Abstract
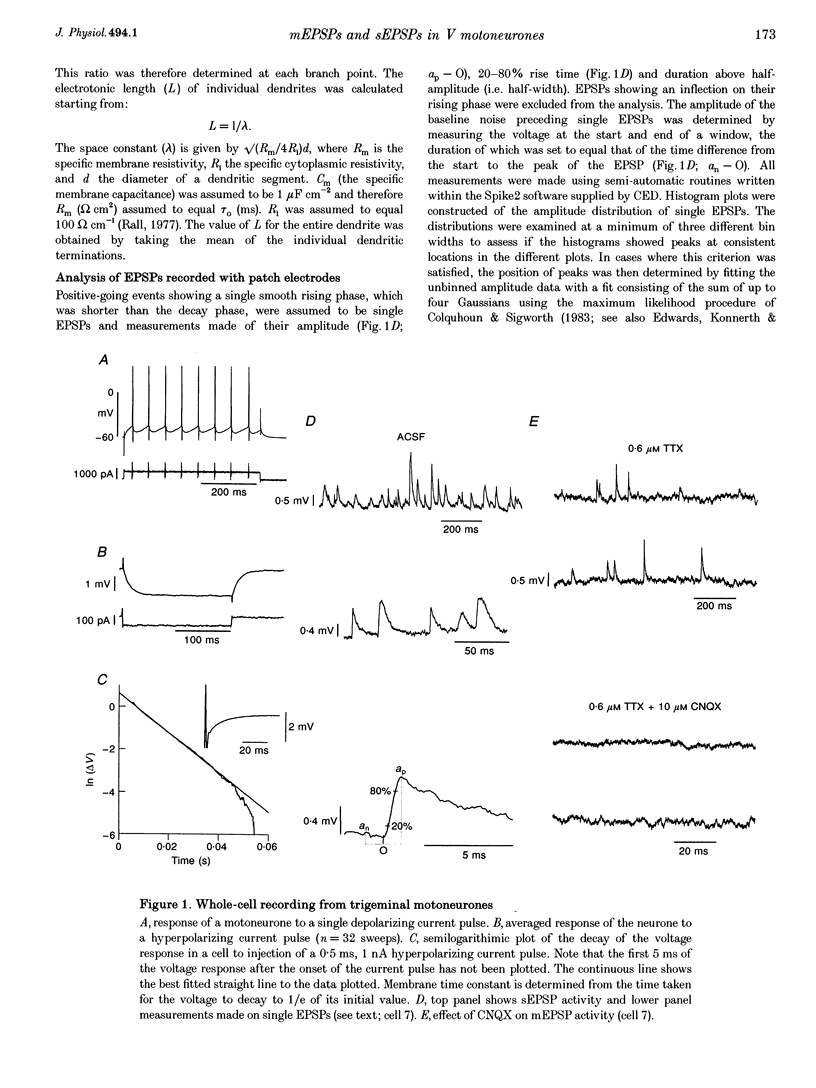
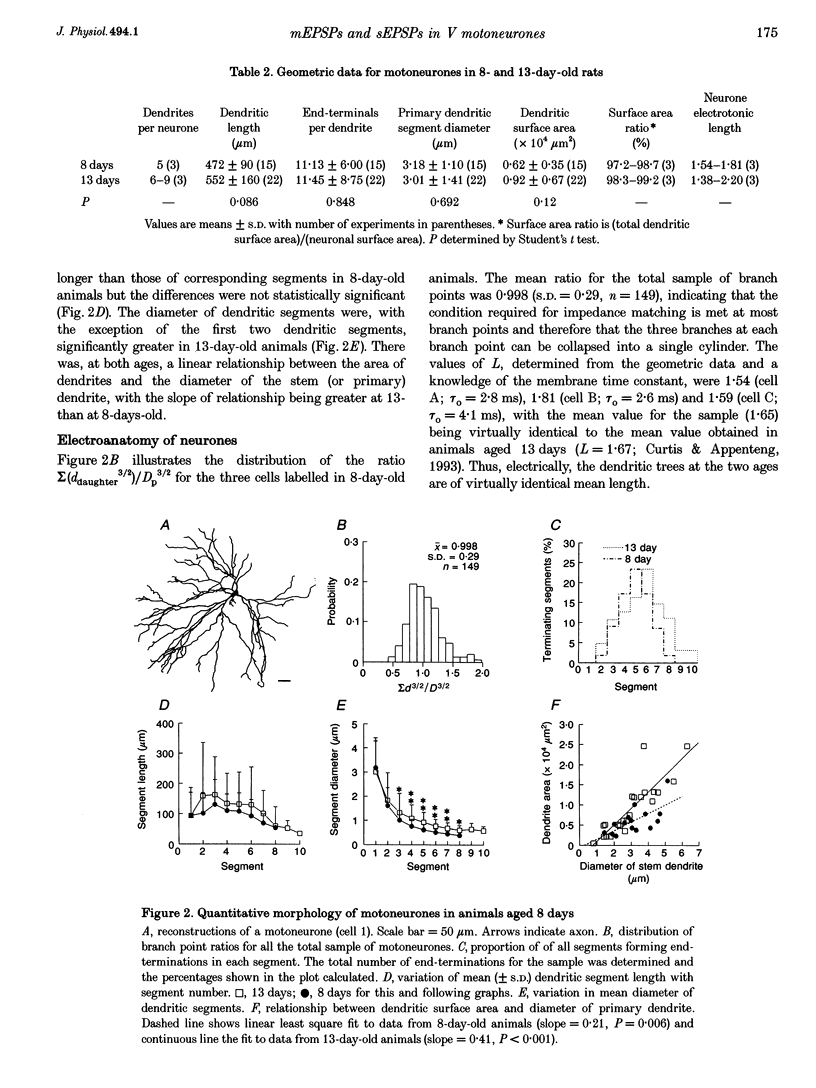
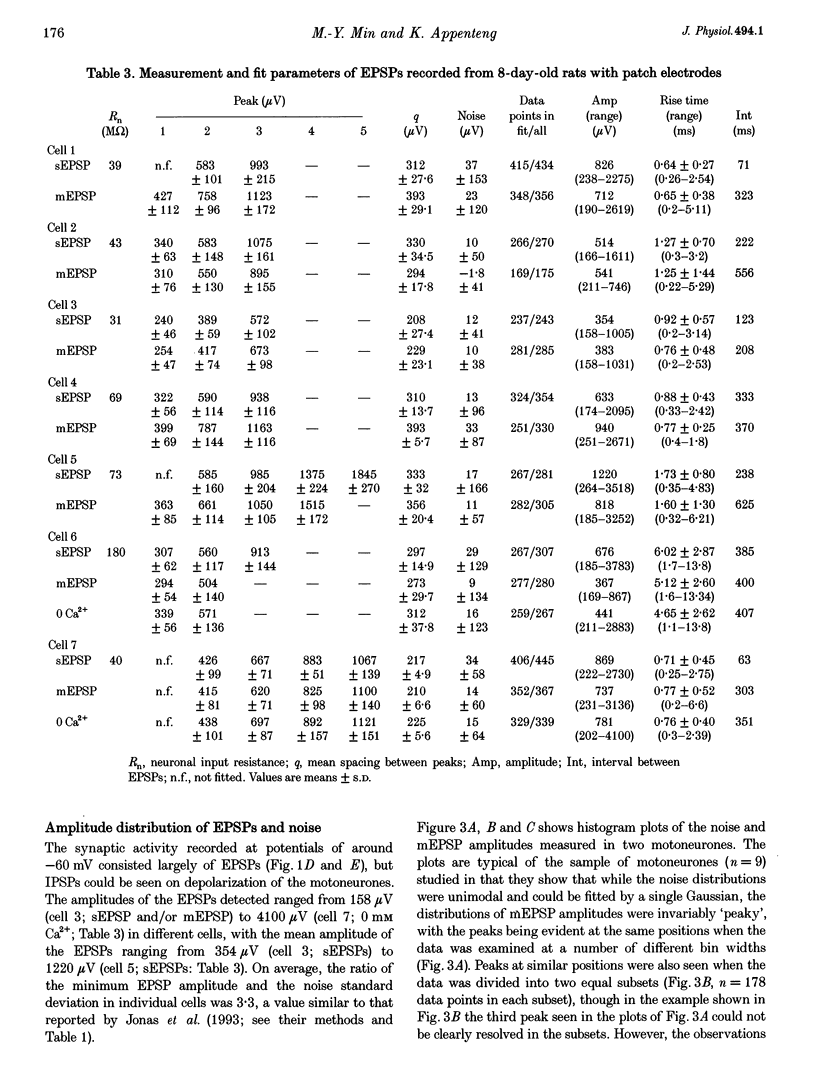
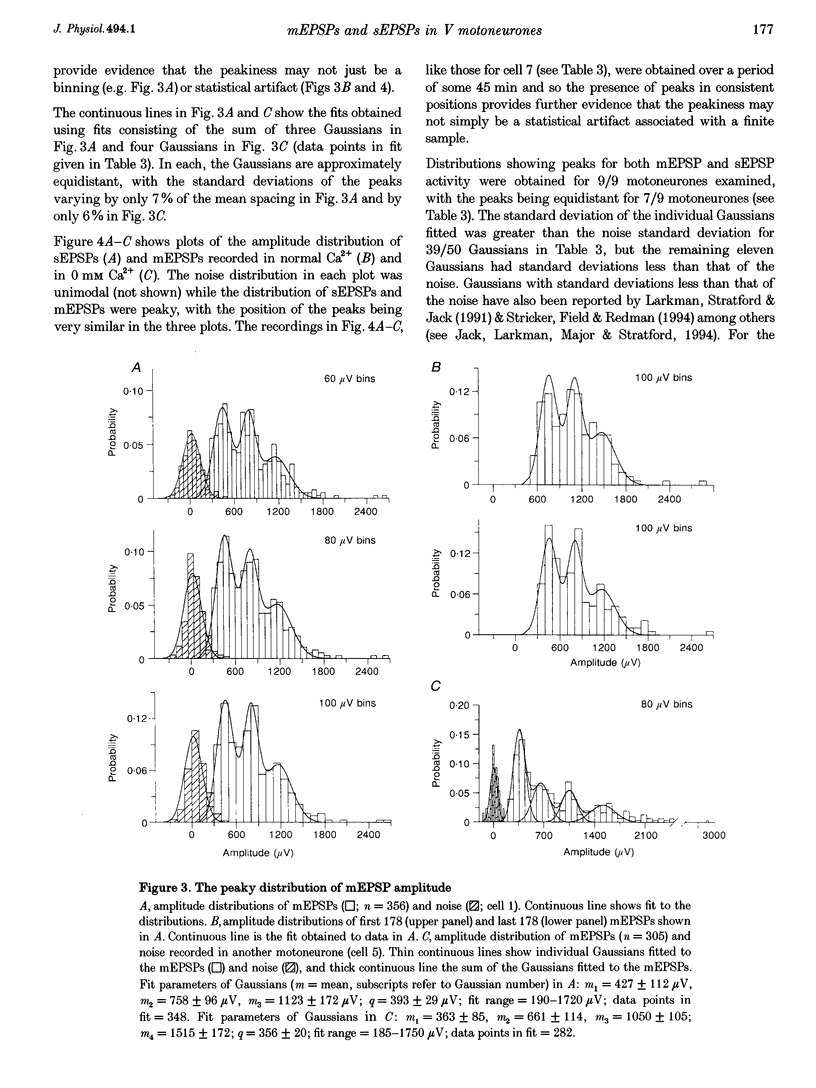
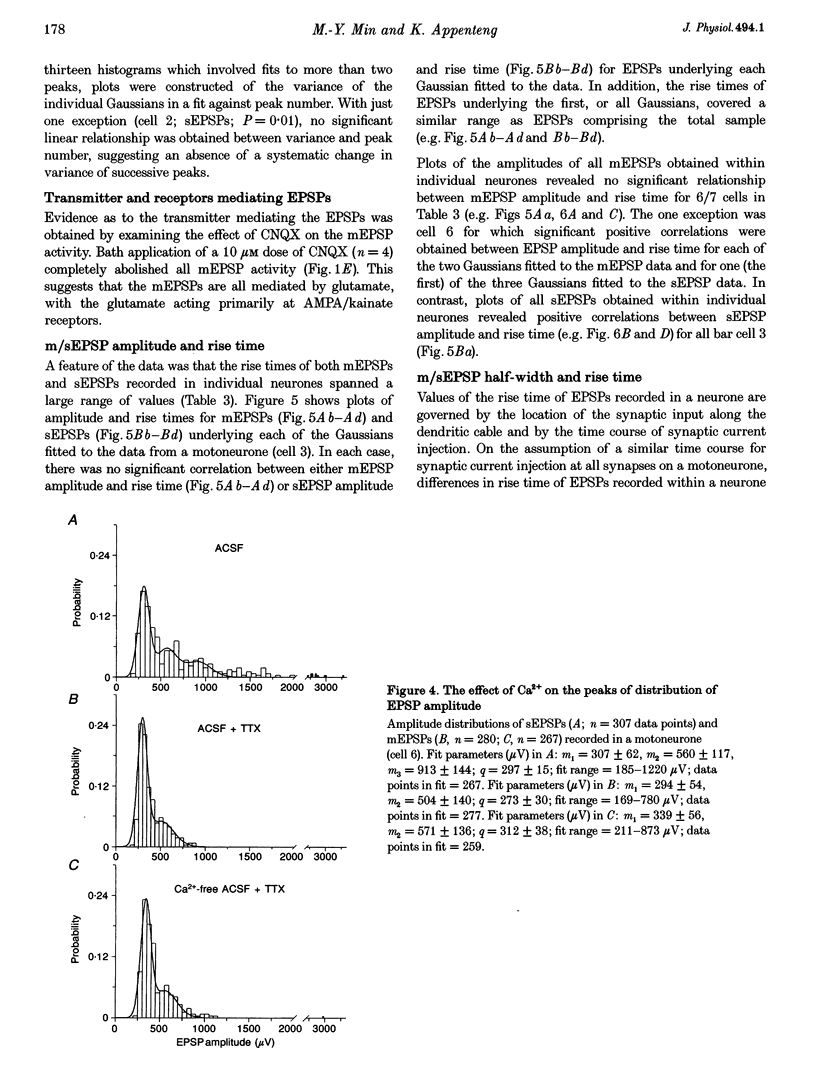
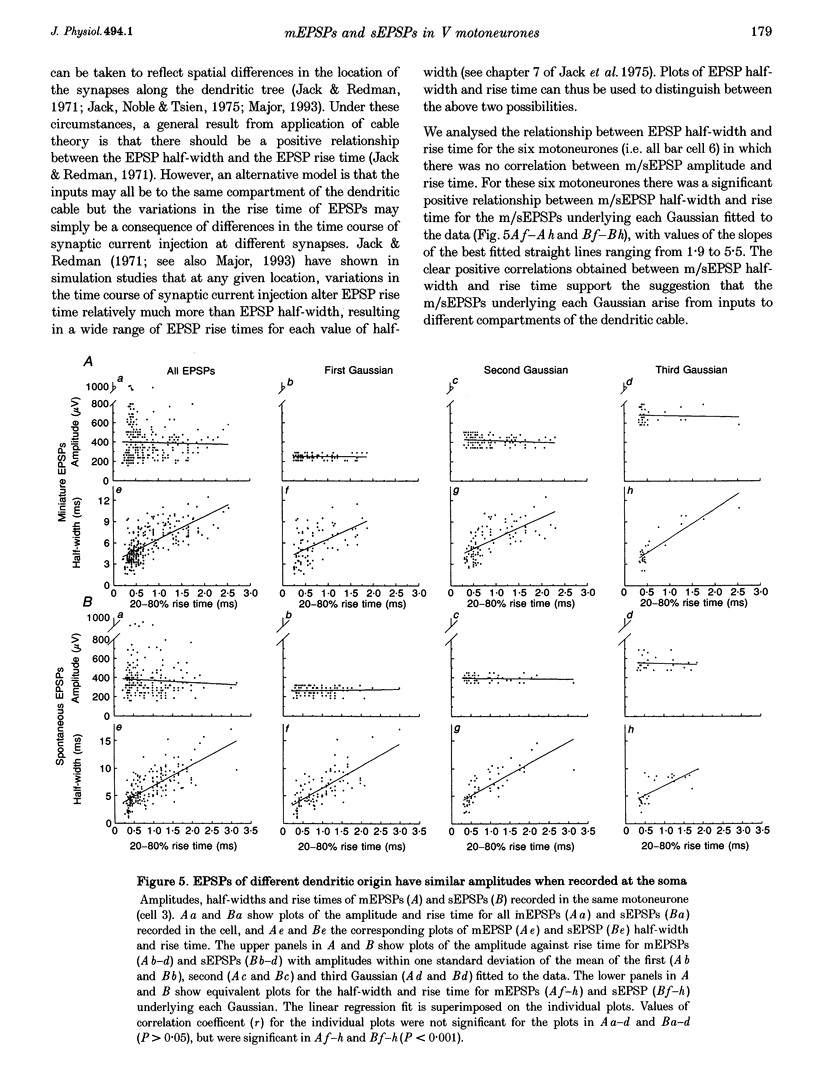
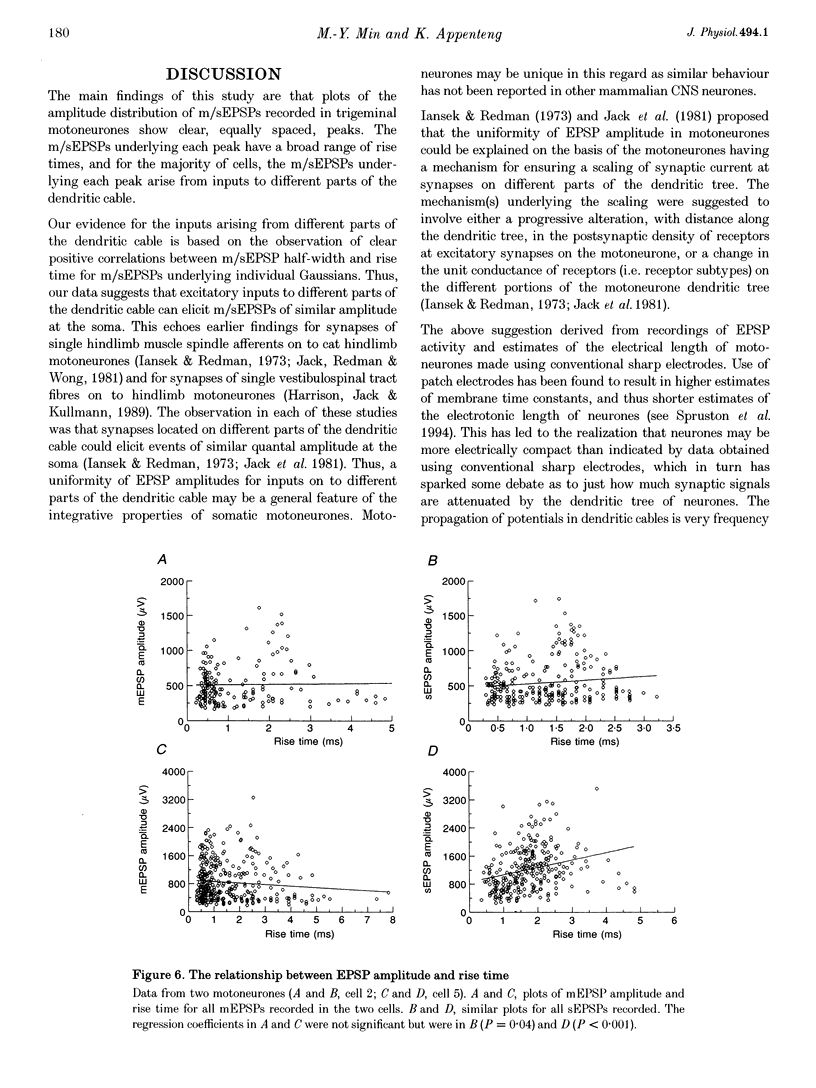
1. The whole-cell variant of the patch recording method has been used to obtain voltage recordings from trigeminal motoneurones in tissue slices (500 microns thick) taken from rats aged 8 days. Membrane properties (input resistance, membrane time constant and rheobase, i.e. threshold current required to elicit an action potential) of the motoneurones were determined and recordings made of the (untriggered) EPSP activity. 2. Untriggered EPSP activity was recorded in standard artificial cerebrospinal fluid (ACSF), ACSF with added tetrodotoxin (TTX) and in nominally Ca(2+)-free ACSF with added TTX. In each case the amplitude distributions of single EPSPs were peaky and could be fitted by a model consisting of the sum of equidistant Gaussians (n = 7/9 cells). In contrast, the amplitude distribution of the noise was always unimodal. 