Abstract
Background
People who use drugs (PWUD) in rural communities increasingly use stimulants, such as methamphetamine and cocaine, with opioids. We examined differences in hepatitis C virus (HCV) testing and treatment history among rural PWUD with opioids, stimulants, and other substance use combinations.
Methods
PWUD were enrolled from ten rural U.S. communities from 2018 to 2020. Participants self-reporting a positive HCV result were asked about their HCV treatment history and drug use history. Drug use was categorized as opioids alone, stimulants alone, both, or other drug(s) within the past 30 days. Prevalence ratios (PR) were yielded using adjusted multivariable log-binomial regression with generalized linear mixed models.
Results
Of the 2,705 PWUD, most reported both opioid and stimulant use (74%); while stimulant-only (12%), opioid-only (11%), and other drug use (2%) were less common. Most (76%) reported receiving HCV testing. Compared to other drug use, those who reported opioid use alone had a lower prevalence of HCV testing (aPR = 0.80; 95% CI: 0.63, 1.02). Among participants (n = 944) who self-reported an HCV diagnosis in their lifetime, 111 (12%) ever took anti-HCV medication; those who used both opioids and stimulants were less likely to have taken anti-HCV medication compared with other drug(s) (aPR = 0.41; 95% CI: 0.19, 0.91).
Conclusions
In this pre-COVID study of U.S. rural PWUD, those who reported opioid use alone had a lower prevalence of reported HCV testing. Those diagnosed with HCV and reported both opioid and stimulant use were less likely to report ever taking anti-HCV medication.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12954-024-01131-6.
Keywords: Opioids, Stimulants, Polysubstance use, HCV, Rural
Background
In the United States (U.S.), the incidence of hepatitis C virus (HCV) has risen in parallel with injection drug use [1, 2]. Despite the high incidence of HCV among people who use drugs (PWUD), estimates of HCV screening rates among this population range from 8–32% [3, 4], with significantly lower rates (6%) [4] in rural areas. The opioid epidemic’s impact on HCV transmission has spurred successful advocacy for routine HCV screening among injection drug-using communities [5] and those who enter substance use treatment [6], as well as analyses to identify counties at risk for outbreaks of HCV and HIV [7]. However, patterns of drug use in rural America continue to evolve. Since the mid-2010s, a “fourth wave” of the opioid epidemic has become apparent, characterized by PWUD intentionally and unintentionally mixing opioids (including analogs such as fentanyl [8] and stimulants (e.g., methamphetamine, cocaine)). Different substance use patterns, such as those who use only opioids versus those who use stimulants and opioids, may influence HCV testing and treatment initiation among PWUD. For example, available substance use treatment options such as medication for opioid use disorders (MOUD) are associated with higher rates of HCV screening [9], and the use of healthcare services is increased among those with methamphetamine-induced psychotic disorders [10]. It is imperative to understand how shifting drug use patterns influence HCV testing and treatment uptake, particularly in rural communities hardest hit by the opioid epidemic’s prior waves.
The HCV continuum of care (CoC) is a useful framework that outlines the steps toward HCV infection clearance; it begins with screening, followed by access to care, receipt of anti-HCV treatment, and achievement of infection cure [11–13]. In a prior study of rural people who inject drugs in Appalachia, among those who tested positive for HCV, 59% contacted a healthcare provider within 18 months of receipt of their positive result, 14% sought HCV treatment, and only 8% reported receiving anti-HCV medications [14] – a proportion 3–4 times lower than the overall U.S. HCV treatment rate [15]. Because HCV specialty providers such as hepatologists, gastroenterologists, and infectious disease physicians are more likely to be in urban areas, rural access to HCV treatment remains extremely limited [16, 17].
There is some evidence that substance use patterns influence the HCV CoC. There is little data on the first step in the HCV CoC, or screening rates, by the types of substances PWUD report using. However, one study of Medicaid enrollees who initiated medication for opioid use disorders found that those with an additional substance use disorder were more likely to receive HCV screening [18]. Completion of the HCV CoC may also vary by the types of substances PWUD report using. In studies of PWUD in New York City and Vancouver, regular cocaine use was associated with loss to follow-up in the HCV CoC [19, 20], which may be due to limited medication treatment options for cocaine use disorder. However, PWUD with publicly-funded insurance may face additional hurdles in accessing treatment. As of 2020, or through the enrollment period of our study, 26% of Medicaid programs had a mandatory minimum sobriety period before authorizing HCV treatment [21]. Although HCV treatment adherence does not appear to be affected by opioid use, conflicting findings make the impact of stimulant use unclear [22–24]. Furthermore, opioids, stimulants, and the use of both result in differing neurological, biological, and psychiatric effects that may uniquely influence HCV treatment completion [25–30]. For example, methamphetamine use is associated with increased depression, anxiety, violent behavior, and suicidality [31], while opioid use is associated with anxiety, phobia, depression, hysteria, and somatization [32].
The association of opioid use, stimulant use, use of both opioids and stimulants, and other drug use on the HCV CoC has not been evaluated in rural areas, where the barriers to HCV care include transportation obstacles and fewer specialized health care providers [33, 34]. Our primary objective was to determine the association between these substance use patterns (i.e., opioids, stimulants) with histories of HCV testing and receipt of anti-HCV treatment among rural PWUD in ten geographically diverse communities. Our secondary objective was to describe the HCV CoC among rural PWUD using self-reported responses to HCV testing and care questions and HCV antibody diagnostic test results. For the first objective, we hypothesized that rural PWUD with recent opioid use would be more likely to report being tested and treated for HCV compared to rural PWUD who reported substance use other than opioids and stimulants. For the second objective, we hypothesized that each step in the HCV CoC would vary by substance use patterns among rural PWUD.
Methods
Participants and procedures
We conducted a cross-sectional study using survey and HCV antibody screening data collected by Rural Opioid Initiative (ROI) sites from 2018 to 2020 and aggregated by the ROI Data Coordinating Center as of December 13, 2022 [35]. The ROI included 8 study sites in rural areas of 10 states: Illinois, Kentucky, Massachusetts, New Hampshire, North Carolina, Ohio, Oregon, Vermont, West Virginia, and Wisconsin, as previously described [36].
