Abstract
Hormonal imbalances during development may have long-lasting effects. Using functional magnetic resonance imaging (fMRI), we compared 14 youths with Congenital Adrenal Hyperplasia (CAH), a genetic disorder of hormonal dysfunction, with 22 healthy controls on memory encoding of emotional faces. Patients remembered fewer faces than controls, particularly fearful faces. FMRI data to successfully encoded fearful faces revealed that males with CAH showed significant activations in amygdala, hippocampus, and anterior cingulate relative to unaffected males, while females with CAH demonstrated deactivations relative to unaffected females in these regions. Findings indicate that steroid abnormalities during development can have important effects on neural correlates of emotional memory.
A growing body of research documents the influence of steroid hormones, such as glucocorticoids and androgens, on development (Hertsgaard, Gunnar, Larson, Brodersen, & Lehman, 1992; Perez-Edgar, Schmidt, Henderson, Schulkin, & Fox, 2008). Perturbations in hormonal levels in utero or during critical periods of development may have organizational effects, that is, create permanent changes in brain structures, organization, or function (Hines, 2008; Owen, Andrews, & Matthews, 2005). These changes may confer vulnerability for psychopathology (Hines, 2008; Owen et al., 2005) and cognitive deficits, especially declarative memory impairments (Cherrier, 2005; Owen et al., 2005). Declarative memory refers to the conscious and voluntary recollection of previously learned information (Milner, Squire, & Kandel, 1998). Glucocorticoids and androgens may influence emotion regulation and declarative memory through their interactions with a large number of their receptors located in the frontal cortex, hippocampus, and amygdala (de Kloet, Joels, & Holsboer, 2005; Nunez, Huppenbauer, McAbee, Juraska, & DonCarlos, 2003; Wilson & Davies, 2007; Handa, Burgess, Kerr, & O’Keefe,1994; Lupien et al., 2005; Roozendaal, 2003), three brain structures implicated in declarative memory function and the processing of emotional information (Davidson, 2002; Milner et al., 1998; Phelps, 2006).
Studies of animal models of pre-natal and early life chronic alterations in glucocorticoids have shown a number of neural alterations in medial temporal structures and prefrontal cortex, as well as on affective and cognitive function. The majority of studies have focused on chronic elevations of glucocorticoid. Briefly, these findings included hippocampal neuronal degeneration, reduced corticosteroid gene expression in amygdala and hippocampus, reduced spine and dendritic density of neurons in the anterior cingulate and orbito-frontal cortices, and diminished corticosteroid gene expression in the prefrontal cortex of rodent or monkey offspring (Matthews, 2001; Patel, Katz, Karssen, & Lyons, 2008; Weinstock, 2008; Welberg & Seckl, 2001). In addition, chronic pre-natal and early life glucocorticoid elevations were also associated with increased anxious-like and depressive-like behaviors and declarative memory deficits in young rodents (Owen et al., 2005; Weinstock, 2008; Welberg & Seckl, 2001). Fewer studies with animals have investigated the effects of chronic glucocorticoid depletion. These studies used a rodent model of early life adrenalectomy (ADX; removal of glucocorticoid). Findings paralleled those of abnormally elevated glucocorticoid, and showed hippocampal neuronal degeneration (Gould, Woolley, & McEwen, 1991; Hashimoto, Marystone, Greenough, & Bohn, 1989; Sloviter, Sollas, Dean, & Neubort, 1993) and impaired fear expression (Moriceau, Roth, Okotoghaide, & Sullivan, 2004; Takahashi, 1994) in juvenile rats. Although no studies have examined the influence of early life ADX on the prefrontal cortex, ADX performed in adult rodents was associated with decreased dendritic length and spine density in the prefrontal cortex (Cerqueira, Taipa, Uylings, Almeida, & Sousa, 2007). Adrenalectomy in adult rodents has also been associated with working memory impairment (Mizoguchi, Ishige, Takeda, Aburada, & Tabira, 2004).
Animal data documenting the organizational effects of androgens are scarcer, but pre-natal and early life levels of androgens were found to influence hippocampal cell function in rodent offspring (Hebbard, King, Malsbury, & Harley, 2003). Pre-natal and early life androgen levels were also shown to influence sex differences in amygdala shape and synaptic function in juvenile rats (Cooke, Hegstrom, Villeneuve, & Breedlove, 1998; Cooke & Woolley, 2005; Wilson & Davies, 2007). Behaviorally, pre-natal and early life androgen levels were reported to reduce social memory function (Hebbard et al., 2003), and to sexually differentiate visual discrimination learning and spatial memory performance in mammal offspring (Hagger & Bachevalier, 1991; Roof, 1993). Unfortunately, no study has assessed the influence of prenatal or early-life levels of androgens on the prefrontal cortex, or on prefrontal cortex-related behaviors.
Despite the large body of animal literature on the organizational effects of glucocorticoids and androgens on neural systems (hippocampus, amygdala, and prefrontal cortex), and on affective/cognitive processes (emotion regulation and declarative memory), very few studies have investigated these effects in humans. Therefore, the neural mechanisms underlying the associations between the organizational effects of steroid abnormalities and the increased risk for emotional dysregulation and declarative memory deficits remain unclear in children. The aim of the present work was to address this gap.
The approach adopted in the present work was to examine the neural substrates of affective and declarative memory processes in children with a history of steroid endocrinopathy and healthy age-matched controls. Alterations in declarative memory and in discrete neural structures (prefrontal cortex, amygdala, and hippocampus) are not only consequences of steroid perturbations (Kuhlmann, Kirschbaum, & Wolf, 2005; Maheu, Merke, et al., 2008; Wolf, 2008), but they also characterize depression and anxiety (Bar-Haim, Lamy, & Glickman, 2005; Bradley, Mogg, & Williams, 1995; Pine et al., 2004; Roberson-Nay et al., 2006). For example, Cushing disease, a syndrome of hypersecretion of glucocorticoid, has been associated with amygdala and hippocampal hyperactivation in youths (Maheu, Mazzone et al., 2008) and affective disturbances, both of manic and depressed types (Haskett, 1985; Hudson, Hudson, Griffing, Melby, & Pope, Jr., 1987; Kelly, 1996; Sonino & Fava, 2001).
Classic Congenital Adrenal Hyperplasia (CAH) is another endocrine disease that can serve as a natural model of the organizational effects of steroid abnormalities (Merke & Bornstein, 2005). Classic CAH is an autosomal recessive genetic disorder consisting of impairment of cortisol biosynthesis and androgen excess. Absence of cortisol leads to the lack of cortisol negative feedback, resulting in overproduction of corticotrophin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH). Treatment is lifetime glucocorticoid replacement; however periods of over- or under-treatment may occur. This alteration of the hypothalamic-pituitary-adrenal (HPA) axis is similar in both sexes. In addition, overproduction of ACTH leads to adrenal androgen excess, which primarily affects females. Previous studies have shown that young girls with CAH exhibit male-like behaviors (aggressive play behaviors, enhanced spatial abilities) compared to their healthy counterparts (Berenbaum, 2001; Cohen-Bendahan, van de Beek, & Berenbaum, 2005). These findings suggested that pre-natal androgen excess masculinizes CAH female brain. We recently demonstrated emotional memory impairments in adolescents with CAH (Maheu, Merke et al., 2008). In addition, we found that children with CAH showed structural (Merke et al., 2003) and functional (Ernst et al., 2007) abnormalities of the amygdala, a key structure in emotion processing. The amygdala was found to be smaller in the CAH group compared to the control group in both males and females (Merke et al., 2003), and, in an independent sample, to be hyper-responsive to negative facial expressions in females, but not in males (Ernst et al., 2007). The present study extends these neuroimaging findings by examining emotional memory to facial stimuli.
