Abstract
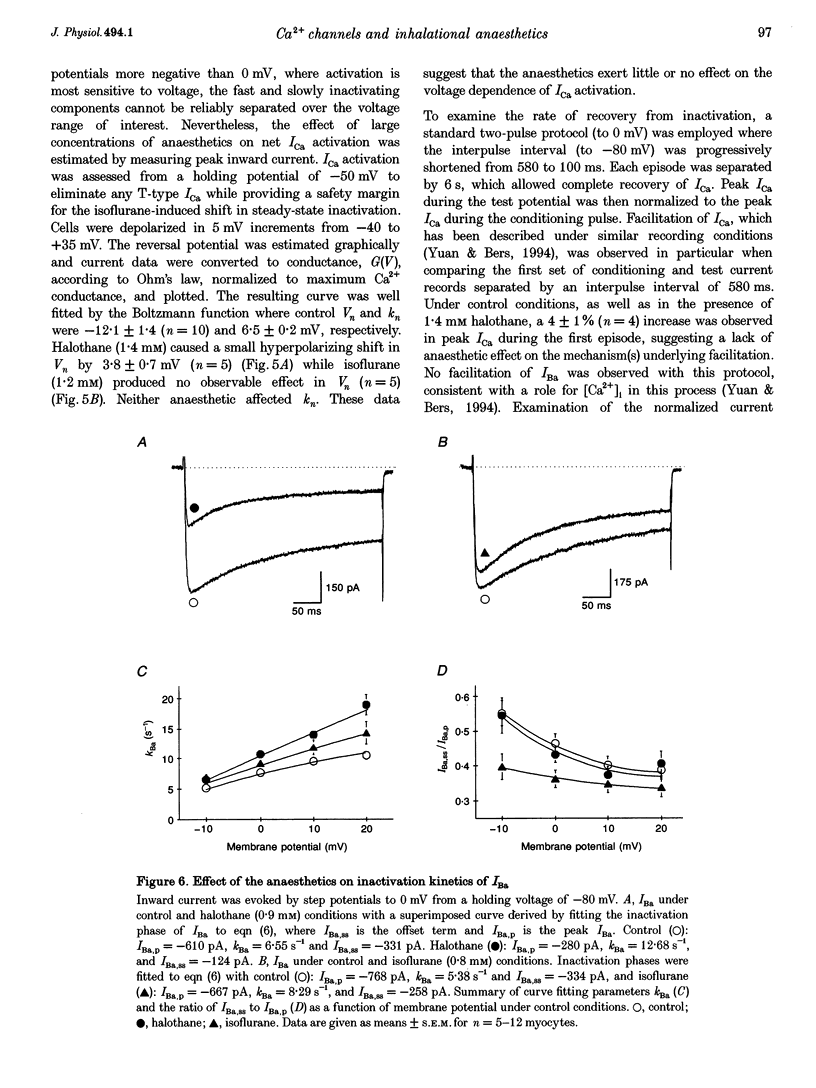
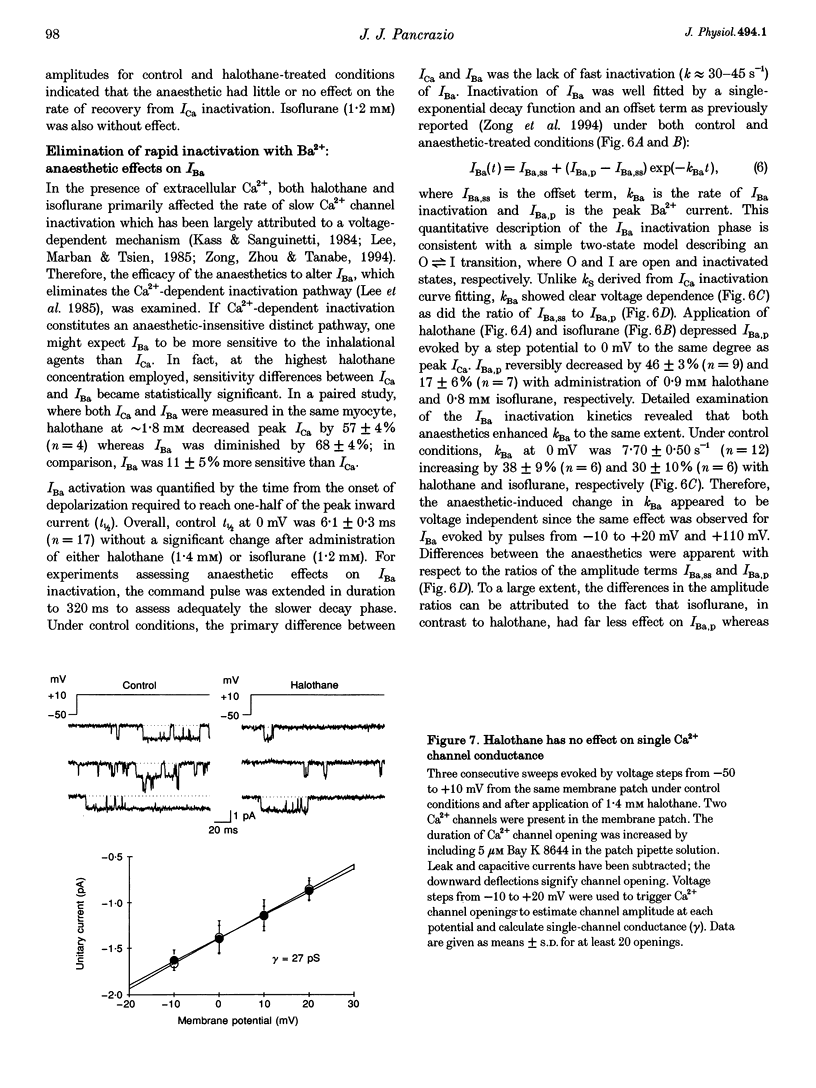
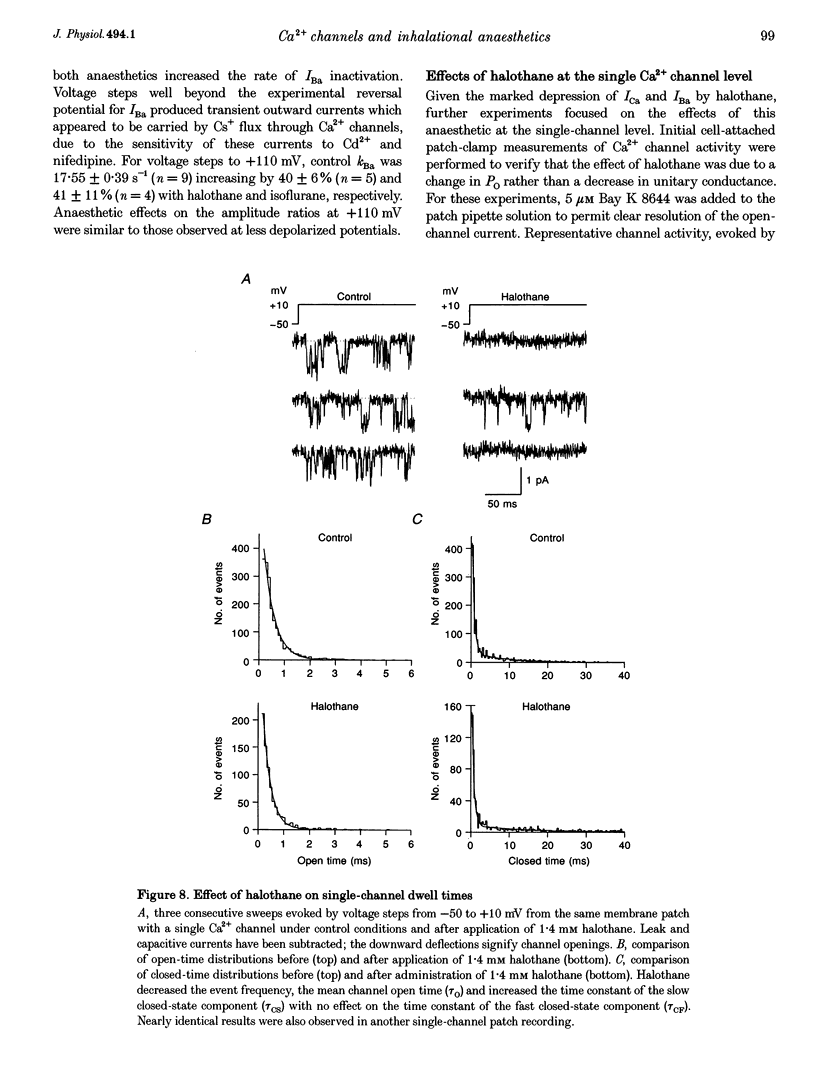
1. The effects of the inhalational anaesthetics halothane and isoflurane on the high-voltage-activated Ca2+ channels were determined in isolated guinea-pig ventricular myocytes using the patch-clamp technique. 2. Recording solutions were equilibrated with inhalational anaesthetic vapour delivered from a calibrated vaporizer set at clinically relevant ranges of partial pressure. Anaesthetic concentrations in solution were determined using gas chromatography. 3. Halothane (0.9 mM in solution) and isoflurane (0.8 mM in solution) decreased peak whole-cell CA2+ current (ICa) by approximately 40 and approximately 20%, respectively, while increasing the apparent rate of inactivation. 4. The sum of fast and slow exponential decay functions was required to fit the inactivation phase of ICa. The anaesthetics preferentially affected the slow component of inactivation while also increasing the rate of slow inactivation. The physiological significance of these effects was addressed by examining ICa evoked by a ventricular action potential waveform. 5. Measurement of the current carried by Ba2+ through Ca2+ channels (IBa) permitted the isolation of the slow component of inactivation. Halothane and isoflurane diminished peak IBa at 0 mV by approximately 45 and approximately 20% respectively, with similar changes in rate and magnitude of the slowly inactivating component as with ICa. 6. Cell-attached patch-clamp measurements of Ca2+ channel activity revealed that halothane did not alter single-channel conductance. Instead, the anaesthetic reduced channel open probability to the same extent as observed during the whole-cell recording, an effect partially due to an increase in null sweeps. In patches with a single channel present, the open-time distribution, fitted by a single exponential, showed a decrease in mean open time. The closed-time distribution, fitted by the sum of slow and fast exponential components, revealed an