Abstract
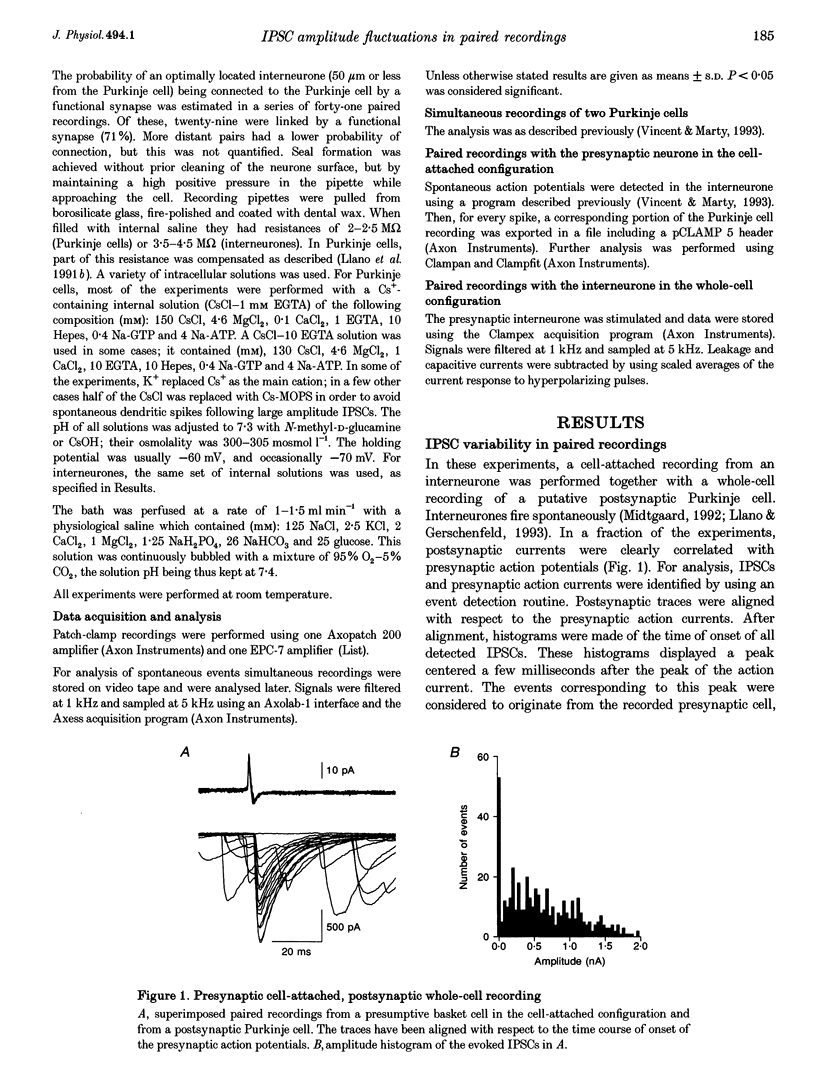
