Abstract
Purpose
The blood urea nitrogen/creatinine ratio (BCR) is an effective marker for disease severity stratification. Its efficacy has been demonstrated under numerous conditions. This study aims to investigate the relationship between BCR and in-hospital mortality in intensive care unit (ICU) patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Patients and Methods
Eligible ICU patients with AECOPD from the eICU database were included in the study. Patients were divided into high-BCR and low-BCR groups on the basis of the optimal cutoff value (22.78) of the ROC curve for predicting in-hospital mortality in AECOPD patients. Propensity score matching (PSM) was used to balance the baseline differences between the high-BCR and low-BCR groups. Multivariate logistic regression was used to analyze the relationship between BCR and in-hospital mortality in ICU patients with AECOPD. Decision curve analysis (DCA) was performed to evaluate the clinical efficacy of each model via multivariate logistic regression.
Results
A total of 3399 eligible ICU patients with AECOPD were included in the study, with 1559 patients in the high-BCR group and 1840 patients in the low-BCR group. After propensity score matching (PSM), 1174 pairs of patients were successfully matched. The results of the multivariate logistic regression revealed that the in-hospital mortality rate for AECOPD patients in the high-BCR subgroup was significantly greater than that in the low-BCR subgroup in both the unmatched and matched cohorts after adjusting for multiple factors. Additionally, DCA demonstrated that the models used in the multivariate logistic regression had effective clinical utility.
Conclusion
The blood urea nitrogen/creatinine ratio (BCR) is an effective predictor of in-hospital mortality in ICU patients with AECOPD. Clinicians can use BCR to identify critically ill ICU patients with AECOPD earlier and implement interventions to improve patient outcomes.
Keywords: acute exacerbation of chronic obstructive pulmonary disease, blood urea nitrogen, creatinine, intensive care unit, hospital mortality
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease that primarily involves chronic bronchitis and emphysema and affects the airways and lungs. It is characterized by persistent airflow limitation resulting from bronchial obstruction, which is not fully reversible, even with bronchodilator treatments.1,2 According to 2019 statistics from the World Health Organization, COPD is now the third leading cause of death globally.3 As the population ages and air quality worsens, the prevalence of COPD is expected to increase, which could increase its economic and social impact.4 Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is characterized by rapid intensification of respiratory symptoms, including increased dyspnea, coughing, and sputum production, in COPD patients. These exacerbations are closely associated with viral and bacterial infections, as well as environmental pollution, making them a significant cause of hospitalization and mortality among COPD patients.5,6 AECOPD often leads to acute respiratory failure, which frequently necessitates endotracheal intubation or invasive mechanical ventilation in the intensive care unit (ICU). It has been reported that 2%–19% of hospitalized AECOPD patients require ICU admission, and the in-hospital mortality rate for these patients in the ICU ranges from 12% to 24%.7,8
BUN is a nitrogenous compound in the plasma that is produced by the liver and excreted through the kidneys. BUN levels are used to assess glomerular filtration function, and are crucial in the diagnosis, treatment, and prognosis of kidney diseases.9 Research indicates that a blood urea nitrogen level ≥7.3 mmol/L serves as an independent risk factor for in-hospital mortality among patients hospitalized with AECOPD.10 Additionally, BUN levels are closely associated with the length of hospital stays in COPD patients.11 BUN levels may be valuable in identifying severe cases of AECOPD and in stratifying patient risk.12 Creatinine (Cr), a metabolic byproduct of creatine and phosphocreatine, is mainly excreted by the kidneys. Elevated blood Cr levels are linked to impaired renal function.13 In patients with COPD, renal involvement and muscle atrophy are significant clinical issues, and both conditions contribute to changes in blood creatinine levels.14,15 Recently, the ratio of BUN to creatinine (BCR) has been recognized as having clinical value in various medical fields. For example, a high BCR is associated with better in-hospital survival in cardiogenic shock patients, whereas a low BCR is an independent risk factor for stroke.16,17 Additionally, a high BCR has been linked to increased mortality in patients with postoperative gastrointestinal tumor fistulas.18 In respiratory medicine, the BCR holds significant clinical value for diagnosing diseases and assessing patient prognosis. The BCR is an effective predictor of mortality risk in COVID-19 patients, supporting personalized treatment approaches.19 A BCR of 97.893 or higher reliably predicts an increased risk of sarcopenia in COPD patients.14 To our knowledge, no studies have yet elucidated the relationship between BCR and mortality among ICU-admitted patients with AECOPD.
Our study aims to explore whether the BUN/Cr ratio can serve as an indicator of mortality among ICU patients with AECOPD. This investigation seeks to determine whether a simple biomarker can facilitate the early identification of critically ill AECOPD patients, potentially enabling more timely and appropriate interventions.
Materials and Methods
Data Source
The data for this study were obtained from the eICU Collaborative Research Database (eICU-CRD). This multicenter database contains deidentified health data for over 200,000 patients who were treated in ICUs across the United States from 2014 to 2015.20 It includes records of critical vital signs, nursing plans, disease severity assessments, diagnostic information, and treatment details. Access to the database was granted to Author Zhiwei Long, with the following credentials: Name ID - 12635073; Record ID - 58,429,392.
Ethics Statement
This study employed a retrospective design, obtained informed consent from the patients, and all the data used were anonymized and de-identified. Since this research does not involve any harm to human subjects, sensitive patient information, or commercial interests, the Ethics Review Committee of the First Affiliated Hospital of Guangzhou Medical University waived the requirement for ethical review.
Study Population
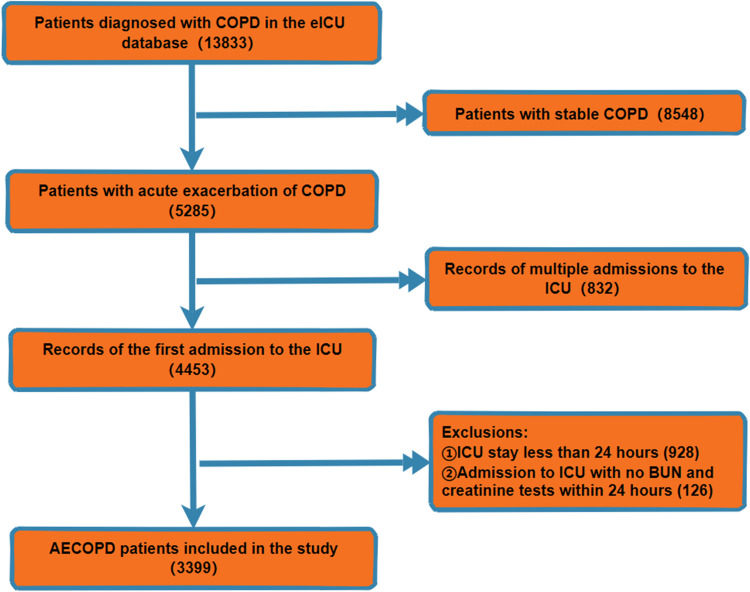
This study included a total of 3399 patients with AECOPD. The inclusion criteria were as follows: (1) participant was diagnosed with AECOPD according to the International Classification of Diseases 10 (ICD-10). The exclusion criteria were as follows: (1) participant had an ICU stay shorter than 24 hours; (2) there was an absence of BUN or creatinine measurements for the participant within the first 24 hours of ICU admission; and (3) for potential participants with multiple ICU admissions, only the first admission was included in the study. Figure 1 illustrates the detailed study population inclusion process.
