Abstract
Recent years have seen significant advances in diagnostic testing of central nervous system (CNS) function and disease. However, there remain challenges in developing a comprehensive suite of non- or minimally invasive assays of neural health and disease progression. Due to the direct connection with the CNS, structural changes in the neural retina, retinal vasculature and morphological changes in retinal immune cells can occur in parallel with disease conditions in the brain. The retina can also, uniquely, be assessed directly and non-invasively. For these reasons, the retina may prove to be an important “window” for revealing and understanding brain disease. In this review, we discuss the gross anatomy of the eye, focusing on the sensory and non-sensory cells of the retina, especially microglia, that lend themselves to diagnosing brain disease by imaging the retina. We include a history of ocular imaging to describe the different imaging approaches undertaken in the past and outline current and emerging technologies including retinal autofluorescence imaging, Raman spectroscopy, and artificial intelligence image analysis. These new technologies show promising potential for retinal imaging to be used as a tool for the diagnosis of brain disorders such as Alzheimer’s disease and others and the assessment of treatment success.
Introduction to the human eye
Imaging of the posterior aspect of the eye has been used for at least 150 years to assess retinal health and provide an early window into vascular conditions such as diabetic retinopathy [54]. More recently, advances in retinal imaging combined with the availability of large-scale retinal datasets has created the new field of ‘oculomics’ [185]. The most promising aspect of the emerging field is the potential to examine the structural, cellular and molecular indicators of brain health (e.g. dementia [11]), neurological disorders (e.g. multiple sclerosis [109, 140]) and systemic diseases (e.g. diabetes [170, 178, 190]) through the eye. Here we provide an overview of ocular anatomy relevant for such imaging and we discuss historical imaging techniques, as well as the latest advances in imaging the retina as a method to better understand eye and brain health.
Gross anatomy of the eye
The eye is an essential part of almost all vertebrates, converting light energy into a chemical signal that the brain translates into an image. In humans, the external eye is composed of a thick collagen layer, comprising the sclera (the white part of the eye that extends posteriorly) and cornea (the transparent part that sits anterior to the iris). Anatomically, there are three intraocular compartments separated by fluid-filled chambers: the anterior, posterior, and vitreous chambers (Fig. 1). The vitreous chamber plays a significant role in maintaining the spherical shape of the eye and ensuring that the retina adheres to the sclera [54]. However, a key property for ocular imaging is that it is transparent to visible and near-infrared wavelengths, allowing the retinal layers to be imaged with minimal interference.
Fig. 1.
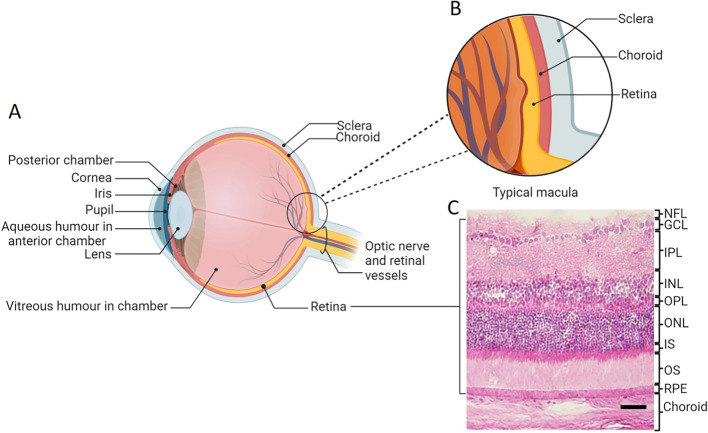
Basic anatomy of the eye. A Schematic diagram of the eye. The eye has three fluid-filled compartments: the anterior, the posterior, and the vitreous chambers. The retina is located posteriorly and comprises the sensory retina and retinal pigment epithelium (RPE). Posterior to the retina is the pigmented, vascular uveal layer comprising the choroid, and posterior to that, the outer collagenous tunic of the sclera. B Magnified schematic diagram of the typical macula. C Hematoxylin and eosin-stained image of the retina. Abbreviations: GCL: ganglion cell layer, INL: inner nuclear layer, IPL: inner plexiform layer, IS: photoreceptor inner segment, NFL: nerve fiber layer, ONL: outer nuclear layer, OPL: outer plexiform layer, OS: photoreceptor outer segment, RPE: retinal pigment epithelium. A, B created with BioRender.com; Toronto, Canada. C Scale bar = 200 µm
Retinal vasculature
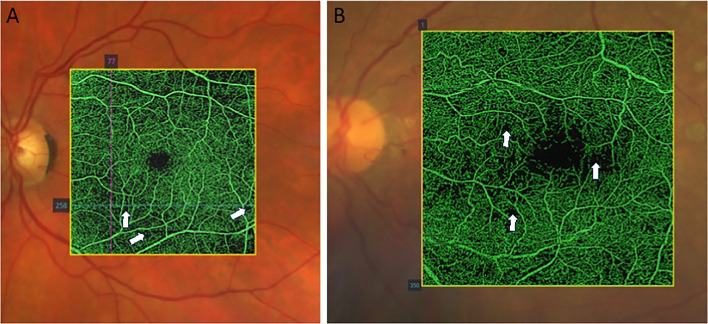
The posterior pole of the eye contains the neural, or sensory, retina, comprising a combination of sensory neurons (e.g., photoreceptors), second and third order neurons (e.g. bipolar cells and ganglion cells) and the retinal pigment epithelium (RPE), which lies on a basement membrane (Bruch’s membrane). Posterior to the retina is the pigmented and highly vascularized choroid. In the human retina, around 65% of the blood supply to the eye enters through the choroid, with 35% entering via the inner retinal vascular system [1]. Study of the retinal vasculature has enabled us to predict the status of vascular disease such as diabetes and hypertension, by assessing structural changes to microvasculature, the presence of hemorrhages, exudates and evidence of capillary dropout or leakage (Fig. 2) [104, 121].
Fig. 2.
Optical Coherence Tomography (OCT) angiographic appearance of diabetic retinopathy. A Areas of capillary rarefaction that do not manifest as gradable diabetic retinopathy. B Significant capillary bed occlusion and dropout in a patient with advanced diabetic retinopathy. Areas of dropout are indicated by white arrowheads. Image: James Armitage, Deakin Collaborative Eye Care Clinic
The sensory retina
The RPE plays an important role in visual function and in the development and progression of retinal diseases and in this sense is also a valuable predictor of retinal health [177]. This monolayer is an integral element of the blood-retina barrier and key in the maintenance of retinal homeostasis [78]. Apically, the long microvilli of the RPE interdigitate with the outer segments of photoreceptors and enable the continuous exchange of nutrients, signaling molecules, and metabolic products, as well as recycling of metabolites via phagocytosis of photoreceptor discs. Basolaterally, the RPE is nourished by the choriocapillaris, which also allows continuous exchange of nutrients, signaling molecules and metabolic products between the retina and blood stream as well as between the RPE and choroid [47, 58, 86, 87]. An important function of the RPE is in trans-epithelial fluid movement. In a normal functioning retina, photoreceptor activity and intraocular pressure stimulate the RPE to absorb water from the subretinal space to the blood. Therefore, the RPE maintains a constant ion composition in the subretinal space during light-induced changes [47, 48]. This process can be disrupted in some diseases. For instance, the hallmark for age-related macular degeneration is the appearance of drusen and atrophy of the RPE [16, 60], which can be readily detected via imaging with broad spectrum white light. In addition, because certain molecules differentially absorb and emit light, fundus autofluorescence (FAF) imaging can further characterize RPE function.
The sensory retina of the human eye itself can be used in image-based disease diagnosis, particularly when tomographic methods are used. For instance, patients with white matter hyperintensity and Parkinson’s disease present with thinner outer retinal layers when visualized with optical coherence tomography (OCT) [212]. The texture of the retinal layers, as seen with OCT, is also an important feature of Alzheimer’s disease (AD) [83]. The sensory retina can be divided into ten layers, each comprising specific cell types with unique roles in light detection and visual processing [1, 117]. Light entering the eye is converted into a biological signal within the photoreceptor outer segments, firstly by changing the membrane potential and thereby the transmission of neurotransmitters across the synaptic cleft. The number of photoreceptors (rods and cones) within this layer can change with retinal diseases. For example, in macular degeneration the number of cones declines, and such changes can be visualized using OCT [194, 196]. Horizontal cells and amacrine cells may provide future targets for imaging-related diagnostics since they have key morphological characteristics that reflect their function [94, 169], and horizontal cells, at least, have been implicated in retinoblastoma in rodent studies [3]. However, there is currently little application of imaging in humans to these cell types. Ganglion cells, on the other hand have been utilized to identify glaucoma, where ganglion cell loss can be identified with OCT [57]. Ganglion cells are the last retinal cells to receive and process visual information before transmitting this information to the brain via the nerve fiber layer towards the optic nerve.
Non-sensory cells of the retina
Among the non-sensory cells of the retina, microglia are key immune cells useful in revealing eye and brain health post-mortem. Microglia primarily reside in both the outer nuclear layer (ONL) and inner plexiform layer (IPL; Fig. 3). However, microglia that are interleukin (IL)-34-dependent, where IL-34 assists their migration through the different retinal layers, are mostly located in the IPL and contribute to cone-driven information processing [134]. Notably, in the event of retinal degeneration or disease, microglia from both the ONL and IPL migrate towards the site of the degenerating pathology [134, 150]. For example, in various retinopathies, such as diabetic retinopathy and age-related macular degeneration, microglia transition into amoeboid-shaped cells and migrate towards degenerating photoreceptors in the ONL [33, 120, 150]. In this regard, the morphology and the location of microglia within specific layers of the retina can be indicative of specific pathologies in post-mortem analyses [79, 134, 152]. Importantly, there is also strong potential for detecting morphological changes in these cells safely with the use of exogenous dyes such as tomato lectin and autofluorescence, as discussed below [22].
Fig. 3.
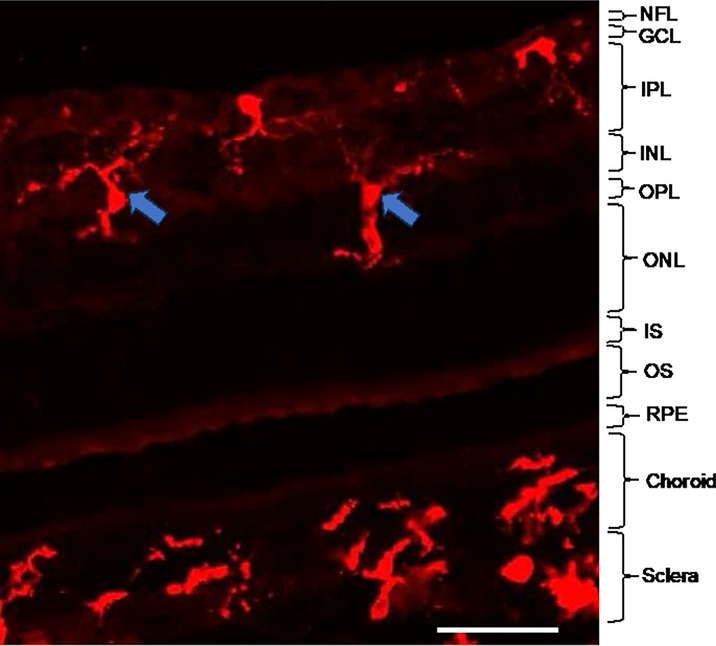
Residence of microglia in the different layers of the retina. Blue arrows indicate example microglia, immunolabelled with ionized calcium binding protein 1 (Iba1; Alexa Fluor-647, red) in the different layers of the retina. Abbreviations: GCL: ganglion cell layer, INL: inner nuclear layer, IPL: inner plexiform layer, IS: photoreceptor inner segment, NFL: nerve fiber layer, ONL: outer nuclear layer, OPL: outer plexiform layer, OS: photoreceptor outer segments. Scale bar = 50 µm
Müller cells are the largest cells in the retina and the principal glial cell, stretching across all retinal layers, with their cell bodies located in the inner nuclear layer (INL). Müller cells primarily provide metabolic and functional support to retinal neurons [23, 141]. For example, they contribute to synthesis of tight junction proteins that help to generate the internal blood-retinal barrier in association with endothelial cells [15]. Being an important part of retina, dysfunction of the Müller cells is the cause of some common retinal diseases such as macular edema, retinal degeneration, glaucoma, and diabetic retinopathy [15, 92]. Fundus imaging and OCT imaging techniques have been used to identify Müller cell dysfunction and diagnose sheen dystrophy, macular disease, and retinal degeneration [9, 138].
