Abstract
Purpose
Transarterial chemoembolization (TACE) is recommended as a standard therapy for intermediate-stage hepatocellular carcinoma (HCC) and is the most widely used first-line treatment for advanced HCC. This study aimed to evaluate the clinical benefits and tolerability of TACE added to a combination of lenvatinib and programmed death-1 (PD-1) inhibitor in patients with unresectable HCC (uHCC).
Patients and Methods
We conducted a retrospective cohort study involving 144 patients with uHCC treated between August 2020 and August 2023. Patients received a combination of lenvatinib and a PD-1 inhibitor with or without TACE (T+L+P, n=81 or L+P, n=63, respectively). The baseline characteristics of the two groups were compared, and propensity score matching (PSM) was used to minimize bias. The study endpoints included overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). Factors influencing survival rates were analyzed using Cox regression, and adverse events (AEs) were documented and assessed.
Results
Before PSM, the T+L+P group showed significantly higher ORR (64.1% vs 44.4%, p < 0.05), longer median PFS (14.3 vs 9.6 months, p < 0.05), and longer median OS (24.6 vs 19.5 months, p < 0.05) compared to the L+P group. Even post-PSM, the T+L+P group showed significantly better OS and PFS compared to the L+P group (mOS: 28.0 vs 17.6 months p=0.0011, mPFS: 15.8 vs 9.3 months, p < 0.05). Univariate and multivariate analyses identified treatment options as independent factors for PFS and OS. The safety profile of the T+L+P regimen was acceptableThe incidence and severity of adverse reactions in the T+L+P group were not significantly different compared to the L+P group (any grade, 90.1 vs 93.6%, p=0.551; grade≥3, 25.9 vs 23.8%, p=0.843).
Keywords: unresectable hepatocellular carcinoma, lenvatinib, transarterial chemoembolization, PD-1 inhibitor, combination therapy, propensity score matching
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and is the third leading cause of cancer-related mortality worldwide.1 Despite the significant progress made in surgical resection, liver transplantation, and ablation, which have shown good potential in HCC treatment and are recommended as primary treatment options for early-stage HCC patients, approximately 70% of patients are diagnosed with HCC at Barcelona Clinic Liver Cancer(BCLC) B or C stages, for whom these methods are not suitable.2–5 However, there are only a few reports on unresectable hepatocellular carcinoma (uHCC) treatment to date. Therefore, exploring effective treatment options has important clinical significance in prolonging the survival of patients with uHCC.
Multi-tyrosine kinase inhibitors (TKIs) sorafenib and lenvatinib have been recommended as first-line therapy for patients with uHCC. However, they provide limited survival benefits. The median overall survival(mOS) with lenvatinib alone was 13.6 months, and with sorafenib was 12.3 months, and the objective response rate (ORR) was 21.4% and 9.2%, respectively.6,7
In the past few years, immune checkpoint inhibitors (ICIs), including programmed death-ligand 1 (PD-L1) inhibitors, have shown promising efficacy and safety in advanced HCC.8–10 However, The CheckMate 459 Phase III clinical trial demonstrated that, compared to monotherapy with sorafenib as first-line treatment for uHCC, ICIs monotherapy did not show significantly better efficacy (16.4 vs 14.7 months, P = 0.075).11 On the other hand, TKIs combined with ICIs have emerged as promising treatment options. Several randomized controlled trials have shown that the combination of anti-PD -(L)1 drugs and TKIs is effective and safe for patients with uHCC, and it is recommended as the first-line treatment.12,13 Although current ICI-based combination therapies have achieved survival benefits in patients with uHCC, the therapeutic response and efficacy are still limited. Therefore, improving the clinical efficacy of combination therapy remains a challenge in the treatment and management of uHCC.14,15
Current guidelines recommend Transarterial chemoembolization (TACE) as the standard therapy for intermediate-stage HCC, and it is the most widely used first-line treatment for advanced HCC, with encouraging tumor response in real-world practice.16,17 However, its efficacy is not satisfactory due to tumor progression, resulting in shorter progression-free survival (PFS). A growing number of studies have suggested that local combined targeting or immunotherapy can improve the survival of patients.18,19 The CHANCE001 trial showed that TACE combined with TKIs and PD-1 inhibitor resulted in a longer mOS compared with TACE alone (19.2 vs 15.7 months) and higher ORR (60.1% vs 32.0%).20 In addition, in another retrospective study, TACE combined with lenvatinib and PD-1 inhibitor also observed better mOS (29.0 vs 17.8 months) and median PFS (16.2 vs 10.2 months) than lenvatinib combined PD-1.21
This study aimed to compare the efficacy and safety of lenvatinib plus PD-1 inhibitor with and without TACE in a single-center and retrospective cohort of uHCC patients.