Figure 1.
Study Population Inclusion.
Variable Extraction
Data was extracted by Structured Query Language (SQL 16.0) and Navicat Premium 16. The extracted data included the following categories: (1) Demographics: age, sex, height, weight, and ethnicity. (2) Vital signs: heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, and oxygen saturation (SpO2). (3) Laboratory indicators: red blood cell distribution width, hemoglobin, white blood cell (WBC), platelet (PLT), potassium, sodium, calcium, glucose, blood urea nitrogen (BUN), creatinine (Cr), pH, PaO2, PaCO2, anion gap, and bicarbonate. (4) Comorbidities: hypertension, diabetes, asthma, congestive heart failure, myocardial infarction, liver disease, gastrointestinal hemorrhage, chronic kidney disease, malignant tumors, sepsis, end-stage renal disease, and acute kidney injury. (5) Treatments: corticosteroids, bronchodilators, invasive ventilation, and dialysis. (6) APACHE-IV score and length of stay in the ICU.
Statistical Analysis
The normality of the data was assessed via the Kolmogorov‒Smirnov test. Depending on the results of this normality test, continuous variables were analyzed via either the t test or the Mann‒Whitney test. For categorical variables, analysis was conducted via either the chi-square test or Fisher’s exact test, as appropriate. A receiver operating characteristic (ROC) curve was used to predict in-hospital mortality among patients with AECOPD. The sensitivity and specificity were calculated, and the Youden index was determined via the following formula: sensitivity + specificity - 1. The optimal cutoff value for the BCR, which maximized the Youden index, was identified as 22.78. Patients were subsequently categorized into high-BCR and low-BCR groups on the basis of this threshold.
Logistic regression was used to compute propensity scores (PSs) for the high-BCR and low-BCR groups. A 1:1 nearest neighbor propensity score matching (PSM) strategy, with a caliper width of 0.02, was implemented to balance the baseline characteristics between the high- and low-BCR groups. This matching process successfully paired 1174 patients. The standardized mean difference (SMD) was calculated post-matching to evaluate the balance achieved. Empirical cumulative distribution function (ECDF) and density plots were generated before and after matching to assess the efficacy of the matching process.
We employed multivariable logistic regression to evaluate the relationship between BCR and in-hospital mortality among ICU-admitted patients with AECOPD. To mitigate multicollinearity, any variable with a variance inflation factor (VIF) greater than 5 was excluded. The analysis was organized into four distinct models. Crude Model: Unadjusted for any variables. Model 1: Adjusted for age, sex, ethnicity, and BMI. Model 2: Further adjusted for comorbidities (hypertension, diabetes, congestive heart failure, myocardial infarction, asthma, liver disease, gastrointestinal hemorrhage, chronic kidney disease, end-stage renal disease, acute kidney injury, malignant tumor, sepsis), in addition to the variables in Model 1. Model 3: Adjusted for all the variables except BUN and creatinine (age, sex, ethnicity, BMI, heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, SpO2, hypertension, diabetes, congestive heart failure, myocardial infarction, asthma, liver disease, gastrointestinal hemorrhage, chronic kidney disease, end-stage renal disease, acute kidney injury, malignant tumor, sepsis, hemoglobin, WBC, PLT, RDW, glucose, sodium, potassium, calcium, pH, PaCO2, PaO2, anion gap, bicarbonate, corticosteroids, bronchodilators, invasive ventilation, dialysis, APACHE-IV score, and ICU length of stay). Furthermore, decision curve analysis (DCA) was performed to assess the stability and clinical utility of each model.
Binary logistic regression was used for subgroup analysis to explore the performance of BCR among various AECOPD patient subgroups, and to identify potential interactions between these subgroups and BCR. Statistical significance was determined by a two-tailed P value of <0.05. All the statistical analyses were conducted via R software, version 4.3.1.
Results
Original Cohort
The study included a total of 3399 AECOPD patients from the eICU database, of whom 297 died in the hospital and 3102 survived. The median age of the patients was 68 years, with males comprising 45.84% of the study group, and 82.94% identifying as Caucasian. Table 1 details the baseline differences between the survivors and nonsurvivors. Nonsurvivors were significantly older than the survivors (p<0.001), had lower BMI values (p<0.001), and a greater proportion were Caucasian (p=0.026). These patients also presented higher respiratory rates and heart rates (p<0.001 and p=0.007, respectively) and lower systolic and diastolic blood pressures (p<0.001). The prevalence of comorbidities such as heart failure (p<0.001), liver disease (p<0.001), gastrointestinal hemorrhage (p<0.001), chronic kidney disease (p=0.046), acute kidney injury (p<0.001), malignant tumors (p=0.010), and sepsis (p<0.001) was significantly greater among the nonsurvivors. The laboratory findings revealed that nonsurvivors had lower hemoglobin levels (p<0.001) and higher white blood cell counts (p<0.001), as well as lower levels of calcium (p<0.001) and bicarbonate (p<0.001), and higher levels of blood urea nitrogen (p<0.001) and creatinine (p<0.001). Within the first 24 hours of ICU admission, a greater percentage of survivors received corticosteroid therapy (p=0.041), whereas nonsurvivors were more likely to undergo invasive ventilation (p<0.001). Furthermore, nonsurvivors had significantly higher APACHE-IV scores (p<0.001) and longer ICU stays (p<0.001).
Table 1.