A brief history of using the eye to reveal general health
Over the past 150 years, retinal imaging has evolved from early identification of basic gross anatomical characteristics to the sophisticated and efficient usage that is common today. We will discuss the early history of retinal imaging, the progression to gold-standard imaging of today, and address what we can expect from emerging technologies in the field of oculomics in the future.
The nineteenth century
The progress of ophthalmology and eye imaging has an almost 300-year history, which began with the earliest large and complex instrumentation with limited diagnostic capacity and has expanded more recently to simpler and more accurate diagnosis capabilities. The understanding that the eye could reflect health was first revealed by Jean Mery in 1704 when he observed that retinal blood vessel changes could be seen through the sclera [192]. The keratoscope was developed by British physician Henry Goode in 1847, enabling the assessment of the anterior surface of the cornea [24]. A few years later, the ophthalmoscope was developed by German physicist and physician Hermann von Helmholtz in 1851 [184], enabling visualization of the retina, optic nerve, vasculature and vitreous humour. This was adapted for use in the clinic by Albrecht von Graefe, who also developed surgical iridectomy as a treatment for glaucoma [80]. History’s first retinal image was published by Dutch ophthalmologist, Adrien van Trigt, in 1853 (Fig. 4) [181] and this was capitalized on in 1886 and 1887 when Jackman and Webster published the first fundus image and then Lucien Howe revealed his own fundus photography from the human eye (Jackman WT 1886; [71]). In 1891, German ophthalmologist, Gerloff, produced the earliest successful image of the retinal blood vessels [1, 62].
Fig. 4.
History’s first image of the retina. [181], first published a retinal image where he successfully showed the optical disc. Image taken from [181]
Discovery and improvement of retinal imaging techniques after the nineteenth century
The twentieth and twenty-first centuries then yielded many new and improved developments in retinal imaging. In particular, the improvement of fundus imaging, the development of OCT, and angiographic imaging are some of the important outcomes for imaging the retina that now assist with diagnosis of disease. Specifically, these imaging modalities expand the current field of oculomics by identifying macroscopic, microscopic and molecular signatures of neurological and systemic diseases in the retina.
Fundus imaging
While the concept of fundus imaging was introduced by von Helmholtz and van Trigt in the mid-1800s, it was not until 1886 that the first fundus image was published by Jackman and Webster [81]. In 1910, Gullstrand developed the fundus camera, which is essentially still used for retinal imaging today due to the high safety profile of the technique. This development earned Gullstrand a Nobel Prize in Physiology or Medicine in 1911 [66]. Fundus cameras were first commercialized in 1926, when the Zeiss Company introduced the Zeiss-Nordenson camera [199]. Early fundus imaging was limited, due to low quality images, long exposure times needed, artifacts caused by eye movement, office-based usage, technician dependency, and its cost [137]. However, it proved to be an important breakthrough for early diagnosis of diseases related to the eye. The originally developed fundus imaging methodology had significant limitations in that it was the two-dimensional representation of a three-dimensional structure. To overcome this limitation, in 1964, Allen first described the concept of stereo fundus photography [5]. In this technique, multiple images are taken from different angles, then a human observer analyzes the images through stereo viewers to perceive a stereoscopic view of retinal form. It is essentially this stereo fundus photography that is still in use today, with its most common usage being in the early detection of diabetic retinopathy through mass-screening (Fig. 5A, B) [27, 91]. This imaging technique also helps to diagnose risk factors for several fatal diseases such as hypertension, cardiovascular disease, diabetes, and myocardial infarction by revealing abnormalities in the retinal blood vessels [36, 197].
Fig. 5.
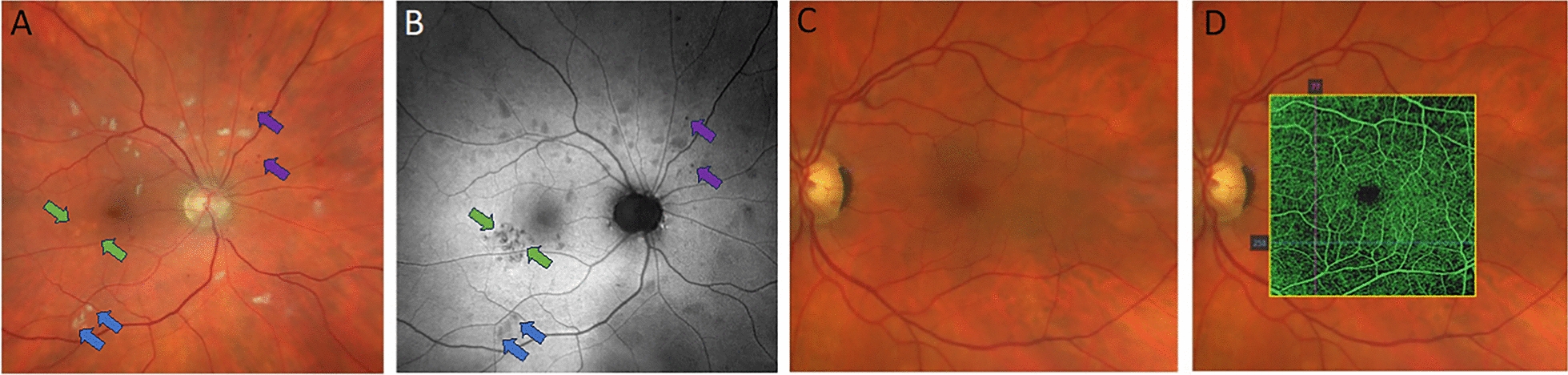
Multimodal imaging. A and B Multimodal imaging of the ocular fundus in a patient with hypertensive retinopathy using Scanning Laser Ophthalmoscopy (Zeiss Clarus 500). A True colour image showing multiple white lesions located in the retinal nerve fibre layer: cotton wool spots (retinal nerve fibre infarction), intraretinal haemorrhages and subtle changes in RPE appearance. B Green fundus autofluorescence image from the same patient highlighting the areas of RPE dysfunction that are difficult to discern on the colour image. A and B Cotton wool spots are indicated by the blue arrows. RPE dysfunction is indicated by the green arrows. Haemorrhages are indicated by purple arrows. C and D Multimodal imaging of the ocular fundus in a patient with type 2 diabetes mellitus. C Image shows a healthy ocular fundus with no evidence of microaneurysms or intraretinal hemorrhages. D The same patient imaged with OCT angiography. There are areas of capillary drop-out visible on angiography that are not detectable with regular fundus imaging. Note D is the same image as shown in Fig. 2B, which is annotated to show areas of capillary dropout. Images: James Armitage, Deakin Collaborative Eye Care Clinic
FAF imaging takes the technology a step further, by taking advantage of the intrinsic fluorescence properties of lipofuscin within the RPE to generate the image. While many conditions are macroscopically visible using broad spectrum white light imaging techniques such as fundus photography, other signs of retinal disease or dysfunction require more specialized imaging techniques that rely on detecting biomarkers of a pathophysiological process (Fig. 5A and B show this effect well). In the retina, microglia play a significant role in maintaining retinal homeostasis, responding to injury, and regulating disease progression. Homeostatic microglia are ramified in shape and interact with the immune effector cells to respond to an immune challenge [32, 189], becoming ameboid in shape and orientating towards the pathogen or injury [82, 155]. Several studies have shown that ameboid retinal microglia are associated with disease states such as age-related macular degeneration, diabetic retinopathy and uveitis [90, 150, 210, 213]. The microglial response to immune challenge produces lysosomal degraded material with autofluorescent properties that may be reflected in FAF and can potentially be used to diagnose and monitor retinal diseases [116]. Of note, hyperfluorescent foci related to microglial activity have been imaged in experimental studies in humans using FAF. Further investigations to characterize these hyperfluorescent foci indicate that they highly correlate with the presence of subretinal microglial and macrophage markers indicative of immune reactivity, with a large proportion of the subretinal microglia being CD16-positive [116, 202]. Microglia have also been shown to infiltrate photoreceptor outer segments where they become highly autofluorescent following an apoptotic event. The microglial autofluorescence profile with FAF, particularly as resolution improves, may be useful as a clinical biomarker in understanding central nervous system (CNS) disease [116, 188].
While widely utilized, fundus imaging currently still has a few limitations. Firstly, this imaging system cannot detect damage or disease at the micro level, i.e., damaged photoreceptors or bipolar cells, and the resolution to detect autofluorescence in specific cells such as microglia is poor. It is also largely restricted to the central 45–60 degrees, which limits understanding of disease processes that affect the peripheral retina including peripheral drusen, peripheral retinal tears, and peripheral cystoid degeneration (Vinay A. Shah 2023). Additionally, the shape of the individual’s eyelashes and eye globe may affect the quality of the image captured. Fundus imaging is also mainly based on qualitative assessment, but for accuracy in disease diagnosis, consistency and quantitative interpretation are required [53, 72, 166, 179].
Angiographic imaging
Another important twentieth century addition to retinal diagnostics has been angiographic imaging (Fig. 5C, D). In 1961, Alvis and Novotny, two students at Indiana University, demonstrated the procedure of retinal fluorescein angiogram imaging [133]. A few years later, in 1967, Gass published their experience of using fluorescein imaging [61]. In this technique, fluorescein dye is injected into the bloodstream. The dye dissolves in plasma and is transported through the vasculature to all organs, including to the retina. This allows an image of the retina to be captured that visualizes dye within the blood, clearly revealing abnormalities to the vasculature [133].
In addition to their role in immune function, microglia play a significant role in blood vessel formation, regulating blood flow and in responding to vascular injury [30, 40],Pialuisa Quiriconi 2024). Animal studies of diabetic retinopathy and other retinal vascular abnormalities have shown that microglia localize at sites of neovascularization and intraretinal hemorrhages [26, 39, 70, 142, 204, 205]. Thus, imaging techniques that combine angiographic imaging with analysis of microglial location and autofluorescence will be informative of retinal disease and vascular issues that may be pertinent to brain health.
However, while useful, this technique has some safety limitations and is costly. Some common side effects include the patient experiencing blurred vision for around three hours and yellowing of the skin, eyes, and urine for up to 48 h. More critically, some patients can experience symptoms of fluorescein hypersensitivity including nausea, vomiting, and allergic reactions (Hospital 2020). For this reason, this technique is not conducted routinely, but is reserved for circumstances where leakage from retinal blood vessels is suspected [200, 211], especially considering that noninvasive vascular imaging methods have been developed based on time-resolved analysis of OCT.