3. All EPSP activity recorded in the presence of TTX was abolished by addition of 6-cyano-7-nitroquinoxaline-2-3-dione (CNQX; 10 microM), suggesting the activity was all mediated by glutamate acting primarily at AMPA/kainate receptors. 4. In the majority of cases, there was no correlation between the amplitude of EPSPs underlying each Gaussian and the EPSP rise time but there was a positive correlation between the EPSP half-width and EPSP rise time. The rise times of EPSPs underlying the first, and all, fitted Gaussians were similar to that for the total sample of EPSPs in each motoneurone. Taken together, this suggests that the EPSPs underlying each Gaussian arise from inputs to different dendritic compartments, and that the range of compartments is similar for EPSPs underlying successive Gaussians. 5. Two conclusions are drawn. First, EPSPs of different dendritic origin have similar amplitudes at the soma. Second, the multimodal distribution of EPSP amplitudes recorded in the presence of TTX raises the possibility that individual boutons may contain multiple release sites, with each perhaps operating on a separate functional group of postsynaptic receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Curtis J. C., Appenteng K. The electrical geometry, electrical properties and synaptic connections onto rat V motoneurones in vitro. J Physiol. 1993 Jun;465:85–119. doi: 10.1113/jphysiol.1993.sp019668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood P. D., Appenteng K., Curtis J. C. Monosynaptic EPSPs elicited by single interneurones and spindle afferents in trigeminal motoneurones of anaesthetized rats. J Physiol. 1992 Sep;455:641–662. doi: 10.1113/jphysiol.1992.sp019320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jack J. J., Kullmann D. M. Monosynaptic EPSPs in cat lumbosacral motoneurones from group Ia afferents and fibres descending in the spinal cord. J Physiol. 