additionally, mothers with a birth interval of more than 24 months had a reduced risk of neonatal and infant mortality compared to those with intervals of less than 24 months, with a 95% confidence level. Birth orders of more than three were associated with 1.23 times higher risk of neonatal mortality and 1.32 times higher risk of infant mortality for C-section deliveries compared to normal deliveries, with these differences being statistically significant. Coming into the household wealth, babies from the poorest background families were at higher risk of dying in the neonatal period compared to babies belonging to the richest background families, in case of both normal as well as C-section birth deliveries (odds = 0.72 vs. 0.29 and odds = 0.87 vs. 0.49). It is possible that the low utilization of maternal healthcare among poor households results from prioritizing basic daily living needs over health care. Moreover, poor households do not have the resources for healthcare expenses, whereas wealthier households can spend a higher proportion of their earnings on healthcare. The unequal distribution of maternal education and wealth status has tended to widen the rural-urban gap in maternal health care utilization, thus risk of babies dying in neo-natal and infant periods were higher in rural areas than the urban areas for both types of birth deliveries. Considering the assistance during birth delivery, the risk of neonatal mortality was higher for births assisted by nurses compared to those assisted by doctors, with the difference being statistically significant at a 95% confidence interval. The Fig. 5 depicts that the risk of infant death increases with stunting and underweight in cases of caesarean deliveries.
Table 4.
Odds ratios (95% confidence level) for neonatal and infant mortality in different sections of birth by demographic and socioeconomic background characteristics in India, 2019-21
| Background Characteristics | Neonatal Mortality | Infant Mortality | ||
|---|---|---|---|---|
| C-section birth | Normal birth | C-section birth | Normal Birth | |
| Place of Delivery | Odds Ratios (CI) | Odds Ratios (CI) | Odds Ratios (CI) | Odds Ratios (CI) |
| Home® | ||||
| Public hospital | 0.60 (0.30–1.20) | 0.97 (0.86–1.1) | 0.51** (0.27–0.92) | 0.95 (0.86–1.05) |
| Private hospital | 0.64 (0.32–1.29) | 1.79*** (1.53–2.09) | 0.55** (0.30–1.01) | 1.51*** (1.32–1.72) |
| Mother age | ||||
| 15–19® | ||||
| 20–24 | 1.19 (0.29–4.98) | 0.74 (0.46–1.23) | 1.53 (0.36–6.37) | 0.71* (0.47–1.05) |
| 25–29 | 0.88 (0.22–3.67) | 0.71 (0.43–1.16) | 1.26 (0.31–5.25) | 0.66** (0.45–0.98) |
| 30+ | 1.06 (0.26–4.45) | 0.73 (0.45–1.20) | 1.53 (0.37–6.35) | 0.67** (0.45–0.99) |
| Preceding birth interval | ||||
| < 24 months® | ||||
| > 24 months | 0.71*** (0.59–0.87) | 0.57*** (0.52–0.62) | 0.66*** (0.56–0.79) | 0.58*** (0.54–0.62) |
| Birth order | ||||
| 1® | ||||
| 2 | 0.74*** (0.63–0.88) | 0.58*** (0.54–0.62) | 0.84** (0.72–0.96) | 0.61*** (0.58–0.65) |
| 3+ | 2.10*** (1.74–2.53) | 1.33*** (1.22–1.46) | 1.93 *** (1.64–2.27) | 1.37*** (1.27–1.47) |
| Place of residence | ||||
| Urban® | ||||
| Rural | 1.10* (0.87–1.39) | 1.18* (1.03–1.34) | 1.09 (0.90–1.33) | 1.09* (0.98–1.21) |
| Household wealth | ||||
| Poorest ® | ||||
| Poorer | 0.72** (0.56–0.94) | 0.87*** (0.79–0.96) | 0.76** (0.59–0.96) | 0.89** (0.82–0.97) |
| Middle | 0.47*** (0.36–0.62) | 0.71*** (0.62–0.80) | 0.58*** (0.46–0.74) | 0.69*** (0.63–0.77) |
| Richer | 0.46 *** (0.34–0.62) | 0.72*** (0.62–0.83) | 0.52*** (0.41–0.68) | 0.69*** (0.62–0.78) |
| Richest | 0.29*** (0.21–0.42) | 0.49*** (0.40–0.60) | 0.32*** (0.24–0.44) | 0.48*** (0.41–0.57) |
| Birth Delivered by | ||||
| Doctor® | ||||
| Nurse | 0.82** (0.69–0.99) | 0.90** (0.82–1.00) | 0.82*** (0.70–0.96) | 0.88*** (0.81–0.95) |
Confounding variables: Maternal education; BMI; Antenatal care and Post-natal care
® Reference Category; Significance level *** p < 0.01, ** p < 0.05, * p < 0.10.