Research staff recruited participants through community outreach using a modified chain-referral approach based on respondent-driven sampling (RDS), useful for recruiting historically stigmatized populations such as PWUD [37, 38]. Research staff recruited “seed” participants using flyers, business cards, and through community outreach activities such as visiting local health departments and community events. These seed participants recruit peers who then recruit others, initiating chains of successive peer-recruitment. Generalized eligibility criteria included reporting the use of any opioid “to get high” or injection of any drug in the past 30 days and being a resident of a study area [36]. The minimum age for enrollment was 18, except for two studies that set the minimum age at 15. Compensation varied by site; participants received cash or a gift card for completing a survey composed of items jointly developed by all ROI sites. The inclusion criteria for our analyses were further narrowed to include only those adult participants (age 18 or older).
Measures
Dependent variables
Our analysis included two primary dependent variables. History of HCV testing was assessed by asking, “Have you ever been tested for HCV before today?” with a dichotomous, yes/no response. Among those who self-reported ever having been told they had HCV, history of anti-HCV treatment was assessed by asking, “Are you taking, or have you already finished prescription medicine to treat your HCV?” with a dichotomous, yes/no response.
Independent variable of interest
The independent variable of interest was participant-reported substance use in the past 30 days. We classified substance use into four patterns: opioid-only; stimulant-only (e.g., methamphetamine, amphetamines, or cocaine); use of both opioids and stimulants (i.e., using both substances separately or together, at least once in the past 30 days); and use of other drugs (e.g., gabapentin, clonidine, or benzodiazepines). Opioid use was defined as using heroin, fentanyl, carfentanil, prescription medications (e.g., oxycodone, Percocet, Percodan, Oxycontin, hydrocodone, Vicodin, Lorcet, Lortab, etc.), buprenorphine, methadone, or synthetic compounds (e.g., U47700) in the past 30 days.
Assessment of covariates for inclusion in adjusted models
We included covariates for assessment based on the literature and a directed acyclic graph (DAG)-informed model for each primary outcome [39]. We generated the DAGs by selecting covariates related to the outcome or exposure variables based on literature and their availability in the ROI dataset. A complete list of covariate definitions and the literature that informed the HCV testing and treatment DAGs are available in Supplemental Material 1.
HCV continuum of care
We described participants’ engagement in the steps of the HCV CoC and potential service delivery gaps by substance use pattern by plotting the prevalence of participants who completed HCV antibody testing at their study visit, participants who tested positive for HCV antibodies among those who completed testing, and the following self-reported steps: (1) ever previously tested for HCV, (2) ever having been told they had HCV, (3) have seen a medical provider in the past six months for HCV infection among those who had been told they have HCV, and, (4) ever taken HCV medication among those who had been told they have HCV. The survey questions do not specify HCV serology; a participant could have responded “yes” to being tested and having HCV, but it is unknown if their testing and results were for chronic HCV (antibody testing) or an active, current infection of HCV (RNA qualitative/quantitative testing). We tested for unadjusted differences by substance use pattern in each step of the HCV CoC by calculating the F statistic and defined a statistical significance difference as α < 0.05.
Statistical analyses
We used two separate models to generate unadjusted and adjusted prevalence estimates of HCV testing history and HCV treatment history, by substance use pattern. We used multivariable log-binomial mixed effects regression to calculate adjusted prevalence ratios (aPR) to measure the association of self-reported HCV testing and treatment with substance use patterns. We implemented modified Poisson models with robust variances [40, 41] on the adjusted and unadjusted HCV treatment log-binomial models due to non-convergence. We used the substance use pattern ‘no use of opioids or stimulants’ as the reference group. To generate PR estimates, we included dichotomous indicator variables for opioid use without stimulant use, stimulant use without opioid use, use of both opioids and stimulants, and use of substances other than opioids or stimulants. We handled missing data by using multiple imputation with a full conditional specification to impute missing values for all covariates. The imputation model included the outcomes of interest (self-reported HCV testing and treatment), exposure of interest (substance use pattern), and all covariates. Among participants who responded to the HCV testing and treatment questions, 5% or less of the observations were missing for each variable, except for the recent use of a syringe services program and health insurance status (15% and 6%, respectively). We adjusted for correlation introduced by RDS clustering using a random effects term for each RDS chain.
In the HCV testing model, the covariates in the final adjustment set included: age; binge drinking in the past 30 days; education; gender; homelessness; race/ethnicity; relationship status; and transportation access. The HCV treatment model, which included fewer covariates due to a smaller sample, included age and gender in the final adjustment set. Injection frequency, use of a syringe services program, engaging in substance use treatment, healthcare stigma, receipt of medical care, and health insurance were mediators in both outcome models and were therefore not included in the final DAG-informed multivariable models to avoid introducing collider bias [42–46]. A mediation analysis for these variables was outside the scope of this study. A future study that conducts these analyses would be of merit and help inform future models.
All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). The Ohio State University Institutional Review Board (IRB) reviewed and deemed this study exempt. All ROI sites had local IRB approval.
Results
Study population
A total of 3,048 participants were enrolled in the ROI study. We excluded 343 for not responding to, “Have you ever tested for HCV before today?” (n = 339) and an age less than 18 years (n = 4). Our final analytical sample for history of HCV testing as the independent variable model included 2,705 participants. Participants from the Wisconsin and combined New England sites (Massachusetts, New Hampshire, and Vermont) had the greatest representation in the analytical sample (27% and 21%, respectively); West Virginia, Oregon, and Illinois were the least represented (6% each). Use of both opioids and stimulants was the most commonly reported substance pattern (74%), followed by stimulant-only (12%), opioid-only (11%), and other drug use (2%).
Among 954 participants who reported having ever been told they “had HCV,” 10 participants had missing responses to, “Are you taking, or have you already finished prescription medicine to treat your HCV?” Therefore, the final analytical sample for history of HCV treatment as the dependent variable model included 944 participants.