Based on the four lines of evidence indicating (1) impaired memory for negative stimuli in adolescents with CAH (Maheu, Merke et al., 2008), (2) the role of the hippocampus and amygdala in emotional memory (LaBar & Cabeza, 2006), (3) structural abnormalities of the amygdala in patients with CAH (Merke et al., 2003), and (4) modulation by sex of amygdala functional alterations in the CAH group (Ernst et al., 2007), we made three predictions. We expected the following: first, impaired emotional memory in patients with CAH relative to healthy controls particularly for negative (i.e., fearful faces) stimuli; second, altered hippocampal and amygdala activations in response to fear-related stimuli in the CAH group relative to healthy controls; and third, a modulation of the neural findings by sex. However, although these previous data may suggest a hyper-functionality in the present study, we did not want to predict the directionality of the effect for three reasons: (1) group differences depend on the specific function being tested (attentional processing vs. memory), (2) previous research indicating an inverted U-function effects of glucocorticoids on memory (Lupien & McEwen, 1997) suggesting either hyper or hypo-functionality, and (3) scarcity of data in this patient group.
METHODS
Subjects
Fourteen adolescents with CAH and 22 healthy subjects completed the study. The present work was an extension of the study by Ernst et al. (2007). Here, we focused the research question on emotional memory rather than on exposure to negative stimuli. We also enlarged the control sample from n = 14 to n = 22 to enhance statistical power and stability of the results. The patient sample was not modified.
Patients with CAH and healthy adolescents did not differ with respect to mean age (CAH group: 13.98 ± 2.76 years; Healthy group: 13.76 ± 2.14 years), sex distribution (CAH: 7 boys, 7 girls; Healthy: 11 boys, 11 girls), puberty level, or Wechsler IQ (CAH: 104 ± 12.79; Healthy: 107 ± 15.63; see Table 1). Patients were initially recruited as part of an ongoing study of the National Institute of Child Health and Human Development/National Institutes of Health and subsequently consented to participate in the current National Institute of Mental Health study. Participants for the healthy control group were recruited by advertisement in the local news-papers. The Institutional Review Board of the NIH approved the study. Parents signed consent forms and adolescents signed assent forms.
TABLE 1.
Demographic Characteristics of Patients With Congenital Adrenal Hyperplasia and Healthy Adolescents
| CAH n = 14 |
Control Children n = 22 |
Statistic | |
|---|---|---|---|
| Age (Mean ± SD) | 13.98 ± 2.76 | 13.76 ± 2.14 | t = .28, ns |
| IQ (Mean ± SD) | 104.08 ± 12.79 | 107.18 ± 15.62 | t = .23, ns |
| Tanner stage | 3.14 ± 1.4 | 3.25 ± 1.33 | t = .61, ns |
| Gender (n, % Female) | 7 (50%) | 11 (50%) |
CAH = Congenital Adrenal Hyperplasia; ns = not significant.
All subjects completed a physical examination as well as neurological and psychiatric assessments. Structured psychiatric interviews were conducted using the structured interview Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version (K-SADS-PL) (Kaufman et al., 1997).
Experimental Paradigm
Emotional memory was assayed by a well-established face-emotion task (Nelson et al., 2003; Roberson-Nay et al., 2006; Maheu et al., 2008). This task comprises face stimuli of 56 actors with different facial expressions, which were derived from standardized sets of gray-scale photographs (Ekman & Friesen, 1976; Tottenham et al., 2009). Each participant viewed 32 different actors, half of these actors were female and the other half male. Each actor was randomly assigned to display one of four facial expressions (happy, angry, fearful, and neutral). For instance, while a given actor might be randomly selected to portray “fear” for one participant, she might be randomly chosen to portray “happiness” for another subject, or “anger” for yet another participant. This design allowed us to control for variability in non-emotional features of the actors (e.g., ethnicity, hair color). A total of 32 “null-event” fixation crosses were included to facilitate data analysis.
Participants saw each actor four times across the paradigm, and each time in a different task condition. However, the emotion displayed by the actor to a given subjects was always the same throughout the task. Each task condition probed a distinct attention condition: In the “threat” attention condition, participants were asked to rate the hostility of the presented face. In the “fear” attention condition, participants were asked how afraid they were of the presented face. In the “non-emotional judgment” condition, participants were asked to rate the width of the nose of the presented face. Finally, in the passive attention condition, the instruction was to simply look at the pictures without making any rating. Responses (1–5 level) were collected on a five-key button box developed by magnetic resonance imaging (MRI) Devices (Waukesha, WI).
Each attention condition was presented in blocks of 10 randomly-ordered stimuli (8 faces and 2 fixation crosses), and each block was presented four times. Order of presentations of condition and facial expression was randomized across participants. The experiment was presented in a single run of 160 trials (4 conditions × 4 block-repeats × 10 stimuli per block) that lasted 14.2 minutes and utilized a rapid event-related design. Each of the four task conditions began with a 3,000 msec instruction screen. Following the instruction screen, the 10 randomly ordered stimulus event trials (8 faces, 2 fixation crosses) each appeared for 4,000 msec. The inter-stimulus interval was displayed as a blank screen that varied from 750 to 1,250 msec (averaging 1,000msec within a 10-trial block). Prior to scanning participants were trained in a MRI simulator to become familiar with the environment and response device. Participants were trained in a practice block prior to scanning and were shown neutral expressions not presented during the MRI version of the task.
After the scanning session, a surprise post-scan memory test was performed outside of the scanner (Nelson et al., 2003). The test allowed data acquired during scanning to be binned based on the memory performance of the subject. In order to avoid explicit mnemonic strategies or other meta-memory processes that might differentially influence the approach to the task, we used an implicit memory task, with subjects being unaware of the post-MRI memory test. This strategy has been used successfully by previous neuroimaging studies designed to examine neural aspects of memory encoding (Alkire, Haier, Fallon, & Cahill, 1998; Buckner, Wheeler, & Sheridan, 2001; Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000; Wagner et al., 1998). In addition, this design mirrors more closely memory-encoding processes elicited during attending to emotionally salient stimuli encountered in everyday life.
Participants viewed 24 previously seen faces during the fMRI acquisition and 24 novel faces not seen previously. However, in contrast to the faces seen during fMRI scanning, all faces in the post-scan memory task were presented with neutral facial expressions. In order to avoid a response bias to faces that were already presented as neutral during the MRI task, and may elicit stronger recognition, these eight images were excluded from presentation during the post-scan memory task. Prior studies find variants of this paradigm difficult, with subjects generally showing better abilities to correctly identify novel faces as novel, possibly since all photographs viewed at recognition are indeed novel. By contrast, identifying photographs of previously seen actors, now depicting neutral expressions, is more difficult (Nelson et al., 2003; Perez-Lopez & Woody, 2001; Pine et al., 2004). Given this tendency to “miss” old actors, measures derived from signal detection theory provide a more precise summary of subjects’ ability to perform the task, as opposed to raw rates of “false-alarms” or “hits.” Specifically, d prime (d’) provides an index of subjects’ ability to separate “signal from noise,” with higher d’ values indicating increasing ability (d’ values range from negative to positive) and positive values signifying higher true signal reporting than false alarm reporting.
Performance Analysis
Accuracy was assessed by computing a signal detection threshold (d’) score. The d’ index is based on the number of correctly (hits) and incorrectly (false alarms) recognized actors. An item was counted as a false alarm when a participant reported remembering an actor that was not previously displayed. Individual d’ indices were calculated for each subject by subtracting z-score for hits from z score for false alarms (Snodgrass & Corwin, 1988). Thus a higher d’ value reflects better memory retrieval. For the statistical analysis, we conducted a repeated-measures ANOVA with the within subjects factor of emotion (fearful, angry, happy) and the two between-subject factors of group (CAH vs. control) and sex (male vs. female) resulting in a 3 × 2 × 2 factorial design. For-post-hoc comparisons, independent t-tests were used. In addition, in order to obtain a measure of the size of the effect of group differences, Cohen’s d was also computed for the behavioral data (Cohen, 1992).
fMRI Data Acquisition
Whole brain oxygen level dependent (BOLD) functional MRI data were acquired on a 3 Tesla General Electric Signa Scanner (Waukesha, Wisconsin). Head movement was restricted using foam padding. Stimuli were presented to subjects through goggles (Avotec Silent Vision Glasses Stuart, FL). Following sagittal localization and manual shimming, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix size of 64 × 64 mm, repetition time (TR) of 2,000 msec, echo time (TE) of 40 msec, field of view (FOV) of 240 mm, and voxels of 3.75 × 3.75 × 5 mm providing whole brain coverage. Images were acquired in 23 contiguous 5-mm axial slices per brain volume positioned parallel to the anterior commissure and posterior commissure (AC-PC) line. All functional data were gathered in a single 14.2-min run for each subject.
