Abstract
Background
Cerebral palsy (CP) is a group of dysfunction syndrome. Spastic CP is the most common form of CP. As a specific treatment, aquatic therapy (AT) can improve spasticity, increase range of motion, and increase muscle strength due to its particular properties.
Objectives
This article aims to review the research status of AT in patients with spastic CP.
Methods
We conducted a wide-ranging review of all existing literature on using AT to intervene with spastic CP from 10 databases from the earliest to May 2024. It follows the methodological framework for conducting a scoping review proposed by the Joanna Briggs Institute. The physical, physiological, and social–psychological functions were summarized and analyzed.
Results
18 articles were included and analyzed. The gross motor ability of patients with spastic CP improved significantly after AT, and walking efficiency was improved; muscle strength showed significant improvement, enhancing the ability to perform daily activities and quality of life. Aerobic forms of exercise are a commonly used treatment for AT, and five weekly interventions are the most effective. Notably, functional improvements were correlated with child age, CP type, and gross motor function classification system grade.
Conclusions
AT can improve the gross motor function, cardiopulmonary function, daily living, and social communication ability of patients with spastic CP. This scoping review can be used as a starting point for future research on AT for children with spastic CP to design the most efficient exercise regimen.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02171-1.
Keywords: Aquatic therapy, Hydrotherapy, Cerebral palsy, Scoping review
Introduction
Cerebral palsy (CP) is a group of non-progressive developmental-motor disorders that occur in a developing fetus or infant brain and continue throughout life [1]. The incidence is as high as 3.4% in developing countries [2]. Surveillance of Cerebral Palsy in Europe categorizes CP into spastic, ataxic, dyskinetic, and mixed CP [3]. Spastic CP can be further classified as unilateral or bilateral [4]. Children with spastic CP have neuromuscular damage during development due to brain lesions or abnormalities, including spasms, increased muscle stiffness, weakness, and muscle contracture [5–7]. 55% of children and adolescents with CP presented with multiple movement disorders of the same limb, the most common movement disorders being spasticity and dystonia (50%), spasticity only (36%), and dystonia only (6%) [8, 9]. Structural and functional changes occur with age, affecting movement, intelligence, and communication[10], further burdening the family and society.
The International Clinical Practice Guidelines [11] recommend that interventions begin with the child's and family's goals and be enjoyable, stimulating, and challenging activities to stimulate the child's interest. Aquatic therapy (AT) is a treatment that uses the properties of water to allow patients to perform exercise therapy in water to relieve their symptoms or improve function [12]. Currently, AT is among the most common physical activities chosen by children with neuromotor disorder and can be used in all phases of disease progression [13, 14]. The underwater environment provides buoyancy, hydrostatic pressure, and other hydrodynamic characteristics [15]. Buoyancy reduces weight, aids postural support [16], and optimizes postural control. The viscous nature of the water provides resistance equal to the degree of force exerted and facilitates muscle strengthening. Hydrostatic pressure activates sensory and motor cortex areas [17, 18], reducing muscle spasms, improving multisensory stimulants' endurance, and promoting blood circulation. The thermal effect of water can alleviate the degree of spasm and improve the range of joint activity in patients [19]. Motor learning and memory are fundamental parts of neurorehabilitation, and neuroplasticity in cortical motor areas after AT intervention enhances functional performance related to motor learning and memory [20], contributing to neurorehabilitation. Although AT benefits patients with CP [21], efficient AT protocols remain uncertain.
A scoping review aims to summarize the range of available research. Recent systematic reviews have only described specific hydrotherapy methods [22] (Halliwick) and comparisons between AT and land-based exercise [23] and have not summarized and analyzed treatment parameters and outcome indicators. To provide a scope of the existing literature, this scoping review was designed to answer the following two main questions: how is AT currently used in patients with spastic CP, and which functions does AT improve in patients with spastic CP? Therefore, the primary purpose of this study was to map the existing research interventions of AT in patients with spastic CP; more specifically, to describe the treatment parameters of AT interventions and to analyze the outcome metrics of the studies.
Materials and methods
The study followed the methodological framework for conducting a scoping review proposed by the Joanna Briggs Institute [24]. A scoping review maps the existing literature or evidence base [25], which can be used to summarize the status of research and identify research gaps [26]. The present study was based on the PRISMA–ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews) checklist [27]. This article has been registered on the Open Science Framework. The registered DOI number is https://doi.org/10.17605/OSF.IO/9CTSX.
Searching for the evidence
A comprehensive search was conducted in May 2024 by two independent reviewers in ten data sources: The Cochrane Library, Embase, PubMed, Web of Science, Scopus, EBSCO, China National Knowledge Infrastructure (CNKI), WanFang Knowledge Service Platform (WanFang), Chinese Biomedical Literature Service System (CBM), and Chinese Scientific Journals Database (VIP). The search period was from database creation to May 10, 2024. Three sets of keywords were used in the search. The first group used the subject terms of CP and all accessible terms from the Mesh Thesaurus, joined by the Boolean search operator “OR”. The second group searched for interventions, using the Mesh subject headings Hydrotherapy and Aquatic Therapy and all related free words, concatenated with the Boolean search operator “OR”. The third group retrieved the type of literature, which was limited to randomized controlled trials (RCTs). There was no time limit for the search. The specific search strategy is shown in Supplementary Table S1.
Selecting the evidence
The selection of results was carried out independently by at least two authors using Endnote 21 software. The languages familiar to both authors were Chinese and English. Inclusion criteria were developed according to the population, intervention, comparison, Outcome, and Study Design (PICOS) principles (18). Eligible studies must meet the following criteria: (1) population: children with spasmodic CP with stable vital signs, under 18 years of age, and with the consent of patients and their families; (2) intervention: any form of AT; (3) comparison: the control group received other interventions except AT; (4) outcome: physical function, physiological function (muscle strength, muscle tension, and cardiopulmonary endurance), and social psychological function; and (5) research design: non-conference papers, dissertations, reviews, and meta-analyses. The first step in study selection consisted of analyzing titles and abstracts; this step included articles written in English and Chinese describing active AT in persons with spastic CP. The following exclusion criteria were applied: (1) repeat article; (2) animal studies; (3) articles describing treatments other than AT and/or with study groups other than persons with spastic CP; and (4) conference papers, dissertations, reviews, or meta-analysis.