Baseline Data of the Original Cohort
| Variable | Total (n=3399) | Survivors (n=3102) | Non-Survivors (n=297) | P-Value |
|---|---|---|---|---|
| Demographic | ||||
| Age | 68.00 (60.00, 76.00) | 68.00 (60.00, 75.00) | 73.00 (66.00, 80.00) | <0.001 |
| Male | 1558 (45.84) | 1424 (45.91) | 134 (45.12) | 0.795 |
| BMI | 27.70 (22.42, 34.31) | 28.07 (22.61, 34.81) | 25.66 (20.70, 30.41) | <0.001 |
| Ethnicity | ||||
| White | 2819 (82.94) | 2556 (82.40) | 263 (88.55) | 0.026 |
| Black | 306 (9) | 289 (9.32) | 17 (5.72) | |
| Others | 274 (8.06) | 257 (8.28) | 17 (5.72) | |
| Vital signs | ||||
| HR (beats/min) | 90.00 (81.00, 99.00) | 90.00 (80.00, 99.00) | 92.00 (86.00, 106.00) | <0.01 |
| RR (beats/min) | 21.00 (18.00, 23.00) | 21.00 (18.00, 23.00) | 21.00 (19.00, 24.00) | 0.007 |
| SBP (mmHg) | 122.26 (110.70, 132.00) | 122.26 (111.18, 132.66) | 114.00 (103.32, 123.44) | <0.001 |
| DBP (mmHg) | 66.90 (60.00, 72.84) | 66.90 (60.33, 73.20) | 63.92 (56.72, 68.87) | <0.001 |
| SpO2 (%) | 96.00 (94.38, 97.55) | 96.00 (94.38, 97.53) | 96.15 (94.53, 97.59) | 0.180 |
| Comorbidities | ||||
| Hypertension | 571 (16.8) | 526 (16.96) | 45 (15.15) | 0.427 |
| Diabetes | 519 (15.27) | 470 (15.15) | 49 (16.50) | 0.538 |
| Congestive heart failure | 672 (19.77) | 610 (19.66) | 62 (20.88) | 0.617 |
| Myocardial infarction | 118 (3.47) | 96 (3.09) | 22 (7.41) | <0.001 |
| Asthma | 157 (4.62) | 146 (4.71) | 11 (3.70) | 0.431 |
| Liver disease | 40 (1.18) | 30 (0.97) | 10 (3.37) | <0.001 |
| Gastrointestinal hemorrhage | 70 (2.06) | 55 (1.77) | 15 (5.05) | <0.001 |
| Chronic kidney disease | 198 (5.83) | 173 (5.58) | 25 (8.42) | 0.046 |
| End stage renal disease | 46 (1.35) | 45 (1.45) | 1 (0.34) | 0.185 |
| Acute renal failure | 439 (12.92) | 362 (11.67) | 77 (25.93) | <0.001 |
| Malignant tumor | 77 (2.27) | 64 (2.06) | 13 (4.38) | 0.010 |
| Sepsis | 532 (15.65) | 443 (14.28) | 89 (29.97) | <0.001 |
| Laboratory | ||||
| Hemoglobin(g/dL) | 11.89 (10.45, 13.25) | 11.90 (10.50, 13.30) | 11.20 (9.80, 12.55) | <0.001 |
| WBC(K/uL) | 10.85 (7.95, 14.20) | 10.70 (7.90, 13.80) | 12.90 (8.50, 18.15) | <0.001 |
| PLT(K/uL) | 213.00 (167.00, 265.00) | 214.00 (167.50, 264.50) | 209.00 (158.00, 273.00) | 0.493 |
| RDW(%) | 15.20 (14.00, 16.25) | 15.15 (14.00, 16.20) | 15.40 (14.10, 16.50) | 0.181 |
| Glucose(mg/dL) | 168.84 (144.45, 170.38) | 168.84 (144.85, 170.00) | 168.84 (139.50, 171.50) | 0.332 |
| Sodium(mmol/L) | 138.50 (135.50, 141.00) | 138.50 (135.67, 141.00) | 138.50 (135.50, 141.00) | 0.399 |
| Potassium(mmol/L) | 4.30 (3.90, 4.68) | 4.30 (3.90, 4.65) | 4.30 (3.90, 4.80) | 0.306 |
| Calcium(mg/dL) | 8.71 (8.30, 9.10) | 8.71 (8.35, 9.10) | 8.52 (8.00, 9.00) | <0.001 |
| PH | 7.30 (7.30, 7.40) | 7.30 (7.30, 7.40) | 7.30 (7.30, 7.40) | 0.966 |
| PaO2(mmHg) | 104.47 (75.69, 112.83) | 104.31 (75.67, 111.42) | 106.57 (75.80, 124.33) | 0.107 |
| PaCO2(mmHg) | 60.25 (48.90, 68.65) | 60.25 (49.00, 69.00) | 60.25 (46.67, 64.50) | 0.113 |
| Anion gap(mEq/L) | 9.79 (7.00, 12.68) | 9.76 (7.00, 12.59) | 10.00 (7.00, 13.20) | 0.354 |
| Bicarbonate(mmol/L) | 29.78 (25.00, 34.00) | 29.78 (25.00, 34.00) | 28.50 (24.50, 33.00) | 0.012 |
| BUN(mg/dL) | 21.50 (15.00, 33.00) | 21.00 (14.50, 32.00) | 30.00 (20.00, 43.00) | <0.001 |
| Cr(mg/dL) | 0.97 (0.70, 1.40) | 0.95 (0.70, 1.35) | 1.14 (0.75, 1.84) | <0.001 |
| BCR | 21.76 (16.50, 28.75) | 21.53 (16.29, 28.30) | 25.13 (18.89, 32.64) | <0.001 |
| Treatment | ||||
| Corticosteroid | 385 (11.33) | 362 (11.67) | 23 (7.74) | 0.041 |
| Bronchodilators | 499 (14.68) | 458 (14.76) | 41 (13.80) | 0.655 |
| Invasive-ventilation | 235 (6.91) | 194 (6.25) | 41 (13.80) | <0.001 |
| Dialysis | 81 (2.38) | 75 (2.42) | 6 (2.02) | 0.668 |
| APACHE-IV | 58.00 (48.00, 64.50) | 58.00 (47.00, 63.00) | 64.00 (58.00, 86.00) | <0.001 |
| ICU stay (day) | 2.73 (1.72, 4.71) | 2.73 (1.72, 4.71) | 4.18 (2.42, 7.19) | <0.001 |
Notes: A p-value less than 0.05 is considered statistically significant.
Abbreviations: BMI, Body Mass Index; HR, Heart Rate; RR, Respiratory Rate; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; RDW, Red Blood Cell Distribution Width; WBC, White Blood Cell; PLT, Platelet; Cr, Creatinine; BUN, Blood urea nitrogen; BCR, BUN to Creatinine ratio.
Before Propensity Score Matching
The ROC curve was plotted to assess the ability of the BCR to predict the in-hospital mortality of AECOPD patients in the ICU. The optimal cutoff value was determined to be 22.78, thereby dividing the original cohort into two groups: a high-BCR group (BCR≥22.78) and a low-BCR group (BCR<22.78). Details of the ROC curve analysis are provided in Table S1 and Figure S1. The baseline characteristics of each group are documented in Table 2. Compared with the low-BCR group, the high-BCR group was significantly older (p<0.001), had a lower proportion of males (p<0.001), had a lower BMI (p<0.001), and had a greater proportion of Caucasians (p<0.001). Vital signs revealed that the high BCR group had a significantly greater heart rate (p=0.048) and lower systolic (p=0.002) and diastolic (p<0.001) blood pressures. Comorbidity analysis revealed significantly lower proportions of asthma and end-stage renal disease in the high-BCR subgroup (p<0.001 for both). The laboratory findings revealed that the high-BCR group had lower hemoglobin levels (p<0.001), higher white blood cell counts (p=0.038), and elevated levels of sodium (p<0.001), potassium (p<0.001), PaCO2 (p<0.001), and bicarbonate (p<0.001), along with a lower anion gap (p<0.001) and higher blood urea nitrogen (p<0.001) and creatinine (p<0.001) levels. The low-BCR group had a significantly greater need for dialysis (p<0.001). Furthermore, the high-BCR group had significantly greater APACHE-IV scores, longer ICU stays, and higher in-hospital mortality rates (all p<0.001).