After echo-planar imaging (EPI) acquisition, a high-resolution T1-weighted anatomical image was acquired to aid with spatial normalization. A standardized magnetization-prepared gradient echo sequence (180 1-mm sagittal slices, FOV = 256, number of excitations (NEX) = 1, TR = 11.4 msec, TE = 4.4 msec, matrix = 256 × 256, time to inversion (TI) = 300, bandwidth = 130 Hz/pixel, 33khz/256 pixels) msec was used to facilitate spatial normalization.
fMRI Processing
All subsequent analyses were conducted with SPM 99 (Wellcome Department of Imaging Neuroscience, University College of London, London, UK) and Matlab 6.1 (The Mathworks Inc., Natick, Massachusetts) in order to more reliably compare the findings to previously published studies using this version of SPM and this specific task (Mueller et al., 2008; McClure et al., 2007, Nelson et al., 2003; Guyer et al., 2008). Preprocessing steps for the functional imaging data included slice time and motion correction, coregistration to the anatomical data and spatial normalization to a Montreal Neurologic Institute (MNI) T1-weighted template image supplied with SPM99. After preprocessing, fMRI images were visually inspected to evaluate the quality of the normalization procedure. Head movement of subjects was analyzed with MedX software (Medical Numerics, Sterling, Virginia) and subjects who moved more than 3.0 mm in any direction were removed from further analysis.
fMRI Analysis
Individual subject-level, event-related response amplitudes were estimated using a general linear model (GLM) for each event type. This study was focused on neural responses to faces that were successfully encoded (remembered on the post-task memory test) and faces that were not successfully encoded (not remembered on the post-task memory test). Given our a priori hypothesis of sex-influenced neural responses to emotional material, sex was modeled in the analysis. A long-standing dilemma regarding fMRI data analyses, or any other functional imaging methodology, is the problem of how best to reconcile behavioral and imaging data. We adopted the strategy that consists of examining functional imaging data only in conditions which showed behavioral group effects. We chose this strategy for two reasons. First, the presence of group differences in behavior reflects the relevance of the task to the condition under study. Second, this strategy limits the number of analyses, which reduces type I errors. In the present work, fearful faces but not angry face, were chosen because of the significant behavioral effects seen between the patient and control groups for the fearful faces only (CAH remembered significantly fewer fearful faces). Moreover, to increase statistical power and to have sufficient trials to compare remembered versus forgotten faces, all emotional faces of a particular type were taken from all attention conditions. Consequently, the main contrast of interest was centered on [remembered fearful faces versus forgotten fearful faces across all attention conditions] influenced by group and sex. Fixation trials served as the implicit baseline.
To further clarify any significant interactions, that is, whether activations resulted from either correctly remembering fearful faces or forgetting them, post-hoc analyses were conducted on remembered fearful faces versus fixation, and forgotten fearful faces versus fixation.
The waveform used to model event-related responses was a rectangular pulse of 4 sec duration (i.e., the duration of each face presentation) convolved with the hemodynamic response function specified in SPM99. Contrast images were created for each subject using pair-wise comparisons of the different event-related BOLD response amplitudes. Before performing group-level analyses, each contrast image was divided by the subject-specific voxel time series mean, generating values proportional to percentage fMRI signal change (Zarahn, Aguirre, & D’Esposito, 1997). These normalized contrast images were then smoothed with an isotropic Gaussian kernel (full-width half-maximum = 11.4) to reduce non-stationarity in the spatial auto-correlation structure produced by the previous step (Friston et al., 2000). For all group-level analyses, the contrast images produced for each participant were fit to a second-level random effects model.
Based on our a priori hypotheses, we used an regions of interest (ROI) approach for the main analysis. Three bilateral ROIs were included: the amygdala, the anterior hippocampus, and the posterior hippocampus. These ROIs were defined using standard, previously validated, anatomical criteria. They were hand-traced on the single MNI template, to which fMRI data were normalized, and then applied to all normalized brains at the group level (Szeszko et al., 1999). We performed voxel-wise tests in these anatomically defined volumes of interest. Consistent with the current standard (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002; Winston, Strange, O’Doherty, & Dolan, 2002), we utilized the Gaussian random field threshold (alpha = .05, corrected) with small volume correction (SVC) implemented in SPM99 (Worsley et al., 1996). Statistical significance of activation in regions of interest was set at p corrected <.05 and cluster size >30 voxels. MNI x, y, z coordinates are reported for significant activation peaks.
Correlations were performed to assess the contribution of memory performance (d’) to the amygdala and anterior and posterior hippocampus activations. Given that we hypothesized effects of sex, these correlations were conducted separately for CAH males, CAH females, control males, and control females.
For completeness, the whole brain analysis is also presented using an uncorrected statistical threshold level of p < .001.
RESULTS
Behavioral Data
The analysis of the behavioral data indicated a significant effect of emotion (F(2,60) = 3.29, p < .05) and a main effect of diagnosis (F(1,30) = 5.49, p < .05) on d’ scores, indicating lower memory scores for the CAH group relative to controls (Table 2). Although there was no emotion by diagnosis interaction, independent t-test comparisons for each emotion type were conducted because of the a priori hypothesis of deficits for negative stimuli in the CAH group versus the control group. In line with predictions, the data indicated significantly fewer remembered items for fearful faces by CAH than by control adolescents (t(33) = 2.04, p < .05, d’ = .73). No other emotion type showed a significant group effect (p > .16). There was no significant interaction of sex with diagnosis, sex with emotion, or main effect of sex (all ps > .21).
TABLE 2.
Predictors of Memory Performance (d’ Values, Hits, Misses, Correct Rejections, and False Alarms) Split by Group and Emotion and the Corresponding Effect Sizes of Group Differences
| Face Emotion Type | Recognition Memory | CAH Patients | Control Children | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| Fearful | hits | 3.64 (.36) | 4.73 (.32) | |
| misses | 4.36 (.36) | 3.27 (.32) | ||
| d’ | .28 (.13) | .67 (.12) | 0.73 | |
| Angry | hits | 3.36 (.52) | 4.05 (.25) | |
| misses | 4.64 (.52) | 3.95 (.25) | ||
| d’ | .06 (.17) | .37 (.10) | 0.54 | |
| Happy | hits | 3.64 (.44) | 4.55 (.26) | |
| misses | 4.36 (.44) | 3.45 (.26) | ||
| d’ | .33 (.12) | .62 (.12) | 0.58 | |
| correct rejections | 15.50 (.91) | 15.45 (.79) | ||
| false alarms | 8.50 (.91) | 8.55 (.79) | ||
| hits total | 10.64 (.98) | 13.32 (.55) | 0.85 |
CAH = Congenital Adrenal Hyperplasia; All values are mean ± SEM. Higher d’ and hits values indicates higher number of successfully remembered items shown for each emotion type and their statistical effect size.
Imaging
Primary (ROI) Analyses
Remembered fearful faces versus forgotten fearful faces.
The primary contrast of interest was centered on remembered versus forgotten fearful faces. In this contrast we found a significant interaction of sex by diagnosis in all a priori ROIs: the left amygdala [MNI: −8 −4 −14], t = 2.40, p = .01, right amygdala [MNI: 22 0 −16], t = 2.12, p = .02, left anterior hippocampus [MNI: −12 −6 −16], t = 2.32, p = .01, left posterior hippocampus [MNI: −30 −26 −10], t = 2.49, p < .01, right anterior hippocampus [MNI: 32 −18 −14], t = 2.63, p < .01, and right posterior hippocampus [MNI: 32 −24 −14], t = 2.81, p < .01. In order to parse out whether the contribution of activations stemmed predominantly from the remembered or the forgotten items, additional analyses were performed on the remembered versus baseline and forgotten versus baseline contrasts. The data revealed that the significant findings were due to group differences in remembered fearful faces versus baseline. No significant findings emerged related to forgotten items. There was no significant main effect of sex or diagnosis.
