Abstract
Objective
This update of a systematic review evaluates the effectiveness of spinal manipulations as a treatment for migraine headaches.
Background
Spinal manipulation therapy (SMT) is sometimes used to treat migraine headaches; however, the biological plausibility and safety of SMT have repeatedly been questioned.
Methods
Amed, Embase, MEDLINE, CINAHL, Mantis, Index to Chiropractic Literature, and Cochrane Central were searched from inception to September 2023. Randomized clinical trials (RCTs) investigating spinal manipulations (performed by various healthcare professionals including physiotherapists, osteopaths, and chiropractors) for treating migraine headaches in human subjects were considered. Other types of manipulative therapy, i.e., cranial, visceral, and soft tissue were excluded. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to evaluate the certainty of evidence.
Results
Three more RCTs were published since our first review; amounting to a total of 6 studies with 645 migraineurs meeting the inclusion criteria. Meta-analysis of six trials showed that, compared with various controls (placebo, drug therapy, usual care), SMT (with or without usual care) has no effect on migraine intensity/severity measured with a range of instruments (standardized mean difference [SMD] − 0.22, 95% confidence intervals [CI] − 0.65 to 0.21, very low certainty evidence), migraine duration (SMD − 0.10; 95% CI − 0.33 to 0.12, 4 trials, low certainty evidence), or emotional quality of life (SMD − 14.47; 95% CI − 31.59 to 2.66, 2 trials, low certainty evidence) at post-intervention. A meta-analysis of two trials showed that compared with various controls, SMT (with or without usual care) increased the risk of AEs (risk ratio [RR] 2.06; 95% CI 1.24 to 3.41, numbers needed to harm = 6; very low certainty evidence). The main reasons for downgrading the evidence were study limitations (studies judged to be at an unclear or high risk of bias), inconsistency (for pain intensity/severity), imprecision (small sizes and wide confidence intervals around effect estimates) and indirectness (methodological and clinical heterogeneity of populations, interventions, and comparators).
Conclusions
The effectiveness of SMT for the treatment of migraines remains unproven. Future, larger, more rigorous, and independently conducted studies might reduce the existing uncertainties.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-024-02719-6.
Keywords: Spinal manipulation, Migraine headache, Effectiveness, Systematic review; Meta-analysis
Background
Migraine affects more than one billion individuals worldwide [1] and the global prevalence rate of the condition is estimated at 14% [2]. The prevalence of migraineurs in general populations ranges in different countries e.g., from 6% in South Korea to 22.4% in Belgium [2, 3]. The Global Burden of Disease (GBD) Study 2019, estimated migraine to be the second most common cause of disability and the leading cause of disability-adjusted life years (DALYs) in young women [4]. Migraine is associated with significant suffering such as severe headaches and autonomic nervous system dysfunction, medical and psychiatric comorbidities, increased healthcare resource use, and poor health-related quality of life [5]. Several risk factors of this complex neurobiological disorder have been identified including hormonal imbalances (estrogen or cortisol dysregulation), female gender, obesity, head trauma, genetic factors, anxiety disorders, chronic stress, and environmental and dietary factors [1, 6]. The pathophysiology of migraine is multidimensional and may involve the trigeminovascular system and brainstem nuclei, the hypothalamus, the thalamus, and the cortex [7, 8, 9, 10]. Adenosine signaling, high-conductance calcium-activated potassium channels [11–13], calcitonin gene-related peptide (CGRP) [14, 15], pituitary adenylate cyclase-activating polypeptide (PACAP) [16] or endothelin have all been implicated in the pathogenesis [17, 18].
Spinal manipulative therapy (SMT) is a manual treatment often practiced by allied health professionals including physiotherapists, chiropractors, and osteopaths. By means of high-velocity, low-amplitude (HVLA) thrusts, SMT (e.g., Diversified, Gonstead, or Toggle Recoil Technique) involves quick pushes to the facet joints to ‘realign vertebrae’, often producing an audible “pop” sound. In contrast, mobilization techniques (e.g., Strain-Counter Strain, Positional Release Technique, or myofascial/soft tissue release) use slower, gentler movements or sustained pressure to gradually stretch and/or relieve tension in muscles and joints of the cervical spine. SMT (not mobilization techniques) is based on the assumption that, by correcting misalignments of the spinal joints, many conditions, including migraine, can be treated effectively. However, biological plausibility, effectiveness, and cost-effectiveness of SMT are doubtful [19, 20]. In addition, several hundred severe complications after upper SMT have been reported including carotid or vertebral artery dissections, strokes, and deaths [21–24].
Several new trials have been published [25–27] since our previous systematic review (SR) was published [28]. Therefore, this article is aimed at updating the evidence base for the effectiveness of SMT for migraine.