HCV CoC by substance use pattern
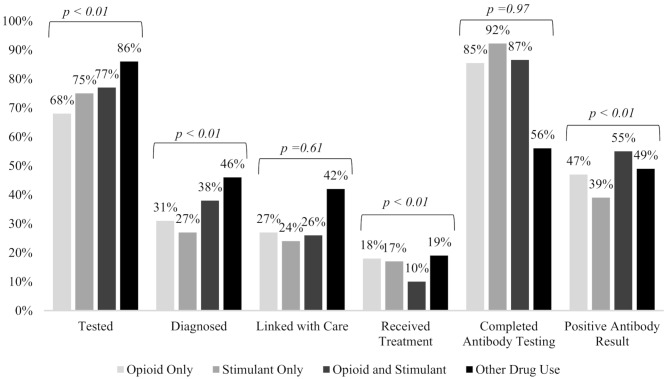
Completion of the following steps in the HCV CoC differed significantly by substance use pattern: testing, diagnosis, receipt of treatment, and antibody status (Fig. 1). Participants who reported other drug use (non-opioid, non-stimulant) were most likely to report past testing for HCV (86%), while those who reported opioid only use were least likely (68%). Of participants who ever received HCV screening, those who reported recent drug use other than opioids and stimulants were most likely to receive an HCV diagnosis (46%), followed by participants who reported both opioid and stimulant use (38%).
Fig. 1.
Hepatitis C virus continuum of care self-reported responses and antibody results among participants in the Rural Opioid Initiative cohort, 2018–2020
Among participants who self-reported ever receiving an HCV diagnosis, those who reported other drug use had the highest prevalence of linking with a medical provider for potential HCV treatment (42%), followed by those who used opioids only (27%) and those who used both opioids and stimulants (26%). Participants who reported other drug use, only opioids, and only stimulants had the highest proportions of ever having taken medication to treat chronic HCV (19%, 18%, and 17%, respectively). In comparison, only 10% of those who reported both opioid and stimulant use reported ever having received treatment.
Participants who completed HCV antibody testing at enrollment were most likely to use stimulants only (92%), compared to only half of those who reported other drug use (56%). As those who reported other drug use were the most likely to ever have been tested for HCV, these participants may have been less likely to complete HCV antibody testing at enrollment compared to their peers. With respect to HCV antibody positivity results captured at enrollment of the ROI study, those who reported opioid and stimulant use had the highest prevalence of HCV positivity (39%), while those who reported stimulant-only use had the lowest prevalence (23%).
HCV testing
Characteristics of Rural Opioid Initiative Participants.
Among the 2,060 participants who reported having previously tested for HCV, most were male (57%), not in a relationship (65%), high school graduates (77%), and non-Hispanic White (84%) (Table 1).
Table 1.
Characteristics of participants in the rural opioid initiative cohort by history of hepatitis C virus testing, 2018–2020
| Total | HCV Testing | |||||
|---|---|---|---|---|---|---|
| Received Testing | Never Received Testing | |||||
| N | % | N | % | N | % | |
| TOTAL | 2705 | 2060 | 76% | 645 | 24% | |
| Age | ||||||
| Median (IQR) | 35 | (28, 43) | 35 | (29, 43) | 33 | (27, 42) |
| Sex | ||||||
| Female | 1159 | 43% | 939 | 46% | 220 | 34% |
| Male | 1534 | 57% | 1111 | 54% | 423 | 66% |
| Transgender | 7 | 0% | 6 | 0% | 1 | 0% |
| Missing | 5 | 0% | 4 | 0% | 1 | 0% |
| Highest level of education completed | ||||||
| Less than high school | 608 | 22% | 424 | 21% | 184 | 29% |
| High school diploma/GED or above | 2094 | 77% | 1636 | 79% | 458 | 71% |
| Missing | 3 | 0% | 0 | 0% | 3 | 0% |
| Marital status | ||||||
| Married/living with partner | 804 | 30% | 604 | 29% | 200 | 31% |
| Single/divorced/ widowed/separated | 1760 | 65% | 1369 | 66% | 391 | 61% |
| Missing | 141 | 5% | 87 | 4% | 54 | 8% |
| Rural Opioid Initiative Site | ||||||
| Illinois | 164 | 6% | 120 | 6% | 44 | 7% |
| Kentucky | 335 | 12% | 246 | 12% | 89 | 14% |
| North Carolina | 341 | 13% | 269 | 13% | 72 | 11% |
| New England1 | 557 | 21% | 435 | 21% | 122 | 19% |
| Ohio | 248 | 9% | 202 | 10% | 46 | 7% |
| Oregon | 163 | 6% | 131 | 6% | 32 | 5% |
| West Virginia | 171 | 6% | 148 | 7% | 23 | 4% |
| Wisconsin | 726 | 27% | 509 | 25% | 217 | 34% |
| Substance use category, past 30 days | ||||||
| Other drug use | 58 | 2% | 50 | 2% | 8 | 1% |
| Opioid without stimulant use | 311 | 11% | 210 | 10% | 101 | 16% |
| Stimulant without opioid use | 328 | 12% | 245 | 12% | 83 | 13% |
| Opioid and stimulant use | 2008 | 74% | 1555 | 75% | 453 | 70% |
| Race/Ethnicity | ||||||
| White, non-Hispanic | 2265 | 84% | 1745 | 85% | 520 | 81% |
| Hispanic/Latino, African American/Black, American Indian, Alaskan Native, Asian/Pacific Islander/Native Hawaiian, African, Other | 411 | 15% | 291 | 14% | 120 | 19% |
| Missing | 29 | 1% | 24 | 1% | 5 | 1% |
| Past 30-day Binge Drinking | ||||||
| Yes | 1277 | 47% | 937 | 45% | 340 | 53% |
| No | 1394 | 52% | 1102 | 53% | 292 | 45% |
| Missing | 34 | 1% | 21 | 1% | 13 | 2% |
| Homeless, past six months | ||||||
| Yes | 1414 | 52% | 1089 | 53% | 325 | 50% |
| No | 1256 | 46% | 949 | 46% | 307 | 48% |
| Missing | 35 | 1% | 22 | 1% | 13 | 2% |
| Access to Transportation | ||||||
| Yes | 1661 | 61% | 1256 | 61% | 405 | 63% |
| No | 899 | 33% | 705 | 34% | 194 | 30% |
| Missing | 145 | 5% | 99 | 5% | 46 | 7% |
1. Defined as Massachusetts, New Hampshire, and Vermont
Association of HCV testing with substance use patterns
In comparing the prevalence of HCV testing between participants’ substance use patterns, participants who used opioids alone were less likely to report ever having been tested for HCV compared to participants who reported other drug use, although this difference was not statistically meaningful (aPR = 0.80; 95% CI: 0.63, 1.02) (Table 2). In general, there was no difference in reporting ever being tested for HCV between the specified substance use patterns involving opioids and/or stimulants compared to those who reported the use of other drugs in adjusted models.