Table 2.
The Baseline Data for the High BCR Group and Low BCR Group Before Propensity Score Matching(PSM)
| Variable | BCR<22.78 (n=1840) | BCR≥22.78 (n=1559) | P-Value |
|---|---|---|---|
| Demographic | |||
| Age | 66.00 (58.00, 74.00) | 70.00 (62.00, 77.00) | <0.001 |
| Male | 914 (49.67) | 644 (41.31) | <0.001 |
| BMI | 28.51 (23.27, 35.32) | 26.81 (21.30, 32.95) | <0.001 |
| Ethnicity | |||
| White | 1460 (79.35) | 1359 (87.17) | <0.001 |
| Black | 221 (12.01) | 85 (5.45) | |
| Others | 159 (8.64) | 115 (7.38) | |
| Vital signs | |||
| HR (beats/min) | 90.00 (80.00, 99.00) | 90.00 (81.00, 99.00) | 0.048 |
| RR (beats/min) | 21.00 (18.00, 23.00) | 21.00 (18.00, 23.00) | 0.932 |
| SBP (mmHg) | 122.26 (111.25, 132.78) | 121.17 (109.94, 130.51) | 0.002 |
| DBP (mmHg) | 66.90 (60.00, 72.84) | 66.64 (59.32, 71.65) | <0.01 |
| SpO2 (%) | 96.00 (94.36, 97.57) | 96.00 (94.41, 97.52) | 0.833 |
| Comorbidities | |||
| Hypertension | 324 (17.61) | 247 (15.84) | 0.170 |
| Diabetes | 282 (15.33) | 237 (15.20) | 0.920 |
| Congestive heart failure | 361 (19.62) | 311 (19.95) | 0.810 |
| Myocardial infarction | 65 (3.53) | 53 (3.40) | 0.833 |
| Asthma | 107 (5.82) | 50 (3.21) | <0.001 |
| Liver disease | 23 (1.25) | 17 (1.09) | 0.667 |
| Gastrointestinal hemorrhage | 32 (1.74) | 38 (2.44) | 0.153 |
| Chronic kidney disease | 110 (5.98) | 88 (5.64) | 0.679 |
| End stage renal disease | 45 (2.45) | 1 (0.06) | <0.001 |
| Acute renal failure | 249 (13.53) | 190 (12.19) | 0.244 |
| Malignant tumor | 42 (2.28) | 35 (2.25) | 0.942 |
| Sepsis | 297 (16.14) | 235 (15.07) | 0.393 |
| Laboratory | |||
| Hemoglobin(g/dL) | 12.00 (10.50, 13.50) | 11.80 (10.40, 13.00) | <0.001 |
| WBC(K/uL) | 10.63 (7.90, 13.87) | 11.10 (8.00, 14.40) | 0.038 |
| PLT(K/uL) | 214.17 (168.00, 267.00) | 212.00 (165.00, 263.50) | 0.147 |
| RDW(%) | 15.10 (13.95, 16.25) | 15.25 (14.10, 16.25) | 0.165 |
| Glucose(mg/dL) | 168.84 (145.19, 168.84) | 168.84 (143.00, 171.71) | 0.432 |
| Sodium(mmol/L) | 138.00 (135.50, 141.00) | 139.00 (136.00, 141.00) | <0.001 |
| Potassium(mmol/L) | 4.25 (3.90, 4.60) | 4.30 (3.95, 4.70) | <0.001 |
| Calcium(mg/dL) | 8.71 (8.30, 9.10) | 8.75 (8.35, 9.13) | 0.121 |
| PH | 7.30 (7.30, 7.40) | 7.30 (7.30, 7.40) | 0.114 |
| PaO2(mmHg) | 107.37 (75.69, 116.66) | 101.00 (75.73, 109.00) | 0.074 |
| PaCO2(mmHg) | 60.25 (46.14, 65.00) | 60.25 (52.00, 72.00) | <0.001 |
| Anion gap(mEq/L) | 10.15 (7.32, 13.06) | 9.00 (6.32, 12.00) | <0.001 |
| Bicarbonate(mmol/L) | 28.12 (24.50, 32.37) | 30.00 (26.84, 35.00) | <0.001 |
| BUN(mg/dL) | 17.00 (12.20, 26.50) | 27.00 (19.84, 40.00) | <0.001 |
| Cr(mg/dL) | 1.03 (0.76, 1.50) | 0.88 (0.64, 1.29) | <0.001 |
| Treatment | |||
| Corticosteroid | 206 (11.20) | 179 (11.48) | 0.793 |
| Bronchodilators | 275 (14.95) | 224 (14.37) | 0.635 |
| Invasive-ventilation | 139 (7.55) | 96 (6.16) | 0.110 |
| Dialysis | 68 (3.70) | 13 (0.83) | <0.001 |
| APACHE-IV | 58.00 (45.00, 62.00) | 58.00 (51.00, 67.00) | <0.001 |
| ICU stay (day) | 2.57 (1.63, 4.36) | 2.93 (1.81, 5.02) | <0.001 |
| In-hospital mortality | 116(6.30) | 181(11.61) | <0.001 |
Notes: A p-value less than 0.05 is considered statistically significant.
Abbreviations: BMI, Body Mass Index; HR, Heart Rate; RR, Respiratory Rate; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; RDW, Red Blood Cell Distribution Width; WBC, White Blood Cell; PLT, Platelet; Cr, Creatinine; BUN, Blood urea nitrogen; BCR, BUN to Creatinine ratio.
After Propensity Score Matching
Propensity scores (PSs) for the high- and low-BCR groups were calculated via logistic regression. Subsequently, 1:1 nearest neighbor propensity score matching (PSM) with a caliper width of 0.02 was performed, successfully pairing 1174 patients between the groups. The efficiency of the match, as shown in Table S2 through the standardized mean difference (SMD) of variables postmatching, all of which were less than 0.1, confirms high-quality matching. Additionally, the empirical cumulative distribution function (ECDF) and density plots for the matched population demonstrated nearly perfect overlap, indicating the high quality of the match (Figures S2 and S3). Table 3 shows the baseline characteristics of the high- and low-BCR groups after matching. The results indicate that, with the exceptions of systolic blood pressure and white blood cell count, all the baseline characteristics were balanced between the high-BCR and low-BCR groups (p>0.05), confirming the effectiveness of the matching process. Notably, creatinine levels were significantly higher in the high-BCR subgroup than in the low-BCR subgroup prior to propensity score matching (p<0.001). However, after matching, creatinine levels were significantly lower in the high-BCR subgroup than in the low-BCR subgroup (p<0.001). This shift suggests that confounding factors in the unmatched cohort may have distorted the true relationship between creatinine levels in the two groups, which PSM effectively corrected.