Materials and Methods
Patients
This retrospective study was approved by the Ethics Committee of the Affiliated Hospital of Guilin Medical College. All patients received their written informed consent during hospitalization. All patients were diagnosed with uHCC by imaging, such as dynamic computed tomography(CT) or magnetic resonance imaging (MRI), according to the guidelines of the BCLC staging system.22 According to the Japanese Society of Hepatology, uHCC is defined as the presence of large solitary or multiple tumors that either extensively affect both lobes of the liver or invade major vessels, such as the main trunk of the portal vein (VP4) and the inferior vena cava.23 This study collected clinical data from patients with uHCC who received TACE combined with lenvatinib and PD-1 (T+L+P) or lenvatinib combined with PD-1 (L+P) treatment at Guilin Medical University between August 2020 and September 2023. Based on whether patients received TACE treatment at enrollment, they were divided into the T+L+P group and the L+P group. Patients who received TACE treatment were given systemic therapy within one month following TACE procedure.
The inclusion criteria were as follows: (1) All patients were diagnosed with uHCC by imaging according to the guidelines of the BCLC staging system; (2) 18–75 years of age; (3) at least one radiologically measurable lesion; (4) Eastern Cooperative Oncology Group (ECOG) performance status score 0–2; (5) Child-Pugh was classified as A or B.
The exclusion criteria were as follows: (1) less than three months of survival time; (2) HCC combined with other malignant tumors; (3) Child-Pugh grade C liver function, uncontrollable ascites, or overt hepatic encephalopathy; (4) incomplete critical clinical data.
TACE Procedures
All patients underwent standardized routine TACE (cTACE). Percutaneous arterial puncture was performed by interventional physicians with five years or more of experience using the Seldinger method. A catheter sheath was placed with a short guide wire, and the catheter was intubated using the X-ray system. The catheter was super-selectively inserted into the tumor-supplying artery and chemoembolized with lipiodol (5–20mL) mixed with oxaliplatin (200 mg), and pirarubicin (20–60 mg). After chemoembolization, the tumor blood supply vessels were blocked, and angiography showed reduced or no tumor staining. After the operation, the catheter was pulled out, the puncture site was pressed, the puncture side limb was immobilized for 12 h, and the patient lay flat for 24 h.
Systemic Therapy Procedures
Patients received 8 mg (bodyweight < 60 kg) or 12 mg (bodyweight ≥ 60 kg) of lenvatinib orally once a day. Within seven days of starting on lenvatinib, they also received a PD-1 inhibitor (camrelizumab, tislelizumab, or sintilimab) intravenously at the standard dose (200 mg), with repeat dosing at 21-day intervals.
Follow Up
At the time of enrollment, all patients had their BCLC stage, physical examination, serological and laboratory tests (such as liver function tests, coagulation function tests, and alpha-fetoprotein (AFP) levels, as well as imaging studies, including contrast-enhanced abdominal CT or MRI and chest CT, documented. Following treatment (in both the T+L+P and L+P groups), all patients underwent monthly follow-ups. Every 3–4 months, contrast-enhanced CT or MRI was conducted according to the mRECIST criteria to evaluate the tumor response, and decisions on whether to repeat TACE were made based on these results.
Clinical Assessments and Toxicity Evaluation
Tumor response assessment, in accordance with the mRECIST criteria, categorizes outcomes based on the findings from Enhanced CT/MRI scans into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The overall response rate (ORR) included both CR and PR. The tumor response was evaluated by contrasted MRI or CT according to mRECIST. Vital signs and clinical laboratory test results were recorded and assessed for the incidence and severity of adverse events based on the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0
Propensity Score Matching (PSM) Analysis
The potential confounding and selection bias between the two groups was reduced by using propensity score matching analysis and 1:1 nearest-neighbor matching without replacement using a caliper width of 0.02. The propensity score was estimated using a logistic regression model fit with the following variables: gender, age, ECOG performance status, hepatitis B virus (HBV), tumor size, tumor number, Child-Pugh grade, BCLC stage, AFP level, macrovascular invasion, and ALBI stage. The standardized mean difference was used to evaluate the covariate balance for the propensity-matched cohorts.
Statistical Analysis
Categorical data were expressed as numbers with percentages. Continuous variables were analyzed using the Student’s t-test or the Mann–Whitney U-test. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. The Log rank test was used to compare differences in PFS and OS between the two groups. Survival curves were plotted using the Kaplan–Meier method. Cox proportional hazard models were used for univariate and multivariate analyses of propensity-matched samples, and a two-tailed P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.1.0; R Project for Statistical Computing, http://www.r-project.org).
Results
Patient Features
We retrospectively gathered clinical data from 153 patients diagnosed with uHCC. Among these, 6 patients survived for less than 3 months, 2 patients were classified as Child-Pugh class C, and 1 patient declined standardized treatment, resulting in incomplete clinical data. As a result, a total of 9 patients were excluded from the analysis. The final cohort comprised 144 patients. Those who initially underwent TACE treatment were allocated to the T+L+P group and commenced systemic therapy within one month following TACE. Patients who started with systemic therapy were placed in the L+P group (Figure 1).