Table 2.
Prevalence ratios of history of hepatitis C virus testing by substance use pattern in rural opioid initiative cohort, 2018–2020
| Substance Use Pattern, past 30 days | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| DAG-Informed Model21 | ||||
| PR | 95% CI | aPR | 95% CI | |
| Other drug use (no opioid or stimulant use) | referent | referent | ||
| Opioid use without stimulant use | 0.78 | (0.60, 1.01) | 0.80 | (0.63, 1.02) |
| Stimulant use without opioid use | 0.88 | (0.67, 1.14) | 0.88 | (0.69, 1.13) |
| Opioid and stimulant use | 0.90 | (0.70, 1.15) | 0.93 | (0.74, 1.16) |
PR, Prevalence Ratio; aPR, adjusted Prevalence Ratio
1. Adjusted for age, binge drinking in the past 30 days, education, gender, homelessness, race, relationship status, and transportation
HCV treatment
Characteristics of participants who self-reported history of HCV
Among participants who self-reported having been told they “had HCV” (n = 944), only 12% reported ever receiving anti-HCV medication (Table 3). Most of those who reported ever receiving treatment were male (53%). Use of both opioids and stimulants was the most reported substance pattern (78%), followed by only opioids (10%), only stimulants (9%), and other drug use (3%). The lowest HCV treatment rates were reported in Kentucky (2%), Oregon (2%), Ohio (6%), and West Virginia (7%) (percentages not shown). In 2016, these four states, excluding Oregon, had Medicaid sobriety restrictions for treatment [47].
Table 3.
Characteristics of the rural opioid initiative cohort by history of hepatitis C virus treatment, 2018–2020
| Self-reported ever having HCV | HCV Treatment | |||||
|---|---|---|---|---|---|---|
| Received Treatment | Never Received Treatment | |||||
| N | % | N | % | N | % | |
| TOTAL | 944 | 111 | 12% | 833 | 88% | |
| Age | ||||||
| Median (IQR) | 36 | (29, 43.5) | 42 | (34, 53) | 35 | (29, 42) |
| Sex | ||||||
| Female | 440 | 47% | 48 | 43% | 392 | 47% |
| Male | 503 | 53% | 63 | 57% | 440 | 53% |
| Transgender | 1 | 0% | 0 | 0% | 1 | 0% |
| Missing | 0 | 0% | 0 | 0% | 0 | 0% |
| Rural Opioid Initiative Site | ||||||
| Illinois | 54 | 6% | 13 | 12% | 41 | 5% |
| Kentucky | 135 | 14% | 3 | 3% | 132 | 16% |
| North Carolina | 124 | 13% | 19 | 17% | 105 | 13% |
| New England1 | 199 | 21% | 33 | 30% | 166 | 20% |
| Ohio | 127 | 13% | 8 | 7% | 119 | 14% |
| Oregon | 45 | 5% | 1 | 1% | 44 | 5% |
| West Virginia | 92 | 10% | 6 | 5% | 86 | 10% |
| Wisconsin | 168 | 18% | 28 | 25% | 140 | 17% |
| Substance use category, past 30 days | ||||||
| No opioid or stimulant use | 26 | 3% | 5 | 5% | 21 | 3% |
| Opioid without stimulant use | 94 | 10% | 17 | 15% | 77 | 9% |
| Stimulant without opioid use | 86 | 9% | 15 | 14% | 71 | 9% |
| Opioid and stimulant use | 738 | 78% | 74 | 67% | 664 | 80% |
1. Defined as Massachusetts, New Hampshire, and Vermont
Association of HCV treatment with substance use patterns
We generated adjusted prevalence ratios to determine if HCV treatment history varied by substance use patterns among participants who reported ever having been told they had HCV (Table 4). Those who used opioids and stimulants were less likely to report ever taking HCV medication compared to those who used other drugs (aPR = 0.41; 95% CI: (0.19, 0.91)). There was no difference in the report of HCV treatment receipt between participants who reported the use of opioids or stimulants alone compared to their peers who used other drugs.
Table 4.
Prevalence ratios of history of hepatitis C virus treatment by substance use pattern, rural opioid initiative cohort, 2018–2020
| Substance Pattern, past 30 days | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| DAG-Informed Model1 | ||||
| PR | 95% CI | aPR | 95% CI | |
| Other drug use (no opioid or stimulant use) | referent | referent | ||
| Opioid use without stimulant use | 0.61 | (0.22, 1.68) | 0.60 | (0.27, 1.32) |
| Stimulant use without opioid use | 0.55 | (0.17, 1.74) | 0.55 | (0.23, 1.34) |
| Opioid and stimulant use | 0.32* | (0.11, 0.95) | 0.41* | (0.19, 0.91) |
PR, Prevalence Ratio; aPR, adjusted Prevalence Ratio
*Associated confidence interval does not include the null value (‘1’)
1. Adjusted for age and gender
Discussion
In this multi-site cohort study of 2,705 people who use drugs in the rural United States, we found differences in the HCV continuum of care (CoC) by substance use patterns; and that participants who recently used both opioids and stimulants were less likely to receive HCV treatment, compared to participants who reported other (non-opioid, non-stimulant) drug use. We explored the associations between the steps in the HCV CoC and the most prevalent substance use patterns among those who use drugs in rural areas. The prevalence along each step in the HCV CoC varied and shifted by self-reported substance use pattern. We found that opioid use alone among rural PWUD was associated with a lower prevalence of prior HCV testing compared to those who reported other drug use, although this comparison was not statistically meaningful. We found that participants who use both opioids and stimulants had a lower prevalence of ever receiving HCV treatment than rural PWUD who did not report opioid or stimulant use. These findings may indicate that those who use both opioids and stimulants may be less likely to engage in HCV treatment compared to those who report recent use of other drugs. Furthermore, the overall reported rate of HCV treatment completion among this population of rural PWUD is insufficient to meet the goals set by the Viral Hepatitis National Strategic Plan for the United States [48] to increase the proportion of people who have cleared hepatitis C infection to 58% by 2025 and 80% by 2030. Rural PWUD must be included in national efforts to reach these goals.