Table 3.
The Baseline Data for the High BCR Group and Low BCR Group After Propensity Score Matching (PSM)
| Variable | BCR<22.78 (n=1174) | BCR≥22.78(n=1174) | P-Value |
|---|---|---|---|
| Demographic | |||
| Age | 69.00 (60.00, 76.00) | 68.00 (61.00, 76.00) | 0.815 |
| Male | 526 (44.80) | 539 (45.91) | 0.590 |
| BMI | 28.15 (23.09, 33.93) | 27.54 (21.95, 33.95) | 0.077 |
| Ethnicity | |||
| White | 1002 (85.35) | 1000 (85.18) | 0.808 |
| Black | 87 (7.41) | 82 (6.98) | |
| Others | 85 (7.24) | 92 (7.84) | |
| Vital signs | |||
| HR (beats/min) | 89.99 (79.23, 99.23) | 89.99 (81.34, 99.20) | 0.078 |
| RR (beats/min) | 21.02 (18.34, 23.47) | 21.00 (18.50, 22.98) | 0.386 |
| SBP (mmHg) | 122.26 (111.30, 131.74) | 121.14 (110.27, 130.37) | 0.047 |
| DBP (mmHg) | 66.90 (60.00, 72.58) | 66.90 (59.81, 72.28) | 0.543 |
| SpO2 (%) | 96.03 (94.34, 97.53) | 96.00 (94.38, 97.43) | 0.762 |
| Comorbidities | |||
| Hypertension | 197 (16.78) | 191 (16.27) | 0.739 |
| Diabetes | 178 (15.16) | 171 (14.57) | 0.685 |
| Congestive heart failure | 242 (20.61) | 229 (19.51) | 0.503 |
| Myocardial infarction | 47 (4.00) | 38 (3.24) | 0.320 |
| Asthma | 46 (3.92) | 47 (4.00) | 0.916 |
| Liver disease | 11 (0.94) | 14 (1.19) | 0.546 |
| Gastrointestinal hemorrhage | 23 (1.96) | 24 (2.04) | 0.883 |
| Chronic kidney disease | 73 (6.22) | 64 (5.45) | 0.428 |
| End stage renal disease | 2 (0.17) | 1 (0.09) | 1.000 |
| Acute renal failure | 157 (13.37) | 148 (12.61) | 0.581 |
| Malignant tumor | 28 (2.39) | 32 (2.73) | 0.601 |
| Sepsis | 176 (14.99) | 176 (14.99) | 1.000 |
| Laboratory | |||
| Hemoglobin(g/dL) | 11.89 (10.40, 13.30) | 11.89 (10.50, 13.15) | 0.383 |
| WBC(K/uL) | 10.50 (7.70, 13.70) | 11.20 (8.11, 14.57) | 0.003 |
| PLT(K/uL) | 211.50 (166.00, 263.00) | 215.00 (165.62, 269.00) | 0.540 |
| RDW(%) | 15.10 (13.91, 16.20) | 15.20 (14.05, 16.20) | 0.201 |
| Glucose(mg/dL) | 168.84 (145.50, 168.84) | 168.84 (144.00, 170.00) | 0.543 |
| Sodium(mmol/L) | 138.50 (136.00, 141.00) | 138.50 (135.50, 141.00) | 0.173 |
| Potassium(mmol/L) | 4.30 (3.90, 4.70) | 4.30 (3.90, 4.70) | 0.799 |
| Calcium(mg/dL) | 8.71 (8.30, 9.15) | 8.71 (8.30, 9.10) | 0.219 |
| PH | 7.30 (7.30, 7.40) | 7.30 (7.30, 7.40) | 0.567 |
| PaO2(mmHg) | 105.35 (75.50, 114.19) | 103.23 (76.00, 108.96) | 0.543 |
| PaCO2(mmHg) | 60.25 (49.42, 68.16) | 60.25 (49.95, 69.00) | 0.915 |
| Anion gap(mEq/L) | 10.00 (6.75, 12.50) | 9.50 (7.00, 12.50) | 0.898 |
| Bicarbonate(mmol/L) | 29.78 (25.50, 34.00) | 29.78 (26.00, 33.50) | 0.954 |
| BUN(mg/dL) | 17.29 (12.50, 26.50) | 27.00 (19.50, 39.00) | <0.001 |
| Cr(mg/dL) | 1.00 (0.75, 1.49) | 0.90 (0.65, 1.30) | <0.001 |
| Treatment | |||
| Corticosteroid | 128 (10.90) | 133 (11.33) | 0.743 |
| Bronchodilators | 155 (13.20) | 167 (14.22) | 0.472 |
| Invasive-ventilation | 82 (6.98) | 66 (5.62) | 0.174 |
| Dialysis | 14 (1.19) | 12 (1.02) | 0.693 |
| APACHE-IV | 58.00 (47.00, 65.00) | 58.00 (49.00, 64.00) | 0.397 |
| ICU stay (day) | 2.70 (1.69, 4.66) | 2.85 (1.77, 4.82) | 0.149 |
| In-hospital mortality | 87 (7.41) | 128 (10.90) | 0.003 |
Notes: A p-value less than 0.05 is considered statistically significant.
Abbreviations: BMI, Body Mass Index; HR, Heart Rate; RR, Respiratory Rate; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; RDW, Red Blood Cell Distribution Width; WBC, White Blood Cell; PLT, Platelet; Cr, Creatinine; BUN, Blood urea nitrogen; BCR, BUN to Creatinine ratio.
