Abstract
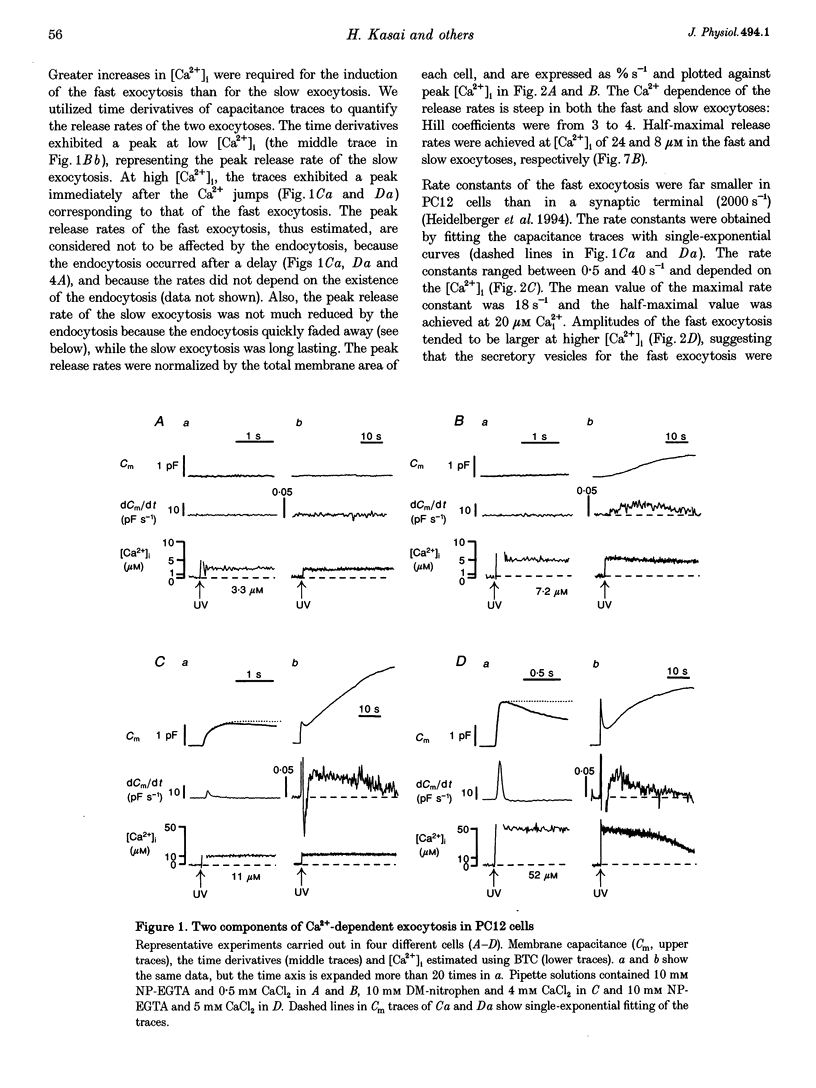
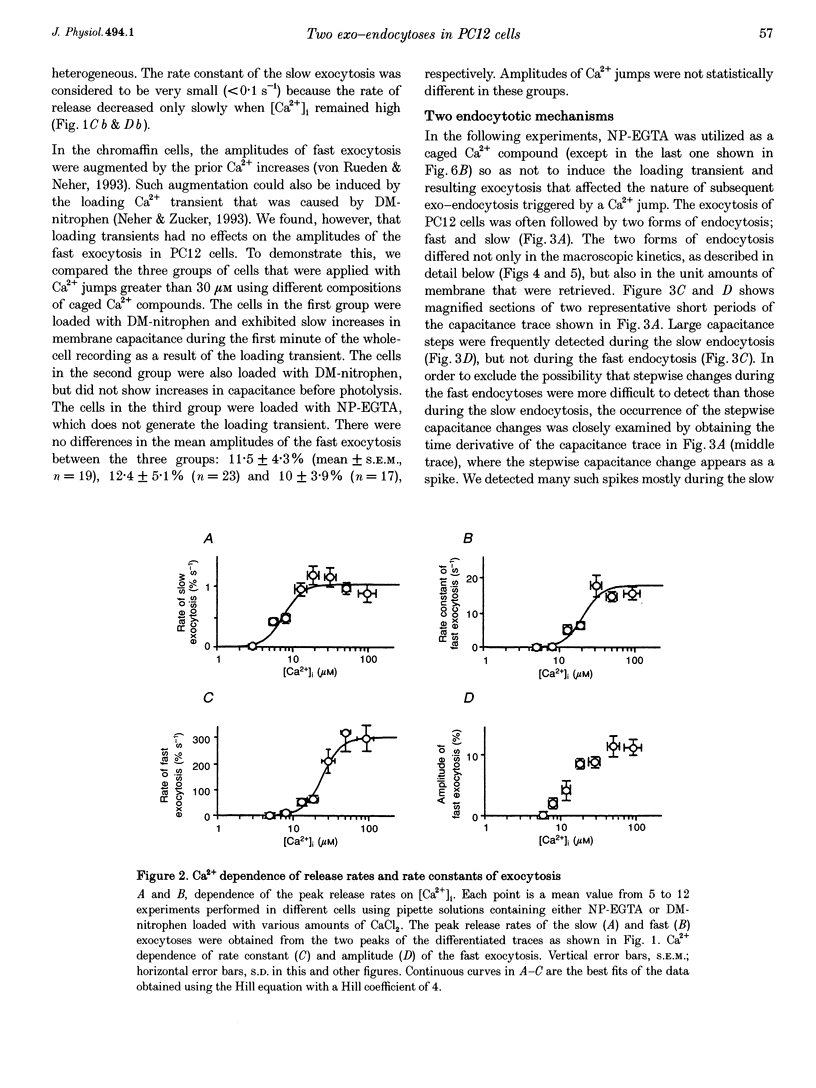
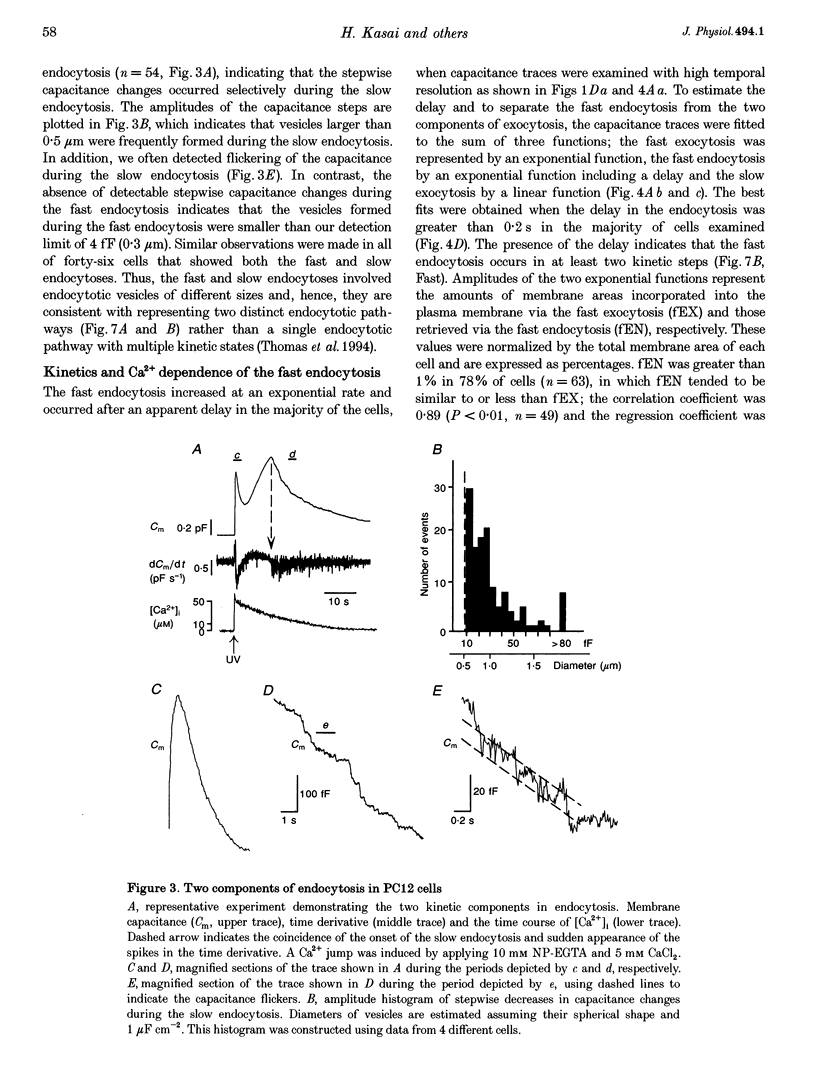
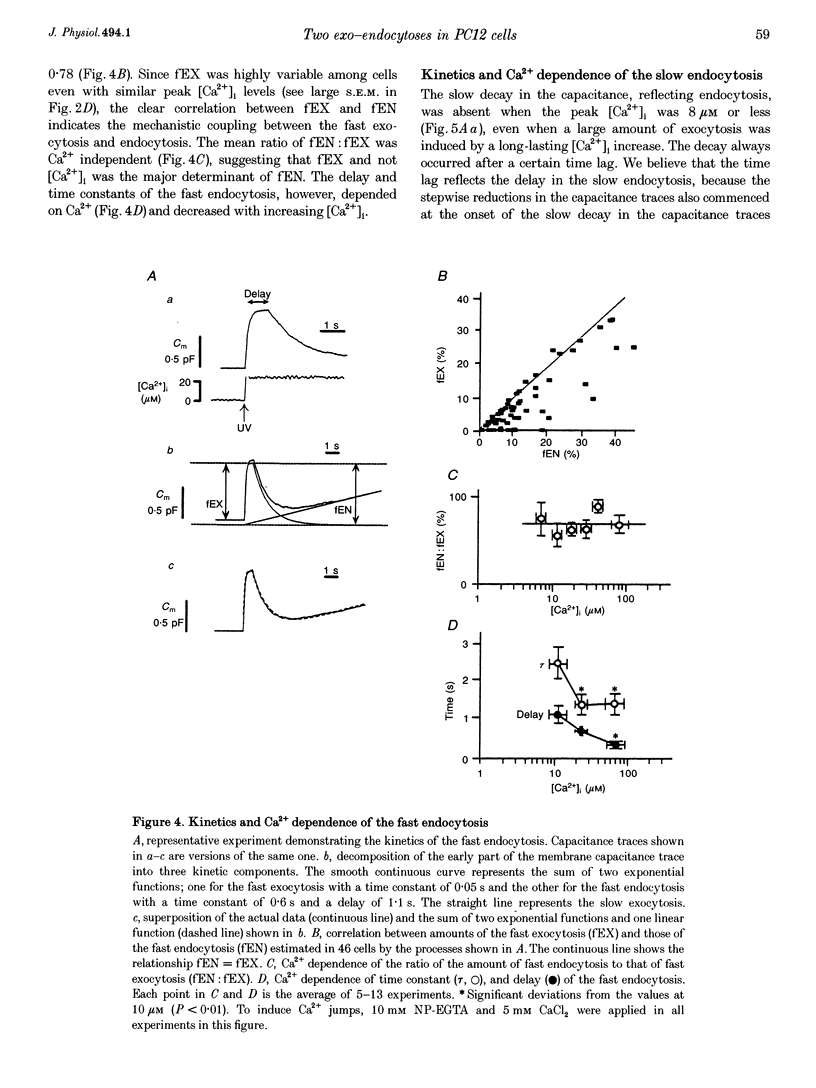
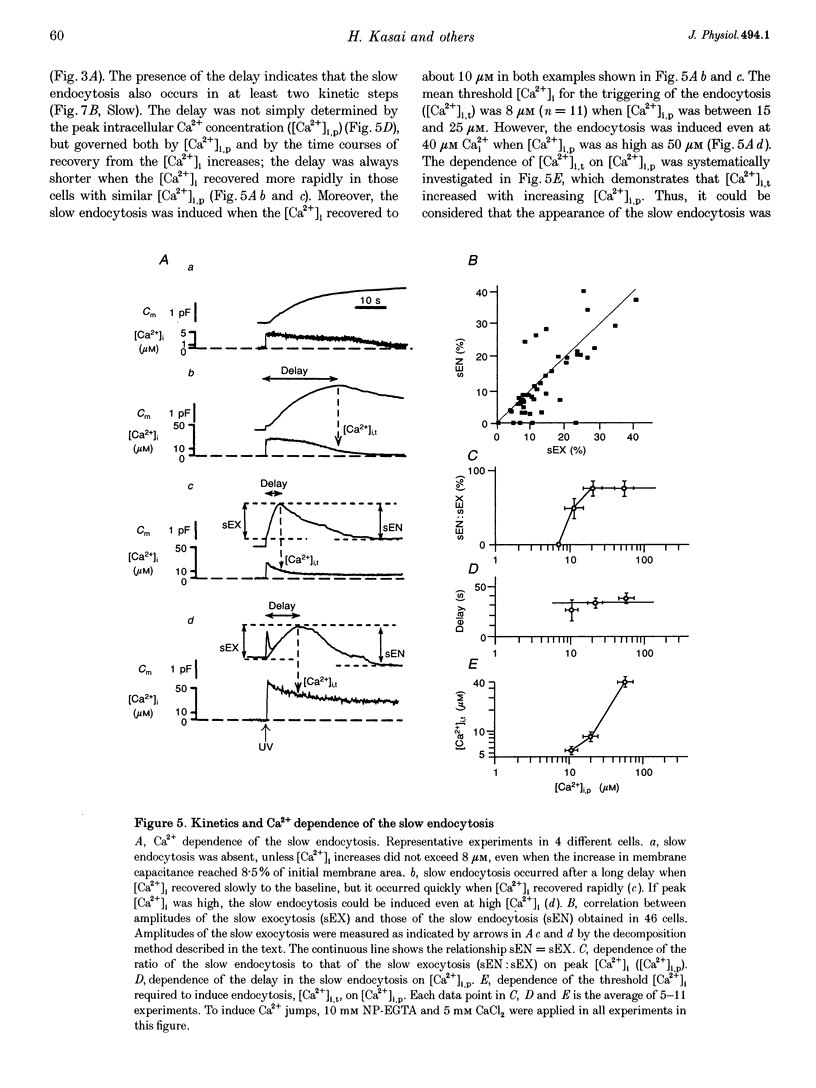
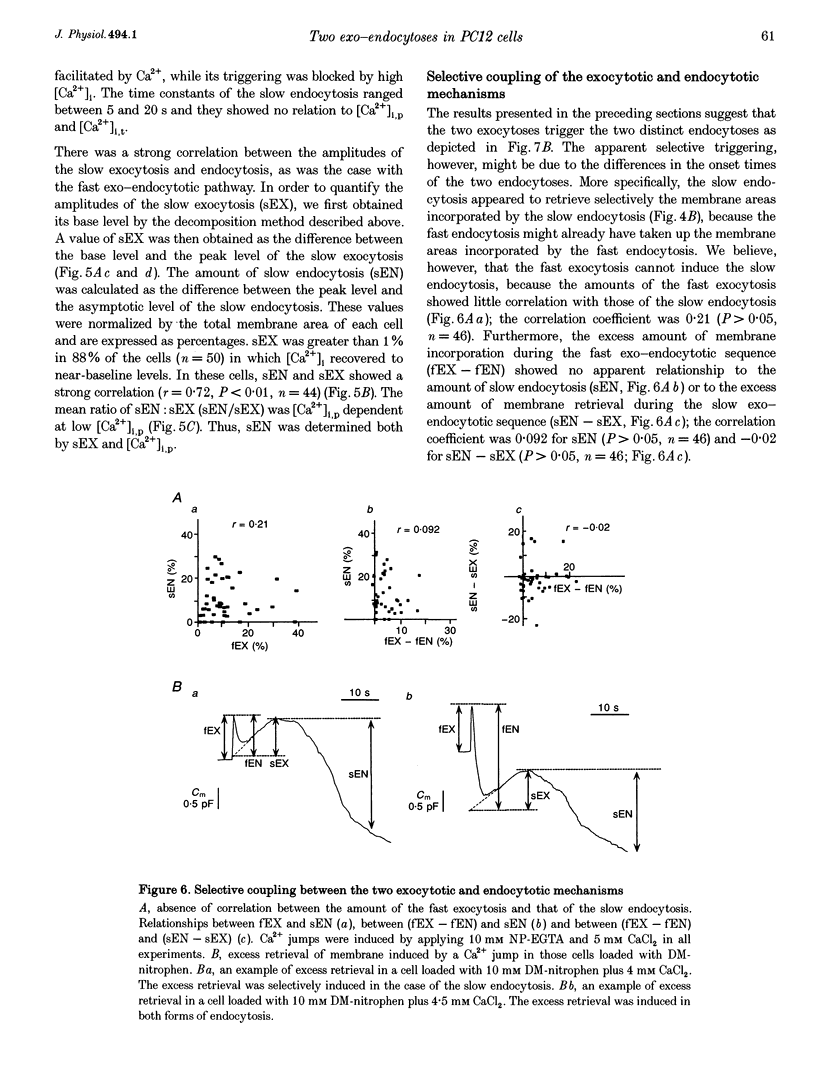
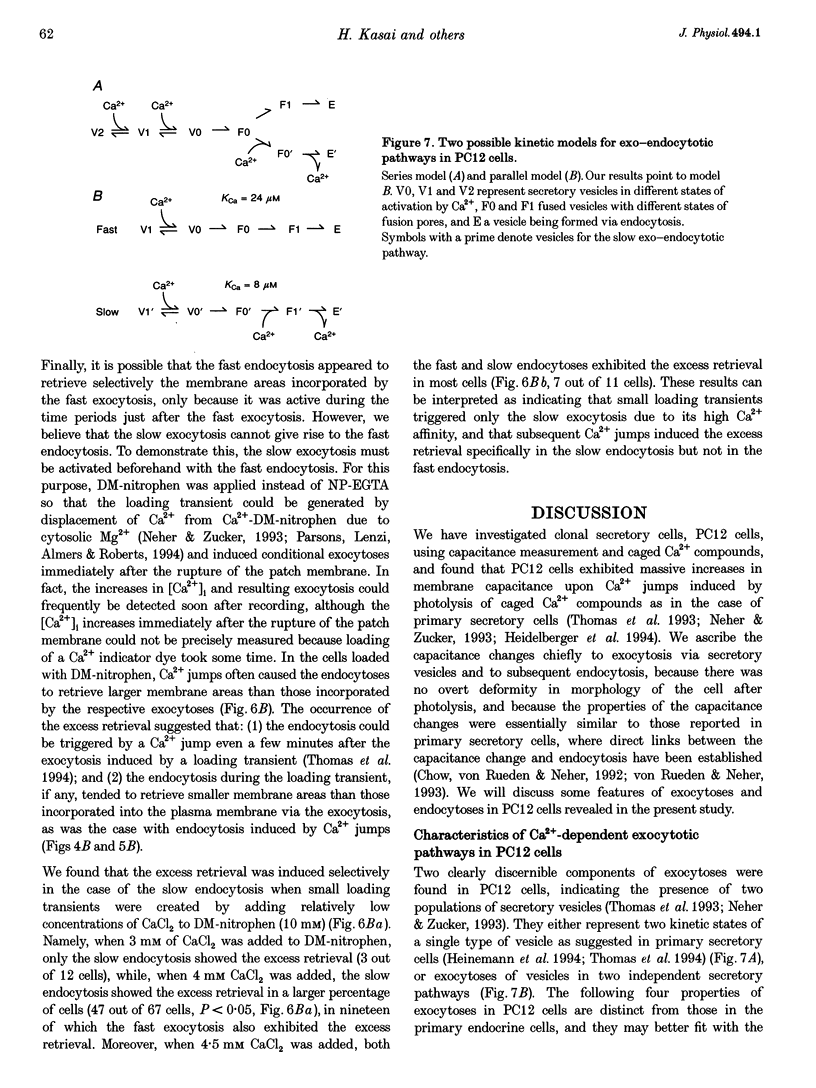
1. Changes in membrane capacitance evoked by the rapid photolysis of a caged Ca2+ compound, DM-nitrophen or nitrophenyl-EGTA, were investigated in undifferentiated PC12 cells. They were interpreted as representing exocytosis and endocytosis. 2. The Ca2+ jumps evoked two components of exocytosis. Slow exocytosis was selectively evoked with small increases in intracellular Ca2+ concentration between 5 and 10 microM, while fast exocytosis preceded the slow one at [Ca2+]i greater than 10 microM. 3. The release rates of the two components of exocytosis depended steeply on [Ca2+]i. A half-maximal release rate was achieved at 8 and 24 microM for the slow and fast exocytoses, respectively. 4. Prior Ca2+ rises did not augment the fast exocytosis. 