Methods
Search strategy and data sources
An update searches were performed in the following electronic databases: Amed, Embase, MEDLINE, CINAHL, Mantis, Index to Chiropractic Literature, and Central (from their respective inceptions to September 2023) using the exact same search terms that had previously been constructed over two concepts: spinal manipulative therapy and migraine headache (without any methodological filters, please refer to the Appendix 1). In addition, bibliographies of all thus identified studies and relevant SRs were scanned for any relevant papers. We searched for English language papers but considered studies published in any language.
Data selection, extraction, and management
The search results from those databases were combined in a single EndNote (20.1) library, and duplicate records of the same reports were removed. Titles and abstracts identified through the electronic database searching were screened by one reviewer (PP) and validated by another (EE). During that initial stage, any references which obviously did not meet the inclusion criteria of the review were excluded. Full papers were obtained for all the potentially relevant references and were examined in detail to determine whether they meet the criteria for inclusion. Again, this was done by one reviewer (PP) and validated by another (EE). With respect to both screening stages, any discrepancies between reviewers were resolved through a consensus. The selection of studies is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. We registered the protocol at www.protocols.io (dx.doi.org/10.17504/protocols.io.q26g7pne9gwz/v1).
The types of data that were extracted included details of the study design, populations, treatments, comparators, outcome measures, and results. Data extraction was performed by one reviewer (PP) and validated by another (EE). Wherever feasible, we attempted to obtain missing data from the original authors; when SDs of continuous outcome data were missing, we calculated them from other statistics, such as 95% CIs, standard errors, or P values.
Eligibility criteria
In this update, we applied the same inclusion and exclusion criteria, i.e., randomized or quasi-randomized trials, testing the effectiveness of SMT in migraine in human subjects; and adhered to the most recent version of The International Classification of Headache Disorders 3rd edition [30]. Any type of control and outcome measures were permissible. For clarity, we excluded participants suffering from other types of headaches, e.g., carcinogenic or tension-type or cluster headaches, as well as observational studies or trials of, e.g., gentle soft tissue mobilizations. Primary outcomes consisted of pain intensity/severity, migraine duration, number of migraine days, disability, quality of life, and adverse effects at follow-ups of up to 12 months. The original data extraction form was used to collect the data. One reviewer performed data extractions (PP), and another (EE) validated the entries. Any disagreements were resolved through a consensus.
Risk of bias assessment
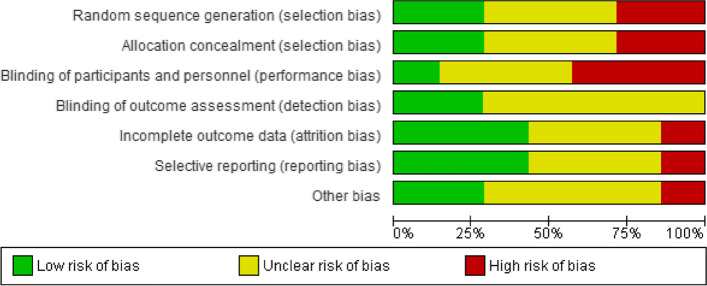
As the Jadad scale is now considered obsolete, we used the Cochrane Risk of Bias tool to assess the methodological risk of bias of all reviewed studies. The Cochrane Handbook for Systematic Reviews of Interventions recommends explicitly reporting the following individual elements for RCTs: random sequence generation; allocation concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data (attrition bias), selective outcome reporting (relevant outcomes reported); other sources of bias (baseline imbalances).
Data synthesis
Where studies were homogeneous enough in terms of populations, interventions, comparators, outcomes, and study designs, we pooled them quantitatively in a meta-analysis. For studies that assessed the same continuous outcomes, we estimated standardized mean differences (for different scales) between groups, along with 95% CIs. For dichotomous outcomes, risk ratios were estimated along with 95% CIs. The results of meta-analyses were displayed in forest plots which provided effect estimates and 95% CIs for each individual study as well as pooled effect estimates and 95% CI. We only combined the results of studies that reported uniform and comparable timing of outcome assessment. All meta-analyses were performed using RevMan 5.4 (desktop version); and adhering to the statistical guidelines described in the Cochrane Handbook, 2022 [31]. We used the Generic Inverse Variance method as some of the trials were of quasi-experimental design and only reported change scores. A random-effects model was chosen as it provides a more conservative estimate of effect. We assessed heterogeneity through a visual inspection of the overlap of forest plots and by calculating the chi-squared, Tau-squared tests and I2 inconsistency statistics. For pooling, if more than one intervention arm was relevant for a single comparison, we compared the relevant SMT arm with the least active control arm to avoid double‐counting data. We performed subgroup analyses by the types of intervention, i.e., osteopathic versus chiropractic SMT. We did not plan to perform any sensitivity analyses given the insufficient number of studies.