Optical laser ophthalmoscopy and optical coherence tomography-based imaging
By 1979, the confocal optical laser ophthalmoscope had been developed. This tool allows a three-dimensional image of the retina to be taken that is up to 100 µm in depth; a significant improvement on earlier technologies albeit that this depth is still relatively shallow in comparison to the whole thickness of the retina, at 300–500 µm [193]. Tomographic imaging became popular after the development of super-luminescent diodes, femtosecond lasers and OCT, which allows the practitioner to derive an accurate three-dimensional image of the retina [76, 182]. Although femtosecond optical ranging (A-scans) and ex vivo OCT were demonstrated in 1987 and 1991, respectively, commercialization of the first ophthalmic OCT device for use in living humans was not finalized until 1996 [56].
Current OCT can image large areas of the retina, including peripheral retina, although, the lateral resolution is still low [85, 206]. Comparison is also difficult between images taken from different machines, due to the absence of a standard value for comparison [156, 201]. However, OCT can be used to diagnose and differentiate between benign nevi and melanomas [158] and is a vital ocular imaging tool for retinal disease diagnosis and progression in disorders such as multiple sclerosis [109, 140].
A key component of OCT imaging is its ability to identify hyperreflective microglia both ex-vivo [92, 125, 146] and in vivo [124, 189]. Improvements in OCT imaging instruments have provided the ability to diagnose and monitor ocular conditions down to the cellular level. For example, dynamic OCT has been shown to distinguish between microglia, macrophages and vitreal hyalocytes in a healthy subject. OCT technology could therefore aid, in the field of oculomics, in identifying specific cell types within the retina supporting better diagnosis and management of ocular disease [144] and from there to brain disease biomarker discovery [68, 88].
Ultrasound imaging
Photography and OCT require the ocular media (cornea, aqueous humour, crystalline lens and vitreous humour) to be transparent so as to not scatter light from the instrument. If the ocular media loses this transparency (e.g. with a cataract or following intraocular hemorrhage) ultrasound imaging may allow visualization of the leakage. Evidence for the use of ultrasound for this purpose was first published by Naor et al. in 2012 [128]. Ultrasound enables real time imaging of blood vessels, tissues, and organs by the use of sound waves [110]. Due to the superficial location of the eye, ultrasound imaging can be used to diagnose eye diseases and monitor the disease pathology [127]. Notably, ultrasound imaging has also been used to detect the transcriptional signal of microglial cells to understand levels of amyloid beta deposition in the APP23 AD mouse model [101]. In addition, ultrasound can be used to detect perturbations to the blood–brain barrier and blood-retinal barrier, as well as microglial changes in the brain and retina [21, 105, 106]. However, this technique has drawbacks in the clinic in that it takes 30 to 45 min to be completed, a trained technician is required, and there may be some risk of thermal damage to the eye [44].
Structural and functional MRI
A further recent addition to diagnostic imaging via the retina is magnetic resonance imaging (MRI), used to visualize retinal morphology and monitor retinal activity [46]. Structural and functional MRI were initially used to detect different layers of the retina in the different animal model studies [31, 35, 163]. Chromium-enhanced in vivo MRI imaging has also been used to detect lipid spatiotemporal profiles in the retina of normal adult rats [29]. In humans, MRI has been used for the non-invasive quantitative measurement of the retinal-choroidal blood flow in the eye of retinal pigmentosa patients [207, 208]. It has also been utilized experimentally to quantify retinal and choroidal blood flow during resting and hand-grip isometric exercise [209]. Apart from the advantage of using this technique, there are some limitations, such as its cost and low spatiotemporal resolution and sensitivity. Improvement of diagnostic algorithms are necessary for more accurate quantitative assessment of the different in vivo aspects in humans, such as retinal blood flow [45].
Second harmonic generation microscopy
In terms of imaging of biological samples, second harmonic generation (SHG) microscopy is one of several recent advances [2]. In the SHG microscopic technique, an excitation laser is used and, in a non-linear process, two lower energy photons are converted to double the incident frequency [25]. This microscopic technique was first used by Freund and Deutsch in 1986 to monitor collagen fibres in the rat tail tendon [55]. Quantitative analysis of the collagen fibrillar structure has also been measured through SHG microscopy [34]. To understand the early progression of glaucoma, the effect of axonal-microtubules on the morphology of retinal nerve fibres has been studied in the rat retina whole mount through the SHG microscopy technique [107]. In addition, the axonal architecture of the inner retinal layer has been monitored in mouse models with this method [122]. In extension to humans, this microscopy technique has been utilized for ex vivo study of the human cornea to understand its morphological changes during infectious processes [175]. Recently this microscopy technique has been used successfully and safely for imaging the anterior segment of a living human eye [10]. However, although it has advantageous applications in the biological sample, it has limitations in penetration and imaging of the eye beyond the cornea and sclera and is currently most informative of structural proteins [34, 139].
Improvement of imaging techniques in the twenty-first century
In the twenty first century, fundus imaging in the clinic is now high quality. To overcome the portability limitations and cost of the imaging, smartphone-based fundus imaging capabilities have been launched, but limitations in image quality and user-friendliness of the image acquisition remain a concern with this technology [84, 174, 195]. Mydriatic, non-mydriatic cameras, and handheld cameras are also routinely used for retinal imaging [98, 118]. Mydriatic cameras are used for in-depth retinal imaging, whereas non-mydriatic cameras can be used for imaging the retina without dilating the pupil, providing a clear picture with high magnification. Ultra-wide field cameras, which can cover almost 82% of the retinal surface, allowing better diagnosis of retinal diseases that impact the peripheral retina are also available. Ocular angiography, pediatric retinal imaging, and OCT are now also much improved over the previous century, particularly since the time needed to capture a retinal image has been reduced. In the era of artificial intelligence (AI), rapid inroads are also being made in retinal image processing and interpretation of the data [98].
The future of eye imaging
Autofluorescence as a tool for disease diagnosis
In the eye, FAF imaging can detect different fluorophores at different wavelengths and this technique has been used in retinal diagnostics for retinitis pigmentosa, age related macular degeneration, macular dystrophies, white dot syndromes, and many other retinal diseases [203]. FAF is also useful to diagnose RPE distress or metabolic challenges, prior to the death of cell [136, 203]. In many FAF devices, one or two optical filters are used to enhance image quality. However, intrinsic fluorophores with similar excitation or emission bands as the target FAF can confound retinal imaging studies and interfere with image clarity [170, 180]. This interference is noticeable in the diagnosis of cataracts and diabetic retinopathy [153, 157]. For example, the yellowed lens in cataractous eyes partially absorbs blue 488 nm light and scatters light differently to non-cataractous eyes, interfering with the detection of autofluorescent macular pigments and thus obscuring diagnostic results of FAF imaging.
On the other hand, the autofluorescence of various retinal structures has significant potential as a diagnostic tool in more than just eye disease. In 2020, Wang et al. showed that the autofluorescence properties of T-cells can be used to distinguish T-cell activity states in a non-destructive manner using human blood samples. In this study, the team used machine learning methods to detect changes in T-cell metabolic co-enzymes including NAD(P)H [191]. Different types of T-cells can also be classified by using autofluorescence lifetime imaging [187]. As such, Walsh et al. used isolated T-cells from peripheral blood and activated them in culture using tetrameric antibodies against the CD2, CD3, and CD28 surface ligands, showing different autofluorescence signals between quiescent and activated T-cells and highlighting fluorescence lifetime imaging as a non-destructive way to understand T-cell activity [187]. Conclusions drawn from measurements of lifetime in the isolated T-cells may be difficult to recapitulate in a biological system, although lifetime has strengths over intensity measurements in circumventing issues of light scattering, excitation light intensity, and focus variability [14]. Notably, NADH and FAD autofluorescence has also been used to identify functional states and distinguish between different immune cell subtypes [102]. In a pain discrimination study, Gosnell et al. used hyperspectral imaging of immune cells combined with AI and deep learning methods to distinguish inflammation in spinal cord in different pain states [64], a methodology that ultimately allowed them to differentiate between control and injured, male and female, spinal cords.
These studies suggest there is potential to use the autofluorescence properties of specific cell types to facilitate disease diagnostics and raise the possibility of doing this via the eye. Notably, post-mitotic cells throughout the CNS, including in the RPE, contain lipofuscin, a pigment that is produced due to oxidative stress. Lipofuscin is composed of more than 20 different fluorophores with optical excitation and emission bands spanning the ultraviolet and visible. In the RPE, lipofuscin is especially detrimental to retinal health as it can generate reactive oxygen species upon photoexcitation with visible light, especially blue light [20, 154]. The best characterized component of retinal lipofuscin is the N-retinyl-N-retinylidene ethanolamine (A2E), which has excitation and emission bands at 430–450 nm and 560–575 nm, respectively [42]. Notably, A2E is indigestible and is accumulated in the lysosomes, the cell’s degradative organelles, in the RPE. Its accumulation interferes with cholesterol metabolism, causes DNA damage, disrupts cell membranes and leads to the generation of the reactive oxygen species [49, 99, 171, 172]. Thus, A2E may be useful as an autofluorescent marker of retinal damage [115]. Also, lipofuscin accumulates in the retina and works as an autofluorescence marker of the retinal damage. Since lipofuscin accumulation occurs in the brain with injury, aging, and disease, it is potentially a marker of brain damage as well.
Potential detection of amyloid beta and diagnosis of Alzheimer’s disease
In recent years, the detection of high levels of amyloid-beta in the superior peripheral quadrant of the retina in AD patients has raised the possibility of using this property for non-invasive imaging to diagnose this disease [37, 96, 108, 129, 167]. During proteolysis of the membrane-bound amyloid precursor protein in AD, amyloid peptide fragments amyloid-beta1-40 and amyloid-beta1-42 are produced and undergo misfolding that lead to eventual plaque formation [160]. Amyloid-beta plaques display bright blue autofluorescence (∼420 nm) under UV (360–370 nm) illumination and a much fainter emission intensity at longer emission wavelengths when excited with blue or green light [176]. These deposits also accumulate with age in the AD retina and cause neurodegeneration in the retinal ganglion cell layer [131]. Crucially, their deposition in the retina precedes that in the brain in transgenic AD mice, suggesting an opportune early diagnostic tool [95]. In vivo hyperspectral imaging of retinal amyloid-beta deposits distinguishes AD patients and those with mild cognitive impairment from healthy controls [69, 111]. Interestingly, patients with early stage mild cognitive impairment show greater changes in hyperspectral imaging signatures than same age healthy controls, suggesting promise for its use as an early diagnostic [126]. In transgenic AD mice, intravenous injections of curcumin—a natural fluorochrome that binds to amyloid-beta plaques—allows high-resolution and specific in vivo imaging for retinal plaques at 550/525 nm excitation and 605/670 nm emission [59, 95]. However, glaucoma and age-related macular degeneration share the same progressive accumulation of extracellular amyloid-beta plaques and intracellular hyperphosphorylated tau protein as AD [165]. The common retinal pathology between AD and other eye-related diseases limits the technique’s specificity at this time. Thus, retinal amyloid-beta imaging for early AD diagnosis is still a developing technique.