Relationship Between BCR and in-Hospital Mortality
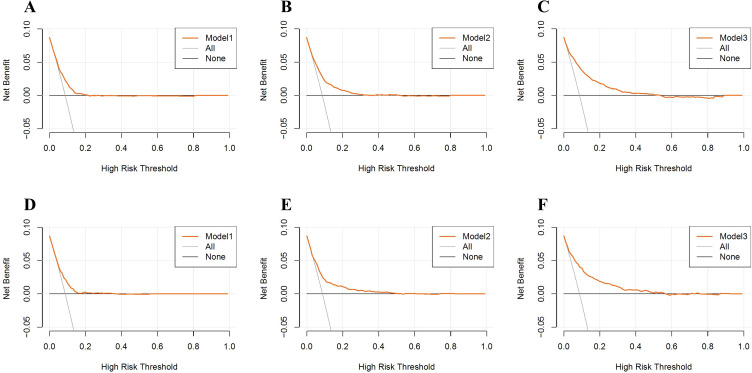
We utilized multivariable logistic regression to examine the association between BCR and in-hospital mortality among ICU patients with AECOPD. Four models were constructed for analysis: Crude model without adjustments; Model 1 adjusted for age, sex, ethnicity, and BMI; Model 2 further adjusted for comorbidities (hypertension, diabetes, congestive heart failure, myocardial infarction, asthma, liver disease, gastrointestinal hemorrhage, chronic kidney disease, end-stage renal disease, acute kidney injury, malignant tumor, sepsis) on top of Model 1; and Model 3 adjusted for all factors (age, sex, ethnicity, BMI, heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, SpO2, comorbidities listed earlier, hemoglobin, white blood cell count, platelet count, RDW, glucose, sodium, potassium, calcium, pH, PaCO2, PaO2, anion gap, bicarbonate, corticosteroid use, bronchodilator use, invasive ventilation, dialysis, APACHE-IV score, and ICU length of stay). To mitigate the risk of collinearity, only variables with a variance inflation factor less than 5 were included in the analyses. The results of the multivariable logistic regression are presented in Table 4. Before propensity score matching (PSM), the results were as follows: unadjusted [OR=1.952 (1.530–2.491), p<0.001]; Model 1 [OR=1.636 (1.274–2.101), p<0.001]; Model 2 [OR=1.693 (1.310–2.188), p<0.001]; Model 3 [OR=1.527 (1.154–2.019), p=0.003]. After PSM, the results were unadjusted [OR=1.529 (1.149–2.034), p=0.004]; Model 1 [OR=1.535 (1.150–2.048), p=0.004]; Model 2 [OR=1.555 (1.156–2.091), p=0.003]; Model 3 [OR=1.620 (1.182–2.220), p=0.003]. These findings indicate that both before and after PSM, the mortality rate among critically ill AECOPD patients in the high-BCR subgroup was significantly higher than that in the low-BCR subgroup. Elevated BCR levels serve as a significant predictor of in-hospital mortality among ICU patients with AECOPD. Additionally, decision curve analysis was employed to assess the clinical utility of Models 1, 2, and 3. As shown in Figure 2, the decision curves for Models 1, 2, and 3 remained consistently above the horizontal line in both the pre- and post-PSM cohorts, indicating stable model performance.
Table 4.
Multivariate Logistic Regression Analysis of the in-Hospital Mortality Rate in ICU Patients with AECOPD
| Crude Model | OR | Model1 | OR | Model2 | OR | Model3 | OR | |
|---|---|---|---|---|---|---|---|---|
| Before PSM | <0.001 | 1.952(1.530–2.491) | <0.001 | 1.636(1.274–2.101) | <0.001 | 1.693(1.310–2.188) | 0.003 | 1.527(1.154–2.019) |
| PSM | 0.004 | 1.529(1.149–2.034) | 0.004 | 1.535(1.150–2.048) | 0.003 | 1.555(1.156–2.091) | 0.003 | 1.620(1.182–2.220) |
Notes: Crude model: Unadjusted for any factors. Model 1: Adjusted for age, gender, ethnicity, and BMI. Model 2: Adjusted for age, gender, ethnicity, BMI, hypertension, diabetes, congestive heart failure, myocardial infarction, asthma, liver disease, gastrointestinal hemorrhage, chronic kidney disease, end-stage renal disease, acute kidney injury, malignant tumor, sepsis. Model 3: Adjusted for age, gender, ethnicity, BMI, heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, SpO2, hypertension, diabetes, congestive heart failure, myocardial infarction, asthma, liver disease, gastrointestinal hemorrhage, chronic kidney disease, end-stage renal disease, acute kidney injury, malignant tumor, sepsis, hemoglobin, WBC, PLT, RDW, glucose, sodium, potassium, calcium, pH, PaCO2, PaO2, anion gap, bicarbonate, corticosteroids, bronchodilators, invasive ventilation, dialysis, APACHE-IV score, ICU length of stay.
Abbreviations: AECOPD, Acute exacerbation of chronic obstructive pulmonary disease; PSM, Propensity score matching.
Figure 2.
Decision curve analysis (DCA) evaluating the clinical utility of model 1, 2, 3 in the original cohort (A–C) and the PSM cohort (D–F).
Notes: “All” represents the net benefit when all patients receive treatment. “None” represents the net benefit when no patients receive treatment. The blue lines represent the net benefits when treatment is administered according to each model.
Subgroup Analysis
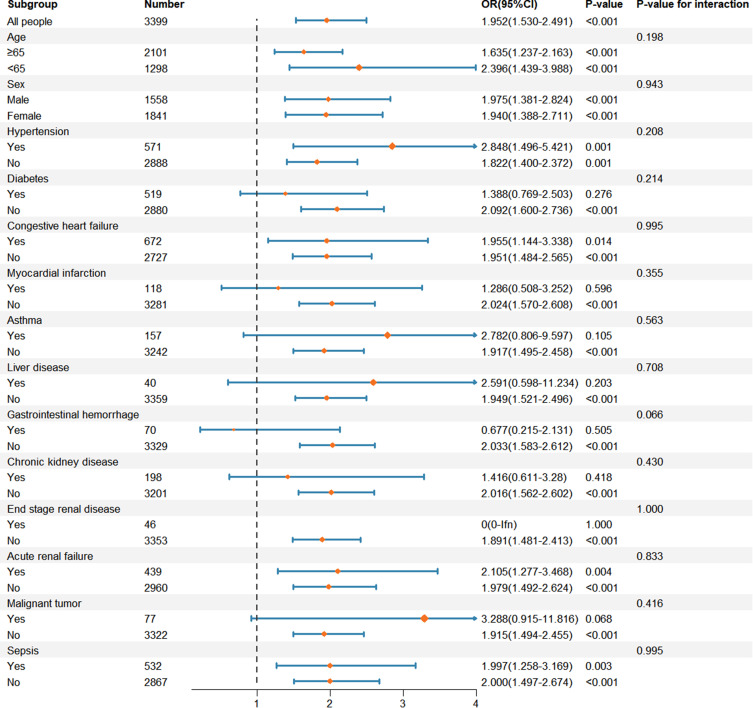
To explore potential interactions, we selected age (≥65 years vs <65 years), sex (male vs female), hypertension (yes vs no), diabetes (yes vs no), congestive heart failure (yes vs no), myocardial infarction (yes vs no), asthma (yes vs no), liver disease (yes vs no), gastrointestinal hemorrhage (yes vs no), chronic kidney disease (yes vs no), end-stage renal disease (yes vs no), acute kidney injury (yes vs no), malignant tumor (yes vs no), and sepsis (yes vs no) as subgroups. The results, presented in Figure 3, indicate that none of these subgroups demonstrated significant interactions with BCR (all p>0.05). These findings underscore the robustness of the BCR as a predictor of in-hospital mortality among AECOPD patients in the ICU.
Figure 3.