Results
Our searches generated a total of 1462 “hits”. After the removal of duplicates, 1142 titles and abstracts were screened for inclusion. Of those, 127 full-text articles were considered potentially relevant. A total of three RCTs have been published since our original review in 2011; and, in total 6 trials with 645 migraineurs were eligible for inclusion (see Fig. 1) [25–27, 32–34]. Their key data are summarized in Table 1. The included studies originated from Australia [33, 34], Norway [27], Spain [26], and the USA [25, 32]. Table 2 lists the adverse effects (AEs) reported in RCTs. Table 3 presents details of the SMT. Table 4 summarizes the details of SMT. Five studies evaluated the effectiveness of chiropractic SMT and one osteopathic SMT [26]. For the results of risk of bias assessments, see Figs. 2 and 3.
Fig. 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only
Table 1.
Randomized controlled studies of spinal manipulations for the treatment of migraines
| First author (year) [reference] | Study design | Participants (n) | Experimental intervention (details see Tab 3) | Control intervention | Primary outcome measure | Main result | Comment |
|---|---|---|---|---|---|---|---|
| Chaibi (2017) [27] | Single-blind, sham-controlled RCT with 3 parallel groups | 104 migraineurs | SMT |
(i) Sham (ii) Pharmacotherapy |
(i) Migraine days per month (ii) Duration (iii) Intensity (iv) Headache Index |
(i) a. Mean (SD) 3.9 (3.1) vs 4.1 (5.7) (p = 0.20) at post-intervention b. 4.5 (3.6) vs 4.6 (5.7) (p = 0.39) at 3 months follow-up c. 4.1 (3.9) vs 5.1 (6.4) (p = 0.65) at 6 months follow-up d. 4.4 (4.2) vs 4.1 (6.0) (p = 0.85) at 12 months follow-up (ii) a. 9.2 (5.8) vs 10.4 (7.0) (p = 0.04) at post-intervention b. 9.5 (6.9) vs 10.6 (7.2) (p = 0.06) at 3 months follow-up c. 7.3 (7.1) vs 11.6 (7.4) (p = 0.12) at 6 months follow-up d. 8.1 (7.3) vs 8.9 (7.7) (p = 0.34) at 12 months follow-up (iii) a. 4.7 (2.8) vs 5.0 (3.0) (p = 0.27) at post-intervention b. 5.0 (3.0) vs 4.9 (2.8) (p = 0.46) at 3 months follow-up c. 4.4 (3.6) vs 5.2 (2.9) (p = 0.69) at 6 months follow-up d. 5.1 (3.5) vs 4.4 (3.2) (p = 0.97) at 12 months follow-up (iv) a. 295.5 (348.1) vs 330.1 (602.3) (p = 0.16) at post-intervention b. 338.0 (350.8) vs 399.6 (582.0) (p = 0.32) at 3 months follow-up c. 313.0 (395.6) vs 402.8 (595.1) (p = 0.56) at 6 months follow-up d. 350.8 (451.6) vs 322.9 (668.8) (p = 0.92) at 12 months follow-up |
Blinding successfully achieved in > 80% of patients |
| Munoz-Gomez (2021) [26] | Single-blind, sham-controlled RCT with 2 parallel groups | 50 migraineurs | OMT | (i) Sham |
(i) Intensity (ii) Frequency (iii) Disability (iv) QoL |
(i) a. Mean (SD) 6.4 (1.0) vs 7.9 (1.1) at 1 month (p < 0.001) b. 6.5 (1.0) vs 7.6 (0.9) at 2 months (p < 0.001) (ii) a. 22.0 (10.9) vs 23.2 (8.9) at 1 month (MD = 1.2; 95% CI − 4.5 to 6.8) b. 20.6 (10.3) vs 23.4 (8.6) at 2 months (MD = 2.8; 95% CI − 2.6 to 8.2) (iii) a. 30.2 (18.1) vs 33.9 (13.9) at 1 month (MD = 3.7; 95% CI − 5.5 to 12.9) b. 24.3 (14.2) vs 34.3 (14.6) at 2 months (MD = 10.0; 95% CI 1.8 to 18.2; p < 0.05) (iv) a. 66.9 (15.0) vs 57.7 (15.8) at 1 month (MD = − 9.3; 95% CI − 18.0 to − 0.5; p < 0.05) b. 66.3 (21.1) vs 58.3 (14.9) at 2 months (MD = − 8.0; 95% CI − 18.4 to 2.4; n.s.) |
Relatively well-designed and reported study |
| Nelson (1998) [32] | Quasi-RCT with 3 parallel groups | 218 patients with migraine | SMT |