Remembered fearful faces versus baseline.
With regard to the amygdala, activation reflecting sex by diagnosis interaction was found to be significant (p < .05) in the right amygdala [MNI: 22 2 −20] and near significant (p = .085) in the left MNI: [−8 −4 −14] amygdala, after correction for multiple comparisons.
With regard to the hippocampus, sex by diagnosis interaction was found to be significant in all four hippocampal regions: left anterior [MNI: −30 −14 −16] t = 3.94, p < .005, left posterior [MNI: −30 −20 −16] t = 3.12, p < .05, right anterior [MNI: 30 −8 −24] t = 2.86, p < .05, and right posterior [MNI: 32 −36 2] t = 3.05, p < .05 (Table 3, Figures 1 and 2).
TABLE 3.
Regional Activations in the Group Comparison of the All Remembered vs. All Forgotten Contrast (Statistical Threshold is Set at p < .05, Uncorrected) and the All Remembered vs. Fixation Contrast (Alpha < .05 for the ROI After Small Volume Correction) for the Three Regions of Interest (ROI) Analyses: Amygdala, Anterior Hippocampus, Posterior Hippocampus as Well as the Volumes for the Corrected (p < .05) Whole Brain Analysis Set at p < .001
| ROIs Fear Remembered vs. Fear Forgotten p < .05 | |||||||
|---|---|---|---|---|---|---|---|
| Boys (CAH—Control) > Girls (CAH—Control) | |||||||
| k (Cluster) | T (Peak) | x,y,z (mm) | P Uncorrected | k (Cluster) | T (Peak) | x,y,z (mm) | P Uncorrected |
| Left amygdala | Right amygdala | ||||||
| 62 | 2.40 | −8 −4 −14 | P = .01 | 116 | 2.12 | 22 0 −16 | P = .02 |
| Left anterior hippocampus | Right anterior hippocampus | ||||||
| 47 | 2.32 | −12 −6 −16 | P = .01 | 70 | 2.63 | 32 −18 −14 | P < .01 |
| Left posterior hippocampus | Right posterior hippocampus | ||||||
| 134 | 2.49 | −30 −26 −10 | P < .01 | 81 | 2.81 | 32 −24 −14 | P < .01 |
| ROIs Fear Remembered vs. Baseline p < .05 | |||||||
| k (Cluster) | T (Peak) | x,y,z (mm) | P Corrected | k (Cluster) | T (Peak) | x,y,z (mm) | P Corrected |
| Left amygdala | Right amygdala | ||||||
| 71 | 2.32 | −8 −4 −14 | P = .085 | 77 | 2.72 | 22 2 −20 | P < .05 |
| Left anterior hippocampus | Right anterior hippocampus | ||||||
| 161 | 3.94 | −30 −14 −16 | P < .005 | 83 | 2.86 | 30 −8 −24 | P < .05 |
| Left posterior hippocampus | Right posterior hippocampus | ||||||
| 238 | 3.12 | −30 −20 −16 | P < .05 | 180 | 3.05 | 32 −36 2 | P < .05 |
| Whole Brain Fear Remembered vs. Baseline p < .001 | |||||||
| Boys (CAH—Control) > Girls (CAH—Control) | Control > CAH | ||||||
| k (Cluster) | T (Peak) | x,y,z (mm) | P Uncorrected | k (Cluster) | T (Peak) | x,y,z (mm) | P Uncorrected |
| Left anterior cingulate | Left fusiform | ||||||
| 483 | 5.83 | −12 48 10 | P < . 001 | 590 | 4.84 | −34 66 −16 | P < .001 |
| Right medial temporal | Left Superior parietal lobule | ||||||
| 281 | 4.33 | 46 −54 0 | P < .001 | 44 | 3.75 | −24 −74 42 | P < .001 |
CAH = Congenital Adrenal Hyperplasia.
FIGURE 1.
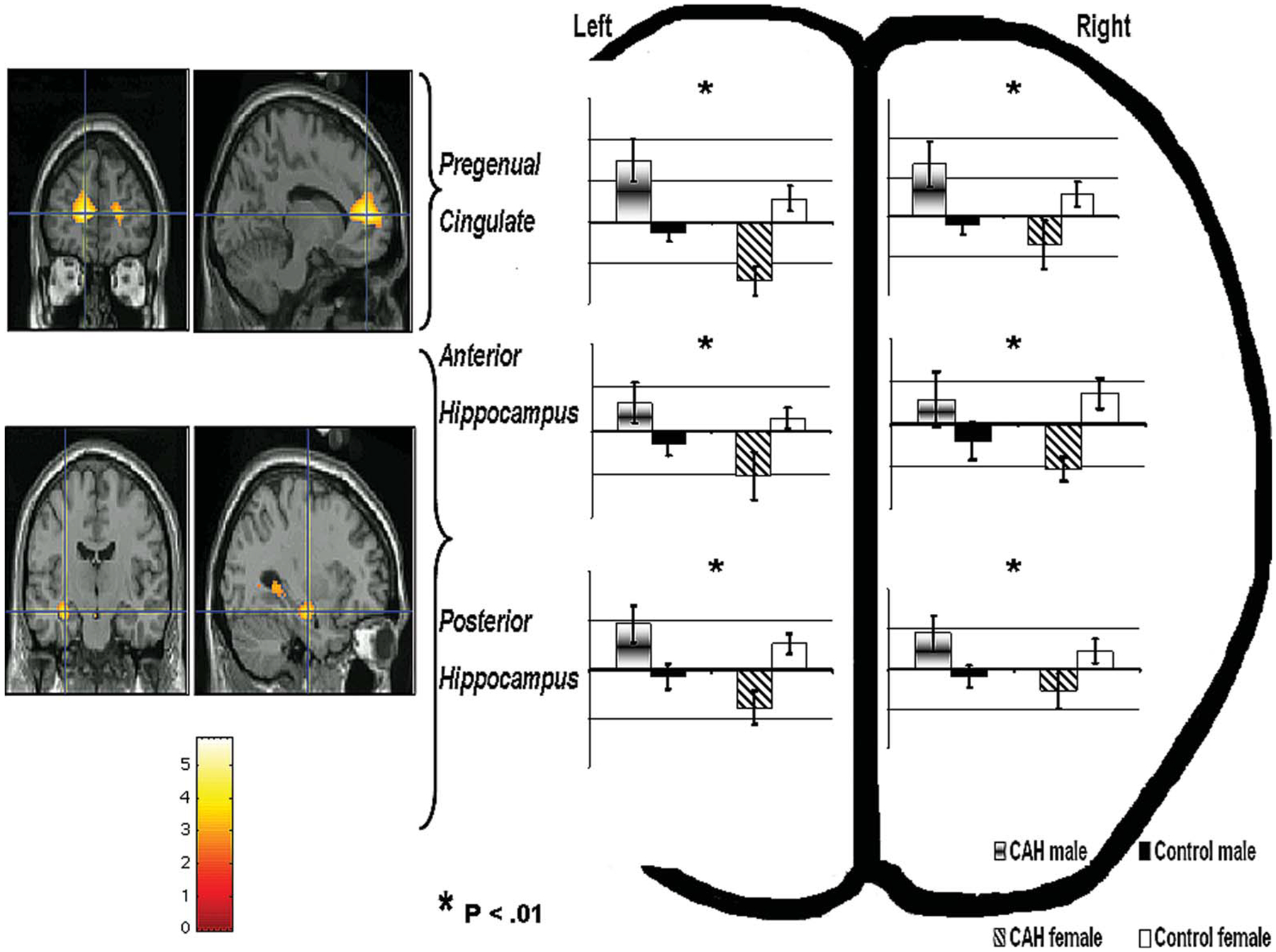
Figure shows the significant sex by group interaction in the anterior cingulate and anterior and posterior hippocampus (left side) and the BOLD signal changes (%) associated with it for each group separately (right side) for the remembered fear faces versus baseline contrast. Bar graphs display peak voxel activations. Error bars denote S.E.M.