Fig. 5.
Association between stunting, underweight and IMR by caesarean delivery
Discussion
The impact of C-section birth deliveries on child health and the higher costs associated with C-section compared to normal deliveries in the private sector are significant public health issues. The present study investigated different birth delivery methods and their effects on child growth and mortality rates in India. The findings revealed that the births delivered in different sections varied across various socio-economic and bio-demographic characteristics in the country. The study found that the total number of birth delivered by C –section was lower than normal section delivery. In public hospitals, doctors generally prefer normal deliveries unless complications arise, such as complicated pregnancies, abnormal labor, or postpartum hemorrhage. In contrast, private institutions report a higher incidence of C-sections, as noted in previous studies [49, 50]. Women aged 30 and older had a higher rate of C-sections compared to those aged 15–19. C-sections were more common among urban residents due to better access to and affordability of medical services in urban areas. Urban women also benefit more from public and private maternal and child health services [51]. Additionally, women from wealthier households more frequently chose C-sections compared to those from poorer households, as household wealth and education contribute to greater female autonomy and decision-making power regarding health [52, 53].
Another significant finding is the impact of neonatal and infant mortality across different birth delivery methods. The study found that neonatal and infant mortality rates were higher for C-sections, particularly among younger mothers (under 20) and those with higher birth orders. Systematic reviews indicate that C-sections can improve maternal, neonatal, and infant survival by 9–16%, although survival rates can vary based on socio-economic factors [54]. Studies in Latin America [24] and the United States [55] have shown that C-sections without clear medical indications double the risk of neonatal death compared to vaginal deliveries. Betran et al. (2018) found that untrained medical staff performing C-sections increases the risk of neonatal death, whereas vaginal deliveries generally present a safer option for both mothers and babies [54]. Prior research indicates that the risk of mortality is notably higher for C-section deliveries after the first week of life [56], and frail infants may have even lower survival rates beyond this period [57].
Regarding child nutritional indicators, C-sections were associated with poorer growth outcomes, including higher rates of stunting, wasting, and underweight. C-sections may also adversely affect breastfeeding practices [58]. The study highlighted that stunting, wasting, and underweight were more prevalent among C-section births compared to normal vaginal deliveries. Previous studies have suggested that C-sections can impair child growth and increase risks such as respiratory problems, frequent illnesses, and issues with food demand and sleep [59]. Chen and Tan found that the risk of child undernutrition was 1.16 times higher for C-section births compared to natural deliveries [5]. Additionally, children born by C-section are more prone to respiratory distress, metabolic issues, and immune system disorders [59]. Our findings also show that anemia is more common among children born by C-section than those born vaginally, aligning with previous studies indicating higher risks of asthma, allergic rhinitis, and atopy in C-section-born children [60].
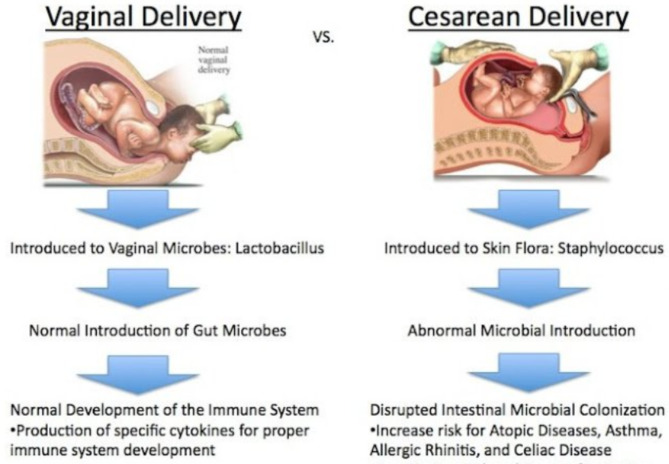
Another key finding is the delayed transfer of breast milk in C-section deliveries compared to vaginal births. According to WHO guidelines, initiating breastfeeding within the first hour is crucial [61]. A compromised immune system in C-section infants makes them more susceptible to infections, affecting growth and development [62]. Evidence indicates that C-section births are negatively associated with timely initiation of breastfeeding, which is significantly linked to child stunting [32, 63–66]. Additionally, infants born via C-section often have gut microbiota issues affecting nutrient absorption, increasing the risk of overweight conditions that persist into early adulthood (Fig. 6) [67]. Similarly, Scott and Binns (2007) found that the onset of lactation was significantly delayed in C-section deliveries compared to normal vaginal deliveries [68]. Thus, C-section deliveries negatively impact the mother-infant relationship compared to natural births [69, 70]. Poor mother-infant relationships can adversely affect child growth, psychological development, and behavioral outcomes [71]. Previous research has reported that normal vaginal deliveries reduce hospital stays, financial costs, and the risk of postpartum complications, while C-sections increase the risk of maternal injury, pain, and gynecological issues, as well as neonatal mortality and malnutrition [72, 73].