(i) Drug therapy (amitriptyline) (ii) SMT + amitriptyline |
(i) Headache index (ii) Severity (iii) Frequency (iv) Medication use |
(i) a. Mean (SD) 11.1 (5.6) vs 8.4 (9.0) at post-treatment b. 10.8 (9.6) vs 12.5 (8.3) at 1 month (ii) a. 4.3 (1.5) vs 4.3 (1.6) at post-treatment b. 4.4 (1.7) vs 4.5 (1.3) at 1 month (iii) a. 37.5 (25.9) vs 26.8 (22.6) at post-treatment b. 36.9 (29.3) vs 40.5 (23.3) at 1 month (iv) a. 1.2 (1.2) vs 0.7 (0.9) at post-treatment b. 1.2 (1.2) vs 1.3 (1.3) at 1 month |
Manipulation group received more attention (14 visits) compared to max. of 3 visits for drug therapy group |
| Parker (1978) [34] | Quasi-RCT with 3 parallel groups | 85 migraine patients |
(i) SMT by chiropractor (ii) SMT by a medical practitioner or physiotherapist |
Mobilization |
(i) Frequency of attacks (ii) Duration of headaches (hours) (iii) Disability (iv) VAS for pain intensity |
(i) Mean = 5.1 vs 5.7 (ii) 19.4 vs 11.9 (iii) 1.8 vs 2.2 (iv) 2.8 vs 4.5 |
(i) Lack of randomization, (ii) Lack of control for placebo effects |
| Rist (2021) [25] | RCT with 2 groups | 61 females with episodic migraines | SMT + UC | UC alone |
(i) Migraine days (ii) Severity (iii) Duration (iv) Disability (MIDAS) (v) (Acute) medication use (vi) QoL (emotional) |
(i) a. mean change = − 1.92; 95% CIs − 3.46 to − 0.37) at weeks 11–14 b. − 1.71; 95% CI − 3.26 to − 0.16 at weeks 15–18 (ii) a. − 0.85 (− 1.77 to 0.06) at weeks 11–14 b. − 0.64 (− 1.65 to 0.38) at weeks 15–18 (iii) a. − 1.48 (− 3.44 to 0.48) at weeks 11–14 b. 0.31 (− 1.96 to 2.57) at weeks 15–18 (iv) a. − 5.58 (− 10.44 to − 0.72) at weeks 11–14 b. − 9.45 (− 17.47 to − 1.43) at weeks 15–18 (v) a. − 1.36 (− 3.48 to 0.76) at weeks 11–14 b. − 0.83 (− 3.20 to 1.53) at weeks 15–18 (vi) a. 3.60 (− 1.10 to 8.30) at weeks 11–14 b. 6.57 (2.78 to 10.36) at weeks 15–18 |
|
| Tuchin (2000) [33] | Quasi-RCT with 2 groups | 127 volunteers | SMT | Placebo (detuned interferential therapy) |
(i) Frequency (ii) Intensity (iii) Duration (iv) Disability (v) Medication use |
(i) mean (SD) = 4.1 (6.55) vs 6.9 (6.6) (p < 0.005) (ii) 6.9 (1.8) vs 6.2 (1.7) n.s (iii) 14.8 (19.8) vs 19.8 (17.7) (p < 0.01) (iv) 13.0 (18.2) vs 15.6 (18.2) (p < 0.05) (v) 9.8 (12.4) vs 16.2 (12.4) (p < 0.001) |
(i) Unequal distribution of patients into groups, (ii) Unjustified blinding (iii) The use of a placebo is not credible |
Legend: CI confidence interval, MD mean difference, MIDAS Migraine Disability Assessment, n.s. no significant between-group differences at post-intervention (unless otherwise specified), OMT osteopathic manipulative therapy, QoL quality of life, SD standard deviation, SMT spinal manipulative therapy, VAS visual analog scale
Table 2.
Adverse effects (AEs) reported in RCTs
| Study (year) | Details of adverse effects reported |
|---|---|
| Chaibi (2017) [27] | Adverse effects were significantly more frequent in the SMT than in the placebo intervention sessions (83/355 vs. 32/348; P < 0.001 |
| Munoz-Gomez (2021) [26] | No serious reported |
| Nelson (1998) [32] | No info provided |
| Parker (1978) [34] | Chiropractic group was more likely to complain of side effects such as neck pain and soreness (P < 0.005) |
| Rist (2021) [25] | Almost twice the amount of overall AEs in the SMT + UC versus UC alone 52% versus 34% |
| Tuchin (2000) [33] | None reported |
Legend: SMT spinal manipulative therapy, UC usual care
Table 3.