Figure 1.
Flow chart of eligible patients enrolled.
A total of 39 pairs of patients met the requirements after PSM. Table 1 summarizes the baseline characteristics of all patients enrolled in this study. Before PSM. The presence of macrovascular invasion and tumor number were significantly different between the two groups (p < 0.05). No significant differences were observed in the other clinical variables. After PSM, no significant differences were found in any of the baseline features between the two groups (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients
| Variables | Before PSM | P value | After PSM | P value | ||
|---|---|---|---|---|---|---|
| T+L+P | L+P | T+L+P | L+P | |||
| n=81,% | n=63,% | n=39,% | n=39,% | |||
| Gender | 0.313 | 0.754 | ||||
| Female | 12 (14.8) | 5 (7.9) | 7 (17.9) | 5 (12.8) | ||
| male | 69 (85.2) | 58 (92.1) | 32 (82.1) | 34 (87.2) | ||
| Age, years | 1 | 1 | ||||
| <65 | 59 (72.8) | 46 (73.0) | 27 (69.2) | 28 (71.8) | ||
| ≥65 | 22 (27.2) | 17 (27.0) | 12 (30.8) | 11 (28.2) | ||
| ECOG -PS score | 0.572 | 0.788 | ||||
| 0 | 64 (79.0) | 53 (84.1) | 29 (74.4) | 31 (79.5) | ||
| 1 | 17 (21.0) | 10 (15.9) | 10 (25.6) | 8 (20.5) | ||
| BCLC stage | 0.110 | 0.820 | ||||
| B | 31 (38.3) | 16 (25.4) | 19 (48.7) | 17 (43.6) | ||
| C | 50 (61.7) | 47 (74.6) | 20 (51.3) | 22 (56.4) | ||
| Child-pugh | 0.52 | 1 | ||||
| A | 64 (79.0) | 46 (73.0) | 30 (76.9) | 30 (76.9) | ||
| B | 17 (21.0) | 17 (27.0) | 9 (23.1) | 9 (23.1) | ||
| AFP,(ng/mL) | 0.966 | 0.485 | ||||
| <400 | 49 (60.5) | 37 (58.7) | 26 (66.7) | 22 (56.4) | ||
| ≥400 | 32 (39.5) | 26 (41.3) | 13 (33.3) | 17 (43.6) | ||
| HBV | 0.664 | 1 | ||||
| Absent | 19 (23.5) | 12 (19.0) | 8 (20.5) | 8 (20.5) | ||
| Present | 62 (76.5) | 51 (81.0) | 31 (79.5) | 31 (79.5) | ||
| Tumor Size (mean (SD)) | 8.38 (±4.67) | 9.26 (±4.25) | 0.244 | 8.14 (±3.81) | 8.46 (±4.12) | 0.726 |
| Tumor size, cm | 0.336 | 1 | ||||
| <10 | 55 (67.9) | 37 (58.7) | 26 (66.7) | 26 (66.7) | ||
| ≥10 | 26 (32.1) | 26 (41.3) | 13 (33.3) | 13 (33.3) | ||
| Tumor number | 0.008 | 1 | ||||
| Multiple | 55 (67.9) | 28 (44.4) | 23 (59.0) | 22 (56.4) | ||
| Single | 26 (32.1) | 35 (55.6) | 16 (41.0) | 17 (43.6) | ||
| Macrovascular invasion | 0.002 | 1 | ||||
| No | 48 (59.3) | 20 (31.7) | 19 (48.7) | 19 (48.7) | ||
| Yes | 33 (40.7) | 43 (68.3) | 20 (51.3) | 20 (51.3) | ||
| ALBI stage | 0.917 | 0.633 | ||||
| 1 | 32 (39.5) | 26 (41.3) | 13 (33.3) | 17 (43.6) | ||
| 2 | 46 (56.8) | 34 (54.0) | 23 (59.0) | 19 (48.7) | ||
| 3 | 3 (3.7) | 3 (4.8) | 3 (7.7) | 3 (7.7) | ||
| ALT, U/L | 0.141 | 1 | ||||
| <40 | 43 (53.1) | 42 (66.7) | 25 (64.1) | 25 (64.1) | ||
| ≥40 | 38 (46.9) | 21 (33.3) | 14 (35.9) | 14 (35.9) | ||
| AST, U/L | 0.88 | 1 | ||||
| <40 | 30 (37.0) | 25 (39.7) | 15 (38.5) | 16 (41.0) | ||
| ≥40 | 51 (63.0) | 38 (60.3) | 24 (61.5) | 23 (59.0) | ||
| TBil | 0.176 | 1 | ||||
| <17 | 61 (75.3) | 40 (63.5) | 28 (71.8) | 28 (71.8) | ||
| ≥17 | 20 (24.7) | 23 (36.5) | 11 (28.2) | 11 (28.2) | ||
| PD-1 inhibitors categories | 0.584 | 0.963 | ||||
| Tislelizumab | 22 (27.2) | 19 (30.2) | 15 (38.5) | 16 (41.0) | ||
| Camrelizumab | 20 (24.7) | 19 (30.2) | 12 (30.8) | 11 (28.2) | ||
| Sintilimab | 39 (48.1) | 25 (39.7) | 12 (30.8) | 12 (30.8) | ||
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; ALT, alanine transaminase; AST, aspartate transaminase; ALBI, albumin-bilirubin; AFP, ɑ-fetoprotein; HBV, Hepatitis B virus; TBil, total bilirubin; T, transarterial chemoembolization(TACE); L, Lenvatinib; P, Programmed death 1.