The second step was to analyze the full texts of the potentially relevant papers. Exclude the following: (1) any articles without intervention; (2) articles with incomplete AT information; (3) any article with incomplete outcome data; (4) any articles that were not original, lacked full text, or were not in English or Chinese; and (5) articles not available in full text. Each paper was included based on the assessment of two independent reviewers. In the case of disagreement, consensus was reached by consulting a third reviewer.
Extracting the evidence
The following descriptive data were obtained from the included articles: first author's name, purpose of research, sample size, age of subjects and type of CP, parameters of the AT intervention treatment, measurement tools and results of outcome indicators, water temperature, and study design. Supplemental Materials Table 2 shows the quality assessment of the included studies.
Results
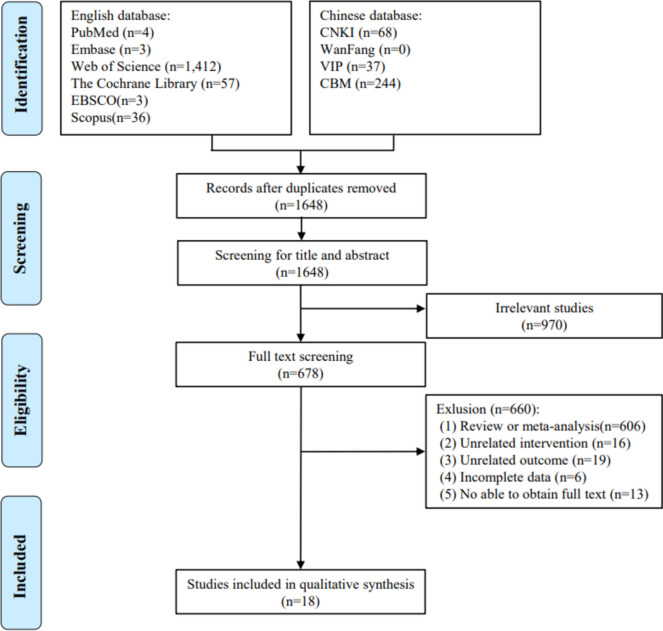
We rigorously searched the ten abovementioned databases according to the inclusion and exclusion criteria and initially obtained 1864 publications. The flowchart of the study screening is shown in Fig. 1. After eliminating duplicates using EndNote 21 software, 1648 articles remained. By reading the titles and abstracts of the articles, articles that did not meet the inclusion criteria were excluded, leaving 678 articles. By reading the complete text, we further excluded 660 articles, including reviews, meta-analyses (n = 606), irrelevant interventions (n = 16), irrelevant endpoints (n = 19), unavailable full text (n = 13), and incomplete data (n = 6). 18 articles ultimately met our study requirements. Of the 18 studies, 12 (67%) were published after 2015. Details on patient attendance and adverse events for each study are shown in Supplemental Materials Table 3.
Fig. 1.
Flow diagram of identified publications
In terms of research types, 12 were clinical RCTs, 2 [28, 29] were case studies, 1 [30] was a research paper, 1 [31] was research reports, 1 [19] was a prospective study, and the other [32] was a single-masked randomized age-stratified crossover study. The results of the analyzed studies were classified into three categories (Supplementary Material Table 4): Functional performance mainly included physical mobility, balance, and walking; physiological domains mainly included cardiorespiratory capacity, body composition, and muscular strength; and psychosocial domains included quality of life, mood, and anxiety. Regarding the types of CP studied, 17 studies dealt only with spastic CP, whereas in one study [33], manual and mixed CP were also involved. Among the 18 studies, 464 children with spastic CP participated in AT, including 47% (218) males and 39% (179) females. Four studies did not report the gender of the participants. Although the inclusion criteria were spastic CP, they were explicitly divided into various studies. There were 1 case of spastic monoplegia, 80 cases of hemiplegia, 165 cases of diplegia, 1 case of triplegia, and 43 cases of quadriplegia. It can be seen that patients with spastic diplegia are more involved in AT. In addition, 5 studies did not report the classification of specific spastic CP. Seventeen studies graded patients participating in the AT site on the Gross Motor Function Classification System (GMFCS).
The average duration of AT was 12 weeks (M = 11.75, SD = 3.09), and two studies did not clarify the duration of AT. These interventions ranged from 6 to 20 weeks, and a single AT treatment duration ranged from 20 to 60 min. Most of the studies used aerobic exercise in the form of warm-up exercise, AT aerobic exercise, and relaxation exercise and combined with other conventional rehabilitation treatment methods. Only two studies [32, 34] have reported the implementation of specific AT methods (Halliwick, Watsu) in the intervention program for children with spastic CP, and two studies have intervention measures for AT combined with sensory integration training [33] and Bobath therapy [35]. Twelve of the 18 studies described the water temperature, ranging from 27.7 ℃ to 40 ℃. Most of the studies were higher than 30 ℃, and only one reached 40 ℃. Three studies indicated that the heart rate index was used to measure the intensity of AT. Two studies used the heart rate reserve or Karvonen formula [36] to calculate the target heart rate. Another study used the percentage of heart rate reserve, and the maximum heart rate was calculated using 220-age. In most studies (n = 11), the frequency of AT was 2–3 times/week. In 4 studies, the frequency of AT was 5 times/week, and only 3 had a frequency of up to once a day. The results of the analysis are most common in the following three categories: functional performance, physiological field, and psychological field. The following will summarize and analyze the three types of research results. A detailed summary is shown in Table 1.
Table 1.