anaesthetic-induced increase in the duration of the slow component with no effect on the fast component. Results are presented in terms of a channel-gating model, and model predictions are examined with a computer simulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke C. W., Rose W. C., Marban E., Wier W. G. Macroscopic and unitary properties of physiological ion flux through T-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992 Oct;456:247–265. doi: 10.1113/jphysiol.1992.sp019335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke C. W., Wier W. G. Ryanodine does not affect calcium current in guinea pig ventricular myocytes in which Ca2+ is buffered. Circ Res. 1991 Mar;68(3):897–902. doi: 10.1161/01.res.68.3.897. [DOI] [PubMed] [Google Scholar]
- Bosnjak Z. J., Supan F. D., Rusch N. J. The effects of halothane, enflurane, and isoflurane on calcium current in isolated canine ventricular cells. Anesthesiology. 1991 Feb;74(2):340–345. doi: 10.1097/00000542-199102000-00022. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Connelly T. J., Coronado R. Activation of the Ca2+ release channel of cardiac sarcoplasmic reticulum by volatile anesthetics. Anesthesiology. 1994 Aug;81(2):459–469. doi: 10.1097/00000542-199408000-00025. [DOI] [PubMed] [Google Scholar]
- Drenger B., Quigg M., Blanck T. J. Volatile anesthetics depress calcium channel blocker binding to bovine cardiac sarcolemma. Anesthesiology. 1991 Jan;74(1):155–165. doi: 10.1097/00000542-199101000-00024. [DOI] [PubMed] [Google Scholar]
- Eskinder H., Rusch N. J., Supan F. D., Kampine J. P., Bosnjak Z. J. The effects of volatile anesthetics on L- and T-type calcium channel currents in canine cardiac Purkinje cells. Anesthesiology. 1991 May;74(5):919–926. doi: 10.1097/00000542-199105000-00018. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirota K., Fujimura J., Wakasugi M., Ito Y. Isoflurane and sevoflurane modulate inactivation kinetics of Ca2+ currents in single bullfrog atrial myocytes. Anesthesiology. 1996 Feb;84(2):377–383. doi: 10.1097/00000542-199602000-00016. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994 Jun;12(6):1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C., 3rd Differential depression of myocardial contractility by halothane and isoflurane in vitro. Anesthesiology. 1986 May;64(5):620–631. doi: 10.1097/00000542-198605000-00013. [DOI] [PubMed] [Google Scholar]
- Lynch C., 3rd Differential depression of myocardial contractility by volatile anesthetics in vitro: comparison with uncouplers of excitation-contraction coupling. J Cardiovasc Pharmacol. 1990 Apr;15(4):655–665. doi: 10.1097/00005344-199004000-00019. [DOI] [PubMed] [Google Scholar]
- Lynch C., 3rd, Frazer M. J. Depressant effects of volatile anesthetics upon rat and amphibian ventricular myocardium: insights into anesthetic mechanisms of action. Anesthesiology. 1989 Mar;70(3):511–522. doi: 10.1097/00000542-198903000-00023. [DOI] [PubMed] [Google Scholar]
- Lynch C., 3rd, Vogel S., Sperelakis N. Halothane depression of myocardial slow action potentials. Anesthesiology. 1981 Oct;55(4):360–368. doi: 10.1097/00000542-198110000-00005. [DOI] [PubMed] [Google Scholar]
- Miao N., Frazer M. J., Lynch C., 3rd Anesthetic actions on calcium uptake and calcium-dependent adenosine triphosphatase activity of cardiac sarcoplasmic reticulum. Adv Pharmacol. 1994;31:145–165. doi: 10.1016/s1054-3589(08)60614-6. [DOI] [PubMed] [Google Scholar]
- Mitra R. L., Morad M. Permeance of Cs+ and Rb+ through the inwardly rectifying K+ channel in guinea pig ventricular myocytes. J Membr Biol. 1991 May;122(1):33–42. doi: 10.1007/BF01872737. [DOI] [PubMed] [Google Scholar]
- Neely A., Olcese R., Wei X., Birnbaumer L., Stefani E. Ca(2+)-dependent inactivation of a cloned cardiac Ca2+ channel alpha 1 subunit (alpha 1C) expressed in Xenopus oocytes. Biophys J. 1994 Jun;66(6):1895–1903. doi: 10.1016/S0006-3495(94)80983-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E., Rüdisüli A., Maurer P., Weingart R. Effects of general anesthetics on current flow across membranes in guinea pig myocytes. Am J Physiol. 1989 Feb;256(2 Pt 1):C273–C281. doi: 10.1152/ajpcell.1989.256.2.C273. [DOI] [PubMed] [Google Scholar]