Subgroup analysis of the relationship between in-hospital mortality and BCR in critically ill AECOPD patients.
Discussion
This retrospective study included 3,399 AECOPD patients from the eICU database. Our research is the first to identify an association between an elevated blood urea nitrogen/creatinine ratio and increased in-hospital mortality among AECOPD patients in the ICU. The large sample size, along with the use of propensity score matching and multivariable logistic regression, significantly reduces potential confounding factors, enhancing the reliability of our results.
Urea nitrogen is the end product of protein metabolism. A retrospective cohort study of 842 patients indicated that elevated BUN levels at emergency admission correlate with increased mortality in AECOPD patients.21 Furthermore, a multicenter retrospective cohort study indicated that when BUN levels were below 40 mg/dL, the length of hospital stay for COPD patients was positively correlated with BUN levels.11 Giri et al reported that critically ill AECOPD patients with BUN levels above 23 mg/dL faced a 1.736-fold greater risk of in-hospital mortality than did those with lower levels.12 These studies suggest an association between high BUN levels and disease severity in COPD patients. Cr is the end product of creatine and phosphocreatine metabolism. Previous studies have shown that low baseline serum Cr concentrations increase the risk of mortality in critically ill patients.22 Kawasaki et al reported that in 124 outpatient COPD patients, serum Cr levels were significantly lower in patients with severe COPD than in mild COPD patients.23 Afzal et al reported that low serum Cr levels in critically ill COPD patients were associated with increased one-year in-hospital mortality.24 Additionally, a prospective cohort study demonstrated that low serum creatinine levels were correlated with sarcopenia and increased hospitalization rates in male outpatient COPD patients.25
The BCR is defined as the ratio of blood urea nitrogen to creatinine. It has been demonstrated to be associated with the onset and poor prognosis of various diseases, and can serve as an independent predictor of mortality. Wang et al reported that BCR was related to all-cause mortality in patients with chronic heart failure combined with renal impairment.26 A prospective cohort study involving 26,379 patients indicated that low BCR levels were an independent high-risk factor for ischemic stroke.17 Liu et al provided epidemiological evidence that high BCR levels increase the risk of coronary artery disease in patients with type 2 diabetes.27 In particular, in the field of respiratory diseases, BCRs serve as valuable biomarkers for disease diagnosis and prognosis assessment. For example, the BCR can distinguish between asthma and heart failure.28 Ma et al suggested that the BCR might be a potential biomarker for in-hospital mortality risk in trauma-related acute respiratory distress syndrome patients.29
This study investigated the relationship between BCR levels and in-hospital mortality among ICU patients with AECOPD. Our research revealed that critically ill AECOPD patients with high BCR levels (≥22.78) faced a 1.952-fold greater risk of in-hospital mortality than did those with low BCR levels (<22.78). This association remained significant after adjusting for confounding factors via multivariable logistic regression and propensity score matching. Currently, the mechanisms underlying the association between elevated BCR levels and increased in-hospital mortality in ICU patients with AECOPD remain unclear. However, several explanations can be proposed. A higher BCR may indicate elevated levels of BUN and lower levels of Cr. Elevated BUN levels may indicate increased inflammation in patients with COPD. Inflammatory responses are crucial in the development and progression of this disease. The levels of inflammatory markers such as TNF-α and IL-6 are significantly elevated in the serum of COPD patients, particularly during acute exacerbations.30 These inflammatory factors may be involved in the proteolysis process, leading to increased BUN levels.31 Furthermore, elevated BUN levels, as the end product of protein metabolism, represent increased metabolic activity. Persistent high metabolism can lead to decreased nutritional status and immune function, which deteriorates patients’ baseline condition and increases the risk of infection.32 Overall, high BUN levels reflect stronger inflammatory responses, poorer baseline conditions, and greater infection risks in AECOPD patients. Lower serum creatinine levels directly indicate reduced muscle mass.33 During acute exacerbations of COPD, circulating TNF-α levels increase sharply, and high TNF-α levels are directly related to acute weight loss and muscle mass reduction.30,34 Therefore, low creatinine levels may also reflect the levels of inflammatory factors in AECOPD patients. Additionally, muscle loss can lead to diaphragm fatigue and reduced respiratory muscle strength, increasing the risk of respiratory failure and consequently increasing mortality in these patients.35 In conclusion, an elevated BCR may predict mortality in critically ill AECOPD patients by indicating higher levels of inflammation, poorer baseline conditions, and an increased risk of muscle atrophy.
Our study has several limitations. First, we identified an association between elevated BCR and increased mortality in critically ill AECOPD patients. The specific mechanisms driving this relationship remain to be determined. This area presents a valuable opportunity for further investigative work. Second, our analysis was based on the blood creatinine and urea nitrogen measurements taken within the first 24 hours of ICU admission. Dynamic monitoring of the BCR could provide a more accurate guide for clinical decision-making throughout a patient’s ICU stay. Finally, the retrospective design of our study may introduce some inherent biases. These findings suggest the value of further exploration through prospective multicenter studies to enhance our understanding of the relationship between BCR and mortality in this patient population.
Conclusion
An elevated BCR is an independent risk factor for in-hospital mortality in ICU patients with AECOPD. Early attention to BCR levels can help clinicians formulate treatment strategies and improve patient outcomes.
Funding Statement
This work was supported by the National Key Research and Development Program (2021YFC0864500, 2023YFC3041700), the National Natural Science Foundation of China (82241003), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515110420), Guangzhou Basic and Applied Basic Research Foundation (Grant No. 202201010420), Guangzhou Science and Technology Plans (No. 202201020513), and Guangxi Natural Science Foundation (2021GXNSFBA220064).
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available in the eICU-CRD dataset, https://physionet.org/content/eicu-crd/2.0/.
Consent for Publication
All participants agreed to publish the article.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; all authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no financial or commercial conflicts of interest.