Efficacy Assessment
Before PSM, CR, PR, SD, and PD were recorded in 10, 42, 16, and 13 patients in the T+ L+P group. CR, PR, SD, and PD were recorded in 6, 22, 23, and 11 patients in the L+P group, respectively. The ORR in the two groups was 64.1% and 44.4%, respectively (p =0.028).
After PSM, CR, PR, SD, and PD were recorded in 4, 21, 8, and 6 patients in the T+ L+P group. CR, PR, SD, and PD were recorded in 4, 11, 14, and 10 patients in the L+P group, respectively. The ORR in the two groups was 64.1% and 38.4%, respectively (p = 0.024) (Table 2).
Table 2.
Tumor Response Before and After PSM
| Variables | Before PSM | P value | After PSM | P value | ||
|---|---|---|---|---|---|---|
| T+L+P | L+P | T+L+P | L+P | |||
| n=81,% | n=63,% | n=39,% | n=39,% | |||
| CR | 11 (13.6) | 6 (9.5) | 4 (10.3) | 3 (7.7) | ||
| PR | 42 (51.9) | 22 (34.9) | 21 (53.8) | 11 (28.2) | ||
| SD | 16 (19.8) | 23 (36.5) | 8 (20.5) | 14 (35.9) | ||
| PD | 13 (16.0) | 11 (17.5) | 6 (15.4) | 10 (25.6) | ||
| ORR | 52 (64.2) | 28 (44.4) | 0.028 | 25 (64.1) | 14 (35.9) | 0.024 |
Abbreviations: PSM, propensity score matching; T, transarterial chemoembolization(TACE); L, lenvatinib; P, Programmed death 1; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate.
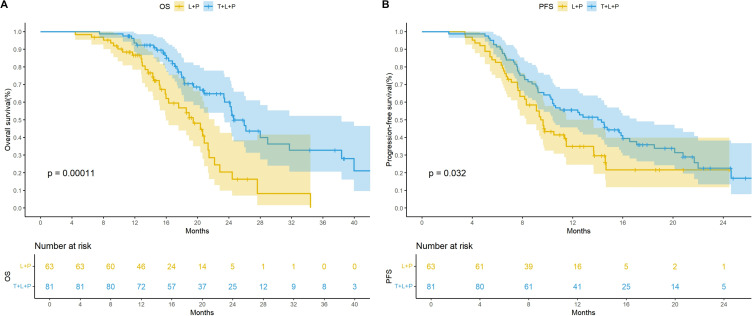
Before PSM, the median OS was 24.6 months (95% CI: 23.5–38.4) in the T+L+P group and 19.5 months (95% CI: 16.0–22.2) in the L+P group. The median PFS was 14.3 months (95% CI: 10.4–17.1) and 9.6 months (95% CI: 8.3–11.5) in the two groups, respectively. Kaplan–Meier curves showed significant differences in the mOS (p = 0.00011) and mPFS (p = 0.032; Figure 2).
Figure 2.
Kaplan-Meier curves for overall survival (A) and progression-free survival before PSM (B) therapy.
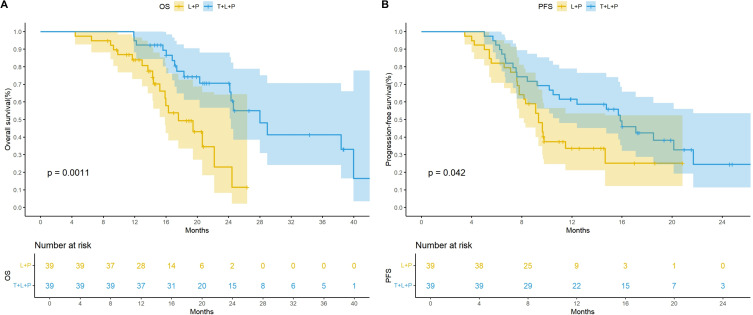
After PSM, the mOS was 28.0 months (95% CI: 24.3– not estimable) in the T+L+P group, while it was 17.6 months (95% CI: 15.9–not estimable) in the L+P group. The mPFS was 15.8 months (95% CI: 11.0– not estimable) and 9.3 months (95% CI: 8.7–not estimable) in the two groups, respectively. The Kaplan–Meier curves showed significant differences in the mOS (p = 0.0011) and mPFS (p = 0.042; Figure 3). Both univariate and multivariate results showed that treatment was an independent prognostic factor for OS and PFS (Tables 3 and 4).