Details of the spinal manipulation intervention
| Study (year) | Details of the chiropractic intervention (direct quote where applicable) |
|---|---|
| Chaibi (2017) [27] | “SMT group received spinal manipulative therapy using the Gonstead method, a specific contact, high-velocity, low-amplitude, short-lever spinal with no post-adjustment recoil that was directed to spinal biomechanical dysfunction (full spine approach) as diagnosed by standard chiropractic tests at each individual treatment session” |
| Munoz-Gomez (2021) [26] | “low velocity and moderate to high amplitude movements were conducted on the neck and upper-trunk joints and sacroiliac joints to force their full range of motion. Specifically, the following techniques were applied bilaterally in all treatment sessions (Dunning et al. 2016): occiput-atlas-axis articulatory manipulation, upper cervical spine (C0–C1) mobilization, middle cervical spine (C2–C7) mobilization in supine, middle cervical spine (C2–C7) mobilization in prone, cervicothoracic junction articulatory manipulation, upper thoracic spine (T2-T6) articulatory manipulation and global sacroiliac joint articulatory manipulation.” |
| Nelson (1998) [32] | Spinal manipulation 14 times over 8 weeks (no more than 2 times per week)—preceded by 5–10 min of massage and/or trigger point therapy |
| Parker (1978) [34] | “ (…) the chiropractors were required to manipulate the cervical spine. […] “The chiropractors were free to manipulate other parts of the spine on members of this group”. Frequency: no more than twice weekly for 2 months |
| Rist (2021) [25] | “All treatments applied in this study were within the scope of chiropractic practice in the Commonwealth of Massachusetts and included: posture correction and spinal stabilization exercises, soft tissue relaxation/release techniques, spinal manipulation and joint mobilization, relaxation techniques, education, stretches, and ergonomic modifications” |
| Tuchin (2000) [33] | “Two months of chiropractic SMT (diversified technique) at vertebral fixations determined by the practitioner (maximum of 16 treatments).” |
Table 4.
Summary of findings for the review’s main comparison
| SMT with or without usual care compared to (least active) control for the treatment of migraines | |||||
|---|---|---|---|---|---|
| Patient or population: migraineurs (all types) Setting: secondary care Intervention: SMT (with or without usual care) Comparison: (least active) control | |||||
| Outcomes | No. of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with (least active) control | Risk difference with SMT | ||||
| Migraine days post-treatment | 472 (4 RCTs) | ⨁◯◯◯ Very lowa,b,c | – | – | SMD 0.24 lower (0.47 lower to 0.02 lower) |
| Migraine duration post-treatment | 312 (4 RCTs) | ⨁◯◯◯ Very lowa,b,c | – | – | SMD 0.11 lower (0.33 lower to 0.12 higher) |
| Intensity/severity post-treatment: measured with VAS scales | 509 (6 RCTs) | ⨁◯◯◯ Very lowa,b,c,d | – | – | SMD 0.22 SD lower (0.65 lower to 0.21 higher) |
| Disability: measured with MIDAS | 234 (3 RCTs) | ⨁◯◯◯ Very lowa,b,c | – | – |
SMD 0.27 lower (0.54 lower to 0.01 lower) |
| Emotional QOL: measured with SF-36 | 111 (2 RCTs) | ⨁◯◯◯ Very lowa,b,e | – | – | SMD 14.47 lower (31.61 lower to 2.68 higher) |
| Adverse effects | 764 (2 RCTs) | ⨁◯◯◯ Very lowa,b,c,d | RR 2.06 (1.24 to 3.41) | 113 per 1000 | 208 more per 1000 (102 more to 368 more) |
CI confidence interval, MIDAS Migraine Disability Assessment, QOL quality of life, RCT randomized controlled trial, RR risk ratio, SF-36 short form 36 questionnaire, SMD standardized mean difference, SMT spinal manipulative therapy, VAS visual analogue scale
GRADE Working Group grades of evidence
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
aUnclear or high risk of bias of the included studies
bClinical and methodological heterogeneity in terms of population, intervention, comparator
cWide confidence intervals around the effect estimate
dSignificant statistical heterogeneity detected
eVery small sample size; wide confidence intervals around the effect estimate
Fig. 2.

Risk of bias summary: review authors’ judgements about each risk of bias item for each included study
Fig. 3.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies
Number of migraine days (post-treatment)
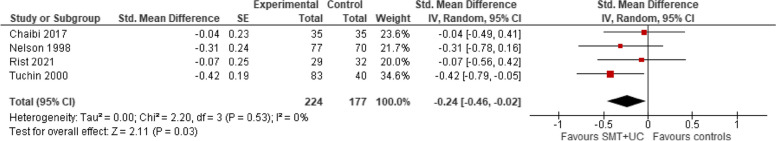
A meta-analysis of four trials showed that, when compared with controls (placebo or usual care), the intervention (with or without the addition of usual care) may reduce slightly the number of migraine days per month (SMD − 0.24; 95% confidence intervals [CI] − 0.47 to − 0.02, very low certainty evidence). There was no evidence of heterogeneity (Tau2 = 0.00; chi2 = 2.06; I2 = 0%, Fig. 4).
Fig. 4.
SMT versus controls: outcome: number of migraine days (post-treatment)
Migraine duration
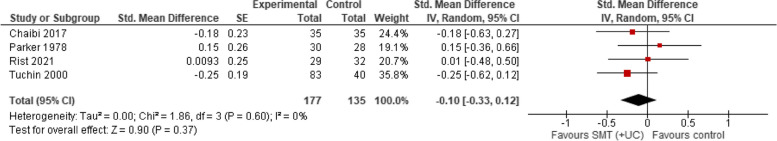
A meta-analysis of four trials showed that compared with various controls (placebo, manipulation, usual care), SMT (with or without usual care) has no effect on migraine duration (SMD − 0.10; 95% CI − 0.33 to 0.12, very low certainty evidence). There was no evidence of heterogeneity (Tau2 = 0.00; chi2 = 1.91, I2 = 0%, Fig. 5).