Study characteristics
| Reference | Study aim | Sample | Intervention programme | Exercise parameters | Water-temperature | Measuring instruments and outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Fragala-Pinkham et al. [28] | To assess the improvement in participants' function after the AT |
n = 2 Case 2: CP-spastic diplegia 7 years old Case 3: CP-right hemiplegia,10 years old |
Case 2: Pool sessions + land sessions Case 3: Pool sessions + land sessions |
2/wk for 60 min for 6 weeks (Total of 8 pool sessions and four land sessions) | NR |
Pre/post-intervention (mean score) Case2: -COPM: Performance ↑6.4, Satisfaction ↑5 -GMFM-66: ↑7.53 -OGS: ↑; −3-min fast walk: Distance (m)↑53.06 -Passive ROM: Ankle DF: L↑11° R↑5° Case3: -COPM: Performance ↑5.25, Satisfaction ↑6.75; -PEDI: ↑56 −3 min fast walk: Distance (m) ↑330 -MMT: ↑ -Passive ROM: Right ankle DF↑15°, Ankle eversion↑5° |
Case study |
| Retarekar et al. [29] | To evaluate the effects of AT for a child with CP |
CP-spastic diplegia n = 1 5 years old GMFCS III |
Warm-Up (5 min) Aerobic Intervention (30–40 min) Cool-Down (5 min) |
3/wk for 12 weeks (at least 1 day of rest between sessions) | 30–32.2 ℃ |
Pre/post-intervention (mean score) -COPM: performance 1.8, satisfaction 2.1 -GMFM-66: ↑2.71 −6MWT: walk distance↑27.1%, walking speed(m/min) ↑9 -Modified EEI: ↓0.92 |
Case study |
| Ballaz et al. [30] | To evaluate the effect of a group aquatic training program on gait efficiency |
spastic CP n = 20 14–21 years old |
Warm-up (10 min) Relay race (15 min) Relaxation (5 min) Aquatic activity (15 min) |
2/wk for 45 min for 10 weeks | 31–32 ℃ |
Pre/post-intervention (mean score) -Modified EEI: ↓0.24, Walking HR: ↓13, Resting HR: ↓4 -Opposite Foot Off (%) ↑3, Foot Off (%) ↑3, Step Length (m)↑0.02, Cadence (step/min) ↓7, Walking Speed (m/s) ↓0.01, Flexion knee strength (Nm/KG) ↑0.007, Extension knee strength (Nm/KG) ↑0.007 -GMFM D↑5, GMFM E↑5 |
Research paper |
| Fragala-Pinkham et al. [31] | To evaluate the effectiveness of the aquatic exercise program |
CP n = 8 6–15 years old GMFCS I (3) GMFCS III (5) |
Warm-up (2–5 min) Aerobic exercise (40–45 min) Strength training (5–10 min) Cool down and stretch (5–10 min) |
2/wk for 60 min for 14 weeks | 31–34 °C |
Pre/post-intervention (mean score) Primary outcome measures -GMFM D & E (% score) ↑8.8 −6 MWT (meters)↑63.7 Secondary outcome measures -SRT I and III (Levels)↑2.31 |
Research report |
| Lai et al. [19] | To investigate the effects of pediatric AT |
spastic CP n = 24 4–12 years old PAT (n = 11); CG (n = 13) |
PAT: PT + AT Warm-up (5–10 min) Pool exercises (40 min): Halliwick Cool down exercises (5–10 min) CG: PT |
CG: PT + OT PAT: PT + OT + AT (2/wk for 60 min for 12 weeks) |
33–36 °C |
Pre/post-intervention (mean score) Primary outcome measures -MAS: Ankle: CG↓0.1 PAT no change, Knee: no change Wrist: CG↓0.4 PAT no change, Elbow: no change -GMFM-66: CG↑0.7 PAT↑5 |
Prospective study |
| Adar et al. [38] | To compare the effects of aquatic exercises and land-based exercises |
spastic CP n = 32 4–17 years old Group 1 (n = 17); Group 2 (n = 15) |
Group 1: warm-up (10 min) + aquatic exercise (50 min) + cool-down (5 min); Group 2: active ROM exercises and stretching exercises (10 min) + aerobic exercise (30 min) + sitting, standing, and gait training (20 min) |
5/wk for 60 min for 6 weeks | 33 °C |
Pre/post-intervention (mean score) -MAS: G1↓ G2↓ -TUG: G1↓1.7 G2: ↓2 -GMFM: G1↑2.1 G2: ↓2 -WeeFIM: G1↑5 G2: ↑7 -Gastrocnemius thickness (mm): G1↑0.01 G2: ↑0.01 -Fascicle length (mm): G1↓1.7 G2: ↓0.8 -Pennation angle (º): G1↓0.7 G2: ↑1.4 -Compressibility ratio: G1↑0.3 G2: ↑0.1 -Child Self Report—PedsQL: G1↑61.3 G2: ↓65.2 -Parent Report—PedsQL: G1↑71.8 G2: ↑9.9 |
RCT |
| E. Tufekcioglu [32] | To compare and examine the effect of aquatic interventions, Watsu vs Immersion |
spastic CP (n = 23) 7.52 ± 2.78 years old WI (n = 12); IW (n = 11) |
Period 1: WI (Watsu therapy), IW (Immersion therapy)10 weeks; Period 2: WI (Immersion therapy), IW (Watsu therapy) 10 weeks |
consisted of a 6-week washout interval during which no treatment; 2/wk for 30 min for 20 weeks | 34 °C |
Period 1 -HRV Time: Mean R-R (ms): WI↑53.84 IW↑39, RMSSD (ms): WI↑12.17 IW↑5.09, pNN50↑8.17 IW13.64(post-intervention) -HF (nu): WI↑6.28 IW↑4.16, LF (nu): WI↑6.95 IW↑13.51 -ROM: Upper: WI↑2.81 IW↑2.02, Lowe: WI↑4.03 IW↑2.35 Period 2 -HRV Time: Mean R-R (ms): WI↑29.25 IW↑39.09, RMSSD (ms): WI↑9.25 IW↑9.91, pNN50↑5.25 IW↑9.64 -HF (nu): WI↑3.95 IW↑7.15; LF (nu): WI↑5.92 IW↑6.07 -ROM: Upper: WI↑0.41 IW↑5.1; Lowe: WI↑1.67 IW↑5.68 |
Single-blinded randomized and age-stratified crossover study |
| Abdelaal and Atia [39] | To evaluate the effectiveness of aquatic aerobic training |
spastic CP AqETG (n = 13) CG (n = 15) |
AqETG: warm-up (10 min), AqET(40 min), cool-down(10 min); CG: TPT |
3/wk for 60 min for 12 weeks | 27.7 ℃ |
Pre/post-intervention (mean scores) -FEV1(%): AqETG↑14.38 CG↑4.6 -FVC (%): AqETG↑15.23 CG↑4.13 -WMA (%): AqETG↑14.38 CG↑1.4 -WSBM: AqETG↑13.38 CG↑0.14 -WTOT: AqETG↑27.77 CG↑1.47 |
RCT |
| Hamed et al. [34] | To compare the effectiveness of the Halliwick aquatic exercise |
spastic CP (n = 34) HCG (n = 17) CEG (n = 17) |
HCG: warm-up (5 min), Halliwick exercises (20 min), cool down (5 min) CEG: conventional exercises |
3/wk for 45 min for 12 weeks | NR |
Pre/post-intervention (mean scores) the five GMFM dimensions -Sitting: HCG↑0.28 CEG↑0.23 -Crawling and kneeling: HCG↑0.23 CEG↑0.21 -Standing: HCG↑0.26 CEG↑0.21 -Walking, jumping, and running: HCG↑0.07 CEG↑0.04 -Total GMFM: HCG↑0.1 CEG↑0.04 |