Figure 3.
Kaplan-Meier curves for overall survival (A) and progression-free survival after PSM (B) therapy.
Table 3.
Univariate and Multivariate Analysis of the Prognostic Factors for OS in All Patients
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender(Male vs Female) | 1(0.43–2.29) | 0.992 | ||
| Age(≥65 vs <65 years) | 1.02(0.5–2.09) | 0.955 | ||
| ECOG-PS(0 vs 1) | 0.79(0.36–1.73) | 0.55 | ||
| BCLC stage(B vs C) | 0.75(0.39–1.44) | 0.391 | ||
| Child pugh(A vs B) | 1.72(0.77–3.82) | 0.183 | ||
| AFP(≥400 vs <400g/mL) | 1.19(0.6–2.36) | 0.611 | ||
| HBV(Present vs Absent) | 2.16(0.75–6.21) | 0.153 | ||
| Tumorsize | 0.96(0.87–1.05) | 0.33 | ||
| Tumorsize(≥10 vs <10cm) | 1.27(0.63–2.57) | 0.507 | ||
| Tumor_number(Single vs Multiple) | 0.7(0.36–1.39) | 0.313 | ||
| Macrovascular invasion(Yes vs No) | 0.94(0.49–1.8) | 0.841 | ||
| ALBI stage | 1.23(0.71–2.13) | 0.461 | ||
| ALT(<40 vs ≥40U/L) | 0.49(0.23–1.05) | 0.036 | 0.75 (0.51–1.22) | 0.022 |
| AST(<40 vs ≥40U/L) | 0.69(0.36–1.34) | 0.275 | ||
| TBil(<17 vs ≥17μmol/L) | 1.46(0.73–2.89) | 0.284 | ||
| Treatment | 0.31(0.15–0.65) | 0.002 | 0.64(0.38–0.84) | 0.0019 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; CI, confidence interval; ALT, alanine transaminase; AST, aspartate transaminase; ALBI, albumin-bilirubin; AFP, ɑ-fetoprotein; HBV, Hepatitis B virus; TBil, total bilirubin.
Table 4.
Univariate and Multivariate Analysis of the Prognostic Factors for PFS in All Patients
| Varaible | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender(Male vs Female) | 1.11(0.52–2.37) | 0.792 | ||
| Age(≥65 vs <65 years) | 0.57(0.3–1.1) | 0.045 | 0.66 (0.41 −0.93) | 0.03 |
| ECOG-PS(0 vs 1) | 0.92(0.48–1.77) | 0.813 | ||
| BCLC stage(B vs C) | 0.93(0.53–1.63) | 0.802 | ||
| Child pugh(A vs B) | 0.67(0.31–1.42) | 0.293 | ||
| AFP(≥400 vs <400g/mL) | 0.62(0.34–1.12) | 0.116 | ||
| HBV(Present vs Absent) | 0.95(0.49–1.87) | 0.892 | ||
| Tumorsize | 1(0.94–1.08) | 0.931 | ||
| Tumorsize(≥10 vs <10cm) | 0.98(0.54–1.76) | 0.934 | ||
| Tumor number(Single vs Multiple) | 1.17(0.66–2.06) | 0.597 | ||
| Macrovascular invasion(Yes vs No) | 0.73(0.42–1.28) | 0.275 | ||
| ALBI stage | 1.01(0.64–1.58) | 0.972 | ||
| ALT(<40 vs ≥40U/L) | 0.74(0.41–1.35) | 0.325 | ||
| AST(<40 vs ≥40U/L) | 0.89(0.51–1.57) | 0.694 | ||
| TBil(<17 vs ≥17μmol/L) | 1.32(0.7–2.47) | 0.393 | ||
| Treatment | 0.55(0.31–0.98) | 0.044 | 0.55(0.31–0.98) | 0.0439 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; CI, confidence interval; ALT, alanine transaminase; AST, aspartate transaminase; ALBI, albumin-bilirubin; AFP, ɑ-fetoprotein; HBV, Hepatitis B virus; TBil, total bilirubin.