Fig. 6.
The process of birth delivery: vaginal vs. caesarean
Conclusion
In conclusion, the study suggests that C-sections deliveries are significantly associated with delayed breastfeeding practices, higher financial burdens and shorter durations of breastfeeding compared to vaginal deliveries. Additionally, C-section delivery may adversely affect child undernutrition as compared to normal vaginal birth. The risk of child undernutrition and mortality is higher in women undergoing emergency C-sections and those with previous C-Sections. Neonatal and infant mortality are also elevated in C-section babies. These findings underscore the importance of a health worker’s visit in providing antenatal and postnatal care. Low coverage of maternal healthcare utilization in rural areas contributes significantly to higher infant and neo-natal mortality associated with C-section deliveries. Therefore, health policies and programs should be aimed at improving reproductive and child health care services, improving the quality of obstetrics services, especially for the C-section. Furthermore, both public and private healthcare institutions should prioritize enhancing the quality of services and promoting vaginal deliveries to reduce child growth issues and mortality rates. The Government of India has launched the National Rural Health Mission (NRHM) which focuses on the states belonging to north, central and northeast regions of the country. In addition, Janani Suraksha Yojna (JSY) under NRHM has been initiated to encourage pregnant women from rural areas to deliver in healthcare institutions. The scheme encourages pregnant women from rural areas to avail themselves of maternal healthcare services (antenatal, natal and postnatal care). However, addressing the unmet needs for maternal healthcare service utilization among specific sub-groups of population (like religion, caste) in rural areas is crucial for further improving the outcomes.
Strength and limitations
The major strength of this study is that it dealt with nationally representative data with large sample size and correlates of normal and C -section birth in public and private institutions. Apart from this, some limitations are there in the large-scale cross-sectional study. First of all the data lack information relating to clinical indications of C-sections such as the study did not cover the distinction between elective and emergency C-sections. Second is that various socioeconomic and bio-demographic factors were included in the study, however, women’s decision-making power also significantly influences the delivery practice, which was not analyzed in the study. The third one is the study did not cover accessibility like the number of primary health care centers, sub-center, and community centers and the quality of health care facilities (number of doctors, number of beds) which might influence health care delivery. Finally, the study did not consider physical barriers such as distance from the health centers, transportation, and road facilities, which can also impact the likelihood of normal birth delivery.
Acknowledgements
This paper uses data from the National Family Health Survey (NFHS). The authors would like to thank International Institute for Population Sciences (IIPS) for providing publicly accessible data.
Author contributions
UD conceptualized and designed the study, performed the data tabulation, and wrote the manuscript. UD and NRR conducted the data analysis, reviewed and edited the manuscript. Both authors have read and approved the final version of the manuscript for publication.
Funding
The authors have not received any specific funds for the publication of this work.
Data availability
Availability of data and material: This research work was performed based on secondary data which is freely available upon request at the IIPS, India website (Source of data: http://rchiips.org/NFHS/index.shtml).