RCT |
| Akinola et al. [37] | To investigate the effect of an aquatic exercise training program |
spastic CP (n = 30) 1–12 years old EG (n = 15) CG (n = 15) |
EG: exercise training in water (stretching, Level 1: 2-point kneeling, Level 2: Sitting, Level 3: Standing, Level 4: Walking) CG: land-based exercise |
Stretching (5 min) each level (15 min) 2/wk for 65 min for 10 weeks |
28 °C and 32 °C |
Pre/post-intervention (GMFM-88 mean scores) EG: -Lying and rolling↑1.03 -Sitting↑1.03 -Crawling and kneeling↑0.8 -Standing↑0.54 -Walking, running, and jumping↑0.27 -Overall gross motor function↑1.43 |
RCT |
| Yufei Ni et al. [41] | To study the effect of hydrotherapy in early rehabilitation |
CP—spastic diplegia (n = 60) < 1.5 years old |
EG: hydrotherapy + PT, OT, ST, ET, acupuncture, and massage CG: PT (Bobath therapy), OT, ST, ET, acupuncture, and massage |
5/wk for 12 weeks | NR |
Pre/post-intervention (mean scores): -BI: CG↑35.79 EG↑44.97 -FMAS: upper limb CG↑28.14 EG↑20.28, lower limbs: CG↑16.83 EG↑10.95 |
RCT |
| Fanxu Song et al. [42] | To observe the effect of hydrotherapy on gross motor function and lower limb muscle strength |
CP—spastic diplegia (n = 60) OG (n = 30, 2.21 ± 1.22 years old); CG (n = 30, 2.57 ± 3.46 years old) |
OG: PT, OT, ST, acupuncture, and massage + Hydrotherapy CG: PT, OT, ST, acupuncture, and massage |
7/wk for 12 weeks | 34–36 ℃ |
Pre/post-intervention (mean scores): -GMFM-88: OG↑11.54 CG↑7.09 -B ultrasonic test results: Quadriceps thickness score (mm) OG↑4.02 CG↑2.46 -MAS score of the quadriceps: OG↓1.45 CG↓0.89 |
RCT |
| Wenwen Luo. [43] | To explore the effect of functional hydrotherapy on children with spastic CP |
spastic CP OG (n = 43; 22.4 ± 8.7 months); CG (n = 43; 22.9 ± 8.5 months) |
OG: Bobath exercise therapy + Functional Hydrotherapy (Adaptive training, Cardiopulmonary conditioning training, Limb motor training) CG: Bobath exercise therapy |
Bobath therapy: 7/wk for 60 min for 12 weeks Functional Hydrotherapy: 5/wk for 60 min for 12 weeks |
NR |
Pre/post-intervention (mean scores): -Biceps surface myoelectric parameter ratio: Iemg (mV·s): OG↑8.6 CG↑4.3, MF(Hz): OG↑12.7 CG↑6.9, CR (%): OG↓6.1 CG↓3.8 -Surface myoelectricity of gastrocnemius muscle: Iemg (mV·s): OG↑11.4 CG↑4.5, MF(Hz): OG↑10.3 CG↑3.4, CR (%): OG↓17.2 CG↓10.8 -GMFM: OG↑20.9 CG↑12.6 -FMFM: OG↑17.1 CG↑13.7 |
RCT |
| Rui Zhang. [33] | To explore the effect of AT combined with sensory integration training |
CP (n = 156) 3–6 years old OG (n = 78) CG (n = 78) |
OG: Sensory integration training + Hydrotherapy CG: Sensory integration training |
Once a day, 6/wk for 12 weeks | NR |
Pre/post-intervention (mean scores): -MAS (adductor): OG↓1.08 CG↓0.69 -BBS: OG↑12.13 CG↑8.3 -GMFM-88: OG↑12.23 CG↑7.68 -WeeFIM: OG↑14.69 CG↑9.73 -MCAVs: OG↑12.66 CG↑7.15 -MCA Vm: OG↑10.53 CG↑6.34 |
RCT |
| Yonghong Zhao et al. [40] | To explore the effects of surfing hydrotherapy combined with aerobic training |
CG (n = 30, 2–6 years old) HG (n = 30, 2–6 years old) |
HG: Routine rehabilitation training + Aquatic aerobics training (5 min warm-up, 40 min aerobic training, 10 min stretch, 5 min slow stretching) CG: Routine rehabilitation training |
2/wk discontinuously for 60 min for 12 weeks | 30–34 °C |
Pre/post-intervention (mean scores): -GMFM: CG↑7.01 HG↑16.19 −6MWT(m): CG↑60.46 HG↑92.14 −10 m return runs(times): CG↑1.9 HG↑3.07 -Brockport improves functional strength: ↑ -BBS (mean scores): CG↑13.47 HG↑13.34 -Muscular tone (°): adductor horn CG↑14.16 HG↑24.67, angle of rouge CG↑8.17 HG↑11.5, dorsiflexion angle of foot CG↓12.83 HG↓11.83 |
RCT |
| Yuting Zou et al. [35] | To investigate the effect of hydrotherapy combined with the Bobath |
spastic CP (n = 80) CG (n = 40) EG (n = 40) |
EG: comprehensive rehabilitation training + hydrotherapy CG: comprehensive rehabilitation training (Bobath therapy, acupuncture, massage, etc.) |
Comprehensive rehabilitation (40 min), Hydrotherapy (15 min); 5–6/wk for 12 weeks | 37–40 ℃ |
Pre/post-intervention (mean scores) -MAS: CG↓0.89 EG↓1.65 -GMFM: CG↑3.83 EG↑8.63 |
RCT |
| Qing Zhu et al. [44] | To explore the effect of hydrotherapy intervention on lower limb motor skills |
spastic CP (n = 110) 2–8 years old CG (n = 55) EG (n = 55) |
EG: conventional treatment + hydrotherapy CG: conventional treatment |
1/d for 20 min for 90 days | NR |
Pre/post-intervention (mean scores): -GMFM: (1) Gross motor score: CG↑6.46 EG↑20.12 (2) Running and jumping score: CG↑2.54 EG↑6.48 (3) Standing score: CG↑4.2 EG↑7.31 -ROM of lower extremity joints: (1) Angle of dorsal flexion: CG↑4.91 EG↑8.97 (2) Angle of popliteal fossa: CG↑3.17 EG↑6.09 (3) Angle of adductor muscle: CG↑3.8 EG↑8.46 |
RCT |
| Fen Gao. [45] | To study the effects of hydrotherapy on lower limbs |
CP-spastic diplegia (n = 78) CG (n = 39) EG (n = 33) |
EG: rehabilitation training therapy + hydrotherapy CG: rehabilitation training |
Rehabilitation training(30 min) hydrotherapy(30 min): 1/d | 35 ℃ |
Pre/post-intervention (mean scores): -GMFM-88: CG↑5.11 EG↑11.8 -Gait parameters: Step size, Step width, Average speed, Step Frequency↑ -ROM: Knee, Ankle, Hip ROM (°): ↑ -Quadriceps femoris thickness (mm): CG↑1.75 EG↑3.6 -MAS: CG↓0.82 EG↓1 -SAS, SDS: ↓ -QOL: CG↑6.84 EG↑9 |
RCT |
↑, increase of the parameter; ↓, decrease of the parameter