Microglia to detect retinal disease
Another key potential avenue for using natural autofluorescence to detect retinal disease is in microglia. Microglia have strong autofluorescence in the visible and near-infrared spectral range [100]. Microglia in the brain, spinal cord and retina respond to immune challenge and injury by engulfing and destroying pathogens and debris, as well as releasing cytokines and other inflammatory factors to make the CNS milieu less amenable to pathogen survival and to recruit other immune cells to the site [130]. In doing so, microglia change their shape, gene expression, and potentially the spectroscopic properties (excitation/emission spectra and lifetime) of their autofluorescence. Under homeostatic conditions, microglia remain ramified with small cell bodies, whereas in the response to an immune challenge, disease, or localized inflammatory conditions, microglia typically have larger cell bodies and are less ramified. As they become more responsive to inflammatory challenge, microglia destroy pathogens or cellular debris via phagocytosis and degradation [41, 130, 168]. Since microglia accumulate debris in their lysosomes during this process, it is likely their autofluorescence profile changes in accordance with disease. In vivo imaging in the aryl hydrocarbon receptor Ahr −/− mouse model of retinal degeneration has revealed changes in microglial autofluorescence properties in this model [89]. Taking this concept even further, brain-centric diseases such as lysosomal storage disorders have already been shown to be reflected in retinal changes in animal models, including in changes in retinal microglia morphology [11], although autofluorescence differences are yet to be tested. These profiles suggest retinal microglia may be a key informant of not only retinal disease, but brain disease as well.
Growing evidence from oculomics studies suggests that during different disease conditions in the retina and brain, observation of microglial changes in the retina can be identified using FAF and OCT imaging techniques, and such information from the eye may reflect brain disease [7, 19, 50, 51]. As discussed, microglia present as hyperreflective foci (HRF) in FAF and OCT images. These HRF can be detected in the retina of individuals with various systemic diseases including multiple sclerosis [148], von Hippel-Lindau disease [145] and diabetic retinopathy and can be considered as an imaging biomarker for microglial activity during these disease states [204]. Although there is debate regarding the origin of retinal HRF, they are thought to be produced through the breakdown of the blood-retinal barrier caused by migrating macrophages, degenerated photoreceptors and deposited protein and lipid [17, 52, 73]. Retinal HRF are highly correlated to microglia changes characterized in various retinal diseases including age-related macular degeneration, uveitis macular oedema, and diabetic macular oedema [17, 65, 135, 186]. In addition, retinal microglial HRF can be visualized over time with dynamic changes in HRF indicating disease progression [75]. The presence of HRF can be considered a clinical biomarker in understanding retinal degeneration and disease, paving the way forward in oculomics. However, there is some controversy surrounding their potential as an indicator of CNS disease. The key limitation is in our lack of understanding of the disease-specific characteristics of HRF. Employing machine learning has the potential to overcome this and allow disease-specific detection with this marker (Midena 2023).
Raman spectroscopy
An emerging disease detection tool for potential use in retinal imaging is Raman spectroscopy. Raman allows us to detect the vibrational frequencies of a molecule of interest to understand the nature of bonding and chemical structures and changes in the presence of specific molecules [97]. In vitro studies using cultured rodent retina have shown that inflammatory changes in the retina can be detected by Raman spectroscopy [119]. Oculomic studies using Raman spectroscopy has also been used to identify early biomarkers of AD in isolated mouse retina [173]. Recently, Alba-Arbalat et al. invented a prototype technology combining Raman spectroscopy and ophthalmology by connecting a spectrophotometer and scanning laser ophthalmoscope and utilizing it to assess retinal molecular changes in human patients with multiple sclerosis [4]. Thus, Raman spectroscopy is potentially useful for non-invasively identifying eye diseases. However, a few important challenges still need to be overcome. Firstly, careful in vivo trials are necessary to validate that patients are safe from the laser wavelengths used. Secondly, it is also essential to make sure that the Raman spectroscopy can differentiate between ophthalmologic diseases with similar pathological and biochemical symptoms. Lastly, high throughput image analysis is necessary, an outcome that may be achieved by the combination of traditional human-based assessment and AI-assisted data analysis tools [103].
Artificial intelligence
“Artificial intelligence” is a buzz term that was first coined by John McCarthy in 1955. It refers to training non-living entities in a specific field to behave like a human [8, 159]. Machine learning is a subset of AI that consists of deep learning, and non-neural networks [149]. Non-neural networks are supervised machine learning algorithms often used in combination with deep neural networks to predict medical outcomes based on multiple variable analysis and give single outputs from multiple variable data trees [63, 151]. On the other hand, deep learning consists of multiple layers of nodes containing variables (such as the pixels of the images, age, and nucleotide changes etc.) that are considered to give a single output [164]. In machine learning approaches, a machine is trained with large set of data that enables the machine to predict a future outcome from those data [38, 149], including from retinal images [28].
As for other fields in recent years, AI has been widely used in the field of the ophthalmology and retinal imaging [159]. To date, AI is used in the diagnosis of several retinal diseases including diabetic retinopathy, age related macular degeneration, glaucoma, cataracts, keratoconus, and diabetic macular edema [43, 77, 105, 106, 132, 162, 190]. Among the different approaches for the AI-assisted diagnosis of diabetic retinopathy, Gulshan et al. have developed two data sets of deep learning algorithms that are 90.3% and 87.0% sensitive, and 98.1% and 98.5% specific [67]. Additional algorithms have been incorporated to validate this AI model targeting people from different ethnic groups, geographical areas, and levels of retinopathy [12, 18, 178]. These approaches have been helpful to improve screening cost and accuracy [198], albeit the field-level application of AI algorithms in rural Thailand has proven challenging due to the poor image quality and speed of image processing. This illustrates the challenges related to implementing this type of technology successfully (Emma Beede 2020). One study conducted with images from the UK Biobank found that 16% of their retinal images were not usable for automated AI approaches to predict disease [114]. Similarly, an analysis of large-scale radiography data sets showed alarming rates of under-diagnosis using AI-based classifications, especially for intersectional under-served subpopulations such as Hispanic females [161]. Undoubtedly, though, the future will see improved algorithms and strategies for their application in the real-world [6]. Human-based AI approaches to diagnostics are also likely to be useful in future applications to pre-clinical models. As such, information gleaned from clinical datasets can be reverse-translated to rodent models for validation of hypotheses or testing of pharmacological interventions [112, 113]. This concept is elaborated on by Ma et al. for the example of glaucoma [113] and has been tested for modelling of schizophrenia-related eye movement abnormalities in marmosets [147].
Concluding statement
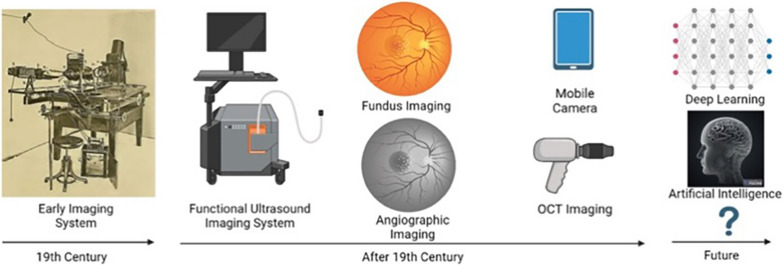
In the eighteenth century, imaging of the eye commenced with very large and complex instrumentation with preliminary quality and emerging diagnostic accuracy. As biomedical sciences have advanced in cooperation with physicists and ophthalmologists, simpler instrumentation has been developed to yield higher quality images (Fig. 6). In the twentieth century, scientists focused on the portability of the instrumentation as well as the resolution and angle of the images acquired. Now, OCT allows us to take more accurate images and fundus imaging, through mobile phone mydriatic and non-mydriatic camera systems, gives us portable options. However, this is not the end of the road for progress in eye imaging systems. Current research in understanding retinal signatures of systemic diseases is seeing an explosion of AI-related techniques for data processing and novel techniques like autofluorescence imaging and Raman spectroscopy promise to deliver the next frontier in eye imaging. An essential advance is likely to see such eye imaging used to diagnose and monitor diseases, injury, and treatment success that occur in deeper less accessible regions of the central nervous system, for example in cases such as AD.
Fig. 6.
Frontier approaches and the future of the eye imaging. Eye imaging systems were complex and cumbersome during their early development. After the nineteenth century, complex imaging instruments were replaced with simple, portable, and accurate machines. Now, hand-held imaging techniques are available, and in the future it is expected that artificial intelligence (AI) and deep learning will significantly reduce the necessity for human intervention and allow accurate diagnosis of eye disease, and brain disease, in a short time. Figure created with BioRender.com; Toronto, Canada. Image of the early imaging system was taken from a blog post [13]
Author contributions
HUB and SJS wrote the main manuscript text. HUB and JA prepared the figures. All authors edited and reviewed the manuscript, contributing unique paragraphs.