Declarations
Ethics approval and consent to participate
This research work was done with secondary data which is freely available at IIPS, India website (Source of data: https://dhsprogram.com/data/dataset/India_Standard-DHS_2020.cfm?fag=0) The study was approved by Institutional Review Board from the International Institute of Population Sciences, Mumbai, and was conducted in accordance with the Declaration of Helsinki. After informing the aim and goal to the study participants, written informed consent was obtained from each participant. All the participants’ information was kept confidential using a coding system. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Levels and trends in child malnutrition, WHO, Geneva. Switzerland, 2018. http://www.who.int/nutgrowthdb/2018-jmebrochure.pdf
- 2.Black, R. E., Victora, C. G., Walker, S. P., Bhutta, Z. A., Christian, P., De Onis,M., … Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. The lancet. 2013;382(9890):427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed]
- 3.Liu, L., Johnson, H. L., Cousens, S., Perin, J., Scott, S., Lawn, J. E., … Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The lancet. 2012;379(9832):2151–2161. 10.1016/S0140-6736(12)60560-1 [DOI] [PubMed]
- 4.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. 10.1016/S0140-6736(07)60032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Tan D. Cesarean section or natural childbirth? Cesarean birth may damage your health. Front Psychol. 2019;10:351. 10.3389/fpsyg.2019.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2012;5:CD003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanardo, V., Pigozzo, A., Wainer, G., Marchesoni, D., Gasparoni, A., Di Fabio, S.,… Trevisanuto, D. Early lactation failure and formula adoption after elective caesarean delivery: cohort study. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2013;98(1):F37-F41. 10.1136/archdischild-2011-301218 [DOI] [PubMed]
- 8.Pérez-Ríos N, Ramos-Valencia G, Ortiz AP. Cesarean delivery as a barrier for breastfeeding initiation: the Puerto Rican experience. J Hum Lactation. 2008;24(3):293–302. 10.1177/0890334408316078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radha K, Devi GP, Manjula RV, Chandrasekharan PA. Study on rising trends of caesarean section (c-section): a bio-sociological effect. IOSR J Dent Med Sci. 2015;14(8):10–3. [Google Scholar]
- 10.Khanal V, Karkee R, Lee AH, Binns CW. Adverse obstetric symptoms and rural–urban difference in cesarean delivery in Rupandehi district, Western Nepal: a cohort study. Reproductive Health. 2016;13(1):1–6. 10.1186/s12978-016-0128-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh P, Hashmi G, Swain PK. High prevalence of cesarean section births in private sector health facilities-analysis of district level household survey-4 (DLHS-4) of India. BMC Public Health. 2018;18(1):1–10. 10.1186/s12889-018-5533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Appropriate technology for birth. Lancet. 1985;2:436–7. [PubMed] [Google Scholar]
- 13.World Health Organization. Joint interregional conference on appropriate technology for birth, fortaleza, Brazil, 22–26 april 1985: summary report. In Joint Interregional Conference on Appropriate Technology for Birth, Fortaleza, Brazil, 22–26 April 1985: summary report. 1985.
- 14.Dumont A, De Bernis L, Bouvier-olle MH, Bréart G, MOMA Study Group. Caesarean section rate for maternal indication in sub-saharan Africa: a systematic review. Lancet. 2001;358(9290):1328–33. 10.1016/S0140-6736(01)06414-5 [DOI] [PubMed] [Google Scholar]
- 15.Althabe F, Sosa C, Belizán JM, Gibbons L, Jacquerioz F, Bergel E. Cesarean section rates and maternal and neonatal mortality in low-, medium‐, and high‐income countries: an ecological study. Birth. 2006;33(4):270–7. 10.1111/j.1523-536X.2006.00118.x [DOI] [PubMed] [Google Scholar]
- 16.Betrán AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, Wagner M. Rates of caesarean section: analysis of global, regional and national estimates. Paediatr Perinat Epidemiol. 2007;21(2):98–113. 10.1111/j.1365-3016.2007.00786.x [DOI] [PubMed] [Google Scholar]
- 17.Lauer JA, Betrán AP, Merialdi M, Wojdyla D. Determinants of caesarean section rates in developed countries: supply, demand and opportunities for control. World Health Rep. 2010;29:1–22. [Google Scholar]
- 18.Maine D, Wardlaw TM, Ward VM. Guidelines for monitoring the availability and use of obstetric services. United Nations Children’s Fund; 1997.
- 19.World Health Organization. WHO statement on caesarean section rates (no. WHO/RHR/15.02). World Health Organization; 2015.