PT: physical Therapy; NR: not reported; COPM: Canadian Occupational Performance Measure; PEDI: Pediatric Evaluation of Disability Inventory; GMFM-66: Gross Motor Function Measure-66; OGS: Observational Gait Scale; EEI: Energy Expenditure Index; ROM: Range of motion; DF: dorsiflexion; 6WMT: 6-Minute Walk Test; GMFCS: Gross Motor Function Classification System; HR: heart rate; SRT: shuttle run test; CG: control group; PAT: pediatric aquatic therapy group; MAS: Modified Ashworth Scale; TUG: Timed Up and GO Test; WeeFIM: the Wee Functional Independence Measure; RCT: randomized controlled trial; PedsQL: the Pediatric Quality of Life; HRV: heart rate variability; Mean R-R: All intervals between adjacent QRS complexes also defined as interbeat intervals; RMSSD: The square root of the mean of the squares of the successive differences between adjacent R-R; Pnn50: The proportion of NN50 divided by the total number of NNs; AqETG: aquatic aerobic exercise training group; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; WOTA: Water Orientation Test Alyn; WMA: WOTA mental adaptation score; WSBM: WOTA skills balance control movement score; WTOT: WOTA total score; TPT: traditional physiotherapy; HCG: the Halliwick concept group; CEG: conventional exercising group; GMFM-88: Gross Motor Function Measure-88; EG: Experimental Group; CG: Control Group; OT: Occupational Therapy; ST: Speech Therapy; ET: Electrical Therapy; BI: Barthel Index; FMAS: Fugl-Meyer assessment scale; OG: Observation group; MF: median frequency; CR: antagonistic muscle contraction rate; FMFM: Fine Motor Function Measure Scale; BBS: Berg Balance Scale; MCA: middle cerebral artery; Vs: systolic velocity; Vm: mean velocity; HG: hydrotherapy group; SAS: Self-rating anxiety scale; SDS: Self-rating depression scale; QOL: Quality of life Score
Functional performance
Evaluation metrics in the domain of functional performance have typically focused on the Gross Motor Function Measure (GMFM) scale, with studies using the GMFM-66 (n = 9) and GMFM-88 (n = 6). Significant differences in GMFM scores were reported in all 12 studies, which reported significant differences in GMFM scores after AT compared to before the intervention. Akinola et al. [37] reported that important differences in the dimensions of GMFM between the experimental and control groups were observed only after the tenth week of intervention. Another study found no significant difference in GMFM comparison between the two groups [38]. Further studies have found that GMFCS levels correlate highly with GMFM parts D and E [31]. For patients with GMFCS III–IV, group aquatic training significantly improved GMFM Part E scores. However, there was no change in GMFM D scores for these patients.
Other evaluation metrics focused on walking ability and walking energy efficiency. The 6-Minute Walk Test and Timed Up and GO Test are commonly used for walking function. Abdelaal et al. [39] showed that AT based on Halliwick's concept was more effective than regular exercise in improving the ability of children with spastic CP aged 3–5 years to perform multiple activities, such as sitting, standing, walking, running, and jumping. A case study [29] observed that after 12 weeks of AT, there was a significantly increased walking endurance, speed, and distance in the 6 Mins Walk Test. However, these improvements waned during the 32 week follow-up period and eventually returned to baseline levels. Another study [31] examined improved walking endurance maintained over the 1 month follow-up period. Improvement in the Timed Up and GO Test did not show a clear advantage of the AT compared to land-based exercise [38]. Retarekar et al. [29] proposed the Modified Energy Expenditure Index, which considers only two factors: waist-to-hip ratio and walking speed. It was found that the Modified Energy Expenditure Index decreased, and walking efficiency improved after AT. The secondary indicators of other studies mainly focused on the shuttle run test, the Brockport manual test, and the Fugl–Meyer assessment scale. In one study, there was no significant difference between the Brockport Manual of Lateral Steps and Curls before and after the intervention at each timepoint [40].
Physiological domain
Seven studies examined muscle tone and thickness improvements when analyzing muscle characteristics. By Modified Ashworth Scale (MAS) measurement, MAS scores of quadriceps femoris [42, 45], gastrocnemius [38], and adductor femoris [33] in children with spastic CP were improved or did not improve [19]. Three studies evaluated muscle thickness after AT and showed improvements in gastrocnemius compressibility ratio [38] and quadriceps thickness [42, 45] after AT treatment. One study concluded that muscle thickness, pinch angle, and fascicle length of the spastic gastrocnemius obtained by ultrasonographic assessment did not correlate with spastic gastrocnemius MAS scores and that the ultrasonographic compressibility ratio may be more sensitive for identifying minor improvements in spastic gastrocnemius spasticity in patients with CP compared with the classic MAS assessment of spastic gastrocnemius [38]. Therefore, muscle compressibility ratio as a new ultrasonographic parameter could be used to assess muscle elasticity in patients with CP.
Three studies [28, 44, 45] evaluated the range of motion (ROM), and the ROM of children with spastic type was improved after AT intervention. There was only one study whose primary outcome indicator was ventilatory function [39]. After the intervention, there was a significant difference in forced vital capacity and forced expiratory volume in 1 s in the experimental group. The study also found an improvement in swimming skills. The AT program can effectively enhance the activity range, circulation, and lung function of patients with CP [19].