5. The fast exocytosis was often followed by a rapid decrease in membrane capacitance, representing endocytosis, after a delay of 0.5-2 s. The speed and delay in the fast endocytosis were Ca2+ dependent. Amounts of the fast endocytosis tended to balance with those of the fast exocytosis evoked by the same Ca2+ jumps. 6. The slow exocytosis was followed by a sluggish endocytosis that was associated with large capacitance steps indicative of secretory processes involving large dense-core vesicles. The onset of the slow endocytosis exhibited a complex Ca2+ dependence. The amounts of the slow endocytosis appeared to parallel those of the slow exocytosis. Prior induction of the slow exocytosis gave rise to selective excess retrieval of membrane during the slow endocytosis. 7. These data indicate the existence of two distinct populations of secretory vesicles in PC12 cells. They seem to couple selectively with specific endocytotic mechanisms. Our data suggest that the two vesicles belong to two distinct secretory pathways.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Bauerfeind R., Régnier-Vigouroux A., Flatmark T., Huttner W. B. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 1993 Jul;11(1):105–121. doi: 10.1016/0896-6273(93)90275-v. [DOI] [PubMed] [Google Scholar]
- Bäck N., Soinila S., Virtanen I. Endocytotic pathways in the melanotroph of the rat pituitary. Histochem J. 1993 Feb;25(2):133–139. doi: 10.1007/BF00157985. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies G. C., Kaplan J. H. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R., Grohovaz F., Valtorta F., Meldolesi J. Neurotransmitter release: fusion or 'kiss-and-run'? Trends Cell Biol. 1994 Jan;4(1):1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature. 1977 Jul 28;268(5618):349–351. doi: 10.1038/268349a0. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989 Feb;108(2):401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Li Y. X., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993 Aug 27;74(4):669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Kasai H., Neher E. Dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels in a mammalian neuroblastoma-glioma cell line. J Physiol. 