Our findings provide insights on HCV screening and care engagement given the shift in substance use patterns among rural PWUD, specifically from the “first wave” of the opioid epidemic that began with prescription opioids to the current “fourth wave” of combined opioid and stimulant use [49]. Due to the significance of the opioid epidemic on HCV transmission, we hypothesized that those who reported only opioid use would be most likely to have reported HCV screening and linkage with HCV care. We were surprised to note that these individuals were actually the least likely to report ever having been tested for HCV – which is the necessary first step to engage in anti-HCV treatment and be cured of the infection. In contrast, participants who did not recently use opioids or stimulants had the highest completion prevalence along each self-reported step of the CoC.
Despite continued recommendations to incorporate HCV screening in opioid use disorder treatment facilities [6, 50], HCV screening was inadequate among participants who used opioids or stimulants in our study population, even though self-reported history of substance use treatment ranged from 68% (stimulant use only) to 82% (opioid use only) among participants (data not shown). Notably, HCV screening rates were highest among participants who engaged in other drug use, such as gabapentin, clonidine, and benzodiazepines. Higher screening rates for participants who engaged in other drugs, which consisted of prescription drugs, may reflect a higher engagement in care with access to screening, warranting exploration. Behaviors that might be associated with increased screening among this population may include different substance use patterns, substance use networks, and engagement with harm reduction resources. Interventions to overcome accessibility barriers to HCV screening and increase awareness of HCV transmission risks are needed to increase screening rates among those who use opioids or stimulants in the rural communities studied.
Our results may indicate two critical service delivery gaps among rural people who use opioids and stimulants: (1) linkage with HCV treatment providers, and (2) linkage to HCV treatment. Those who used other drugs had the highest prevalence of self-reporting having been treated for HCV, and those with recent opioid use had the second lowest prevalence. Among PWUD in Australia, those who used methamphetamine had lower odds of initiating HCV treatment [24]. Of PWUD in an Australian national survey, those who reported methamphetamine as the most injected drug were less likely to complete HCV screening than participants who mainly injected heroin, which may indicate that those who reported methamphetamine use may be less likely to engage in services or receive harm reduction messaging [51]. Expanding HCV care to primary care settings is recommended to improve treatment initiation and completion [52]. Despite the differences in Australia’s and the US’s healthcare systems, unique factors related to substance use patterns likely influence HCV care. Further research is needed to determine the causes of service delivery gaps such as provider and system barriers, or if those who use opioids and stimulants are less likely to seek healthcare, or both.
Multilevel social factors may be responsible for the lower likelihood of receiving HCV treatment among those who reported using both opioids and stimulants compared to rural PWUD who reported no use of either, as well as the low overall receipt of HCV treatment among the entire study sample. Historically, many patients were required to adhere to sobriety requirements to receive HCV treatment, especially those insured by state Medicaid programs that covered the majority (64%) of our study participants [47]. In 2016, of the states included in this study, Ohio, Kentucky, West Virginia, Wisconsin, Illinois, and Vermont had Medicaid programs that required a period of sobriety from drugs or alcohol, whereas Oregon, North Carolina, New Hampshire, and Massachusetts did not have sobriety restrictions for treatment [47]. By 2020, Ohio, Vermont, Massachusetts, and Wisconsin had removed their Medicaid sobriety restrictions [21]. Due to variations in Medicaid sobriety requirements among states in the New England ROI site, unmeasured differences in HCV treatment may have minimally biased and reduced the magnitude of association between substance use pattern and receipt of HCV treatment. Despite changes to the Medicaid sobriety requirements, disparities in receiving HCV treatment persist [15, 53, 54]. At the societal level, a misalignment may exist between policy and practice (i.e., policy uptake) among HCV treatment providers. In circumstances where no treatment restrictions exist, providers may withhold HCV treatment due to misconceptions about treatment adherence or until PWUD first receive Medicaid-assisted therapy [55, 56]. These low treatment rates may indicate persistent bias and stigmatizing beliefs towards PWUD among HCV treatment providers and staff due to perceiving behaviors of manipulation, violence, and poor motivation compared to non-PWUD patients, while lacking adequate training to serve PWUD [55, 57]. Individual level factors of those who use both opioids and stimulants may also likely contribute to their lower prevalence of HCV treatment. These factors may include apathy towards a health problem (i.e., HCV) that does not feel as immediate as competing priorities such as homelessness and managing withdrawals [58, 59] or the need to reduce their polysubstance use to a single substance prior to initiating HCV treatment [60]. A multilevel approach is likely required to eliminate gaps in HCV service among rural PWUD, such as the removal of prior authorization requirements and additional state-level Medicaid restrictions [61], eradicating provider stigma towards PWUD, improving linkage to treatment, and addressing short-term needs of PWUD to improve HCV knowledge retention and motivation for treatment initiation are all imperative to reverse the HCV epidemic.