Psychosocial domain
Outcome assessments in the psychosocial domain focused primarily on activities of daily living, quality of life (QOL), and anxiety and depression, such as the Pediatric Quality-of-Life Inventory–CP, the Wee Functional Independence Measure, the Canadian Occupational Performance Measure, and so on. Through the Pediatric Quality-of-Life Inventory–CP assessment, most of the items in the children’s self-report and parent agency report were significantly improved after AT intervention [38], and the other study [19] only evaluated and compared the parent agency report part and found no significant difference. As assessed by the Wee Functional Independence Measure, a study showed that both water and land training improved activities of daily living in children with spastic CP. In another study, the independence of hydrotherapy combined with sensory integration training intervention was improved. Through the Canadian Occupational Performance Measure assessment, Retarekar et al. [29] found that parents believed that their children’s ability to act in family and community environments was significantly improved, and the improvement was maintained 13 weeks after the end of the intervention.
Discussion
The purpose of this scoping review was to summarize and analyze the existing interventions and functional improvements in patients with spastic CP treated with AT. 18 papers were ultimately screened to provide a detailed summary of subject characteristics, study intervention parameters, and measures of outcome indicators. The World Health Organization considers limb motor function as the main rehabilitation goal for children with CP [46]. At present, most of the studies on AT in patients with spastic CP are RCTs (72%), but most of the studies have the problems of short intervention time and small sample size, resulting in low-quality research. Therefore, future studies should design larger-scale and appropriate trials to determine the best AT treatment regimen.
AT has a positive impact on the functional performance, physiological domain, and psychological domain of children with spastic CP. There are differences in the timepoint of improvement in GMFM scores or no improvement, which might be associated with the duration of intervention. AT plays an active role in enhancing the gross motor function of children with CP. The research discovers that especially for children with GMFCS level II and spastic diplegia, this could be because patients with mild functional impairments have more chances to engage in aquatic sports, thereby achieving better improvement in motor function [19]. The overall responsiveness of the GMFM-66 was better than that of the GMFM-88 in terms of correlation with therapist judgment, with scores > 1.6 indicating clinically meaningful improvement [47]. Ballington et al. [16] discovered that the Halliwick therapy resulted in a clinically meaningful improvement of 4.25 points in mean GMFM-66 scores for gross motor function in children with CP, consistent with the conclusions reached in this scoping review. One aspect of the functional improvement may be due to the Halliwick therapy’s focus on trunk rotation and core stability [48], which is more conducive to improvements in gross motor function. On the other hand, it may be due to the thermal effect of water, hydrostatic pressure, and viscosity. Warm water raises the body's core temperature, which relaxes muscles and relieves spasms. Hydrostatic pressure redistributes body fluids, increasing central blood volume, which reduces peripheral vascular resistance and promotes systemic circulation. Viscosity leads to resistance, providing resistance equal to the child's strength, facilitating improved muscle strength, and balancing body posture.
The improvement of walking ability is manifested in endurance, speed, distance, and efficiency. Thabet et al. [49] found that children with spastic hemiplegia showed improvements in gait parameters such as average walking speed, stride length (on the healthy and the affected side), and stride duration after both water exercise and treadmill training. Another study also proved improvements in walking speed and metabolic cost in children with CP after AT [50]. Children with spastic CP showed a significant reduction in walking speed, gait cycle time, and percentage of standing phase while walking in water but no change in gait rhythm [51]. This may explain the improvement in walking ability after AT. Buoyancy provides weight support, increases dynamic balance, and permits longer single-leg support. The increased ability to single-support the phase increases stride duration and enhances stability in children with CP. Improvements in walking efficiency are due to enhanced cardiorespiratory fitness after AT. Study has shown that water immersion also induces physiological responses in the heart and respiratory system, including increased cardiac output and improved cardiorespiratory function [15]. Possible benefits of adaptive AT include improved cardiorespiratory endurance, strength, coordination, and swimming skills [52]. This scoping review observed that AT intervention significantly improved cardiopulmonary ventilation in patients with spastic CP. Improvements in lung function were due to improved respiratory muscle strength and increased thoracic ROM after AT [53]. It is worth noting that improvements in ROM have been found in studies combining land-based and aquatic exercise. Therefore, follow-up studies are necessary to compare the effects of land-based and aquatic exercises further and determine which method is responsible for the improvements in ROM.
This study found improvements in activities of daily living and QOL in children with spasmodic CP during both water and land exercise. GMFCS grade was negatively correlated with total QOL [54]. Social participation level, physical activity performance, and walking ability were positively associated with QOL [55]. It was found that intervening in childhood with CP to reduce psychological difficulties and stress, especially pain, may then have long-term effects on QOL [56]. This study found that AT can benefit motor performance, walking ability, and cardiorespiratory endurance, which carried over to other aspects of the child's life, such as quality of survival. One possible reason for the improvement in activities of daily living and QOL is the ideal water environment. The water provides support, reduces joint load, and improves limb control. Water exercise is more fun for children, increases confidence, reduces resistance to complex tasks [28], and increases training motivation [29].
Limitations
To include as wide a range of literature as possible, two authors have minimized selection bias using detailed search terms and search formulas and independent screening.
Conclusion
The AT can improve physical function, physiological function, and psychosocial aspects of patients with spastic CP. In particular, lower limb muscle strength, lower limb joint mobility, and lower limb muscle tone were improved to varying degrees, and enhanced lower limb function led to improved walking ability, further improving activities of daily living and independence. Although the included studies analyzed many aspects of improvement, little consideration was given to aspects of fine motor ability and cardiorespiratory endurance. This scoping review's summary of the existing literature can be used as a starting point for future research on AT interventions for patients with spastic CP and to standardize the parameters to design the most efficient exercise regimen.
Supplementary Information
Acknowledgements
We thank all the staff and participants in this study for their contribution.
Author contributions
AMX: conceptualization, methodology, investigation, formal analysis, writing—original draft, writing—review & editing; YXF: investigation, data curation, methodology, writing—original draft; CSW: formal analysis, methodology, writing-review & editing; DH: resources, supervision; writing—review & editing JMQ: investigation, validation; writing—original draft; RXZ: investigation, validation; writing—original draft; LW: funding acquisition, supervision, writing—review & editing; CLF: conceptualization, funding acquisition, resources, writing—review & editing; QZ: funding acquisition, supervision, investigation, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Beijing Hospitals Authority Youth Programme, code QML20232202.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aomeng Xiang and Yanxin Fu have contributed equally to this work.
Contributor Information
Liang Wu, Email: 1972wuliang@sina.com.
Chunliang Fan, Email: fanchunliang1109@126.com.