- Pancrazio J. J. Ion channel events simulated with the program SIMSTATE. Comput Methods Programs Biomed. 1995 Feb;46(2):165–174. doi: 10.1016/0169-2607(94)01618-p. [DOI] [PubMed] [Google Scholar]
- Pancrazio J. J., Johnson P. A., Lynch C., 3rd A major role for calcium-dependent potassium current in action potential repolarization in adrenal chromaffin cells. Brain Res. 1994 Dec 30;668(1-2):246–251. doi: 10.1016/0006-8993(94)90531-2. [DOI] [PubMed] [Google Scholar]
- Pancrazio J. J. PCS: an IBM-compatible microcomputer program for the analysis and display of voltage-clamp data. Comput Methods Programs Biomed. 1993 Jul;40(3):175–180. doi: 10.1016/0169-2607(93)90055-p. [DOI] [PubMed] [Google Scholar]
- Park W. K., Pancrazio J. J., Lynch C., 3rd Mechanical and electrophysiological effects of protamine on isolated ventricular myocardium: evidence for calcium overload. Cardiovasc Res. 1994 Apr;28(4):505–514. doi: 10.1093/cvr/28.4.505. [DOI] [PubMed] [Google Scholar]
- Qin Z., Szabo G., Cafiso D. S. Anesthetics reduce the magnitude of the membrane dipole potential. Measurements in lipid vesicles using voltage-sensitive spin probes. Biochemistry. 1995 Apr 25;34(16):5536–5543. doi: 10.1021/bi00016a027. [DOI] [PubMed] [Google Scholar]
- Richard S., Charnet P., Nerbonne J. M. Interconversion between distinct gating pathways of the high threshold calcium channel in rat ventricular myocytes. J Physiol. 1993 Mar;462:197–228. doi: 10.1113/jphysiol.1993.sp019551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S., Tiaho F., Charnet P., Nargeot J., Nerbonne J. M. Two pathways for Ca2+ channel gating differentially modulated by physiological stimuli. Am J Physiol. 1990 Jun;258(6 Pt 2):H1872–H1881. doi: 10.1152/ajpheart.1990.258.6.H1872. [DOI] [PubMed] [Google Scholar]