It is important, both clinically and for public health, to continue studying the relationship between substance use patterns and HCV care to address unique barriers to care and prevent ongoing transmission. We cannot confirm the effect of substance use patterns on the HCV CoC as the data were not constructed to include temporality (i.e., longitudinally), nor can we accurately evaluate if substance use patterns affect the healthcare-seeking behaviors of HCV screening and treatment. Our findings related to non-injection drug use of stimulants and other drugs are limited by the study’s eligibility criteria of any opioid use, regardless of route, and those who reported injection of any substance. This likely led to exclusions of people who used substances other than opioids without injection in our analyses, potentially biasing our results towards rural PWUD with reported opioid use. Similarly, based on the eligibility criteria, participants who reported non-opioid use may be more likely to report recent injection drug use. In general, most of our sample included participants with a reported history of injection drug use (92%) and within the past 30 days (86%) (data not shown). Our results are also limited by the self-reporting of substance use pattern classifications, as those who reported only opioid, stimulant, or other drug use may have unintentionally engaged in polysubstance use of one or more other substances. Further, the ROI cohort includes a geographically diverse sample of rural counties, but still, our results may not be generalizable to all rural U.S. communities. We found that participants who only used stimulants were the least likely to report seeing a medical provider for HCV, while those who used both opioids and stimulants were the least likely to report receipt of HCV treatment. In the context of tailoring clinical care to a patient’s substance use pattern, rapid HCV care may be more critical for those who use stimulants and opioids, as this population may use more frequently compared to those who use either substance alone [62]. Also, there are no FDA-approved medication assisted treatment options for stimulants [63], and there is a greater likelihood of overlap with other potential risks for transmission, such as sharing drug use equipment [64] and engaging in unprotected sex [65]. From a medical care perspective, it needs to be determined if HCV treatment providers in rural communities are not adhering to treatment policies by refusing treatment to those who use both opioids and stimulants or if the participants who use both are less likely to engage in care. Examining both factors would help inform public health efforts, particularly in rural settings with sparse HCV care resources [4].
Using a recruitment method based on RDS may have introduced selection bias [66]. While we cannot mitigate this limitation, this may have introduced a bias similar to the ‘healthy worker’ bias, meaning those who enrolled in this study were open to meeting with a research team to improve health outcomes for PWUD in their community and may be more likely to seek medical care or engage in behaviors to improve their health, compared to participants who did not use the local syringe services program or attend community events. If this selection bias is present, it may have influenced the HCV CoC proportions to be higher than in the rural PWUD population. Further, this selection bias would not impact our comparisons between those who did and did not engage in HCV testing and treatment by substance use pattern. Selection bias may have also been introduced in the HCV CoC, as those who reported other drug use were the most likely to ever have been tested for HCV, likely leading to a lower prevalence of HCV antibody testing at enrollment compared to their peers.
Conclusions
Our study described the differences in the HCV CoC by substance use pattern among rural PWUD in 10 U.S. rural communities. The history of HCV testing varied significantly by substance use pattern among rural PWUD, and those who reported only opioid use were the least likely to report ever being tested for HCV. We also found that rural PWUD who reported ever having been told they had HCV and who recently used both opioids and stimulants were less likely to report ever receiving HCV medication compared to rural PWUD who did not report recent use of opioids or stimulants. The next steps should include a more in-depth evaluation of the barriers to HCV treatment among those who use both stimulants and opioids and longitudinal studies to discern the effect of substance use patterns on the HCV CoC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- aPR
Adjusted Prevalence Ratio
- CI
Confidence Interval
- DAG
Directed Acyclic Graph
- PWUD
People who use drugs
- PR
Prevalence Ratio
- ROI
Rural Opioid Initiative
- U.S.
United States
Author contributions
ATE was responsible for conceptualizing the manuscript and writing the original draft. ATE, DK, and KEL developed the methodology. ATE and DK conducted the formal analysis. DK, WCM, and KEL supervised the analysis and manuscript development process. ATE, DK, WCM, JF, CBH, LSM, PDF, KL, JT, AMY, PTK, MTP, WJ, RPW, DB, DR, and KEL contributed to the review and editing process. ATE, DK, WCM, JF, PDF, JT, HC, PTK, WJ, VFG, GS, and KEL provided funding acquisition. All authors read and approved the final manuscript.
Funding
This publication is based upon data collected and/or methods developed as part of the Rural Opioid Initiative (ROI), a multi-site study with a common protocol which was developed collaboratively by investigators at eight research institutions and at the National Institute of Drug Abuse (NIDA), the Appalachian Regional Commission (ARC), the Centers for Disease Control and Prevention (CDC), and the Substance Abuse and Mental Health Services Administration (SAMHSA). Research presented in this publication is the result of secondary data harmonization and analysis and supported by grant U24DA048538 from NIDA. Primary data collection was supported by grants UG3DA044829/UH3DA044829, UG3DA044798/UH3DA044798, UG3DA044830/UH3DA044830, UG3DA044823/UH3DA044823, UG3DA044822/UH3DA044822, UG3DA044831/UH3DA044831, UG3DA044825, UG3DA044826/UH3DA044826, and U24DA044801 co-funded by NIDA, ARC, CDC, and SAMHSA. The authors thank the other ROI investigators and their teams, the ROI Executive Steering Committee chair, Dr. Holly Hagan, the NIDA Science Officer, Dr. Richard Jenkins, and, particularly, the participants of the individual ROI studies for their valuable contributions. A full list of participating ROI investigators and institutions can be found on the ROI website at http://ruralopioidinitiative.org/studies.html. Additional funding for this publication includes NIDA F31DA054752 (ATE), NIDA K01DA048174 (KEL), NIDA R01DA052214 (DK and WCM), NIDA R01DA047334 and U54GM104942 (JF), NIDA F30DA050423 (DLB), and NCATS U01TR002631 (PTK).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Each site received approval from their corresponding Institutional Review Board and all data are protected by a Certificate of Confidentiality.