Qin Zhang, Email: 2767348230@qq.com.
References
- 1.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 2.McIntyre S, Goldsmith S, Webb A, Ehlinger V, Hollung SJ, McConnell K, et al. Global prevalence of cerebral palsy: a systematic analysis. Dev Med Child Neurol. 2022;64:1494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christine C, Dolk H, Platt MJ, Colver A, Prasauskiene A, Krägeloh-Mann I. Recommendations from the SCPE collaborative group for defining and classifying cerebral palsy. Dev Med Child Neurol Suppl. 2007;109:35–8. [DOI] [PubMed] [Google Scholar]
- 4.Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213–20. [PubMed] [Google Scholar]
- 5.Mockford M, Caulton JM. The pathophysiological basis of weakness in children with cerebral palsy. Pediatr Phys Ther. 2010;22:222–33. [DOI] [PubMed] [Google Scholar]
- 6.Gillett JG, Boyd RN, Carty CP, Barber LA. The impact of strength training on skeletal muscle morphology and architecture in children and adolescents with spastic cerebral palsy: a systematic review. Res Dev Disabil. 2016;56:183–96. [DOI] [PubMed] [Google Scholar]
- 7.Barrett RS, Lichtwark GA. Gross muscle morphology and structure in spastic cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52:794–804. [DOI] [PubMed] [Google Scholar]
- 8.Dar H, Stewart K, McIntyre S, Paget S. Multiple motor disorders in cerebral palsy. Dev Med Child Neurol. 2024;66:317–25. [DOI] [PubMed] [Google Scholar]
- 9.Liguori S, Young VM, Arienti C, Pollini E, Patrini M, Gimigliano F, et al. Overview of cochrane systematic reviews for rehabilitation interventions in individuals with cerebral palsy: a mapping synthesis. Dev Med Child Neurol. 2023;65:1280–91. [DOI] [PubMed] [Google Scholar]
- 10.Gibson N, Blackmore AM, Chang AB, Cooper MS, Jaffe A, Kong W-R, et al. Prevention and management of respiratory disease in young people with cerebral palsy: consensus statement. Dev Med Child Neurol. 2021;63:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackman M, Sakzewski L, Morgan C, Boyd RN, Brennan SE, Langdon K, et al. Interventions to improve physical function for children and young people with cerebral palsy: international clinical practice guideline. Dev Med Child Neurol. 2022;64:536–49. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Wang J, Wang Y, Fang J, Cong F, Qin Xu, et al. Hydrotherapy rehabilitation technology expert consensus. Chin J Rehabilit Med. 2019;34:756–60. [Google Scholar]
- 13.Brunton LK, Bartlett DJ. Description of exercise participation of adolescents with cerebral palsy across a 4 year period. Pediatr Phys Ther. 2010;22:180–7. [DOI] [PubMed] [Google Scholar]
- 14.Ogonowska-Slodownik A, de Lima AAR, Cordeiro L, Morgulec-Adamowicz N, Alonso-Fraile M, Güeita-Rodríguez J. Aquatic therapy for persons with neuromuscular diseases—a scoping review. J Neuromuscul Dis. 2022;9:237–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker BE. Aquatic therapy: scientific foundations and clinical rehabilitation applications. PM R. 2009;1:859–72. [DOI] [PubMed] [Google Scholar]
- 16.Ballington SJ, Naidoo R. The carry-over effect of an aquatic-based intervention in children with cerebral palsy. Afr J Disabil. 2018;7:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato D, Onishi H, Yamashiro K, Iwabe T, Shimoyama Y, Maruyama A. Water immersion to the femur level affects cerebral cortical activity in humans: functional near-infrared spectroscopy study. Brain Topogr. 2012;25:220–7. [DOI] [PubMed] [Google Scholar]
- 18.Sato D, Yamashiro K, Onishi H, Shimoyama Y, Yoshida T, Maruyama A. The effect of water immersion on short-latency somatosensory evoked potentials in human. BMC Neurosci. 2012;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai C-J, Liu W-Y, Yang T-F, Chen C-L, Wu C-Y, Chan R-C. Pediatric aquatic therapy on motor function and enjoyment in children diagnosed with cerebral palsy of various motor severities. J Child Neurol. 2015;30:200–8. [DOI] [PubMed] [Google Scholar]
- 20.Sato D, Yamashiro K, Yamazaki Y, Ikarashi K, Onishi H, Baba Y, et al. Priming effects of water immersion on paired associative stimulation-induced neural plasticity in the primary motor cortex. Int J Environ Res Publ Health. 2019;17:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Blanco E, Merino-Andrés J, Aguilar-Soto B, García YC, Puente-Villalba M, Pérez-Corrales J, et al. Influence of aquatic therapy in children and youth with cerebral palsy: a qualitative case study in a special education school. Int J Environ Res Publ Health. 2020;17:3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapia C, Constanzo J, González V, Barría RM. The effectiveness of aquatic therapy based on the halliwick concept in children with cerebral palsy: a systematic review. Dev Neurorehabil. 2023;26:371–6. [DOI] [PubMed] [Google Scholar]
- 23.Shariat A, Najafabadi MG, Dos Santos IK, Anastasio AT, Milajerdi HR, Hassanzadeh G, et al. The effectiveness of aquatic therapy on motor and social skill as well as executive function in children with neurodevelopmental disorder: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2024;105:1000–7. [DOI] [PubMed] [Google Scholar]
- 24.Jordan PZ. JBI manual for evidence synthesis. 2020. https://api.semanticscholar.org/CorpusID:243267797
- 25.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 26.Armstrong R, Hall BJ, Doyle J, Waters E. Cochrane update. “Scoping the scope” of a cochrane review. J Publ Health (Oxf). 2011;33:147–50. [DOI] [PubMed] [Google Scholar]
- 27.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 28.Fragala-Pinkham MA, Dumas HM, Barlow CA, Pasternak A. An aquatic physical therapy program at a pediatric rehabilitation hospital: a case series. Pediatr Phys Ther. 2009;21:68–78. [DOI] [PubMed] [Google Scholar]
- 29.Retarekar R, Fragala-Pinkham MA, Townsend EL. Effects of aquatic aerobic exercise for a child with cerebral palsy: single-subject design. Pediatr Phys Ther. 2009;21:336–44. [DOI] [PubMed] [Google Scholar]