FIGURE 2.
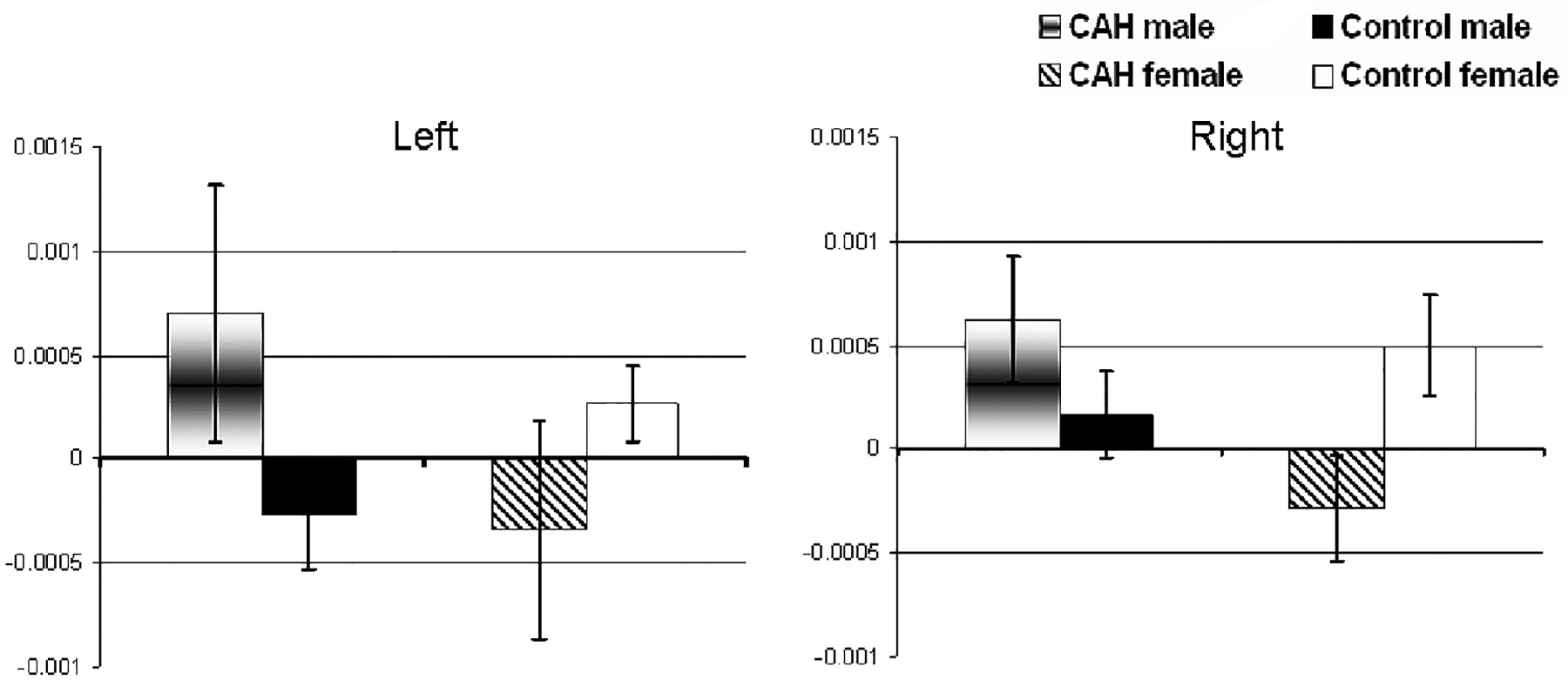
Amygdala mean peak voxel activations (S.E.M.) for the remembered fear faces versus baseline contrast in each group separately. These peak activations were identified previously in the contrast of sex by group interaction.
In order to clarify the sex by diagnosis interaction, direct analyses of BOLD signal changes were performed between males with CAH and healthy males, and between females with CAH and healthy females. These analyses revealed that CAH males had significantly higher BOLD signal changes than healthy males in all the ROIs sampled: left amygdala [MNI: −10 −2 −14] t = 2.10, right amygdala [MNI: 36 0 −24] t = 2.58, p < .05, left anterior hippocampus [MNI: −32 −12 −14] t = 2.82, p < .05, left posterior hippocampus [MNI: −32 −20 −14] t = 2.11, p < .05, right anterior hippocampus [34 −6 −24] t = 2.51, p < .05, and right posterior hippocampus [MNI: 40 −24 −8] t = 2.70. Conversely, relative to healthy females, females with CAH exhibited lower BOLD activations in the hippocampal ROIs: left anterior hippocampus [MNI: −28 −18 −20] t = 2.42, p < .05, left posterior hippocampus [MNI: −14 −34 −2] t = 2.64, p < .05, right anterior hippocampus [MNI: 18 −8 −16] t = 1.72, p < .05 and right posterior hippocampus [MNI: 16 −32 −2] t = 1.86, p < .05. The amygdalae did not differ by diagnosis in the female group. Finally, there were no significant main effects of diagnosis or sex in any of the ROIs examined.
Correlations
Several significant correlations (p < .05) were found between scores of memory performance for fearful remembered faces (d’) and ROI activations. The pattern, however, was mixed.
Healthy females showed a negative correlation between memory performance and hippocampal activation (left anterior (t = 2.57, MNI: −32 −18 −20), left posterior (t = 2.37, MNI: −32 −20 −18) and right posterior hippocampus (t = 3.79, MNI: 34 −36 −6)). The higher the hippocampal activation was, the worse was memory performance in healthy females. CAH females showed no significant correlations with performance in these regions.
CAH males, similarly to healthy females, showed a negative correlation between memory performance and activation of hippocampal ROIs, including left posterior (t = 2.03, MNI: −24 −26 −8), right anterior (t = 4.49, MNI: 28 −18 −2), and right posterior (t = 3.76,MNI: 28 −20 −12) regions. They also showed a negative correlation with the right amygdala (t = 3.24, MNI: 26 −2 −24). In contrast, healthy males showed that better encoding of fearful faces was associated with greater activation of the left anterior (t = 4.41, MNI: −24 −14 −12) and posterior (t = 4.83, MNI: −12 −38 8) hippocampus.
Whole Brain Imaging Analysis
Remembered fearful faces versus forgotten fearful faces.
Whole brain analysis of the remembered fearful faces versus forgotten fearful faces contrast did not show any significant effects at the uncorrected threshold level of p < .001.
Remembered fearful faces versus baseline (Table 3).
At the uncorrected threshold of p < .001, whole brain analysis of the contrast of remembered fearful faces versus baseline revealed a number of significant areas of activations to the interaction sex by diagnosis. In addition to those of the medial temporal cortex that included amygdala and hippocampus, these activations included left pregenual cingulate cortex [MNI: 12 48 10] t = 5.83 and the right medial temporal lobe [MNI: 46 −54 0] t = 4.33.
With regards to main effects, control adolescents relative to patients with CAH showed stronger activations in the left fusiform gyrus [MNI: −34 66 −16] t = 4.84 and the left superior parietal lobule [MNI: −24 −74 42] t = 3.75. There were no significant regions more activated by the CAH than by the control group.
To clarify the sex by diagnosis interaction, follow-up analyses were conducted in the male group separately from the female group. Compared to healthy males, males with CAH showed higher BOLD signal changes in left [MNI: −12 48 10] t = 3.88, p < . 05 and right [MNI: 0 54 4] t = 1.85, p < .05 pregenual cingulate cortices. Compared to healthy females, females with CAH exhibited lower BOLD changes in left [MNI: −8 46 10] t = 2.57, p < .05 and right [MNI: 0 54 10] t = 1.86, p < .05 pregenual cingulate cortices.