Adverse Events
Table 5 lists the treatment-related adverse events (TRAEs) for the two groups. There were no significant differences between the T+L+P group and L+P with respect to rates of TRAEs. Fever, hypertension, and nausea were the most common TRAEs. TACE-related adverse events(AEs), such as fever and abdominal pain, were controllable and stabilized after a short period of treatment. Twenty-one patients (25.9%) in the T+ L+P group had grade 3 or higher AEs, while fifteen patients (23.8%) in the L+P group had grade 3 or higher AEs. In the T+ L+P group, TRAEs led to a reduction in the lenvatinib dose in 13 (16.0%) patients and discontinuation of the PD1 inhibitor in four (4.9%) patients. However, in the L+P group, TRAEs led to a reduction in the lenvatinib dose in seven patients (11.1%) and an discontinuations in the PD-1 inhibitor used in two (3.1%) patients. No treatment-related death occurred in either group (Table 5).
Table 5.
Treatment-Related Adverse Events
| Adverse events | Any Grade | P value | ≥ Grade 3 | P value | ||
|---|---|---|---|---|---|---|
| T+L+P | L+P | T+L+P | L+P | |||
| n=81,% | n=63,% | n=81,% | n=63,% | |||
| Total | 73 (90.1) | 59 (93.6) | 0.551 | 21 (25.9) | 15 (23.8) | 0.843 |
| Fever | 29 (35.8) | 0 (0.0) | <0.001 | 0 | 0 | – |
| Nausea | 26 (32.1) | 6 (9.5) | 0.002 | 0 | 0 | – |
| Abdominal pain | 7 (8.6) | 0 (0.0) | 0.045 | 0 | 0 | – |
| Hypertension | 27 (33.3) | 21 (33.3) | 1 | 3 (3.7) | 1 (1.6) | 0.798 |
| Diarrhea | 12 (14.8) | 17 (27.0) | 0.11 | 1 (1.2) | 0 (0.0) | 1 |
| Fatigue | 9 (11.1) | 8 (12.7) | 0.974 | 3 (3.7) | 0 (0.0) | 0.339 |
| Rash | 8 (9.9) | 5 (7.9) | 0.912 | 1 (1.2) | 0 (0.0) | 1 |
| Decreased appetite | 17 (21.0) | 13 (20.6) | 1 | 2 (2.5) | 1 (1.6) | 1 |
| Abnormal liver function | 17 (21.0) | 12 (19.0) | 0.937 | 0 | 0 | – |
| Hypothyroidism | 8 (9.9) | 7 (11.1) | 1 | 8 (9.9) | 7 (11.1) | 1 |
| Hyperthyroidism | 4 (4.9) | 2 (3.2) | 0.916 | 4 (4.9) | 2 (3.2) | 0.916 |
| Hypoalbuminemia | 10 (12.3) | 9 (14.3) | 0.926 | 0 | 0 | – |
| Thrombocytopenia | 5 (6.2) | 3 (4.8) | 1 | 0 | 0 | – |
| Neutropenia | 8 (9.9) | 6 (9.5) | 1 | 1 (1.2) | 1 (1.6) | 1 |
| Lymphopenia | 3 (3.7) | 3 (4.8) | 1 | 0 (0.0) | 2 (3.2) | 0.37 |
| Leukocytopenia | 5 (6.2) | 7 (11.1) | 0.447 | 1 (1.2) | 3 (4.8) | 0.443 |
Abbreviations: T, transarterial chemoembolization(TACE); L, Lenvatinib; P, Programmed death 1.
Discussion
Our findings suggest that T+L+P provides better survival benefits than the L+P alone in patients with BCLC stage B or C uHCC and moderate liver function. The T+L+P treatment regimen demonstrated a significant extension in mOS (28.0 vs 17.6 months), mPFS (15.8 vs 9.3 months), and an improvement in ORR (64.1% vs 38.2%) compared to the L+P regimen. Furthermore, no substantial differences in adverse effects were observed between the two treatment groups, with all toxicity being controllable. Therefore, the addition of TACE to a combination of lenvatinib and PD-1 inhibitor may be a better treatment option for patients with uHCC.