- 20.Ye J, Betrán AP, Guerrero Vela M, Souza JP, Zhang J. Searching for the optimal rate of medically necessary cesarean delivery. Birth. 2014;41(3):237–44. 10.1111/birt.12104 [DOI] [PubMed] [Google Scholar]
- 21.UNICEF. The state of the world’s children 2009: maternal and newborn health. Unicef. 2008;9.
- 22.Berhan Y, Berhan A. Skilled health personnel attended delivery as a proxy indicator for maternal and perinatal mortality: a systematic review. Ethiop J Health Sci. 2014;24:69–80. 10.4314/ejhs.v24i0.7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314(21):2271–9. 10.1001/jama.2015.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned repeat cesarean section at term and adverse childhood health outcomes: a record-linkage study. PLoS Med. 2016;13(3):e1001973. 10.1371/journal.pmed.1001973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polidano C, Zhu A, Bornstein JC. The relation between cesarean birth and child cognitive development. Sci Rep. 2017;7(1):1–10. 10.1038/s41598-017-10831-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardwell CR, Stene LC, Joner G, Cinek O, Svensson J, Goldacre MJ, Patterson CC. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–35. 10.1007/s00125-008-0941-z [DOI] [PubMed] [Google Scholar]
- 27.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208(4):249–54. 10.1016/j.ajog.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 28.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between caesarean section and childhood asthma. Clin Experimental Allergy. 2008;38(4):629–33. 10.1111/j.1365-2222.2007.02780.x [DOI] [PubMed] [Google Scholar]
- 29.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes. 2013;37(7):893–9. 10.1038/ijo.2012.195 [DOI] [PubMed] [Google Scholar]
- 30.Taras H, Potts-Datema W. Chronic health conditions and student performance at school. J Sch Health. 2005;75(7):255–66. 10.1111/j.1746-1561.2005.tb06686.x [DOI] [PubMed] [Google Scholar]
- 31.Taras H, Potts-Datema W. Obesity and student performance at school. J Sch Health. 2005;75(8):291–5. 10.1111/j.1746-1561.2005.00040.x [DOI] [PubMed] [Google Scholar]
- 32.Rowe-Murray HJ, Fisher JR. Baby friendly hospital practices: cesarean section is a persistent barrier to early initiation of breastfeeding. Birth. 2002;29(2):124–31. 10.1046/j.1523-536X.2002.00172.x [DOI] [PubMed] [Google Scholar]
- 33.Ghana statistical service (GSS), Ghana health service (GHS), and ICF international. 2015. Ghana Demographic and Health Survey 2014, GSS, GHS, and ICF International, Rockville, MD, USA, 2015.
- 34.Service GS. Ghana 2003: results from the demographic and health survey. Stud Fam Plann. 2003;36:158–62. [DOI] [PubMed] [Google Scholar]
- 35.Khadem N, Khadivzadeh T. The intelligence quotient of school aged children delivered by cesarean section and vaginal delivery. Iran J Nurs Midwifery Res. 2010;15(3):135. [PMC free article] [PubMed] [Google Scholar]
- 36.Tian YT. China is not the world’s first cesarean section rate. Beijing: Guangming Daily. 2017.
- 37.Nakamura-Pereira M, do, Carmo Leal M, Esteves-Pereira AP, Domingues RMSM, Torres JA, Dias MAB, Moreira ME. Use of Robson classification to assess cesarean section rate in Brazil: the role of source of payment for childbirth. Reproductive health. 2016;13(3):245–256. 10.1186/s12978-016-0228-7 [DOI] [PMC free article] [PubMed]
- 38.International Institute for Population Sciences (IIPS); ICF. National Family Health Survey (NFHS-4), 2015–2016: India; IIPS: Mumbai, India. 2017; Available online: http://rchiips.org/NFHS/NFHS4Reports/India.pdf (accessed on 12 February 2018).