DISCUSSION
Adolescents with CAH, characterized by in utero absence of cortisol in both males and females, and by in utero excessive testosterone in females, showed impaired memory recall particularly of fearful faces. This memory impairment was not influenced by sex. Furthermore, in line with predictions, the key findings revealed abnormal responses of the subcortical neural network underlying emotional memory in the CAH group. Amygdala and hippocampus responses to successfully encoded fearful faces were influenced by CAH status as a function of sex. Girls with CAH showed abnormally low amygdala and hippocampal responses to remembered fearful faces, whereas boys with CAH showed abnormally high responses in these regions relative to their healthy counterparts. Finally, an exploratory whole brain analysis indicated a similar pattern of activation in the pregenual ACC, a structure bridging cognitive and emotional processes.
This is the first study to address the neural correlates of emotional memory in CAH. Previous research has examined neurocognitive function of women with CAH, and found no impairment on verbal or short-term memory (Malouf, Migeon, Carson, Petrucci, & Wisniewski, 2006). However, this research did not extend cognitive testing to emotional material. The prediction of a hormonal impact on emotional features of cognitive function is supported by (1) the well-recognized modulation of mood by sex and stress (i.e., glucocorticoid) steroids (McEwen, 2008; Shively & Bethea, 2004); (2) the presence of steroid receptors in structures involved in emotional processes (de Kloet et al., 2005; Lupien et al., 2005; Wilson & Davies, 2007); (3) findings of alterations of hippocampal structure and function following exposure to chronic early life steroid dysfunctions (excess or depletion) in offspring (Hebbard et al., 2003; Owen et al., 2005; Welberg & Seckl, 2001); (4) and finally evidence of abnormalities within the amygdala in youths with steroid-related endocrine disorders including CAH (Ernst et al., 2007; Merke et al., 2003) and Cushing Syndrome (Bourdeau, Bard, Forget, Boulanger, Cohen, & Lacroix, 2005; Maheu, Mazzone, et al., 2008).
Consistent with this notion, we recently reported, in a sample of adolescents with CAH, memory impairments influenced by the emotional valence of stimuli (Maheu, Merke, et al., 2008). This deficit affected negative stimuli but not positive stimuli. In the present work, we also found memory impairment predominantly to negatively valenced stimuli (fearful faces), even though we employed stimuli that were different from those used by Maheu et al. (expressive faces vs. International Affective Picture System, IAPS). In both studies, memory performance was affected by CAH status, that is, worse performance for CAH relative to controls, but this CAH-related effect was not influenced by sex. Although the relatively low hit rate of about 30–45% should be viewed with caution, the findings are in line with previous studies in healthy adult and adolescent volunteers using the same task (Nelson et al., 2003) as well as CAH and age-matched controls using the IAPS stimuli set (Maheu et al., 2008) for emotional stimuli.
This absence of sex effect on performance is in contrast to the neuroimaging findings. Indeed, the direction of abnormal activation in regions of interest differed between males and females with CAH. Females with CAH, who are exposed to high androgens in utero, showed hypoactivation, whereas males with CAH showed hyperactivation in the amygdala, hippocampus and pregenual cingulate cortex relative to their respective healthy counterparts. The fact that both hyper- and hypo-activation of these ROIs are associated with abnormal performance suggests that the relationship between memory function and neural activation may follow an inverted U curve. Too low or too high activation of these regions may be associated with impaired memory. This sex effect also suggests that in utero androgen excess, the hormonal difference between affected females and males, may alter the neural consequences of in utero glucocorticoid deficiency.
Our data suggest that steroid imbalances have organizational effects on the brain, defined as permanent alterations in brain structure, organization, or function. We observed perturbations in the amygdala, hippocampus, and pregenual cingulate cortex in adolescents with CAH. All three brain structures possess androgen and glucocorticoid receptors (Handa et al., 1994; Nunez et al., 2003; Roozendaal, 2003; Lupien & Lepage, 2001; Wilson & Davies, 2007). In addition, pre-natal and early-life steroid (glucocorticoid and androgen) excesses or depletions have been shown to be associated with neuronal degeneration, alterations in steroid gene expression, and dendritic and synaptic deteriorations in these regions (Cerqueira et al., 2007; Cooke et al., 1998; Hashimoto et al., 1989; Owen et al., 2005; Weinstock, 2008). Moreover, pre-natal and early-life androgen and glucocorticoid levels were also shown to influence emotion regulation and memory performance in mammal offspring (Moriceau et al., 2004; Owen et al., 2005; Weinstock, 2008; Hebbard et al., 2003), as well as in youths with CAH (Maheu, Merke, et al., 2008; Mueller et al., 2008). Thus, pre-natal excess in androgen levels and/or prenatal depletion in glucocorticoid levels may have influenced the functional development of amygdala, hippocampus and pregenual cingulate cortex, and associated memory for threat stimuli in patients with CAH.
Pre-natal and early life alterations in CRH and ACTH could also explain our findings. Patients with CAH show hypersecretion of CRH and ACTH, due to lack of glucocorticoid feedback on the hypothalamus-pituitary-adrenal (HPA) axis (Merke & Bornstein, 2005). CRH and the melanocortin-4 receptors (MC4R), to which ACTH binds, are abundantly expressed in the amygdala, hippocampus, and frontal cortex (Aguilera, Nikodemova, Wynn, & Catt, 2004; Chaki & Okuyama, 2005; Gantz & Fong, 2003), and acute elevations in CRH and cerebral ACTH levels were shown to influence emotional learning as well as anxiety-like and depression-like behaviors (Chaki, Ogawa, Toda, Funakoshi, & Okuyama, 2003; Chaki & Okuyama, 2005; Croiset, Nijsen, & Kamphuis, 2000; Gulpinar & Yegen, 2004; Jaferi & Bhatnagar, 2007; McGaugh, 1983; Roozendaal, Brunson, Holloway, McGaugh, & Baram, 2002; Yamano et al., 2004). Finally, the possible influence of lifetime glucocorticoid treatment on neuronal function in the patients with CAH must be acknowledged. Intermittent periods of over- or under-treatment may have influenced the functional development of the frontal cortex, amygdala, and hippocampus, leading to memory deficits and neuronal dysfunctions in the CAH group.
Similarly to previous work (Ernst et al., 2007), findings were qualitatively different for males and females. In the present work, girls with CAH showed hypoactivation and boys with CAH showed hyperactivation in all three structures. The direction of these abnormalities detected during memory encoding is opposite to that reported in our previous work during exposure to negative stimuli, which examined neural processes during attentional processing (Ernst et al., 2007). Indeed, during exposure to negative stimuli, girls with CAH showed abnormally high activation of the amygdala relative to healthy girls, whereas boys with CAH did not differ from healthy boys. These opposite findings suggest that the direction of functional perturbations depend on the process being challenged. More work will be needed to confirm these findings. Together with our previous findings of reduction of amygdala volume in both male and female CAH patients compared to healthy children (Merke et al., 2003), the present study supports amygdala dysfunction in CAH, particularly in females, and suggests that early disturbances in the steroid hormonal milieu may affect emotional processing. The modulation by sex status of CAH-related neural abnormalities is important as it suggests a contribution of androgen imbalance to the observed dysfunctions. The dearth of data on the relative effects of sex and stress steroids on amygdala function makes further interpretations highly speculative. However, recent data have reported that supraphysiological levels of androgens were associated with decreased amygdala activation and deficits in declarative memory (Maki et al., 2007). However, these findings were observed in elderly men (66–86 years), and may not be relevant to findings in adolescents with early exposure to excess androgen. However, they hint to detrimental effects of androgen excess on the functioning of key memory-related structures during memory encoding. Finally, considering that amygdala has been shown to influence hippocampus activity during the emotional memory process (Richardson, Strange, & Dolan, 2004; Roozendaal, 2003), the alteration in the hippocampus observed here could be partially explained through its interaction with the amygdala. However the increase in activation of hippocampus in CAH male could be also a compensatory effect to try to reach better performance during the memory task.