Fig. 5.
SMT versus controls: outcome: migraine duration (post-treatment)
Quality of life (emotional)
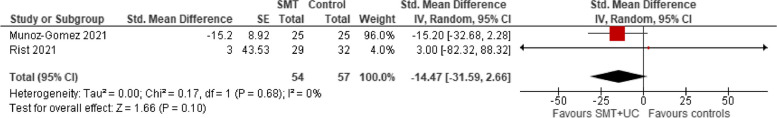
A meta-analysis of two trials showed that compared with various controls (placebo or usual care), SMT (with or without usual care) has no effect on quality of life (QoL) (SMD − 14.47, 95% CI − 31.59 to 2.66, very low certainty evidence). There was no evidence of heterogeneity (Tau2 = 0.00; chi2 = 0.17, I2 = 0%, Fig. 6). There was insufficient reporting for the remaining QoL domains.
Fig. 6.
SMT versus controls: outcome: emotional QoL (post-treatment)
Disability
A meta-analysis of three trials showed that compared with various controls (placebo, manipulation, usual care), SMT (with or without usual care) slightly reduces disability (SMD − 0.27, 95% CI − 0.54 to − 0.01, very low certainty evidence). There was no evidence of heterogeneity (Tau2 = 0.00; chi2 = 1.63, I2 = 0%, Fig. 7).
Fig. 7.
SMT versus controls: outcome: disability (post-treatment)
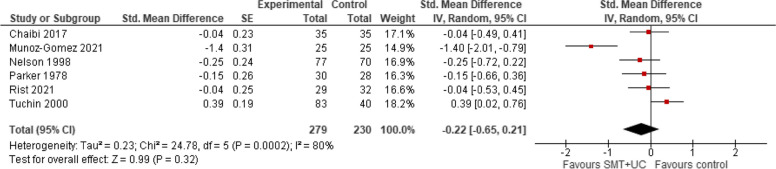
Intensity/severity post-treatment
A meta-analysis of six trials showed that compared with various controls (placebo, manipulation, usual care), SMT (with or without usual care) has no effect on migraine intensity/severity measured with a range of instruments (SMD − 0.22, 95% CI − 0.65 to 0.21, very low certainty evidence). There was evidence of considerable heterogeneity (Tau2 = 0.23; chi2 = 23.75, I2 = 79%, Fig. 8).
Fig. 8.
SMT versus controls: outcome: intensity/severity (post-treatment)
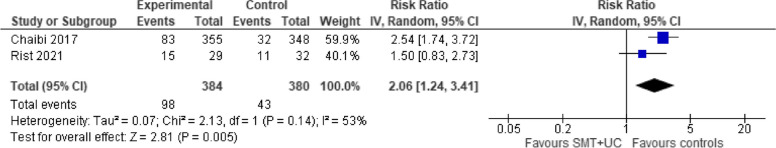
Safety
A meta-analysis of two trials showed that compared with various controls (placebo, pharmacotherapy, usual care), SMT (with or without usual care) increased the risk of AEs (RR 2.06; 95% CI 1.24 to 3.41, very low certainty evidence). There was evidence of significant heterogeneity (Tau2 = 0.07; chi2 = 2.13, I2 = 53%, Fig. 9).
Fig. 9.
SMT versus controls: outcome: adverse effects
Subgroup analyses
A subgroup analysis by the type of intervention, i.e., chiropractic versus osteopathic SMT for the outcome of pain intensity/severity showed a reduced amount of heterogeneity (I2 = 79% vs I2 = 28%, Fig. 10); and greater effect size for the osteopathic SMT.
Fig. 10.
Subgroup analysis (chiropractic vs osteopathic SMT) versus controls: outcome: intensity/severity (post-treatment)
Discussion
This SR was aimed at updating and critically evaluating the available evidence for the effectiveness of SMT in treating migraines. Three studies were published since our first SR [28], amounting to a total of six trials (with less than 600 participants, over the past 45 years) meeting the eligibility criteria [25–27, 32–34]. The results of the new three trials were contradictory and failed to eliminate the existing uncertainties.
Quality of the evidence
Using the GRADE approach, the quality of the evidence was judged to be very low for all outcomes. Reasons for downgrading the evidence included study limitations (the majority of trials (66.6%) had serious methodological flaws, indirectness (this pertained to the duration, frequency, and intensity of experimental and the control interventions, and outcome measures as well the populations, i.e., chronic vs episodic migraine vs healthy individuals vs females only), imprecision (small or very small sample sizes; wide confidence intervals around the effect estimates) and inconsistency (significant statistical heterogeneity).
We noticed several improvements when compared with the findings of our previous SR [28]. For example, two studies (33%) attempted to control for placebo effects by implementing a sham procedure. The results of these sham-controlled RCTs were, however, contradictory [26, 27]. The new RCTs had also larger sample sizes as well as better quality of reporting and adhering to International Headache Society guidelines for migraine prevention trials.