- Stevens W. C., Cromwell T. H., Halsey M. J., Eger E. I., 2nd, Shakespeare T. F., Bahlman S. H. The cardiovascular effects of a new inhalation anesthetic, Forane, in human volunteers at constant arterial carbon dioxide tension. Anesthesiology. 1971 Jul;35(1):8–16. doi: 10.1097/00000542-197107000-00005. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Puttick R. M., Terrar D. A. The effects of propofol and enflurane on single calcium channel currents of guinea-pig isolated ventricular myocytes. Br J Pharmacol. 1994 Apr;111(4):1147–1153. doi: 10.1111/j.1476-5381.1994.tb14865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrar D. A., Victory J. G. Effects of halothane on membrane currents associated with contraction in single myocytes isolated from guinea-pig ventricle. Br J Pharmacol. 1988 Jun;94(2):500–508. doi: 10.1111/j.1476-5381.1988.tb11553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrar D. A., Victory J. G. Isoflurane depresses membrane currents associated with contraction in myocytes isolated from guinea-pig ventricle. Anesthesiology. 1988 Nov;69(5):742–749. doi: 10.1097/00000542-198811000-00017. [DOI] [PubMed] [Google Scholar]
- Wheeler D. M., Rice R. T., Hansford R. G., Lakatta E. G. The effect of halothane on the free intracellular calcium concentration of isolated rat heart cells. Anesthesiology. 1988 Oct;69(4):578–583. doi: 10.1097/00000542-198810000-00019. [DOI] [PubMed] [Google Scholar]
- Wilde D. W. Isoflurane reduces Ca++ channel current and accelerates current decay in guinea pig portal vein smooth muscle cells. J Pharmacol Exp Ther. 1994 Dec;271(3):1159–1166. [PubMed] [Google Scholar]
- Wilde D. W., Knight P. R., Sheth N., Williams B. A. Halothane alters control of intracellular Ca2+ mobilization in single rat ventricular myocytes. Anesthesiology. 1991 Dec;75(6):1075–1086. doi: 10.1097/00000542-199112000-00020. [DOI] [PubMed] [Google Scholar]
- Yuan W., Bers D. M. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994 Sep;267(3 Pt 2):H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- Zong S., Zhou J., Tanabe T. Molecular determinants of calcium-dependent inactivation in cardiac L-type calcium channels. Biochem Biophys Res Commun. 1994 Jun 30;201(3):1117–1123. doi: 10.1006/bbrc.1994.1821. [DOI] [PubMed] [Google Scholar]