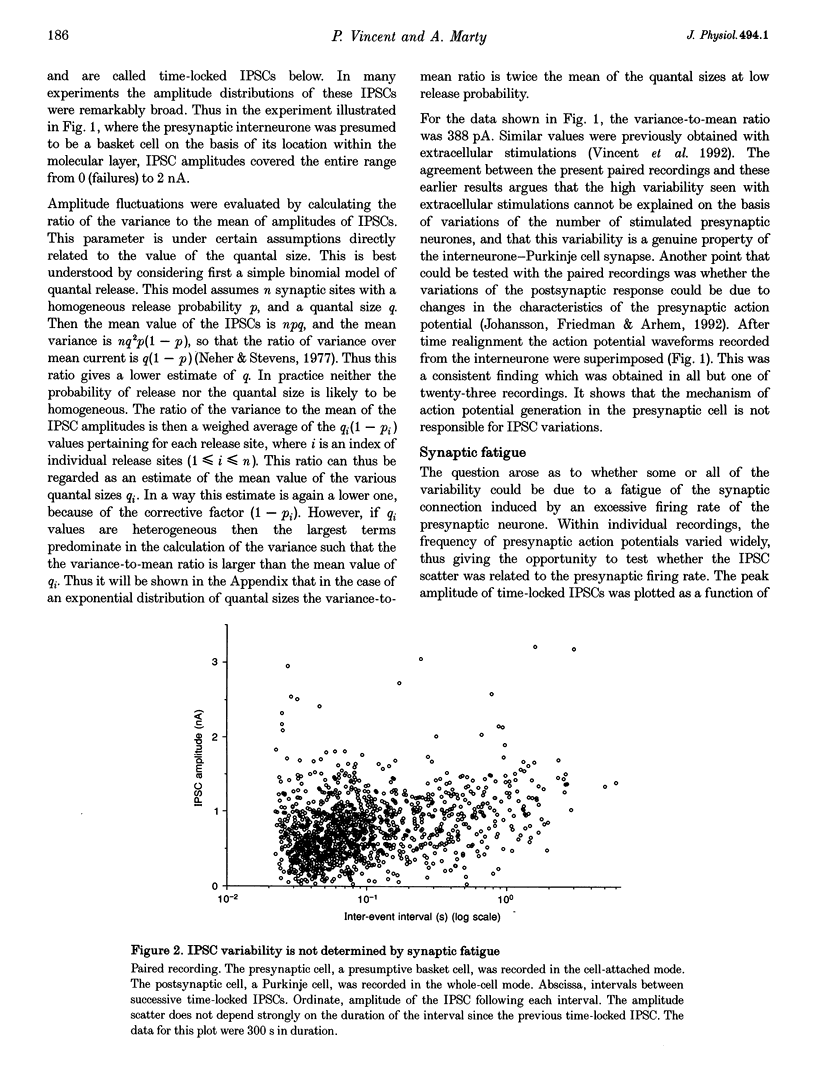
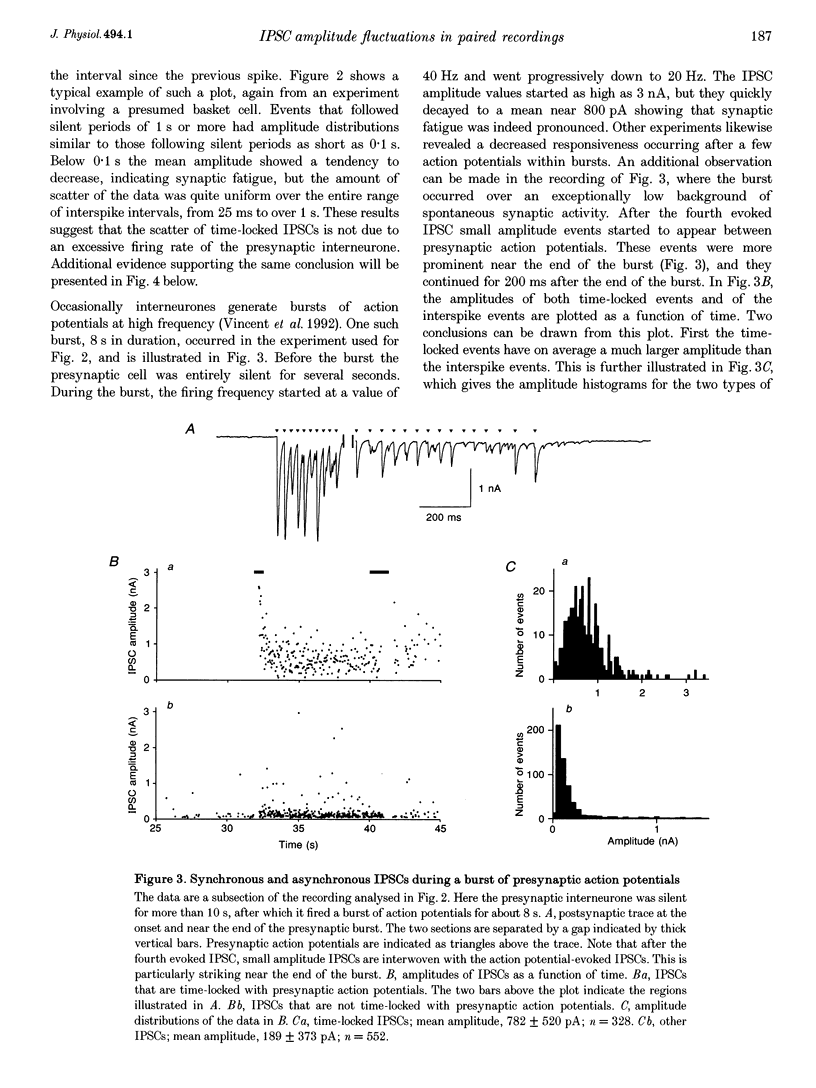