Previously, several studies reported the clinical outcomes of combining TACE, TKIs, and PD-1 inhibitors in the treatment of HCC patients. Yang et al assessed the effectiveness of TACE in combination with TKIs and camrelizumab for patients with uHCC. The results demonstrated an ORR of 64.5%, a disease control rate (DCR) of 77.4%, and a mPFS of 6.5 months.24 Meanwhile, Guo et al conducted a study, which evaluated the efficacy of TACE combined with lenvatinib and PD-1 treatments in 96 patients with uHCC. The result showed an ORR of 68.8% and a mOS of 30.4 months.25 Synthesis of previous relevant literature, the combination of TACE lenvatinib, and PD-1 inhibitors for the treatment of patients with uHCC resulting an ORR of 26.1%-87.2%, DCR of 70–100%, mPFS of 6.3–22.5 months, and mOS of 15.7–29.0 months.26
Our findings align closely with those reported in their studies. Compared to the CHANCE001 trial, we observed significantly extended OS (28.0 vs 19.2 months) and PFS (15.8 vs 9.3 months). These results may be related to the following factors: 1) A lower proportion of patients in our study had BCLC stage C (51.3% vs 65.8%); 2) Our study did not include patients who had previously received HCC-related treatments; 3) The TACE procedures in our study were performed by physicians at a single center using conventional TACE, rather than multi-center conventional TACE or drug-eluting bead TACE.20 In addition, two studies aimed to compare the clinical benefit and tolerability of lenvatinib, PD-1 inhibitor, and TACE versus lenvatinib and PD-1 inhibitor in uHCC patients, the results illustrated that patients who received triple therapy had better OS and PFS than those who received dual therapy.21,27 In comparison to their study, our research implemented PSM and excluded patients with a history of HCC-related treatments, and we achieved similar results. The favorable results of T+L+P is more effective than L+P may be due to a synergistic anti-tumor effect between the different therapies. First, TACE induces hypoxia in the tumor tissue, which results in tumor cell necrosis and apoptosis, but also promotes tumor angiogenesis, which leads to recurrence and metastasis after TACE.28 Lenvatinib inhibits the kinase activity of VEGF receptors (VEGFR-12, 3), thereby reducing the upregulation of proangiogenic factors following TACE. This effect helps to decrease the probability of recurrence following TACE.29 Second, TACE can effectively activate the immune system by releasing neoantigens and immune-related inflammatory factors, providing a microenvironment for immunotherapy, thereby enhancing the anti-tumor activity of drugs and improving the tumor control rate.15,30 Lenvatinib is a multi-kinase inhibitor with antiproliferative and antiangiogenitic activities. It modulates the tumor immune microenvironment, thereby enhancing the immune response to PD-1 inhibitors in HCC.31 We observed that macrovascular invasion was statistically different between the two groups in the baseline characteristics prior to PSM. According to the literature,32 HCC patients with macrovascular invasion generally have a poorer prognosis. However, these patients still show a favorable response to systemic treatment (mOS: 24.0 months; mPFS: 13.8 months; ORR: 58.7%).33 To minimize data bias and the impact of confounding factors, we conducted PSM and performed univariate and multivariate analyses to determine the factors influencing patient survival and treatment outcomes. Univariate and multivariate analyses both demonstrated that the treatment strategy is an independent prognostic factor for OS and PFS. Therefore, adding TACE to lenvatinib and PD-1 inhibitor may be a beneficial therapeutic option for patients with HCC. Univariate and multivariate analyses revealed that combination therapy was an independent prognostic factor for OS and PFS. Therefore, adding TACE to lenvatinib and PD-1 inhibitor may be a beneficial therapeutic option for patients with HCC.
In this study, the toxicity associated with the T+ L+P treatment regimen were deemed acceptable Notably, there were no fatalities due to adverse reactions in either the T+L+P group or the L+P group during the treatment period. Consistent with previous studies,21,34 similar AEs were observed in the T+L+P and L+P therapy groups, such as fever, hypertension, and nausea.
This study has some limitations. First, because of the retrospective nature of the study, a selection bias cannot be ruled out despite the PSM. Second, the types of PD-1 inhibitors used in this study were inconsistent due to patients’ treatment decisions. However, based on previous studies, different PD-1 inhibitors showed similar clinical benefits.35,36 Third, only a single center was included in the study. As a result, the data collected after PSM is limited. Our findings need to be confirmed by randomized controlled studies with a larger sample size. In this study, all HCC patients assessed were from a Chinese population with a high prevalence of HBV infection. Our results indicate that the T+L+P treatment regimen is more effective than the L+P regimen, suggesting it could be a preferred option for uHCC patients. However, given the limited sample size and the single-center design of the study, further large-scale prospective trials are necessary to validate these findings.
Conclusion
The addition of TACE to a combination of lenvatinib and PD-1 inhibitor resulted in better clinical outcomes than lenvatinib plus PD-1 inhibitor alone in patients with uHCC. This triplet therapy regimen (T+L+P) has been shown to prolong OS and PFS in patients with uHCC, while also enhancing the ORR. Moreover, the adverse events (AEs) associated with this treatment were within an acceptable and manageable range. These findings indicate that the T+L+P regimen could serve as a preferred therapeutic option for patients with uHCC.
Acknowledgments
We would like to express our gratitude to all participants and researchers for their contributions to this study.
Funding Statement
This study was financially supported by the Guangxi Medical and health key discipline construction project, Guangxi medical and healthcare appropriate technology development and promotion and application projects (grant number: S2022132), Guangxi Natural Science Foundation (grant number: 2022JJA140009) and Self-financed scientific research projects of Guangxi Autonomous Region Health and Wellness Commission(Z-C20230840).