Funding
This project was supported by funding from the European Union (EU) Joint Program on Neurodegenerative Disease (JPND) Grant: (SOLID JPND2021-650-233 via the National Health and Medical Research Council (NHMRC)-2014629) an NHMRC Ideas Grant (NHMRC-2019196) to SJS, an Australian Research Council Discovery Project (ARC; DP230101331) to SJS and BdR, as well as an RMIT University Research Stipend Scholarship to HUB and an RMIT University Vice Chancellor's Ph.D Scholarship to MS.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abramoff MD, Garvin MK, Sonka M. Retinal imaging and image analysis. IEEE Rev Biomed Eng. 2010;3:169–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghigh A, Bancelin S, Rivard M, Pinsard M, Ibrahim H, Legare F. Second harmonic generation microscopy: a powerful tool for bio-imaging. Biophys Rev. 2023;15:43–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS, Dyer MA. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alba-Arbalat S, Andorra M, Sanchez-Dalmau B, Camos-Carreras A, Dotti-Boada M, Pulido-Valdeolivas I, Llufriu S, Blanco Y, Sepulveda M, Saiz A, Batet O, Bilbao I, Torre I, Amat-Roldan I, Martinez-Lapiscina EH, Villoslada P. In Vivo molecular changes in the retina of patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2021;62:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen L. Ocular fundus photography: suggestions for achieving consistently good pictures and instructions for stereoscopic photography. Am J Ophthalmol. 1964;57:13–28. [PubMed] [Google Scholar]
- 6.Alshammri R, Alharbi G, Alharbi E, Almubark I. Machine learning approaches to identify Parkinson’s disease using voice signal features. Front Artif Intell. 2023;6:1084001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altay L, Scholz P, Schick T, Felsch M, Hoyng CB, den Hollander AI, Langmann T, Fauser S. Association of hyperreflective foci present in early forms of age-related macular degeneration with known age-related macular degeneration risk polymorphisms. Invest Ophthalmol Vis Sci. 2016;57:4315–20. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GW, Lorch AC. A(eye): a review of current applications of artificial intelligence and machine learning in ophthalmology. Int Ophthalmol Clin. 2020;60:57–71. [DOI] [PubMed] [Google Scholar]
- 9.Arrigo A, Perra C, Aragona E, Giusto D, Doglioni C, Pierro L, Giordano Resti A, Bandello F, Battaglia Parodi M. Extrafoveal Muller cells detection in vivo in the human retina: a pilot study based on optical coherence tomography. Exp Eye Res. 2020;199: 108183. [DOI] [PubMed] [Google Scholar]
- 10.Avila FJ, Gambin A, Artal P, Bueno JM. In vivo two-photon microscopy of the human eye. Sci Rep. 2019;9:10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beard H, Chidlow G, Neumann D, Nazri N, Douglass M, Trim PJ, Snel MF, Casson RJ, Hemsley KM. Is the eye a window to the brain in Sanfilippo syndrome? Acta Neuropathol Commun. 2020;8:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellemo V, Lim ZW, Lim G, Nguyen QD, Xie Y, Yip MYT, Hamzah H, Ho J, Lee XQ, Hsu W, Lee ML, Musonda L, Chandran M, Chipalo-Mutati G, Muma M, Tan GSW, Sivaprasad S, Menon G, Wong TY, Ting DSW. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: a clinical validation study. Lancet Digit Health. 2019;1:e35–44. [DOI] [PubMed] [Google Scholar]
- 13.Bennett TJ. 2013. History of ophthalmic photography blog. In.: Ophthalmic photographers' society eye imaging experts
- 14.Berezin MY, Achilefu S. Fluorescence lifetime measurements and biological imaging. Chem Rev. 2010;110:2641–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair CJ. ’Geographic atrophy of the retinal pigment epithelium. A manifestation of senile macular degeneration. Arch Ophthalmol. 1975;93:19–25. [DOI] [PubMed] [Google Scholar]
- 17.Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C, Vienna Diabetic Retinopathy Research Group. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116:914–20. [DOI] [PubMed] [Google Scholar]
- 18.Bora A, Balasubramanian S, Babenko B, Virmani S, Venugopalan S, Mitani A, de Oliveira Marinho G, Cuadros J, Ruamviboonsuk P, Corrado GS, Peng L, Webster DR, Varadarajan AV, Hammel N, Liu Y, Bavishi P. Predicting the risk of developing diabetic retinopathy using deep learning. Lancet Digit Health. 2021;3:e10–9. [DOI] [PubMed] [Google Scholar]
- 19.Borrelli E, Zuccaro B, Zucchiatti I, Parravano M, Querques L, Costanzo E, Sacconi R, Prascina F, Scarinci F, Bandello F, Querques G. Optical coherence tomography parameters as predictors of treatment response to eplerenone in central serous chorioretinopathy. J Clin Med. 2019;8:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993;19:201–4. [DOI] [PubMed] [Google Scholar]
- 21.Bourdin A, Ortoli M, Karadayi R, Przegralek L, Sennlaub F, Bodaghi B, Guillonneau X, Carpentier A, Touhami S. Efficacy and safety of low-intensity pulsed ultrasound-induced blood-retinal barrier opening in mice. Pharmaceutics. 2023;15:1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brawek B, Olmedillas Del Moral M, Garaschuk O. In Vivo visualization of microglia using tomato lectin. Methods Mol Biol. 2019;2034:165–75. [DOI] [PubMed] [Google Scholar]
- 23.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. [DOI] [PubMed] [Google Scholar]
- 24.Brody J, Waller S, Wagoner M. Corneal topography: history, technique, and clinical uses. Int Ophthalmol Clin. 1994;34:197–207. [DOI] [PubMed] [Google Scholar]
- 25.Campagnola P. Second harmonic generation imaging microscopy: applications to diseases diagnostics. Anal Chem. 2011;83:3224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canonica J, Foxton R, Garrido MG, Lin CM, Uhles S, Shanmugam S, Antonetti DA, Abcouwer SF, Westenskow PD. Delineating effects of angiopoietin-2 inhibition on vascular permeability and inflammation in models of retinal neovascularization and ischemia/reperfusion. Front Cell Neurosci. 2023;17:1192464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavallerano J, Lawrence MG, Zimmer-Galler I, Bauman W, Bursell S, Gardner WK, Horton M, Hildebrand L, Federman J, Carnahan L, Kuzmak P, Peters JM, Darkins A, Ahmed J, Aiello LM, Aiello LP, Buck G, Cheng YL, Cunningham D, Goodall E, Hope N, Huang E, Hubbard L, Janczewski M, Lewis JW, Matsuzaki H, McVeigh FL, Motzno J, Parker-Taillon D, Read R, Soliz P, Szirth B, Vigersky RA, Ward T. Telehealth practice recommendations for diabetic retinopathy. Telemed J E Health. 2004;10:469–82. [DOI] [PubMed] [Google Scholar]
- 28.Cen LP, Ji J, Lin JW, Ju ST, Lin HJ, Li TP, Wang Y, Yang JF, Liu YF, Tan S, Tan L, Li D, Wang Y, Zheng D, Xiong Y, Wu H, Jiang J, Wu Z, Huang D, Shi T, Chen B, Yang J, Zhang X, Luo L, Huang C, Zhang G, Huang Y, Ng TK, Chen H, Chen W, Pang CP, Zhang M. Automatic detection of 39 fundus diseases and conditions in retinal photographs using deep neural networks. Nat Commun. 2021;12:4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan KC, Fan SJ, Zhou IY, Wu EX. In vivo chromium-enhanced MRI of the retina. Magn Reson Med. 2012;68:1202–10. [DOI] [PubMed] [Google Scholar]
- 30.Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–602. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Wang Q, Zhang H, Yang X, Wang J, Berkowitz BA, Wickline SA, Song SK. In vivo quantification of T1, T2, and apparent diffusion coefficient in the mouse retina at 11.74T. Magn Reson Med. 2008;59:731–8. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10:27–39. [DOI] [PubMed] [Google Scholar]
- 33.Chen M, Xu H. Parainflammation, chronic inflammation, and age-related macular degeneration. J Leukoc Biol. 2015;98:713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc. 2012;7:654–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thule PM, Olson DE, Duong TQ. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci USA. 2006;103:17525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung N, Liew G, Lindley RI, Liu EY, Wang JJ, Hand P, Baker M, Mitchell P, Wong TY, Retina Multi-Center, and Group Stroke Study Collaborative. Retinal fractals and acute lacunar stroke. Ann Neurol. 2010;68:107–11. [DOI] [PubMed] [Google Scholar]
- 37.Christinaki E, Kulenovic H, Hadoux X, Baldassini N, Van Eijgen J, De Groef L, Stalmans I, van Wijngaarden P. Retinal imaging biomarkers of neurodegenerative diseases. Clin Exp Optom. 2022;105:194–204. [DOI] [PubMed] [Google Scholar]
- 38.Consejo A, Melcer T, Rozema JJ. Introduction to machine learning for ophthalmologists. Semin Ophthalmol. 2019;34:19–41. [DOI] [PubMed] [Google Scholar]
- 39.Crespo-Garcia S, Reichhart N, Hernandez-Matas C, Zabulis X, Kociok N, Brockmann C, Joussen AM, Strauss O. In vivo analysis of the time and spatial activation pattern of microglia in the retina following laser-induced choroidal neovascularization. Exp Eye Res. 2015;139:13–21. [DOI] [PubMed] [Google Scholar]
- 40.Csaszar E, Lenart N, Cserep C, Kornyei Z, Fekete R, Posfai B, Balazsfi D, Hangya B, Schwarcz AD, Szabadits E, Szollosi D, Szigeti K, Mathe D, West BL, Sviatko K, Bras AR, Mariani JC, Kliewer A, Lenkei Z, Hricisak L, Benyo Z, Baranyi M, Sperlagh B, Menyhart A, Farkas E, Denes A. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J Exp Med. 2022;219:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. [DOI] [PubMed] [Google Scholar]
- 42.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–66. [PubMed] [Google Scholar]
- 43.Dos Santos VA, Schmetterer L, Stegmann H, Pfister M, Messner A, Schmidinger G, Garhofer G, Werkmeister RM. CorneaNet: fast segmentation of cornea OCT scans of healthy and keratoconic eyes using deep learning. Biomed Opt Express. 2019;10:622–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duck FA. Medical and non-medical protection standards for ultrasound and infrasound. Prog Biophys Mol Biol. 2007;93:176–91. [DOI] [PubMed] [Google Scholar]
- 45.Duong TQ. Magnetic resonance imaging of the retina: a brief historical and future perspective. Saudi J Ophthalmol. 2011;25:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duong TQ, Muir ER. Magnetic resonance imaging of the retina. Jpn J Ophthalmol. 2009;53:352–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edelman JL, Miller SS. Epinephrine stimulates fluid absorption across bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1991;32:3033–40. [PubMed] [Google Scholar]
- 48.Emma Beede, Elizabeth Baylor, Fred Hersch, Anna Iurchenko, Lauren Wilcox, Paisan Ruamviboonsuk, Laura M. Vardoulakis. 2020. “A Human-Centered Evaluation of a Deep Learning System Deployed in Clinics for the Detection of Diabetic Retinopathy”. In CHI '20: Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, 1–12. Honolulu HI USA: Association for Computing Machinery.
- 49.Feng J, Chen X, Sun X, Wang F, Sun X. Expression of endoplasmic reticulum stress markers GRP78 and CHOP induced by oxidative stress in blue light-mediated damage of A2E-containing retinal pigment epithelium cells. Ophthalmic Res. 2014;52:224–33. [DOI] [PubMed] [Google Scholar]
- 50.Fragiotta S, Abdolrahimzadeh S, Dolz-Marco R, Sakurada Y, Gal-Or O, Scuderi G. Significance of hyperreflective foci as an optical coherence tomography biomarker in retinal diseases: characterization and clinical implications. J Ophthalmol. 2021;2021:6096017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fragiotta S, Rossi T, Cutini A, Grenga PL, Vingolo EM. Predictive factors for development of neovascular age-related macular degeneration: a spectral-domain optical coherence Tomography Study. Retina. 2018;38:245–52. [DOI] [PubMed] [Google Scholar]
- 52.Framme C, Wolf S, Wolf-Schnurrbusch U. Small dense particles in the retina observable by spectral-domain optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:5965–9. [DOI] [PubMed] [Google Scholar]
- 53.Frampton GK, Kalita N, Payne L, Colquitt JL, Loveman E, Downes SM, Lotery AJ. Fundus autofluorescence imaging: systematic review of test accuracy for the diagnosis and monitoring of retinal conditions. Eye. 2017;31:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francis AW, Wanek J, Lim JI, Shahidi M. Enface thickness mapping and reflectance imaging of retinal layers in diabetic retinopathy. PLoS ONE. 2015;10: e0145628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freund I, Deutsch M. Second-harmonic microscopy of biological tissue. Opt Lett. 1986;11:94. [DOI] [PubMed] [Google Scholar]
- 56.Fujimoto, J., and E. Swanson. 2016. 'The Development, Commercialization, and Impact of Optical Coherence Tomography', Invest Ophthalmol Vis Sci, 57: OCT1-OCT13. [DOI] [PMC free article] [PubMed]
- 57.Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8:117–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallemore RP, Hughes BA, Miller SS. Retinal pigment epithelial transport mechanisms and their contributions to the electroretinogram. Prog Retinal Eye Res. 1997;16:509–66. [Google Scholar]
- 59.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–104. [DOI] [PubMed] [Google Scholar]
- 60.Gass JD. Drusen and disciform macular detachment and degeneration. Trans Am Ophthalmol Soc. 1972;70:409–36. [PMC free article] [PubMed] [Google Scholar]
- 61.Gass JD, Sever RJ, Sparks D, Goren J. A combined technique of fluorescein funduscopy and angiography of the eye. Arch Ophthalmol. 1967;78:455–61. [DOI] [PubMed] [Google Scholar]
- 62.Gerloff O. Uber die photographie des Augenhintergrundes. Klin Monatsblätter Augenheilkunde. 1891;5:397–403. [Google Scholar]
- 63.Ghods A, Cook DJ. A survey of deep network techniques all classifiers can adopt. Data Min Knowl Discov. 2021;35:46–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gosnell ME, Staikopoulos V, Anwer AG, Mahbub SB, Hutchinson MR, Mustafa S, Goldys EM. Autofluorescent imprint of chronic constriction nerve injury identified by deep learning. Neurobiol Dis. 2021;160: 105528. [DOI] [PubMed] [Google Scholar]
- 65.Grewal DS, O’Sullivan ML, Kron M, Jaffe GJ. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol. 2017;177:116–25. [DOI] [PubMed] [Google Scholar]
- 66.Gullstrand A. Neue methoden der reflexlosen ophthalmoskopie. Berichte Deutsche Ophthalmologische Gesellschaft. 1910;6:42. [Google Scholar]
- 67.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, Venugopalan S, Widner K, Madams T, Cuadros J, Kim R, Raman R, Nelson PC, Mega JL, Webster DR. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10. [DOI] [PubMed] [Google Scholar]
- 68.Gupta AK, Meng R, Modi YS, Srinivasan VJ. Imaging human macular pigments with visible light optical coherence tomography and superluminescent diodes. Opt Lett. 2023;48:4737–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hadoux X, Hui F, Lim JKH, Masters CL, Pebay A, Chevalier S, Ha J, Loi S, Fowler CJ, Rowe C, Villemagne VL, Taylor EN, Fluke C, Soucy JP, Lesage F, Sylvestre JP, Rosa-Neto P, Mathotaarachchi S, Gauthier S, Nasreddine ZS, Arbour JD, Rheaume MA, Beaulieu S, Dirani M, Nguyen CTO, Bui BV, Williamson R, Crowston JG, van Wijngaarden P. Non-invasive in vivo hyperspectral imaging of the retina for potential biomarker use in Alzheimer’s disease. Nat Commun. 2019;10:4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hikage F, Lennikov A, Mukwaya A, Lachota M, Ida Y, Utheim TP, Chen DF, Huang H, Ohguro H. NF-kappaB activation in retinal microglia is involved in the inflammatory and neovascularization signaling in laser-induced choroidal neovascularization in mice. Exp Cell Res. 2021;403: 112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hildred RB. A brief history on the development of ophthalmic retinal photography into digital imaging. J Audiov Media Med. 1990;13:101–5. [DOI] [PubMed] [Google Scholar]
- 72.Hogg RE, Silva R, Staurenghi G, Murphy G, Santos AR, Rosina C, Chakravarthy U. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology. 2014;121:1748–55. [DOI] [PubMed] [Google Scholar]
- 73.Horii T, Murakami T, Nishijima K, Akagi T, Uji A, Arakawa N, Muraoka Y, Yoshimura N. Relationship between fluorescein pooling and optical coherence tomographic reflectivity of cystoid spaces in diabetic macular edema. Ophthalmology. 2012;119:1047–55. [DOI] [PubMed] [Google Scholar]
- 74.Hospital, The Royal Victorian Eye and Ear. 2020. Retinal angiogram,. https://eyeandear.org.au/patients-visitors/fact-sheets/retinal-angiogram/. Accessed 03 May 2023.