In addition to the a priori regions, the left anterior pregenual cingulate (ACC) also presented a significant sex by diagnosis interaction for remembered fearful faces. Liberzon et al. (2007) showed that the same rostral ACC area was activated in combat patients with posttraumatic stress disorder (PTSD) but not in controls in response to emotional challenges. PTSD has previously been linked to perturbed cortisol levels in adult (Liberzon et al., 2007) and pediatric (Carrion et al., 2002) patients. Moreover, Kennedy et al. (2006) reported reduced ACC activation (pregenual, BA32) in women with Major Depression who did not respond to antidepressant treatment, but elevated ACC activation in healthy females during mentalizing about sad personal events. Although the study by Kennedy et al. (2006) did not examine the impact of the processing of negative emotional events in males, her findings are consistent with the current data in that female patients showed decreased neural responses in this region relative to healthy females. Our findings thus suggest a potential impact of steroid dysfunction on the pregenual ACC in CAH and point to a potential link to psychiatric disorders.
A number of limitations should be mentioned. First, the sample of patients with CAH was relatively small. However, group differences were detected at both behavioral and neural levels, suggesting that the findings are robust. A second shortcoming of the study is that the model of CAH cannot unequivocally dissociate effects of glucocorticoids from effects of androgens, although in utero androgen excess primarily affects females. The present work stands as a first attempt to study early steroid abnormalities and their consequences on neural development in humans. This work is important to follow because of studies indicating a contribution of early steroid abnormalities to the development of mood and anxiety disorders (Goodyer, Herbert, Tamplin, & Altham, 2000a, 2000b; Herbert et al., 1996). A third shortcoming is that despite the use of an age- and sex-matched control group, the healthy group does not permit to control for the effects of a chronic illness on brain development. Fourth, it is possible that structural differences may have influenced the functional imaging findings. However, given that morphological changes were observed in a previous study in both males and females (Merke et al, 2003), it appears unlikely that these morphological changes would account for the differences in sex-related perturbations.
Despite these limitations, this is the first study, to our knowledge, to address the neurobiological network involved in emotional memory for social threat cues, that is, fear faces, in children with CAH, a congenital disorder of steroid dysfunction. Memory for socially negative cues is critical for avoiding potentially dangerous situations, and deficits in memorizing such cues can be deleterious for adaptive behaviors. Major questions remain regarding the hormonal mechanisms responsible for the findings. With the help of animal models, future work can help clarify these mechanisms. Research in humans with organizational hormonal disturbances is warranted to map the neural deficits and associated neurocognitive processes resulting from early steroid imbalance.
ACKNOWLEDGMENTS
We thank the staff of the NIH MR Center for making this study possible. FSM was supported by a postdoctoral fellowship from the Fonds de la recherche en sante du Quebec (FRSQ). This work was supported in part by the intramural program of NICHD and NIMH, NIH.
Contributor Information
Luigi Mazzone, Division of Child Neurology and Psychiatry, University of Catania, Catania, Italy.
Sven C. Mueller, NIH/DHHS, Bethesda, Maryland
Francoise Maheu, Research Centre of the CHU Ste-Justine, University of Montreal, Montreal, Canada.
Carol VanRyzin, Clinical Center, NICHHD/DHSS, Bethesda, Maryland.
Deborah P. Merke, Clinical Center, NICHHD/DHSS, Bethesda, Maryland
Monique Ernst, NIH/DHSS, Bethesda, Maryland.
REFERENCES
- Aguilera G, Nikodemova M, Wynn PC, & Catt KJ (2004). Corticotropin releasing hormone receptors: two decades later. Peptides, 25(3), 319–329. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Fallon JH, & Cahill L (1998). Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proceedings of the National Academy of Sciences of the U S A, 95(24), 14506–14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, & Glickman S (2005). Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition, 59(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA (2001). Cognitive function in congenital adrenal hyperplasia. Endocrinology and Metabolism Clinics of North America, 30(1), 173–192. [DOI] [PubMed] [Google Scholar]
- Bourdeau I, Bard C, Forget H, Boulanger Y, Cohen H, & Lacroix A (2005). Cognitive function and cerebral assessment in patients who have Cushing’s syndrome. Endocrinology and Metabolism Clinics of North America, 34(2), 357–369, ix. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, & Williams R (1995). Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behaviour Research and Therapy, 33(7), 755–770. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, & Sheridan MA (2001). Encoding processes during retrieval tasks. Journal of Cognitive Neuroscience, 13(3), 406–415. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, & Cahill L (2000). Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience, 20(19), RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, & Reiss AL (2002). Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry, 51(7), 575–582. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, & Sousa N (2007). Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cerebral Cortex, 17(9), 1998–2006. [DOI] [PubMed] [Google Scholar]
- Chaki S, Ogawa S, Toda Y, Funakoshi T, & Okuyama S (2003). Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. European Journal of Pharmacology, 474(1), 95–101. [DOI] [PubMed] [Google Scholar]
- Chaki S, & Okuyama S (2005). Involvement of melanocortin-4 receptor in anxiety and depression. Peptides, 26(10), 1952–1964. [DOI] [PubMed] [Google Scholar]
- Cherrier MM (2005). Androgens and cognitive function. Journal of Endocrinological Investigation, 28(3 Suppl), 65–75. [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155–159. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, & Berenbaum SA (2005). Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neuroscience and Biobehavioral Reviews, 29(2), 353–384. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, & Breedlove SM (1998). Sexual differentiation of the vertebrate brain: Principles and mechanisms. Frontiers in Neuroendocrinology, 19(4), 323–362. [DOI] [PubMed] [Google Scholar]
- Cooke BM, & Woolley CS (2005). Sexually dimorphic synaptic organization of the medial amygdala. Journal of Neuroscience, 25(46), 10759–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croiset G, Nijsen MJ, & Kamphuis PJ (2000). Role of corticotropin-releasing factor, vasopressin and the autonomic nervous system in learning and memory. European Journal of Pharmacology, 405(1–3), 225–234. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2002). Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry, 51(1), 68–80. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, & Holsboer F (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. [DOI] [PubMed] [Google Scholar]
- Ekman P, & Friesen W (1976). Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Ernst M, Maheu FS, Schroth E, Hardin J, Golan LG, Cameron J, et al. (2007). Amygdala function in adolescents with congenital adrenal hyperplasia: A model for the study of early steroid abnormalities. Neuropsychologia, 45(9), 2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Mechelli A, Turner R, & Price CJ (2000). Nonlinear responses in fMRI: The Balloon model, Volterra kernels, and other hemodynamics. Neuroimage, 12, 466–477. [DOI] [PubMed] [Google Scholar]
- Gantz I, & Fong TM (2003). The melanocortin system. American Journal of Physiology. Endocrinology and Metabolism, 284(3), E468–474. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, & Altham PM (2000a). First-episode major depression in adolescents. Affective, cognitive and endocrine characteristics of risk status and predictors of onset. British Journal of Psychiatry, 176, 142–149. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, & Altham PM (2000b). Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British Journal of Psychiatry, 177, 499–504. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, & McEwen BS (1991). Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. Journal of Comparative Neurology, 313(3), 479–485. [DOI] [PubMed] [Google Scholar]
- Gulpinar MA, & Yegen BC (2004). The physiology of learning and memory: Role of peptides and stress. Current Protein and Peptide Science, 5(6), 457–473. [DOI] [PubMed] [Google Scholar]
- Hagger C, & Bachevalier J (1991). Visual habit formation in 3-month-old monkeys (Macaca mulatta): Reversal of sex difference following neonatal manipulations of androgens. Behavioural Brain Research, 45(1), 57–63. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, & O’Keefe JA (1994). Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior, 28(4), 464–476. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, & Weinberger DR (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage, 17(1), 317–323. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Marystone JF, Greenough WT, & Bohn MC (1989). Neonatal adrenalectomy alters dendritic branching of hippocampal granule cells. Experimental Neurology, 104(1), 62–67. [DOI] [PubMed] [Google Scholar]
- Haskett RF (1985). Diagnostic categorization of psychiatric disturbance in Cushing’s syndrome. American Journal of Psychiatry, 142(8), 911–916. [DOI] [PubMed] [Google Scholar]