Abbreviations
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CR, complete response; CT, computed tomography; ECOG-PS, Eastern Cooperative Oncology Group- performance status; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; HR, hazard ratio; CI, confidence interval; mOS, median OS; mPFS, median PFS; ORR, objective response rate; OS, overall survival; CR, complete response; PD-1, programmed death 1; PFS, progression-free survival; mRECIST, Response Evaluation Criteria in Solid Tumors; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TRAE, treatment-related adverse effect.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Institutional Ethics Committee of Guilin Medical University Research Ethics Committee in accordance with the 1975 Declaration of Helsinki. All patients signed written informed consent for their clinical data prior to the procedure.
Consent for Publication
The article was published with the consent of all the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest in the research and publication of this article.
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incide- nce and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi: 10.6004/jnccn.2021.0022 [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Shen T, Li J, et al. Clinical practice guideline on liver transplantation for hepatocellular carcinoma in China (2021 edition). Chin Med J. 2022;135(24):2911–2913. (). doi: 10.1097/CM9.0000000000002515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang Y-K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 8.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 10.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. doi: 10.1016/S1470-2045(20)30011-5 [DOI] [PubMed] [Google Scholar]
- 11.Sangro B, Park J, Finn R, et al. LBA-3 CheckMate 459: long-term (minimum follow-up 33.6 months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Oncol. 2020; 31:S241–2. [Google Scholar]
- 12.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 14.Wu C-J, Lee P-C, Hung Y-W, et al. Lenvatinib plus pembrolizumab for systemic therapy-naïve and -experienced unresectable hepatocellular carcinoma. Cancer Immunol Immunother. 2022; 71(11):2631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng A-L, Hsu C, Chan SL, Choo S-P, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72(2):307–319. doi: 10.1016/j.jhep.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 17.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subseq- uent selective TACE. Liver Cancer. 2019;8(5):299–311. doi: 10.1159/000502905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chem- oembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi: 10.2147/JHC.S332420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu HD, Li HL, Huang MS, et al. CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8(1):58. doi: 10.1038/s41392-022-01235-0 PMID: 36750721; PMCID: PMC9905571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin Y, Zhang X, Liu N, et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int. 2023. 17:753–764. doi: 10.1007/s12072-023-10502-3 [DOI] [PubMed] [Google Scholar]
- 22.EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–364. doi: 10.1159/000327577 [DOI] [PubMed] [Google Scholar]
- 24.Yang F, Yang J, Xiang W, et al. Safety and efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for hepatocellular carcinoma. Front Oncol. 2022;11:657512. doi: 10.3389/fonc.2021.657512 PMID: 35096555; PMCID: PMC8792047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo P, Pi X, Gao F, et al. Transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors for patients with unresectable hepatocellular carcinoma: a propensity score matching study. Front Oncol. 2022;12:945915. doi: 10.3389/fonc.2022.945915 PMID: 36338683; PMCID: PMC9630329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Wu J, Li S, et al. Effect of transcatheter arterial chemoembolization combined with lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a treatment with Chinese characteristics. Biosci Trends. 2024;18(1):42–48. doi: 10.5582/bst.2023.01326 Epub 2024 Feb 8. PMID: 38325823. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zhao M, Han G, Han X, Shi J, Mi L. Transarterial chemoembolization combined with PD-1 inhibitors plus lenvatinib showed impr- oved efficacy for treatment of unresectable hepatocellular carcinoma compared with PD-1 inhibitors plus lenvatinib. Technol Cancer Res Treat. 2023;22:15330338231166765. doi: 10.1177/15330338231166765 PMID: 37161343; PMCID: PMC10185979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1alpha and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res. 2016;8(4):297–302. doi: 10.14740/jocmr2496w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. HepIntl. 2021;15(3):663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi: 10.1038/s41575-020-00395-0 [DOI] [PubMed] [Google Scholar]
- 31.Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi: 10.1111/cas.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PawarodeA V, SriuranpongV E, Sriuranpong V, Kullavanijaya P, Patt YZ. al. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients[J]. AmJ Clin Oncol. 1998;21(4):386–391. doi: 10.1097/00000421-199808000-00014 [DOI] [PubMed] [Google Scholar]
- 33.Zhu M, Liu Z, Chen S, et al. Sintilimab plus bevacizumab combined with radiotherapy as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a multicenter, single-arm, phase 2 study. Hepatology. 2024;80(4):807–815. [Epub 2024 Feb 15. PMID: 38358542]. doi: 10.1097/HEP.0000000000000776 [DOI] [PubMed] [Google Scholar]
- 34.Qu S, Zhang X, Wu Y, et al. Efficacy and safety of TACE combined with lenvatinib Plus PD-1 inhibitors compared with TACE alone for unresectable hepatocellular carcinoma patients: a prospective cohort study. Front Oncol. 2022;12:874473. doi: 10.3389/fonc.2022.874473 PMID: 35530353; PMCID: PMC9068979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Hu X, Li Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus assoc- iated hepatocellular carcinoma patients. Annals Transl Med. 2020;8(18):1187. doi: 10.21037/atm-20-6063 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. doi: 10.1186/s12943-021-01489-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon reasonable request.