- 75.Huang CH, Yang CH, Lai YJ, Hsiao CK, Hou YC, Yang CM, Chen TC. Hyperreflective foci as important prognostic indicators of progression of retinitis pigmentosa. Retina. 2022;42:388–95. [DOI] [PubMed] [Google Scholar]
- 76.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991;254:1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang DK, Hsu CC, Chang KJ, Chao D, Sun CH, Jheng YC, Yarmishyn AA, Wu JC, Tsai CY, Wang ML, Peng CH, Chien KH, Kao CL, Lin TC, Woung LC, Chen SJ, Chiou SH. Artificial intelligence-based decision-making for age-related macular degeneration. Theranostics. 2019;9:232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ignatova I, Frolov R, Nymark S. The retinal pigment epithelium displays electrical excitability and lateral signal spreading. BMC Biol. 2023;21:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Indaram M, Ma W, Zhao L, Fariss RN, Rodriguez IR, Wong WT. 7-Ketocholesterol increases retinal microglial migration, activation, and angiogenicity: a potential pathogenic mechanism underlying age-related macular degeneration. Sci Rep. 2015;5:9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ivanisevic M, Stanic R, Ivanisevic P, Vukovic A. Albrecht von Graefe (1828–1870) and his contributions to the development of ophthalmology. Int Ophthalmol. 2020;40:1029–33. [DOI] [PubMed] [Google Scholar]
- 81.Jackman WT, Webster JD. On photographing the retina of the living human eye. Philadel Photogr. 1886;23:340–1. [Google Scholar]
- 82.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. [DOI] [PubMed] [Google Scholar]
- 83.Janez-Garcia L, Bachtoula O, Salobrar-Garcia E, de Hoz R, Ramirez AI, Gil P, Ramirez JM, Janez-Escalada L. Roughness of retinal layers in Alzheimer’s disease. Sci Rep. 2021;11:11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jansen LG, Schultz T, Holz FG, Finger RP, Wintergerst MWM. Smartphone-based fundus imaging: applications and adapters. Ophthalmologe. 2022;119:112–26. [DOI] [PubMed] [Google Scholar]
- 85.Kalra G, Pichi F, Kumar Menia N, Shroff D, Phasukkijwatana N, Aggarwal K, Agarwal A. Recent advances in wide field and ultrawide field optical coherence tomography angiography in retinochoroidal pathologies. Expert Rev Med Devices. 2021;18:375–86. [DOI] [PubMed] [Google Scholar]
- 86.Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–47. [DOI] [PubMed] [Google Scholar]
- 87.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5:71–81. [DOI] [PubMed] [Google Scholar]
- 88.Kelly D, Coen RF, Akuffo KO, Beatty S, Dennison J, Moran R, Stack J, Howard AN, Mulcahy R, Nolan JM. Cognitive function and its relationship with macular pigment optical density and serum concentrations of its constituent carotenoids. J Alzheimers Dis. 2015;48:261–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim SY, Yang HJ, Chang YS, Kim JW, Brooks M, Chew EY, Wong WT, Fariss RN, Rachel RA, Cogliati T, Qian H, Swaroop A. Deletion of aryl hydrocarbon receptor AHR in mice leads to subretinal accumulation of microglia and RPE atrophy. Invest Ophthalmol Vis Sci. 2014;55:6031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinuthia UM, Wolf A, Langmann T. Microglia and inflammatory responses in diabetic retinopathy. Front Immunol. 2020;11: 564077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinyoun JL, Martin DC, Fujimoto WY, Leonetti DL. Ophthalmoscopy versus fundus photographs for detecting and grading diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992;33:1888–93. [PubMed] [Google Scholar]
- 92.Kobat SG, Turgut B. Importance of Muller Cells. Beyoglu Eye J. 2020;5:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohlfaerber T, Pieper M, Munter M, Holzhausen C, Ahrens M, Idel C, Bruchhage KL, Leichtle A, Konig P, Huttmann G, Schulz-Hildebrandt H. Dynamic microscopic optical coherence tomography to visualize the morphological and functional micro-anatomy of the airways. Biomed Opt Express. 2022;13:3211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Philos Trans R Soc Lond B Biol Sci. 1970;258:261–83. [DOI] [PubMed] [Google Scholar]
- 95.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(Suppl 1):S204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, Kile SJ, Blanco A, Fuchs DT, Ashfaq A, Frautschy S, Cole GM, Miller CA, Hinton DR, Verdooner SR, Black KL, Koronyo-Hamaoui M. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnan CVRKS. A new type of secondary radiation. Nature. 1928;121:501–2. [Google Scholar]
- 98.Kulkarni S, Deshpande M. Recent advances in retinal imaging and diagnostics. Commun Eye Health. 2019;32:S9–10. [PMC free article] [PubMed] [Google Scholar]
- 99.Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007;104:11026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lei L, Tzekov R, Tang S, Kaushal S. Accumulation and autofluorescence of phagocytized rod outer segment material in macrophages and microglial cells. Mol Vis. 2012;18:103–13. [PMC free article] [PubMed] [Google Scholar]
- 101.Leinenga G, Bodea LG, Schroder J, Sun G, Zhou Y, Song J, Grubman A, Polo JM, Gotz J. Transcriptional signature in microglia isolated from an Alzheimer’s disease mouse model treated with scanning ultrasound. Bioeng Transl Med. 2023;8: e10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lemire S, Thoma OM, Kreiss L, Volkl S, Friedrich O, Neurath MF, Schurmann S, Waldner MJ. Natural NADH and FAD autofluorescence as label-free biomarkers for discriminating subtypes and functional states of immune cells. Int J Mol Sci. 2022;23:2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, Yan P, Li Y, Han M, Zeng Q, Li J, Yu Z, Zhang D, Chen X. Harnessing the power of Raman spectroscopic imaging for ophthalmology. Front Chem. 2023;11:1211121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li LJ, Ikram MK, Wong TY. Retinal vascular imaging in early life: insights into processes and risk of cardiovascular disease. J Physiol. 2016;594:2175–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Teng X, Yang C, Wang Y, Wang L, Dai Y, Sun H, Li J. Ultrasound controlled anti-inflammatory polarization of platelet decorated microglia for targeted ischemic stroke therapy. Angew Chem Int Ed Engl. 2021;60:5083–90. [DOI] [PubMed] [Google Scholar]
- 106.Li Z, Jiang J, Chen K, Chen Q, Zheng Q, Liu X, Weng H, Wu S, Chen W. Preventing corneal blindness caused by keratitis using artificial intelligence. Nat Commun. 2021;12:3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lim H, Danias J. Effect of axonal micro-tubules on the morphology of retinal nerve fibers studied by second-harmonic generation. J Biomed Opt. 2012;17: 110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lim JK, Li QX, He Z, Vingrys AJ, Wong VH, Currier N, Mullen J, Bui BV, Nguyen CT. The eye as a biomarker for Alzheimer’s disease. Front Neurosci. 2016;10:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin TY, Motamedi S, Asseyer S, Chien C, Saidha S, Calabresi PA, Fitzgerald KC, Samadzadeh S, Villoslada P, Llufriu S, Green AJ, Preiningerova JL, Petzold A, Leocani L, Garcia-Martin E, Oreja-Guevara C, Outteryck O, Vermersch P, Balcer LJ, Kenney R, Albrecht P, Aktas O, Costello F, Frederiksen J, Uccelli A, Cellerino M, Frohman EM, Frohman TC, Bellmann-Strobl J, Schmitz-Hubsch T, Ruprecht K, Brandt AU, Zimmermann HG, Paul F. Individual prognostication of disease activity and disability worsening in multiple sclerosis with retinal layer thickness z scores. Neurol Neuroimmunol Neuroinflamm. 2024;11: e200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu J, Chen Y, Wang G, Lv Q, Yang Y, Wang J, Zhang P, Liu J, Xie Y, Zhang L, Xie M. Ultrasound molecular imaging of acute cardiac transplantation rejection using nanobubbles targeted to T lymphocytes. Biomaterials. 2018;162:200–7. [DOI] [PubMed] [Google Scholar]
- 111.Loffler KU, Edward DP, Tso MO. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest Ophthalmol Vis Sci. 1995;36:24–31. [PubMed] [Google Scholar]
- 112.Ma D, Deng W, Khera Z, Sajitha TA, Wang X, Wollstein G, Schuman JS, Lee S, Shi H, Ju MJ, Matsubara J, Beg MF, Sarunic M, Sappington RM, Chan KC. Early inner plexiform layer thinning and retinal nerve fiber layer thickening in excitotoxic retinal injury using deep learning-assisted optical coherence tomography. Acta Neuropathol Commun. 2024;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma D, Pasquale LR, Girard MJA, Leung CKS, Jia Y, Sarunic MV, Sappington RM, Chan KC. Reverse translation of artificial intelligence in glaucoma: connecting basic science with clinical applications. Front Ophthalmol. 2023;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.MacGillivray TJ, Cameron JR, Zhang Q, El-Medany A, Mulholland C, Sheng Z, Dhillon B, Doubal FN, Foster PJ, Trucco E, Sudlow C, U. K. Biobank Eye, and Consortium Vision. Suitability of UK biobank retinal images for automatic analysis of morphometric properties of the vasculature. PLoS ONE. 2015;10:e0127914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maeda A, Golczak M, Chen Y, Okano K, Kohno H, Shiose S, Ishikawa K, Harte W, Palczewska G, Maeda T, Palczewski K. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2011;8:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Makabe K, Sugita S, Mandai M, Futatsugi Y, Takahashi M. Microglia dynamics in retinitis pigmentosa model: formation of fundus whitening and autofluorescence as an indicator of activity of retinal degeneration. Sci Rep. 2020;10:14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malhotra A, Minja FJ, Crum A, Burrowes D. Ocular anatomy and cross-sectional imaging of the eye. Semin Ultrasound CT MR. 2011;32:2–13. [DOI] [PubMed] [Google Scholar]
- 118.Vilela MAP, Valença FM, Barreto PKM, Amaral CEV, Pellanda LC. Agreement between retinal images obtained via smartphones and images obtained with retinal cameras or fundoscopic exams—systematic review and meta-analysis. Clinical opthalmology. 2018;12:2581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marro M, Taubes A, Abernathy A, Balint S, Moreno B, Sanchez-Dalmau B, Martinez-Lapiscina EH, Amat-Roldan I, Petrov D, Villoslada P. Dynamic molecular monitoring of retina inflammation by in vivo Raman spectroscopy coupled with multivariate analysis. J Biophotonics. 2014;7:724–34. [DOI] [PubMed] [Google Scholar]