The risk–benefit ratio
Only three studies (50%) reported adverse effects (AEs). We found that participants in the SMT groups had a twofold increased risk of experiencing an AEs when compared with controls such as pharmacotherapy, placebo, or usual care [25, 27, 34]. Given the lack of (or very small) specific therapeutic effects, even the slightest risk of carotid or vertebral artery dissections, strokes or deaths inevitably shifts the risk–benefit ratio towards the negative.
Comparing with other SRs
A recent SR and meta-analysis concluded that SMT “may be an effective therapeutic technique to reduce migraine days and pain/intensity [35]. The credibility of this conclusion is, however, questionable. The authors pooled studies of disparate interventions such as SMT with osteopathic (gentle) soft-tissue techniques [36, 37]. There are a few potential gaps in the literature concerning the efficacy and safety of SMT in migraineurs. For instance, while only a handful of studies examine short-term safety, long-term data on AEs of SMT, especially repeated manipulations, is limited [38]. Secondly, as SMT techniques vary considerably, and studies often lack standardization of the specific methods used (and are poorly reported), which makes it difficult to compare results across studies [39]. Thirdly, evidence is limited regarding how different patient subgroups (e.g., individuals with comorbidities, age and gender differences, or chronic vs acute migraine sufferers) respond to SMT.
Strengths and limitations
Our SR has several strengths including the comprehensive search strategy, strictly adhering to the Cochrane Handbook and original eligibility criteria; statistical pooling of all outcomes; and critical evaluation of the evidence including the GRADE criteria. However, our SR also has several limitations. Firstly, although our searches were comprehensive, we cannot guarantee that all relevant articles were located. Secondly, there was a considerable amount of statistical heterogeneity for one outcome, i.e., migraine severity/intensity that stemmed from clinical and methodological differences in populations and interventions tested. We aimed to minimize these biases by running subgroup analyses, but it is conceivable that residual biases remain.
Conclusion/implications for future research
The effects of SMT on migraines are uncertain mainly due to very low-quality evidence. Therefore, from the clinical point of view, SMT cannot be considered an evidence-based therapy for this condition. As many studies on SMT for migraines are small, have limited follow-up, or lack rigorous methodology, hence better quality and larger RCTs are needed to reduce the existing uncertainties. Also, while SMT is often compared with inactive treatments, there is limited research comparing it directly with other established headache treatments, such as pharmacotherapy, (e.g., Triptans, Gepants, Ditans, Monoclonal Antibodies, OnabotulinumtoxinA) lifestyle changes, (e.g., regular sleep, exercise, nutrition/hydration) physical therapy, or cognitive behavioral therapy.
Supplementary Information
Supplementary Material 1. Appendix 1. Search strategy for MEDLINE
Acknowledgements
None.
Authors’ contributions
Conceptualization: P.P. and E.E.; methodology: P.P.; validation: E.E.; formal analysis: P.P.; investigation: P.P.; resources: P.P.; data curation: P.P.; writing—original draft preparation: P.P.; writing—review and editing: P.P., A.K., and E.E.; visualization: P.P.; supervision: P.P.; project administration: P.P. All authors have read and agreed to the published version of the manuscript.
Funding
There was no funding for this research.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amiri P, et al. Migraine: a review on its history, global epidemiology, risk factors, and comorbidities. Front Neurol. 2021;12:800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safiri S, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain. 2022;163(2):e293–309. [DOI] [PubMed] [Google Scholar]
- 3.Kim KM, et al. Prevalence, disability, and management patterns of migraine in korea: nationwide survey data from 2009 and 2018. J Clin Neurol. 2021;17(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner TJ, et al. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631–49. [DOI] [PubMed] [Google Scholar]
- 6.Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache. 2006;46(9):1334–43. [DOI] [PubMed] [Google Scholar]
- 7.Charles A, Brennan KC. The neurobiology of migraine. Handb Clin Neurol. 2010;97:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goadsby PJ, et al. Neurobiology of migraine. Neuroscience. 2009;161(2):327–41. [DOI] [PubMed] [Google Scholar]
- 9.Puledda F, et al. Migraine: from pathophysiology to treatment. J Neurol. 2023;270(7):3654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross EC, et al. The metabolic face of migraine - from pathophysiology to treatment. Nat Rev Neurol. 2019;15(11):627–43. [DOI] [PubMed] [Google Scholar]