- 30.Ballaz L, Plamondon S, Lemay M. Group aquatic training improves gait efficiency in adolescents with cerebral palsy. Disabil Rehabil. 2011;33:1616–24. [DOI] [PubMed] [Google Scholar]
- 31.Fragala-Pinkham MA, Smith HJ, Lombard KA, Barlow C, O’Neil ME. Aquatic aerobic exercise for children with cerebral palsy: a pilot intervention study. Physiother Theory Pract. 2014;30:69–78. [DOI] [PubMed] [Google Scholar]
- 32.Tufekcioglu E. The effects of Watsu therapy on autonomic cardiovascular modulation and flexibility of children with cerebral palsy. Baltic J Health Phys Act. 2020;12:21–32. [Google Scholar]
- 33.Rui Z. Observation on the application of hydrotherapy combined with sensory integration training in the rehabilitation of children with cerebral palsy. Chin J Conval Med. 2018;27:1016–9. [Google Scholar]
- 34.Hamed SA, ElMeligie MM, Kentiba E. The effects of Halliwick aquatic exercises on gross motor function of children aged from 3 to 5 years with spastic cerebral palsy. Pedag Phys Culture And Sport. 2023;27:24–31. [Google Scholar]
- 35.Yuting Z, Huijia Z, Mengping T, Jihong H, Can L, Gongping L, et al. Clinical observation on the treatment of spastic cerebral palsy by hydrotherapy with bobath therapy. J Chin Phys. 2014;16:1252–4. [Google Scholar]
- 36.Epstein LH, Paluch RA, Kalakanis LE, Goldfield GS, Cerny FJ, Roemmich JN. How much activity do youth get? A quantitative review of heart-rate measured activity. Pediatrics. 2001;108:E44. [DOI] [PubMed] [Google Scholar]
- 37.Akinola BI, Gbiri CA, Odebiyi DO. Effect of a 10 week aquatic exercise training program on gross motor function in children with spastic cerebral palsy. Glob Pediatr Health. 2019. 10.1177/2333794X19857378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adar S, Dündar Ü, Demirdal ÜS, Ulaşlı AM, Toktaş H, Solak Ö. The effect of aquatic exercise on spasticity, quality of life, and motor function in cerebral palsy. Turk J Phys Med Rehabil. 2017;63:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdelaal AAM, Atia DT. Efficacy of aquatic exercise on pulmonary function and aquatic skills performance in older children with cerebral palsy. Random Contr Stud Physiother Q. 2023;31:81–6. [Google Scholar]
- 40.Zhao Y, Wen C, Qi Y, Shi N, Li E. Effects of aerobic hydrotherapy on movement, balance function and muscle tension of spastic cerebral palsy children. Chin J Rehabilit. 2021;36:93–7. [Google Scholar]
- 41.Yufei N, Qiang L, Jianhua C, Xiaoli D. Study on the clinical effect of hydrotherapy in early rehabilitation treatment of children with spastic diplegia. Asia-Pac Traditional Med. 2014;10:93–4. [Google Scholar]
- 42.Fanxu S, Xiaojie L, Chunfeng C, Jiajing H. Effect of hydrotherapy on gross motor function and lower limb muscle strength and muscle tension in children with spastic diplegia cerebral palsy. Chin Pediatr Integr Tradit West Med. 2015;7:331–3. [Google Scholar]
- 43.Wenwen L. Analysis of the effect of functional hydrotherapy on muscle strength and motor function in children with spastic cerebral palsy. Chin J Conval Med. 2018;27:909–11. [Google Scholar]
- 44.Qing Z, Lufen L, Shuang M. Effect of hydrotherapy intervention on lower limb motor skills of children with spastic cerebral palsy. Qingdao Med J. 2022;54:202–4. [Google Scholar]
- 45.Fen G. Analysis of the effect of hydrotherapy on gross motor function and lower limb muscle strength dystonia in children with spastic diplegic cerebral palsy. Med Health. 2022;10:39–42. [Google Scholar]
- 46.Huang C, Chen Y, Chen G, Xie Y, Mo J, Li K, et al. Efficacy and safety of core stability training on gait of children with cerebral palsy: a protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2020;99: e18609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H-Y, Yang YH. Evaluating the responsiveness of 2 versions of the gross motor function measure for children with cerebral palsy. Arch Phys Med Rehabil. 2006;87:51–6. [DOI] [PubMed] [Google Scholar]
- 48.Garcia M, Joares E, Silva M, Bissolotti R, Oliveira S, Battistella L. The Halliwick concept, inclusion and participation through aquatic functional activities. Acta Fisiátr. 2012;19:142–50. [Google Scholar]
- 49.Thabet NS, Zaky NA, Banoub MB. Underwater exercises versus treadmill training on gait in children with spastic hemiparetic cerebral palsy. Int J Physiother Res. 2017;5:2385–91. [Google Scholar]
- 50.Getz M, Hutzler Y, Vermeer A, Yarom Y, Unnithan VB. The effect of aquatic and land-based training on the metabolic cost of walking and motor performance in children with cerebral palsy: a pilot study. Int Sch Res Not. 2012;2012:1–8. [Google Scholar]
- 51.Phothirook P, Amatachaya S, Peungsuwan P. Muscle activity and co-activation of gait cycle during walking in water and on land in people with spastic cerebral palsy. Int J Environ Res Public Health. 2023;20:1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fragala-Pinkham M, O’Neil ME, Haley SM. Summative evaluation of a pilot aquatic exercise program for children with disabilities. Disabil Health J. 2010;3:162–70. [DOI] [PubMed] [Google Scholar]
- 53.Lee HY, Cha YJ, Kim K. The effect of feedback respiratory training on pulmonary function of children with cerebral palsy: a randomized controlled preliminary report. Clin Rehabil. 2014;28:965–71. [DOI] [PubMed] [Google Scholar]
- 54.Blasco M, García-Galant M, Laporta-Hoyos O, Ballester-Plané J, Jorba-Bertran A, Caldú X, et al. Factors related to quality of life in children with cerebral palsy. Pediatr Neurol. 2023;141:101–8. [DOI] [PubMed] [Google Scholar]
- 55.Omura J, Fuentes M, Bjornson K. Participation in daily life: influence on quality of life in ambulatory children with cerebral palsy. PM R. 2018;10:1185–91. [DOI] [PubMed] [Google Scholar]
- 56.Colver A, Rapp M, Eisemann N, Ehlinger V, Thyen U, Dickinson HO, et al. Self-reported quality of life of adolescents with cerebral palsy: a cross-sectional and longitudinal analysis. Lancet. 2015;385:705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.