- 120.Masuda T, Shimazawa M, Hara H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid Med Cell Longev. 2017;2017:9208489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Sharrett AR, Klein BE, Wang JJ, Chambless LE, Wong TY. Risk prediction of coronary heart disease based on retinal vascular caliber (from the atherosclerosis risk in communities [ARIC] study). Am J Cardiol. 2008;102:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meah A, Boodram V, Bucinca-Cupallari F, Lim H. Axonal architecture of the mouse inner retina revealed by second harmonic generation. PNAS Nexus. 2022;1:pgac160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Torresin T, Lupidi M, Frizziero L, Toto L, Covello G, Midena G, Pilotto E, Figus M, Mariotti C, Midena E. OCT hyperreflective retinal foci as sign of microglial activation in diabetic retinopathy: an AI automatic quantification approach. Investig Ophthalmol Vis Sci. 2023;64:1288. [Google Scholar]
- 124.Miller EB, Karlen SJ, Ronning KE, Burns ME. Tracking distinct microglia subpopulations with photoconvertible Dendra2 in vivo. J Neuroinflammation. 2021;18:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miller EB, Zhang P, Ching K, Pugh EN Jr, Burns ME. In vivo imaging reveals transient microglia recruitment and functional recovery of photoreceptor signaling after injury. Proc Natl Acad Sci USA. 2019;116:16603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.More SS, Beach JM, McClelland C, Mokhtarzadeh A, Vince R. In Vivo assessment of retinal biomarkers by hyperspectral imaging: early detection of Alzheimer’s disease. ACS Chem Neurosci. 2019;10:4492–501. [DOI] [PubMed] [Google Scholar]
- 127.Nagaraju RM, Gurushankar G, Bhimarao, and B. Kadakola. Efficacy of high frequency ultrasound in localization and characterization of orbital lesions. J Clin Diagn Res. 2015;9:TC01-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Naor O, Hertzberg Y, Zemel E, Kimmel E, Shoham S. Towards multifocal ultrasonic neural stimulation II: design considerations for an acoustic retinal prosthesis. J Neural Eng. 2012;9: 026006. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen CTO, Hui F, Charng J, Velaedan S, van Koeverden AK, Lim JKH, He Z, Wong VHY, Vingrys AJ, Bui BV, Ivarsson M. Retinal biomarkers provide “insight” into cortical pharmacology and disease. Pharmacol Ther. 2017;175:151–77. [DOI] [PubMed] [Google Scholar]
- 130.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. [DOI] [PubMed] [Google Scholar]
- 131.Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49:5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niwas SI, Lin W, Bai X, Kwoh CK, Jay Kuo CC, Sng CC, Aquino MC, Chew PT. Automated anterior segment OCT image analysis for angle closure glaucoma mechanisms classification. Comput Methods Programs Biomed. 2016;130:65–75. [DOI] [PubMed] [Google Scholar]
- 133.Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24:82–6. [DOI] [PubMed] [Google Scholar]
- 134.O’Koren EG, Yu C, Klingeborn M, Wong AYW, Prigge CL, Mathew R, Kalnitsky J, Msallam RA, Silvin A, Kay JN, Bowes Rickman C, Arshavsky VY, Ginhoux F, Merad M, Saban DR. Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity. 2019;50(723–37): e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ogino K, Murakami T, Tsujikawa A, Miyamoto K, Sakamoto A, Ota M, Yoshimura N. Characteristics of optical coherence tomographic hyperreflective foci in retinal vein occlusion. Retina. 2012;32:77–85. [DOI] [PubMed] [Google Scholar]
- 136.Oishi A, Miyata M, Numa S, Otsuka Y, Oishi M, Tsujikawa A. Wide-field fundus autofluorescence imaging in patients with hereditary retinal degeneration: a literature review. Int J Retina Vitreous. 2019;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Panwar N, Huang P, Lee J, Keane PA, Chuan TS, Richhariya A, Teoh S, Lim TH, Agrawal R. Fundus photography in the 21st century–a review of recent technological advances and their implications for worldwide healthcare. Telemed J E Health. 2016;22:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Parida H, Kannan NB, Rathinam SR. Imaging of Muller cell sheen dystrophy. Indian J Ophthalmol. 2020;68:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park CY, Lee JK, Chuck RS. Second harmonic generation imaging analysis of collagen arrangement in human cornea. Invest Ophthalmol Vis Sci. 2015;56:5622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Patil SA, Joseph B, Tagliani P, Sastre-Garriga J, Montalban X, Vidal-Jordana A, Galetta SL, Balcer LJ, Kenney RC. Longitudinal stability of inter-eye differences in optical coherence tomography measures for identifying unilateral optic nerve lesions in multiple sclerosis. J Neurol Sci. 2023;449: 120669. [DOI] [PubMed] [Google Scholar]
- 141.Pfeiffer-Guglielmi B, Francke M, Reichenbach A, Fleckenstein B, Jung G, Hamprecht B. Glycogen phosphorylase isozyme pattern in mammalian retinal Muller (glial) cells and in astrocytes of retina and optic nerve. Glia. 2005;49:84–95. [DOI] [PubMed] [Google Scholar]
- 142.Phipps JA, Vessey KA, Brandli A, Nag N, Tran MX, Jobling AI, Fletcher EL. The role of angiotensin II/AT1 receptor signaling in regulating retinal microglial activation. Invest Ophthalmol Vis Sci. 2018;59:487–98. [DOI] [PubMed] [Google Scholar]
- 143.Quiriconi P, Hristov V, Aburaya M, Greferath U, Jobling AI, Fletcher EL. The role of microglia in the development of diabetic retinopathy. Metabol Health Dis. 2024;2:14. [Google Scholar]
- 144.Pichi F, Neri P, Moreno-Rodriguez L, Carreno E. Dancing in the eye: dynamic optical coherence tomography to distinguish different retinal microglia populations. Int Ophthalmol. 2024;44:165. [DOI] [PubMed] [Google Scholar]
- 145.Pilotto E, Torresin T, Bacelle ML, De Moja G, Ferrara AM, Zovato S, Midena G, Midena E. Hyper-reflective retinal foci as possible in vivo imaging biomarker of microglia activation in von Hippel-Lindau disease. PLoS ONE. 2022;17: e0272318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pollreisz A, Kunze LE, Brunner E, Drexler W, Schmidt-Erfurth U, Pircher M. Quantitative assessment of retinal microglia by volumetric adaptive optics OCT in eyes with diabetic retinopathy. Investig Ophthalmol Visual Sci. 2024;65:2178. [Google Scholar]
- 147.Polyakova Z, Iwase M, Hashimoto R, Yoshida M. The effect of ketamine on eye movement characteristics during free-viewing of natural images in common marmosets. Front Neurosci. 2022;16:1012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Puthenparampil M, Torresin T, Franciotta S, Marin A, De Napoli F, Mauceri VA, Miante S, Pilotto E, Midena E, Gallo P. Hyper-reflecting foci in multiple sclerosis retina associate with macrophage/microglia-derived cytokines in cerebrospinal fluid. Front Immunol. 2022;13: 852183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rajkomar A, Dean J, Kohane I. Machine learning in medicine reply. N Engl J Med. 2019;380:2589–90. [DOI] [PubMed] [Google Scholar]
- 150.Rashid K, Akhtar-Schaefer I, Langmann T. Microglia in retinal degeneration. Front Immunol. 2019;10:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rashidi HH, Tran NK, Betts EV, Howell LP, Green R. Artificial intelligence and machine learning in pathology: the present landscape of supervised methods. Acad Pathol. 2019;6:2374289519873088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rodriguez IR, Clark ME, Lee JW, Curcio CA. 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp Eye Res. 2014;128:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rovati L, Fankhauser F, Docchio F, Van Best J. Diabetic retinopathy assessed by dynamic light scattering and corneal autofluorescence. J Biomed Opt. 1998;3:357–63. [DOI] [PubMed] [Google Scholar]
- 154.Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. “Blue light-induced reactivity of retinal age pigment In vitro generation of oxygen-reactive species.” J Biol Chem. 1995;270:18825–30. [DOI] [PubMed] [Google Scholar]
- 155.Rui Y, Zhang M, Lee DMW, Snyder VC, Raghuraman R, Gofas-Salas E, Mece P, Yadav S, Tiruveedhula P, Grieve K, Sahel JA, Errera MH, Rossi EA. Label-free imaging of inflammation at the level of single cells in the living human eye. Ophthalmol Sci. 2024;4: 100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Salas M, Augustin M, Ginner L, Kumar A, Baumann B, Leitgeb R, Drexler W, Prager S, Hafner J, Schmidt-Erfurth U, Pircher M. Visualization of micro-capillaries using optical coherence tomography angiography with and without adaptive optics. Biomed Opt Express. 2017;8:207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sasamoto Y, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Nishida K. Effect of cataract in evaluation of macular pigment optical density by autofluorescence spectrometry. Invest Ophthalmol Vis Sci. 2011;52:927–32. [DOI] [PubMed] [Google Scholar]
- 158.Say EA, Shah SU, Ferenczy S, Shields CL. Optical coherence tomography of retinal and choroidal tumors. J Ophthalmol. 2011;2011: 385058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunovic H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1–29. [DOI] [PubMed] [Google Scholar]
- 160.Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6:1054–61. [DOI] [PubMed] [Google Scholar]
- 161.Seyyed-Kalantari L, Zhang H, McDermott MBA, Chen IY, Ghassemi M. Underdiagnosis bias of artificial intelligence algorithms applied to chest radiographs in under-served patient populations. Nat Med. 2021;27:2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shahriari MH, Sabbaghi H, Asadi F, Hosseini A, Khorrami Z. Artificial intelligence in screening, diagnosis, and classification of diabetic macular edema: a systematic review. Surv Ophthalmol. 2023;68:42–53. [DOI] [PubMed] [Google Scholar]
- 163.Shen Q, Cheng H, Pardue MT, Chang TF, Nair G, Vo VT, Shonat RD, Duong TQ. Magnetic resonance imaging of tissue and vascular layers in the cat retina. J Magn Reson Imaging. 2006;23:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111:1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sivak JM. The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Invest Ophthalmol Vis Sci. 2013;54:871–80. [DOI] [PubMed] [Google Scholar]