- 11.Al-Karagholi MA, Hakbilen CC, Ashina M. The role of high-conductance calcium-activated potassium channel in headache and migraine pathophysiology. Basic Clin Pharmacol Toxicol. 2022;131(5):347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokoti L, Al-Karagholi MA, Ashina M. Latest insights into the pathophysiology of migraine: the ATP-sensitive potassium channels. Curr Pain Headache Rep. 2020;24(12):77. [DOI] [PubMed] [Google Scholar]
- 13.Lengyel M, et al. TRESK background potassium channel modifies the TRPV1-mediated nociceptor excitability in sensory neurons. Cephalalgia. 2021;41(7):827–38. [DOI] [PubMed] [Google Scholar]
- 14.Summ O, et al. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain. 2010;133(9):2540–8. [DOI] [PubMed] [Google Scholar]
- 15.Santos-Lasaosa S, et al. Calcitonin gene-related peptide in migraine: from pathophysiology to treatment. Neurologia (Engl Ed). 2022;37(5):390–402. [DOI] [PubMed] [Google Scholar]
- 16.Zagami AS, Edvinsson L, Goadsby PJ. Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol. 2014;1(12):1036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thuraiaiyah J, et al. Involvement of adenosine signaling pathway in migraine pathophysiology: a systematic review of clinical studies. Cephalalgia. 2022;42(8):781–92. [DOI] [PubMed] [Google Scholar]
- 18.Forland-Schill A, Berring-Uldum A, Debes NM. Migraine pathophysiology in children and adolescents: a review of the literature. J Child Neurol. 2022;37(7):642–51. [DOI] [PubMed] [Google Scholar]
- 19.Mirtz TA, et al. An epidemiological examination of the subluxation construct using Hill’s criteria of causation. Chiropr Osteopat. 2009;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vautravers P, Maigne JY. Cervical spine manipulation: risks–benefit–assessment. Rev Neurol (Paris). 2003;159(11):1064–6. [PubMed] [Google Scholar]
- 21.Smith WS, et al. Spinal manipulative therapy is an independent risk factor for vertebral artery dissection. Neurology. 2003;60(9):1424–8. [DOI] [PubMed] [Google Scholar]
- 22.Ernst E. Deaths after chiropractic: a review of published cases. Int J Clin Pract. 2010;64(8):1162–5. [DOI] [PubMed] [Google Scholar]
- 23.John S, Tavee J. Bilateral diaphragmatic paralysis due to cervical chiropractic manipulation. Neurologist. 2015;19(3):65–7. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell DM, Bondy SJ, Williams JI. Chiropractic manipulation and stroke: a population-based case-control study. Stroke. 2001;32(5):1054–60. [DOI] [PubMed] [Google Scholar]
- 25.Rist PM, et al. Multimodal chiropractic care for migraine: a pilot randomized controlled trial. Cephalalgia. 2021;41(3):318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz-Gomez E, et al. Effectiveness of a manual therapy protocol based on articulatory techniques in migraine patients. A randomized controlled trial. Musculoskelet Sci Pract. 2021;54:102386. [DOI] [PubMed] [Google Scholar]
- 27.Chaibi A, et al. Chiropractic spinal manipulative therapy for migraine: a three-armed, single-blinded, placebo, randomized controlled trial. Eur J Neurol. 2017;24(1):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posadzki P, Ernst E. Spinal manipulations for the treatment of migraine: a systematic review of randomized clinical trials. Cephalalgia. 2011;31(8):964–70. [DOI] [PubMed] [Google Scholar]
- 29.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia, 2018. 38(1): p. 1–211. [DOI] [PubMed]
- 31.Higgins JPT, T.J., Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). . Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. . 2022; Available from: www.training.cochrane.org/handbook.
- 32.Nelson CF, et al. The efficacy of spinal manipulation, amitriptyline and the combination of both therapies for the prophylaxis of migraine headache. J Manipulative Physiol Ther. 1998;21(8):511–9. [PubMed] [Google Scholar]
- 33.Tuchin PJ. The efficacy of chiropractic spinal manipulative therapy (SMT) in the treatment of migraine A pilot study. Australas Chiropr Osteopathy. 1997;6(2):41–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Parker GB, Tupling H, Pryor DS. A controlled trial of cervical manipulation of migraine. Aust N Z J Med. 1978;8(6):589–93. [DOI] [PubMed] [Google Scholar]
- 35.Rist PM, et al. The impact of spinal manipulation on migraine pain and disability: a systematic review and meta-analysis. Headache. 2019;59(4):532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerritelli F, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-Armed randomized controlled trial. Complement Ther Med. 2015;23(2):149–56. [DOI] [PubMed] [Google Scholar]
- 37.Voigt K, et al. Efficacy of osteopathic manipulative treatment of female patients with migraine: results of a randomized controlled trial. J Altern Complement Med. 2011;17(3):225–30. [DOI] [PubMed] [Google Scholar]
- 38.Ernst E, Posadzki P. Reporting of adverse effects in randomised clinical trials of chiropractic manipulations: a systematic review. N Z Med J. 2012;125(1353):87–140. [PubMed] [Google Scholar]
- 39.Ernst E, Posadzki P. An independent review of NCCAM-funded studies of chiropractic. Clin Rheumatol. 2011;30(5):593–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Appendix 1. Search strategy for MEDLINE
Data Availability Statement
All data generated or analyzed during this study are included in this published article.