References
- 1.Kahnert K, Jörres RA, Behr J, Welte T. The diagnosis and treatment of COPD and its comorbidities. Dtsch Arztebl Int. 2023;120(25):434–444. doi: 10.3238/arztebl.m2023.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lareau SC, Fahy B, Meek P, Wang A. Chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2019;199(1):P1–P2. doi: 10.1164/rccm.1991P1 [DOI] [PubMed] [Google Scholar]
- 3.Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi: 10.1016/s0140-6736(22)00470-6 [DOI] [PubMed] [Google Scholar]
- 4.López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi: 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 5.Ritchie AI, Wedzicha JA. Definition, causes, pathogenesis, and consequences of chronic obstructive pulmonary disease exacerbations. Clin Chest Med. 2020;41(3):421–438. doi: 10.1016/j.ccm.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology. 2016;21(7):1152–1165. doi: 10.1111/resp.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prediletto I, Giancotti G, Nava S. COPD exacerbation: why it is important to avoid icu admission. J Clin Med. 2023;12(10):3369. doi: 10.3390/jcm12103369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinellu A, Zinellu E, Mangoni AA, et al. Clinical significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute exacerbations of COPD: present and future. Eur Respir Rev. 2022;31(166):220095. doi: 10.1183/16000617.0095-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Sun H, Liu J. Bun level is associated with cancer prevalence. Eur J Med Res. 2023;28(1):213. doi: 10.1186/s40001-023-01186-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Qin Y, Zhou C, et al. Elevated bun upon admission as a predictor of in-hospital mortality among patients with acute exacerbation of COPD: a secondary analysis of multicenter cohort study. Int J Chron Obstruct Pulmon Dis. 2023;18:1445–1455. doi: 10.2147/COPD.S412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du J, Niu J, Ma L, Sui Y, Wang S. Association between blood urea nitrogen levels and length of stay in patients with pneumonic chronic obstructive pulmonary disease exacerbation: a secondary analysis based on a multicentre, retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2022;17:2847–2856. doi: 10.2147/COPD.S381872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giri M, He L, Hu T, et al. Blood urea nitrogen is associated with in-hospital mortality in critically ill patients with acute exacerbation of chronic obstructive pulmonary disease: a propensity score matching analysis. J Clin Med. 2022;11(22):6709. doi: 10.3390/jcm11226709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rosa S, Samoni S, Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Crit Care. 2016;20(1):69. doi: 10.1186/s13054-016-1218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao H, Wang J, Zou X, Zhang K, Zhou J, Chen M. High blood urea nitrogen to creatinine ratio is associated with increased risk of sarcopenia in patients with chronic obstructive pulmonary disease. Exp Gerontol. 2022;169:111960. doi: 10.1016/j.exger.2022.111960 [DOI] [PubMed] [Google Scholar]
- 15.Sorino C, Scichilone N, Pedone C, Negri S, Visca D, Spanevello A. When kidneys and lungs suffer together. J Nephrol. 2019;32(5):699–707. doi: 10.1007/s40620-018-00563-1 [DOI] [PubMed] [Google Scholar]
- 16.Lin HL, Chen CW, Lu CY, et al. High preoperative ratio of blood urea nitrogen to creatinine increased mortality in gastrointestinal cancer patients who developed postoperative enteric fistulas. Kaohsiung J Med Sci. 2012;28(8):418–422. doi: 10.1016/j.kjms.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 17.Peng R, Liu K, Li W, et al. Blood urea nitrogen, blood urea nitrogen to creatinine ratio and incident stroke: the Dongfeng-Tongji cohort. Atherosclerosis. 2021;333:1–8. doi: 10.1016/j.atherosclerosis.2021.08.011 [DOI] [PubMed] [Google Scholar]
- 18.Sun D, Wei C, Li Z. Blood urea nitrogen to creatinine ratio is associated with in-hospital mortality among critically ill patients with cardiogenic shock. BMC Cardiovasc Disord. 2022;22(1):258. doi: 10.1186/s12872-022-02692-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q, Wang Y, Zhao X, et al. Diagnostic performance of a blood urea nitrogen to creatinine ratio-based nomogram for predicting in-hospital mortality in covid-19 patients. Risk Manag Healthc Policy. 2021;14:117–128. doi: 10.2147/rmhp.S278365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eicu collaborative research database, a freely available multi-center database for critical care research. Sci Data. 2018;5(1):180178. doi: 10.1038/sdata.2018.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Chen L, Zheng H, Wu S, Wang S. The association of blood urea nitrogen levels upon emergency admission with mortality in acute exacerbation of chronic obstructive pulmonary disease. Chron Respir Dis. 2021;18:14799731211060051. doi: 10.1177/14799731211060051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartin-Ceba R, Afessa B, Gajic O. Low baseline serum creatinine concentration predicts mortality in critically ill patients independent of body mass index. Crit Care Med. 2007;35(10):2420–2423. doi: 10.1097/01.ccm.0000281856.78526.f4 [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki Y, Nishiki K, Nojiri M, et al. Prognostic value of the serum creatinine/cystatin c ratio in patients with chronic obstructive pulmonary disease. Respir Investig. 2024;62(1):143–149. doi: 10.1016/j.resinv.2023.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Afzal AB, Khalid S, Baksi S. Association between low serum creatinine and mortality in patients with severe chronic obstructive pulmonary disease. Cureus. 2022;14(9):e29376. doi: 10.7759/cureus.29376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai K, Tanaka A, Homma T, et al. Serum creatinine/cystatin c ratio as a surrogate marker for sarcopenia in patients with chronic obstructive pulmonary disease. Clin Nutr. 2021;40(3):1274–1280. doi: 10.1016/j.clnu.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Xu X, Shi S, et al. Blood urea nitrogen to creatinine ratio and long-term survival in patients with chronic heart failure. Eur J Med Res. 2023;28(1):343. doi: 10.1186/s40001-023-01066-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Ma G, Tong C, et al. Elevated blood urea nitrogen-to-creatinine ratio increased the risk of coronary artery disease in patients living with type 2 diabetes mellitus. BMC Endocr Disord. 2022;22(1):50. doi: 10.1186/s12902-022-00954-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Zhou L, Zhang Y. Diagnostic values of blood urea nitrogen (bun), creatinine (cr), and the ratio of bun to cr for distinguishing heart failure from asthma and chronic obstructive pulmonary disease. Comput Math Methods Med. 2022;2022:4586458. doi: 10.1155/2022/4586458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Lin S, Xie Y, et al. Association between bun/creatinine ratio and the risk of in-hospital mortality in patients with trauma-related acute respiratory distress syndrome: a single-centre retrospective cohort from the mimic database. BMJ Open. 2023;13(4):e069345. doi: 10.1136/bmjopen-2022-069345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng Z, Ke X, Gong S, et al. Blood urea nitrogen to serum albumin ratio: a good predictor of in-hospital and 90-day all-cause mortality in patients with acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med. 2022;22(1):476. doi: 10.1186/s12890-022-02258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beier K, Eppanapally S, Bazick HS, et al. Elevation of blood urea nitrogen is predictive of long-term mortality in critically ill patients independent of “normal” creatinine. Crit Care Med. 2011;39(2):305–313. doi: 10.1097/CCM.0b013e3181ffe22a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin c. Clin J Am Soc Nephrol. 2008;3(2):348–354. doi: 10.2215/cjn.02870707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langen RC, Gosker HR, Remels AH, Schols AM. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int J Biochem Cell Biol. 2013;45(10):2245–2256. doi: 10.1016/j.biocel.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Su R, Hu R, et al. Sarcopenia index as a predictor of clinical outcomes among older adult patients with acute exacerbation of chronic obstructive pulmonary disease: a cross-sectional study. BMC Geriatr. 2023;23(1):89. doi: 10.1186/s12877-023-03784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the eICU-CRD dataset, https://physionet.org/content/eicu-crd/2.0/.