- 39.Gebremedhin S. Trend and socio-demographic differentials of caesarean section rate in Addis Ababa, Ethiopia: analysis based on Ethiopia demographic and health surveys data. Reproductive Health. 2014;11(1):1–6. 10.1186/1742-4755-11-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang BS, Zhou LF, Coulter D, Liang H, Zhong Y, Guo YN, Gao ES. Effects of caesarean section on maternal health in low risk nulliparous women: a prospective matched cohort study in Shanghai, China. BMC Pregnancy Childbirth. 2010;10(1):1–10. 10.1186/1471-2393-10-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burrows LJ, Meyn LA, Weber AM. Maternal morbidity associated with vaginal versus cesarean delivery. Obstet Gynecol. 2004;103(5):907–12. [DOI] [PubMed] [Google Scholar]
- 42.Farrell SA. Cesarean section versus forceps-assisted vaginal birth: it’s time to include pelvic injury in the risk–benefit equation. CMAJ. 2002;166(3):337–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Huang X, Wang ML, Jing J. The effect of cesarean section on neuropsychiatric development in children. Foreign Med Sci. 2004;15:4–6. 10.3969/j.issn.1673-5293.2004.01.002 [Google Scholar]
- 44.WHO MULTICENTRE GROWTH REFERENCE STUDY GROUP. WHO Child Growth standards: based on length/height-for age, weight-for-age, weight-for-age and body mass index for age-methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 45.Das U, Chaplot B, Azamathulla HM. The role of place of delivery in preventing neonatal and infant mortality rate in India. Geographies. 2021;1(1):47–62. https://www.mdpi.com/2673-7086/1/1/4# [Google Scholar]
- 46.Srivastava S, Chaurasia H, Singh KJK, Chaudhary P. Exploring the spatial patterns of cesarean section delivery in India: evidence from National Family Health Survey-4. Clin Epidemiol Global Health. 2020;8(2):414–22. 10.1016/j.cegh.2019.09.012 [Google Scholar]
- 47.Paixao, E. S., Bottomley, C., Pescarini, J. M., Wong, K. L., Cardim, L. L., Ribeiro Silva, R. D. C., … Campbell, O. M. Associations between cesarean delivery and child mortality: A national record linkage longitudinal study of 17.8 million births in Brazil. PLoS medicine. 2021;18(10):e1003791. 10.1371/journal.pmed.1003791 [DOI] [PMC free article] [PubMed]
- 48.Elnakib S, Abdel-Tawab N, Orbay D, Hassanein N. Medical and non-medical reasons for cesarean section delivery in Egypt: a hospital-based retrospective study. BMC Pregnancy Childbirth. 2019;19(1):1–11. 10.1186/s12884-019-2558-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahlen HG, Tracy S, Tracy M, Bisits A, Brown C, Thornton C. Rates of obstetric intervention among low-risk women giving birth in private and public hospitals in NSW: a population-based descriptive study. BMJ Open. 2012;2:e001723. 10.1136/bmjopen-2012-001723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Einarsdóttir K, Haggar F, Pereira G, et al. Role of public and private funding in the rising caesarean section rate: a cohort study. BMJ Open. 2013;3:e002789. 10.1136/bmjopen-2013-002789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamal SM. Preference for institutional delivery and caesarean sections in Bangladesh. J Health Popul Nutr. 2013;31(1):96. 10.3329/jhpn.v31i1.14754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamal SM. Factors affecting utilization of skilled maternity care services among married adolescents in Bangladesh. Asian Popul Stud. 2009;5(2):153–70. 10.1080/17441730902992075 [Google Scholar]
- 53.Caldwell JC, Celik Y, Hotchkiss DR. Maternal education as a factor in child mortality. World Health Forum. The socio-economic determinants of maternal health care utilization in Turkey. Soc Sci Med. 2000;50:1797–806. [DOI] [PubMed]
- 54.Betran AP, Torloni MR, Zhang J, et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod Health. 2015;12:57. 10.1186/s12978-015-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDorman MF, Declercq E, Menacker F, Malloy MH. Infant and neonatal mortality for primary cesarean and vaginal births to women with no indicated risk, United States, 1998–2001 birth cohorts. Birth. 2006;33(3):175–82. 10.1111/j.1523-536X.2006.00102.x [DOI] [PubMed] [Google Scholar]