1989 May;412:43–63. doi: 10.1113/jphysiol.1989.sp017603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K., Armstrong W. E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988 Aug;25(1):1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. The amplitude, time course and charge of unitary excitatory post-synaptic potentials evoked in spinal motoneurone dendrites. J Physiol. 1973 Nov;234(3):665–688. doi: 10.1113/jphysiol.1973.sp010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Larkman A. U., Major G., Stratford K. J. Quantal analysis of the synaptic excitation of CA1 hippocampal pyramidal cells. Adv Second Messenger Phosphoprotein Res. 1994;29:275–299. doi: 10.1016/s1040-7952(06)80021-2. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. The propagation of transient potentials in some linear cable structures. J Physiol. 1971 Jun;215(2):283–320. doi: 10.1113/jphysiol.1971.sp009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Lev-Tov A. Patch clamp analysis of excitatory synapses in mammalian spinal cord slices. Pflugers Arch. 1990 Nov;417(3):285–290. doi: 10.1007/BF00370994. [DOI] [PubMed] [Google Scholar]
- Korn H., Bausela F., Charpier S., Faber D. S. Synaptic noise and multiquantal release at dendritic synapses. J Neurophysiol. 1993 Sep;70(3):1249–1254. doi: 10.1152/jn.1993.70.3.1249. [DOI] [PubMed] [Google Scholar]
- Korn H., Sur C., Charpier S., Legendre P., Faber D. S. The one-vesicle hypothesis and multivesicular release. Adv Second Messenger Phosphoprotein Res. 1994;29:301–322. doi: 10.1016/s1040-7952(06)80022-4. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Larkman A., Stratford K., Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991 Mar 28;350(6316):344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Liu G., Feldman J. L. Quantal synaptic transmission in phrenic motor nucleus. J Neurophysiol. 1992 Oct;68(4):1468–1471. doi: 10.1152/jn.1992.68.4.1468. [DOI] [PubMed] [Google Scholar]
- Major G. Solutions for transients in arbitrarily branching cables: III. Voltage clamp problems. Biophys J. 1993 Jul;65(1):469–491. doi: 10.1016/S0006-3495(93)81039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jaffe D. B., Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci. 1994 Apr;17(4):161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Spruston N., Johnston D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol. 1992 Mar;67(3):508–529. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- Stricker C., Field A. C., Redman S. Probabilistic secretion of quanta at excitatory synapses on CA1 pyramidal neurons. Adv Second Messenger Phosphoprotein Res. 1994;29:323–340. doi: 10.1016/s1040-7952(06)80023-6. [DOI] [PubMed] [Google Scholar]
- Ulrich D., Lüscher H. R. Miniature excitatory synaptic currents corrected for dendritic cable properties reveal quantal size and variance. J Neurophysiol. 1993 May;69(5):1769–1773. doi: 10.1152/jn.1993.69.5.1769. [DOI] [PubMed] [Google Scholar]
- Walmsley B. Central synaptic transmission: studies at the connection between primary afferent fibres and dorsal spinocerebellar tract (DSCT) neurones in Clarke's column of the spinal cord. Prog Neurobiol. 1991;36(5):391–423. doi: 10.1016/0301-0082(91)90017-u. [DOI] [PubMed] [Google Scholar]