1992 Mar;448:161–188. doi: 10.1113/jphysiol.1992.sp019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Storage and release of neurotransmitters. Cell. 1993 Jan;72 (Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Knoll G., Plattner H., Nordmann J. J. Exo-endocytosis in isolated peptidergic nerve terminals occurs in the sub-second range. Biosci Rep. 1992 Dec;12(6):495–501. doi: 10.1007/BF01122037. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Inooka G., Li Y. X., Miyashita Y., Kasai H. Agonist-induced localized Ca2+ spikes directly triggering exocytotic secretion in exocrine pancreas. EMBO J. 1993 Aug;12(8):3017–3022. doi: 10.1002/j.1460-2075.1993.tb05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. M., Heuser J. E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984 Feb;98(2):685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta M., Inagaki N., Nemoto Y., Matsukura S., Takahashi M., Seino S. Synaptotagmin III is a novel isoform of rat synaptotagmin expressed in endocrine and neuronal cells. J Biol Chem. 1994 Apr 22;269(16):11675–11678. [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Parsons T. D., Lenzi D., Almers W., Roberts W. M. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994 Oct;13(4):875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Ramaswami M., Krishnan K. S., Kelly R. B. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994 Aug;13(2):363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Rebois R. V., Reynolds E. E., Toll L., Howard B. D. Storage of dopamine and acetylcholine in granules of PC12, a clonal pheochromocytoma cell line. Biochemistry. 1980 Mar 18;19(6):1240–1248. doi: 10.1021/bi00547a031. [DOI] [PubMed] [Google Scholar]
- Rosenboom H., Lindau M. Exo-endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals internally perfused with high calcium concentrations. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5267–5271. doi: 10.1073/pnas.91.12.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Thomas P., Lee A. K., Wong J. G., Almers W. A triggered mechanism retrieves membrane in seconds after Ca(2+)-stimulated exocytosis in single pituitary cells. J Cell Biol. 1994 Mar;124(5):667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Lee A. K., Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993 Jul;11(1):93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Torda M., Meldolesi J. The effect of alpha-latrotoxin on the neurosecretory PC12 cell line: electron microscopy and cytotoxicity studies. Neuroscience. 1983 Nov;10(3):1011–1024. doi: 10.1016/0306-4522(83)90239-7. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Effects of photolabile calcium chelators on fluorescent calcium indicators. Cell Calcium. 1992 Jan;13(1):29–40. doi: 10.1016/0143-4160(92)90027-p. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Petersen O. W., Olsnes S., Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H., Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994 Feb 24;367(6465):735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- von Rüden L., Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993 Nov 12;262(5136):1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]