- 56.Paixao, E. S., Bottomley, C., Pescarini, J. M., Wong, K. L., Cardim, L. L., Ribeiro Silva, R. D. C., … Campbell, O. M. Associations between cesarean delivery and child mortality: a national record linkage longitudinal study of 17.8 million births in Brazil. PLoS medicine. 2021;18(10):e1003791. 10.1371/journal.pmed.1003791 [DOI] [PMC free article] [PubMed]
- 57.Hernán MA. The hazards of hazard ratios. Epidemiology (Cambridge, Mass.). 2010;21(1)13. 10.1097/EDE.0b013e3181c1ea43 [DOI] [PMC free article] [PubMed]
- 58.Saaka M, Hammond AY. Caesarean section delivery and risk of poor childhood growth. J Nutr Metabolism. 2020;2020. 10.1155/2020/6432754 [DOI] [PMC free article] [PubMed]
- 59.Rahman M, Khan N, Rahman A, Alam M, Khan A. Long-term effects of caesarean delivery on health and behavioural outcomes of the mother and child in Bangladesh. J Health Popul Nutr. 2022;41(1):1–7. 10.1186/s41043-022-00326-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roduit, C., Scholtens, S., de Jongste, J. C., Wijga, A. H., Gerritsen, J., Postma,D. S., … Smit, H. A. Asthma at 8 years of age in children born by caesarean section. Thorax. 2009;64(2):107–113. 10.1136/thx.2008.100875 [DOI] [PubMed]
- 61.WHO, U., USAID, A., &, AED U. Indicators for assessing infant and young child feeding practices. Geneva: World Health Organization; 2008. [Google Scholar]
- 62.Belachew A. Timely initiation of breastfeeding and associated factors among mothers of infants age 0–6 months old in Bahir Dar City, Northwest, Ethiopia, 2017: a community based cross-sectional study. Int Breastfeed J. 2019;14:1–6. 10.1186/s13006-018-0196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tewabe T. Timely initiation of breastfeeding and associated factors among mothers in Motta town, East Gojjam Zone, Amhara regional state, Ethiopia, 2015: a cross-sectional study. BMC Pregnancy Childbirth. 2016;16(1):1–7. 10.1186/s12884-016-1108-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esteves TMB, Daumas RP, Oliveira MICD, Andrade CA D. F. D., Leite IC. Factors associated to breastfeeding in the first hour of life: systematic review. Revista de saude publica. 2014;48:697–708. 10.1590/S0034-8910.2014048005278 [DOI] [PMC free article] [PubMed]
- 65.Prior E, Santhakumaran S, Gale C, Philipps LH, Modi N, Hyde MJ. Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am J Clin Nutr. 2012;95(5):1113–35. 10.3945/ajcn.111.030254 [DOI] [PubMed] [Google Scholar]
- 66.Batiro B, Demissie T, Halala Y, Anjulo AA. Determinants of stunting among children aged 6–59 months at Kindo Didaye Woreda, Wolaita Zone, Southern Ethiopia: unmatched case control study. PLoS ONE. 2017;12(12):e0189106. 10.1371/journal.pone.0189106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tun, H. M., Bridgman, S. L., Chari, R., Field, C. J., Guttman, D. S., Becker, A. B.,… Kozyrskyj, A. L. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA pediatrics. 2018;172(4):368–377 10.1001/jamapediatrics.2017.5535 [DOI] [PMC free article] [PubMed]
- 68.Scott JA, Binns CW, Oddy WH. Predictors of delayed onset of lactation. Matern Child Nutr. 2007;3(3):186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simons CJR, Ritchie SK, Mullett MD. Relationships between parental ratings of infant temperament, risk status and delivery method. J Paedia Health Care. 1992;6:240–5. 10.1016/0891-5245(92)90021-U [DOI] [PubMed] [Google Scholar]
- 70.Hillan E. Caesarean section: psychosocial effects. Nurs Stand. 1991;50:30–3. 10.7748/ns.5.50.30.s35 [DOI] [PubMed] [Google Scholar]
- 71.Di SM. The study on 4–7 years old cesarean section children’s behavioral characteristics. Master’s Thesis. Shanghai: East China Normal University. 2009.
- 72.Sandall, J., Tribe, R. M., Avery, L., Mola, G., Visser, G. H., Homer, C. S., … Temmerman,M. Short-term and long-term effects of caesarean section on the health of women and children. The Lancet. 2018;392(10155):1349–1357. 10.1016/S0140-6736(18)31930-5 [DOI] [PubMed]
- 73.Sufang G, Padmadas SS, Fengmin Z, Brown JJ, Stones RW. Delivery settings and caesarean section rates in China. Bull World Health Organ. 2007;85:755–62. 10.2471/BLT.06.035808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and material: This research work was performed based on secondary data which is freely available upon request at the IIPS, India website (Source of data: http://rchiips.org/NFHS/index.shtml).