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, Holtzman D. Increases in Acute Hepatitis C virus infection related to a growing opioid epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, Serrecchia J, Blankenship S, Ward JW, Holtzman D, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged =30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012</at. MMWR Morb Mortal Wkly Rep. 2015;64:453–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Westgard LK, Sato T, Bradford WS, Eaton EF, Pilcher F, Hale AJ, Singh D, Martin M, Appa AA, Meyer JP, et al. National HIV and HCV Screening Rates for Hospitalized people who use drugs are suboptimal and heterogeneous across 11 US hospitals. Open Forum Infect Dis. 2024;11:ofae204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull-Otterson L, Huang YA, Zhu W, King H, Edlin BR, Hoover KW. Human immunodeficiency Virus and Hepatitis C virus infection testing among commercially insured persons who inject drugs, United States, 2010–2017. J Infect Dis. 2020;222:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for Hepatitis C screening among adults - United States, 2020. MMWR Recomm Rep. 2020;69:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration. Screening and Treatment of Viral Hepatitis in People with Substance Use Disorders. 2021. [PubMed]
- 7.Van Handel MM, Rose CE, Hallisey EJ, Kolling JL, Zibbell JE, Lewis B, Bohm MK, Jones CM, Flanagan BE, Siddiqi AE, et al. County-Level Vulnerability Assessment for Rapid Dissemination of HIV or HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr. 2016;73:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry. 2021;34:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S, Healy S, Shapoval L, Forthal S, Neighbors CJ. Hepatitis C Virus Screening among Medicaid-Insured individuals with opioid Use Disorder across Substance Use Disorder Treatment settings. Subst Use Misuse. 2021;56:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R. Methamphetamine Treatment Project Corporate A: clinical course and outcomes of methamphetamine-dependent adults with psychosis. J Subst Abuse Treat. 2008;35:445–50. [DOI] [PubMed] [Google Scholar]
- 11.Linas BP, Barter DM, Leff JA, Assoumou SA, Salomon JA, Weinstein MC, Kim AY, Schackman BR. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS ONE. 2014;9:e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris MD, Mirzazadeh A, Evans JL, Briceno A, Coffin P, Hahn JA, Page KA. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: low number treated. Drug Alcohol Depend. 2019;198:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsui JI, Miller CM, Scott JD, Corcorran MA, Dombrowski JC, Glick SN. Hepatitis C continuum of care and utilization of healthcare and harm reduction services among persons who inject drugs in Seattle. Drug Alcohol Depend. 2019;195:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens DB, Young AM, Havens JR. Healthcare contact and treatment uptake following hepatitis C virus screening and counseling among rural Appalachian people who use drugs. Int J Drug Policy. 2017;47:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson WW, Symum H, Sandul A, Dhsc, Gupta N, Patel P, Nelson N, Mermin J, Wester C. Vital signs: Hepatitis C treatment among insured adults - United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2022;71:1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.M M, A K, T G, al. E: the 2014 update of the Rural-Urban Chartbook. (Rural Health Reform Policy Research Center ed.; 2014.
- 17.Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PLoS ONE. 2013;8:e84826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahrens K, Sharbaugh M, Jarlenski MP, Tang L, Allen L, Austin AE, Barnes AJ, Burns ME, Clark S, Zivin K, et al. Prevalence of testing for human immunodeficiency virus, Hepatitis B Virus, and Hepatitis C Virus among Medicaid enrollees treated with medications for opioid use disorder in 11 States, 2016–2019. Clin Infect Dis. 2023;76:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winetsky D, Burack D, Antoniou P, Garcia B, Gordon P, Scherer M. Psychosocial factors and the Care Cascade for Hepatitis C Treatment Colocated at a Syringe Service Program. J Infect Dis. 2020;222:S392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Socias ME, Ti L, Wood E, Nosova E, Hull M, Hayashi K, Debeck K, Milloy MJ. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int. 2019;39:1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters P, Greenwald R, Ninburg M, Simmons A. State policies limiting Progress towards HCV Elimination in the U.S.: Center for Health Law and Policy Innovation; 2020. [Google Scholar]
- 22.Riley DE, Liu L, Cohen B, Robinson S, Groessl EJ, Ho SB. Characteristics and impact of methamphetamine use in patients with chronic hepatitis C. J Addict Med. 2014;8:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham EB, Hajarizadeh B, Amin J, Litwin AH, Gane E, Cooper C, Lacombe K, Hellard M, Read P, Powis J et al. Adherence to once-daily and twice-daily direct acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clin Infect Dis 2019. [DOI] [PubMed]
- 24.Alavi M, Grebely J, Micallef M, Dunlop AJ, Balcomb AC, Day CA, Treloar C, Bath N, Haber PS, Dore GJ. Enhancing treatment for Hepatitis CiOSSSG: Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clin Infect Dis. 2013;57(Suppl 2):S62–69. [DOI] [PubMed] [Google Scholar]
- 25.Lamonica AK, Boeri M. An exploration of the relationship between the use of methamphetamine and prescription drugs. J Ethnogr Qual Res. 2012;6:160. [PMC free article] [PubMed] [Google Scholar]
- 26.Branch SY, Beckstead MJ. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J Neurophysiol. 2012;108:802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassilev P, Avvisati R, Koya E, Badiani A. Distinct Populations of Neurons Activated by Heroin and Cocaine in the Striatum as Assessed by catFISH. eNeuro 2020, 7. [DOI] [PMC free article] [PubMed]
- 28.Hekmat S, Mehrjerdi Z, Moradi A, Ekhtiari H, Bakhshi S. Cognitive flexibility, attention and speed of Mental Processing in Opioid and Methamphetamine addicts in comparison with non-addicts. Basic Clin Neurosci. 2011;2:12–9. [Google Scholar]
- 29.Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychol Rev. 2007;17:317–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M. Methamphetamine Treatment P: Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–90. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadi J, Toobaee S, Kharras M, Radmehr M. Psychiatric disorders in opioid dependants. Int J Soc Psychiatry. 2003;49:185–91. [DOI] [PubMed] [Google Scholar]
- 33.Schranz AJ, Barrett J, Hurt CB, Malvestutto C, Miller WC. Challenges facing a rural opioid epidemic: treatment and Prevention of HIV and Hepatitis C. Curr HIV/AIDS Rep. 2018;15:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Socias ME, Karamouzian M, Parent S, Barletta J, Bird K, Ti L. Integrated models of care for people who inject drugs and live with hepatitis C virus: a systematic review. Int J Drug Policy. 2019;72:146–59. [DOI] [PubMed] [Google Scholar]
- 35.National Rural Opioid Initiative. HIV, HCV and Related comorbidities in Rural communities affected by Opioid Injection Drug Epidemics in the United States: Building systems for Prevention, Treatment and Control (UG3/UH3). NIDA; 2018.