- 166.Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:5495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Snyder PJ, Alber J, Alt C, Bain LJ, Bouma BE, Bouwman FH, DeBuc DC, Campbell MCW, Carrillo MC, Chew EY, Cordeiro MF, Duenas MR, Fernandez BM, Koronyo-Hamaoui M, La Morgia C, Carare RO, Sadda SR, van Wijngaarden P, Snyder HM. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement. 2021;17:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sominsky L, De Luca S, Spencer SJ. Microglia: Key players in neurodevelopment and neuronal plasticity. Int J Biochem Cell Biol. 2018;94:56–60. [DOI] [PubMed] [Google Scholar]
- 169.Song PI, Matsui JI, Dowling JE. Morphological types and connectivity of horizontal cells found in the adult zebrafish (Danio rerio) retina. J Comp Neurol. 2008;506:328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sparrow JM, Bron AJ, Brown NA, Neil HA. Autofluorescence of the crystalline lens in early and late onset diabetes. Br J Ophthalmol. 1992;76:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sparrow JR, Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Invest Ophthalmol Vis Sci. 2001;42:1356–62. [PubMed] [Google Scholar]
- 172.Sparrow JR, Cai B, Jang YP, Zhou J, Nakanishi K. A2E, a fluorophore of RPE lipofuscin, can destabilize membrane. Adv Exp Med Biol. 2006;572:63–8. [DOI] [PubMed] [Google Scholar]
- 173.Stiebing C, Jahn IJ, Schmitt M, Keijzer N, Kleemann R, Kiliaan AJ, Drexler W, Leitgeb RA, Popp J. Biochemical characterization of mouse retina of an Alzheimer’s disease model by raman spectroscopy. ACS Chem Neurosci. 2020;11:3301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Straub J, Sprowl RA. Technical and optical aspects of smartphone-based fundus photography: possibilities and limitations in practice. Ophthalmologe. 2022;119:127–35. [DOI] [PubMed] [Google Scholar]
- 175.Tan HY, Sun Y, Lo W, Teng SW, Wu RJ, Jee SH, Lin WC, Hsiao CH, Lin HC, Chen YF, Ma DH, Huang SC, Lin SJ, Dong CY. Multiphoton fluorescence and second harmonic generation microscopy for imaging infectious keratitis. J Biomed Opt. 2007;12: 024013. [DOI] [PubMed] [Google Scholar]
- 176.Thal DR, Ghebremedhin E, Haass C, Schultz C. UV light-induced autofluorescence of full-length Abeta-protein deposits in the human brain. Clin Neuropathol. 2002;21:35–40. [PubMed] [Google Scholar]
- 177.Thompson DA, Gal A. Genetic defects in vitamin A metabolism of the retinal pigment epithelium. Dev Ophthalmol. 2003;37:141–54. [DOI] [PubMed] [Google Scholar]
- 178.Ting DSW, Cheung CY, Lim G, Tan GSW, Quang ND, Gan A, Hamzah H, Garcia-Franco R, San Yeo IY, Lee SY, Wong EYM, Sabanayagam C, Baskaran M, Ibrahim F, Tan NC, Finkelstein EA, Lamoureux EL, Wong IY, Bressler NM, Sivaprasad S, Varma R, Jonas JB, He MG, Cheng CY, Cheung GCM, Aung T, Hsu W, Lee ML, Wong TY. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ueda-Arakawa N, Ooto S, Tsujikawa A, Yamashiro K, Oishi A, Yoshimura N. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina. 2013;33:490–7. [DOI] [PubMed] [Google Scholar]
- 180.Van Schaik HJ, Alkemade C, Swart W, Van Best JA. Autofluorescence of the diabetic and healthy human cornea in vivo at different excitation wavelengths. Exp Eye Res. 1999;68:1–8. [DOI] [PubMed] [Google Scholar]
- 181.Van Trigt, AC. 1853. 'Trajecti ad Rhenum', Dissertatio ophthalmologica inauguralis de speculo oculi.
- 182.van Velthoven ME, Faber DJ, Verbraak FD, van Leeuwen TG, de Smet MD. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res. 2007;26:57–77. [DOI] [PubMed] [Google Scholar]
- 183.Vinay A. Shah, Robert A Hyde, Alexander Engelmann, Jennifer I Lim, Jay Chhablani, Peter A.Karth, Nikhila Khandwala, Cassie Huang. 2023. 'Peripheral Retinal Degenerations', American Academy of Ophthalmology. https://eyewiki.aao.org/Peripheral_Retinal_Degenerations. Accessed 3 Dec.
- 184.von Helmholtz, HLF. 1851. 'Beschreibung eines Augen-Spiegels', A Farstnerische Verlagsbuchhandlung.
- 185.Wagner SK, Fu DJ, Faes L, Liu X, Huemer J, Khalid H, Ferraz D, Korot E, Kelly C, Balaskas K, Denniston AK, Keane PA. Insights into systemic disease through retinal imaging-based oculomics. Transl Vis Sci Technol. 2020;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Waldstein SM, Vogl WD, Bogunovic H, Sadeghipour A, Riedl S, Schmidt-Erfurth U. Characterization of Drusen and hyperreflective foci as biomarkers for disease progression in age-related macular degeneration using artificial intelligence in optical coherence tomography. JAMA Ophthalmol. 2020;138:740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Walsh AJ, Mueller KP, Tweed K, Jones I, Walsh CM, Piscopo NJ, Niemi NM, Pagliarini DJ, Saha K, Skala MC. Classification of T-cell activation via autofluorescence lifetime imaging. Nat Biomed Eng. 2021;5:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang NK, Lai CC, Liu CH, Yeh LK, Chou CL, Kong J, Nagasaki T, Tsang SH, Chien CL. Origin of fundus hyperautofluorescent spots and their role in retinal degeneration in a mouse model of Goldmann-Favre syndrome. Dis Model Mech. 2013;6:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang X, Zhao L, Zhang J, Fariss RN, Ma W, Kretschmer F, Wang M, Qian HH, Badea TC, Diamond JS, Gan WB, Roger JE, Wong WT. Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J Neurosci. 2016;36:2827–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang YL, Yang JY, Yang JY, Zhao XY, Chen YX, Yu WH. Progress of artificial intelligence in diabetic retinopathy screening. Diabetes Metab Res Rev. 2021;37: e3414. [DOI] [PubMed] [Google Scholar]
- 191.Wang ZJ, Walsh AJ, Skala MC, Gitter A. Classifying T cell activity in autofluorescence intensity images with convolutional neural networks. J Biophotonics. 2020;13: e201960050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nguyen CL, Wayenborgh JP. Hermann von Helmholtz: the ophthalmoscope and some of his other contributions to ophthalmology. Hist Ophthal Intern. 2015;1:165–77. [Google Scholar]
- 193.Webb RH, Hughes GW. Scanning laser ophthalmoscope. IEEE Trans Biomed Eng. 1981;28:488–92. [DOI] [PubMed] [Google Scholar]
- 194.Whitmore SS, DeLuca AP, Andorf JL, Cheng JL, Mansoor M, Fortenbach CR, Critser DB, Russell JF, Stone EM, Han IC. Modeling rod and cone photoreceptor cell survival in vivo using optical coherence tomography. Sci Rep. 2023;13:6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wintergerst MWM, Mishra DK, Hartmann L, Shah P, Konana VK, Sagar P, Berger M, Murali K, Holz FG, Shanmugam MP, Finger RP. Diabetic retinopathy screening using smartphone-based fundus imaging in India. Ophthalmology. 2020;127:1529–38. [DOI] [PubMed] [Google Scholar]
- 196.Wolfing JI, Chung M, Carroll J, Roorda A, Williams DR. High-resolution retinal imaging of cone-rod dystrophy. Ophthalmology. 2006;113(1019): e1. [DOI] [PubMed] [Google Scholar]
- 197.Wong TY, Cheung N, Islam FM, Klein R, Criqui MH, Cotch MF, Carr JJ, Klein BE, Sharrett AR. Relation of retinopathy to coronary artery calcification: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2008;167:51–8. [DOI] [PubMed] [Google Scholar]
- 198.Xie Y, Nguyen QD, Hamzah H, Lim G, Bellemo V, Gunasekeran DV, Yip MYT, Qi Lee X, Hsu W, Li Lee M, Tan CS, Tym Wong H, Lamoureux EL, Tan GSW, Wong TY, Finkelstein EA, Ting DSW. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet Digit Health. 2020;2:e240–9. [DOI] [PubMed] [Google Scholar]
- 199.Yannuzzi LA. The retinal atlas. Elsevier: New York; 2010. [Google Scholar]
- 200.Young LH, Kim J, Yakin M, Lin H, Dao DT, Kodati S, Sharma S, Lee AY, Lee CS, Sen HN. Automated detection of vascular leakage in fluorescein angiography—a proof of concept. Transl Vis Sci Technol. 2022;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen Y, Shi Z, Shen Y. Eye damage due to cosmetic ultrasound treatment: a case report. BMC Ophthalmol. 2018;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yuksel S, Aredo B, Zegeye Y, Zhao CX, Tang M, Li X, Hulleman JD, Gautron L, Ludwig S, Moresco EMY, Butovich IA, Beutler BA, Ufret-Vincenty RL. Forward genetic screening using fundus spot scale identifies an essential role for Lipe in murine retinal homeostasis. Commun Biol. 2023;6:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retina Vitreous. 2016;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–32. [DOI] [PubMed] [Google Scholar]
- 205.Zhang LY, Pan J, Mamtilahun M, Zhu Y, Wang L, Venkatesh A, Shi R, Tu X, Jin K, Wang Y, Zhang Z, Yang GY. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics. 2020;10:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang Q, Rezaei KA, Saraf SS, Chu Z, Wang F, Wang RK. Ultra-wide optical coherence tomography angiography in diabetic retinopathy. Quant Imaging Med Surg. 2018;8:743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang Y, Harrison JM, Nateras OS, Chalfin S, Duong TQ. Decreased retinal-choroidal blood flow in retinitis pigmentosa as measured by MRI. Doc Ophthalmol. 2013;126:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang Y, Nateras OS, Peng Q, Kuranov RV, Harrison JM, Milner TE, Duong TQ. Lamina-specific anatomic magnetic resonance imaging of the human retina. Invest Ophthalmol Vis Sci. 2011;52:7232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang Y, Nateras OS, Peng Q, Rosende CA, Duong TQ. Blood flow MRI of the human retina/choroid during rest and isometric exercise. Invest Ophthalmol Vis Sci. 2012;53:4299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhao N, Hao XN, Huang JM, Song ZM, Tao Y. Crosstalk between microglia and muller glia in the age-related macular degeneration: role and therapeutic value of neuroinflammation. Aging Dis. 2024;15:1132–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhao Y, MacCormick IJ, Parry DG, Leach S, Beare NA, Harding SP, Zheng Y. Automated detection of leakage in fluorescein angiography images with application to malarial retinopathy. Sci Rep. 2015;5:10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao Y, Zhao J, Gu Y, Chen B, Guo J, Xie J, Yan Q, Ma Y, Wu Y, Zhang J, Lu Q, Liu J. Outer Retinal layer thickness changes in white matter hyperintensity and Parkinson’s disease. Front Neurosci. 2021;15: 741651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou R, Horai R, Silver PB, Mattapallil MJ, Zarate-Blades CR, Chong WP, Chen J, Rigden RC, Villasmil R, Caspi RR. The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. J Immunol. 2012;188:1742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.