- Hebbard PC, King RR, Malsbury CW, & Harley CW (2003). Two organizational effects of pubertal testosterone in male rats: Transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Experimental Neurology, 182(2), 470–475. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Altham PM, Pearson J, Secher SM, & Shiers HM (1996). Adrenal secretion and major depression in 8- to 16-year-olds, II. Influence of co-morbidity at presentation. Psychological Medicine, 26(2), 257–263. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Larson M, Brodersen L, & Lehman H (1992). First time experiences in infancy: When they appear pleasant, do they activate adrenocortical stress response? Developmental Psychobiology, 25, 319–334. [DOI] [PubMed] [Google Scholar]
- Hines M (2008). Early androgen influences on human neural and behavioural development. Early Human Development, 84(12), 805–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hudson MS, Griffing GT, Melby JC, & Pope HG Jr. (1987). Phenomenology and family history of affective disorder in Cushing’s disease. American Journal of Psychiatry, 144(7), 951–953. [DOI] [PubMed] [Google Scholar]
- Jaferi A, & Bhatnagar S (2007). Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Research, 1186, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kelly WF (1996). Psychiatric aspects of Cushing’s syndrome. QJM: An International Journal of Medicine, 89(7), 543–551. [DOI] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, & Zubieta JK (2006). Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Archives of General Psychiatry, 63, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Kirschbaum C, & Wolf OT (2005). Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiology Learning and Memory, 83(2), 158–162. [DOI] [PubMed] [Google Scholar]
- LaBar KS, & Cabeza R (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7(1), 54–64. [DOI] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, & Taylor SF (2007). Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. American Journal of Psychiatry, 164(8), 1250–1258. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, et al. (2005). Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology, 30(3), 225–242. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, & Lepage M (2001). Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioural Brain Research, 127(1–2), 137–158. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, & McEwen BS (1997). The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Research Reviews, 24, 1–27. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Mazzone L, Merke DP, Keil MF, Stratakis CA, Pine DS, et al. (2008). Altered amygdala and hippocampus function in adolescents with hypercortisolemia: A functional magnetic resonance imaging study of Cushing syndrome. Development and Psychopathology, 20(4), 1177–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Merke DP, Schroth EA, Keil MF, Hardin J, Poeth K, et al. (2008). Steroid abnormalities and the developing brain: Declarative memory for emotionally arousing and neutral material in children with congenital adrenal hyperplasia. Psychoneuroendocrinology, 33(2), 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Ernst M, London ED, Mordecai KL, Perschler P, Durso SC, et al. (2007). Intramuscular testosterone treatment in elderly men: Evidence of memory decline and altered brain function. Journal of Clinical Endocrinology and Metabolism, 92(11), 4107–4114. [DOI] [PubMed] [Google Scholar]
- Malouf MA, Migeon CJ, Carson KA, Petrucci L, & Wisniewski AB (2006). Cognitive outcome in adult women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hormone Research, 65(3), 142–150. [DOI] [PubMed] [Google Scholar]
- Matthews SG (2001). Antenatal glucocorticoids and the developing brain: Mechanisms of action. Seminars in Neonatology, 6(4), 309–317. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583(2–3), 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL (1983). Hormonal influences on memory. Annual Review of Psychology, 34, 297–323. [DOI] [PubMed] [Google Scholar]
- Merke DP, & Bornstein SR (2005). Congenital adrenal hyperplasia. Lancet, 365(9477), 2125–2136. [DOI] [PubMed] [Google Scholar]
- Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, & Giedd JN (2003). Children with classic congenital adrenal hyperplasia have decreased amygdala volume: Potential prenatal and postnatal hormonal effects. Journal of Clinical Endocrinology and Metabolism, 88(4), 1760–1765. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, & Kandel ER (1998). Cognitive neuroscience and the study of memory. Neuron, 20(3), 445–468. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda S, Aburada M, & Tabira T (2004). Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. Journal of Neuroscience, 24(24), 5492–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, & Sullivan RM (2004). Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience, 22(5–6), 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. (2008). Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology, 33, 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, et al. (2003). Developmental differences in neuronal engagement during implicit encoding of emotional faces: An event-related fMRI study. Journal of Child Psychology and Psychiatry, 44(7), 1015–1024. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Huppenbauer CB, McAbee MD, Juraska JM, & DonCarlos LL (2003). Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. Journal of Neurobiology, 56(3), 293–302. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, & Matthews SG (2005). Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neuroscience and Biobehavioral Reviews, 29(2), 209–226. [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, & Lyons DM (2008). Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology, 33(3), 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Schmidt LA, Henderson HA, Schulkin J, & Fox NA (2008). Salivary cortisol levels and infant temperament shape developmental trajectories in boys at risk for behavioral maladjustment. Psychoneuroendocrinology, 33(7), 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lopez JR, & Woody SR (2001). Memory for facial expressions in social phobia. Behaviour Research and Therapy, 39(8), 967–975. [DOI] [PubMed] [Google Scholar]
- Phelps EA (2006). Emotion and cognition: Insights from studies of the human amygdala. Annual Review Psychology, 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Pine DS, Lissek S, Klein RG, Mannuzza S, Moulton JL 3rd, Guardino M, et al. (2004). Face-memory and emotion: Associations with major depression in children and adolescents. Journal of Child Psychology and Psychiatry, 45(7), 1199–1208. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, & Dolan RJ (2004). Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience, 7(3), 278–285. [DOI] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, et al. (2006). Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biological Psychiatry, 60(9), 966–973. [DOI] [PubMed] [Google Scholar]
- Roof RL (1993). Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behavioural Brain Research, 53(1–2), 1–10. [DOI] [PubMed] [Google Scholar]
- Roozendaal B (2003). Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Progress in Neuropsychopharmacology and Biological Psychiatry, 27(8), 1213–1223. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, & Baram TZ (2002). Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proceedings of the National Academy of Sciences of the U S A, 99(21), 13908–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, & Bethea CL (2004). Cognition, mood disorders, and sex hormones. Institute of Laboratory Animal Resources Journal, 45(2), 189–199. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Sollas AL, Dean E, & Neubort S (1993). Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: characterization of an in vivo model of controlled neuronal death. Journal of Comparative Neurology, 330(3), 324–336. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, & Corwin J (1988). Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General, 117, 34–50. [DOI] [PubMed] [Google Scholar]
- Sonino N, & Fava GA (2001). Psychiatric disorders associated with Cushing’s syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs, 15(5), 361–373. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, et al. (1999). Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of General Psychiatry, 56(10), 913–919. [DOI] [PubMed] [Google Scholar]
- Takahashi LK (1994). Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Brain Research Developmental Brain Research, 81(1), 121–127. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. (2009). NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. (1998). Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science, 281(5380), 1188–1191. [DOI] [PubMed] [Google Scholar]
- Weinstock M (2008). The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews, 32(6), 1073–1086. [DOI] [PubMed] [Google Scholar]
- Welberg LA, & Seckl JR (2001). Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology, 13(2), 113–128. [DOI] [PubMed] [Google Scholar]
- Wilson CA, & Davies DC (2007). The control of sexual differentiation of the reproductive system and brain. Reproduction, 133, 311–359. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, & Dolan RJ (2002). Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience, 5(3), 277–283. [DOI] [PubMed] [Google Scholar]
- Wolf OT (2008). The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychologica (Amst), 127(3), 513–531. [DOI] [PubMed] [Google Scholar]
- Worsley KM, Marrett S, Neelin P, Vandal AC, Friston KJ, & Evans AC (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4, 58–73. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, et al. (2004). Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. Journal of Veterinary Medical Science, 66(11), 1323–1327. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, & D’Esposito M (1997). Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. Neuroimage, 5(3), 179–197. [DOI] [PubMed] [Google Scholar]