- 36.Jenkins RA, Whitney BM, Nance RM, Allen TM, Cooper HLF, Feinberg J, Fredericksen R, Friedmann PD, Go VF, Jenkins WD, et al. The Rural Opioid Initiative Consortium description: providing evidence to Understand the Fourth Wave of the Opioid Crisis. Addict Sci Clin Pract. 2022;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston LG, Sabin K. Sampling hard-to-Reach populations with Respondent Driven Sampling. Methodological Innovations Online. 2010;5:38–48. [Google Scholar]
- 38.Douglas DH. Respondent-driven sampling: a New Approach to the study of hidden populations. Soc Probl. 1997;44:174–99. [Google Scholar]
- 39.Glymour M, Pearl J, Jewell NP. Causal inference in statistics: a primer. Wiley; 2016.
- 40.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 41.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. In.; 1967.
- 42.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42:1511–9. [DOI] [PubMed] [Google Scholar]
- 43.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, Poole C. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–84. [DOI] [PubMed] [Google Scholar]
- 45.Hernán MA. Invited Commentary: Selection Bias without colliders. Am J Epidemiol. 2017;185:1048–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groenwold RHH, Palmer TM, Tilling K. To adjust or not to adjust? When a confounder is only measured after exposure. Epidemiology 2021, 32. [DOI] [PMC free article] [PubMed]
- 47.Campbell CA, Canary L, Smith N, Teshale E, Ryerson AB, Ward JW. State HCV incidence and policies related to HCV Preventive and Treatment Services for Persons Who Inject Drugs - United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2017;66:465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services. Viral Hepatitis National Strategic Plan for the United States: A Roadmap to Elimination (2021–2025). Washington, DC; 2020.
- 49.Ciccarone D, Mars S, Rosenblum D, Unick J. Of speedballs and goofballs: Stimulants and the 4th wave of the opioid crisis 2019.
- 50.Ko JY, Haight SC, Schillie SF, Bohm MK, Dietz PM. National Trends in Hepatitis C Infection by opioid use disorder status among pregnant women at delivery hospitalization - United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2019;68:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibbs D, Price O, Grebely J, Larney S, Sutherland R, Read P, Butler K, Degenhardt L, Peacock A. Hepatitis C virus cascade of care among people who inject drugs in Australia: factors associated with testing and treatment in a universal healthcare system. Drug Alcohol Depend. 2021;228:109050. [DOI] [PubMed] [Google Scholar]
- 52.Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, Parish B, Burke T, Pak W, Dunkelberg J, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Academies. A National Strategy for the Elimination of Hepatitis B and C. In A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Edited by Strom BL, Buckley GJ. Washington (DC); 2017. [PubMed]
- 54.Nephew LD, Wang Y, Mohamed K, Nichols D, Rawl SM, Orman E, Desai AP, Patidar KR, Ghabril M, Chalasani N, Kasting ML. Removal of medicaid restrictions were associated with increased hepatitis C virus treatment rates, but disparities persist. J Viral Hepat. 2022;29:366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park H, Brown C, Wilson DL, Huang PL, Hernandez-Con P, Horne P, Goodin A, Joseph A, Segal R, Cabrera R, Cook RL. Clinician barriers, perceptions, and practices in treating patients with hepatitis C virus and substance use disorder in the United States. Prev Med Rep. 2023;32:102138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trooskin SB, Dore G, Kostman J. We must do Better: addressing HCV Treatment barriers in persons who inject drugs in the United States. J Infect Dis. 2020;222:S773–81. [DOI] [PubMed] [Google Scholar]
- 57.Zwick J, Appleseth H, Arndt S. Stigma: how it affects the substance use disorder patient. Subst Abuse Treat Prev Policy. 2020;15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Motavalli D, Taylor JL, Childs E, Valente PK, Salhaney P, Olson J, Biancarelli DL, Edeza A, Earlywine JJ, Marshall BDL, et al. Health is on the Back Burner: Multilevel barriers and facilitators to Primary Care among people who inject drugs. J Gen Intern Med. 2021;36:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsui JI, Barry MP, Austin EJ, Sweek EW, Tung E, Hansen RN, Ninburg M, Scott JD, Glick SN, Williams EC. Treat my whole person, not just my condition’: qualitative explorations of hepatitis C care delivery preferences among people who inject drugs. Addict Sci Clin Pract. 2021;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.I want to get better, but… identifying the perceptions and experiences of people who inject drugs with respect to evolving hepatitis C virus treatments. Int J Equity Health. 2021;20:81. [DOI] [PMC free article] [PubMed]
- 61.Hepatitis C. State of Medicaid Access: Hepatitis C: State of Medicaid Access. 2024.
- 62.Al-Tayyib A, Koester S, Langegger S, Raville L. Heroin and methamphetamine injection: an Emerging Drug Use Pattern. Subst Use Misuse. 2017;52:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan B, Kondo K, Ayers C, Freeman M, Montgomery J, Paynter R, Kansagara D. In Pharmacotherapy for Stimulant Use Disorders: A Systematic Review. Washington (DC); 2018: VA Evidence-based Synthesis Program Reports]. [PubMed]
- 64.Rezaei O, Ghiasvand H, Higgs P, Noroozi A, Noroozi M, Rezaei F, Armoon B, Bayani A. Factors associated with injecting-related risk behaviors among people who inject drugs: a systematic review and meta-analysis study. J Addict Dis 2020:1–18. [DOI] [PubMed]
- 65.Cepeda JA, Vickerman P, Bruneau J, Zang G, Borquez A, Farrell M, Degenhardt L, Martin NK. Estimating the contribution of stimulant injection to HIV and HCV epidemics among people who inject drugs and implications for harm reduction: a modeling analysis. Drug Alcohol Depend. 2020;213:108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCreesh N, Frost SD, Seeley J, Katongole J, Tarsh MN, Ndunguse R, Jichi F, Lunel NL, Maher D, Johnston LG, et al. Evaluation of respondent-driven sampling. Epidemiology. 2012;23:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.