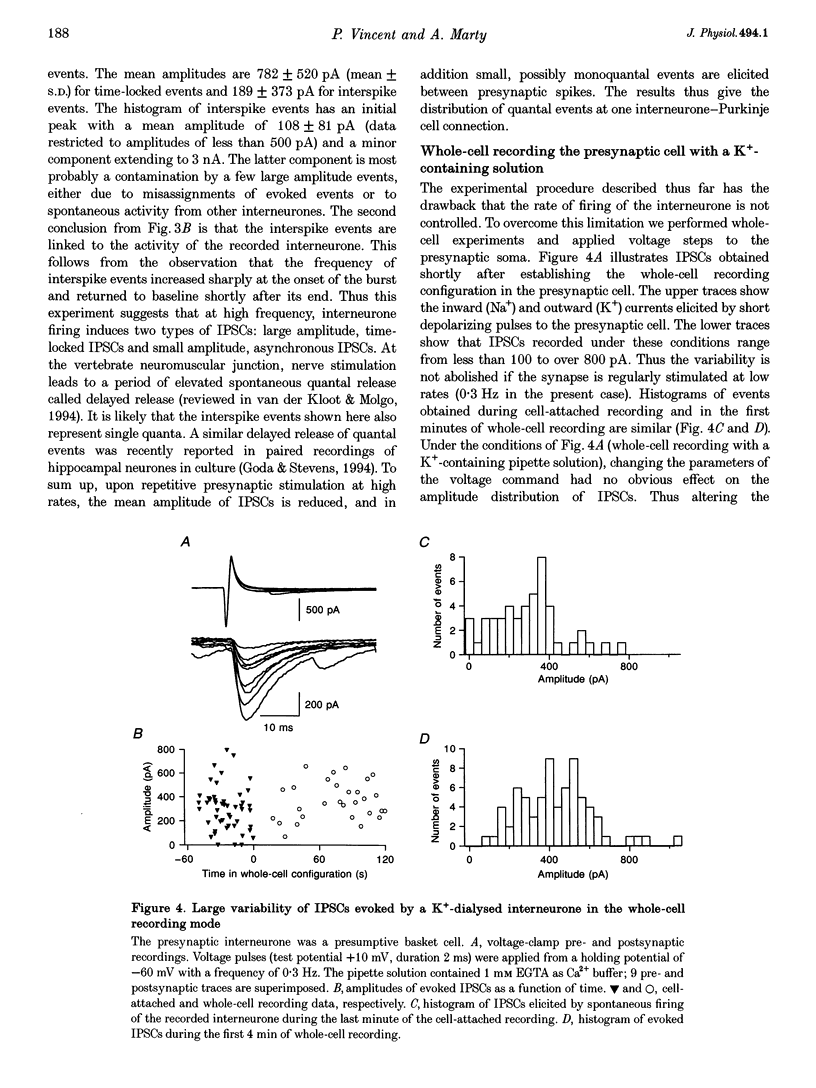
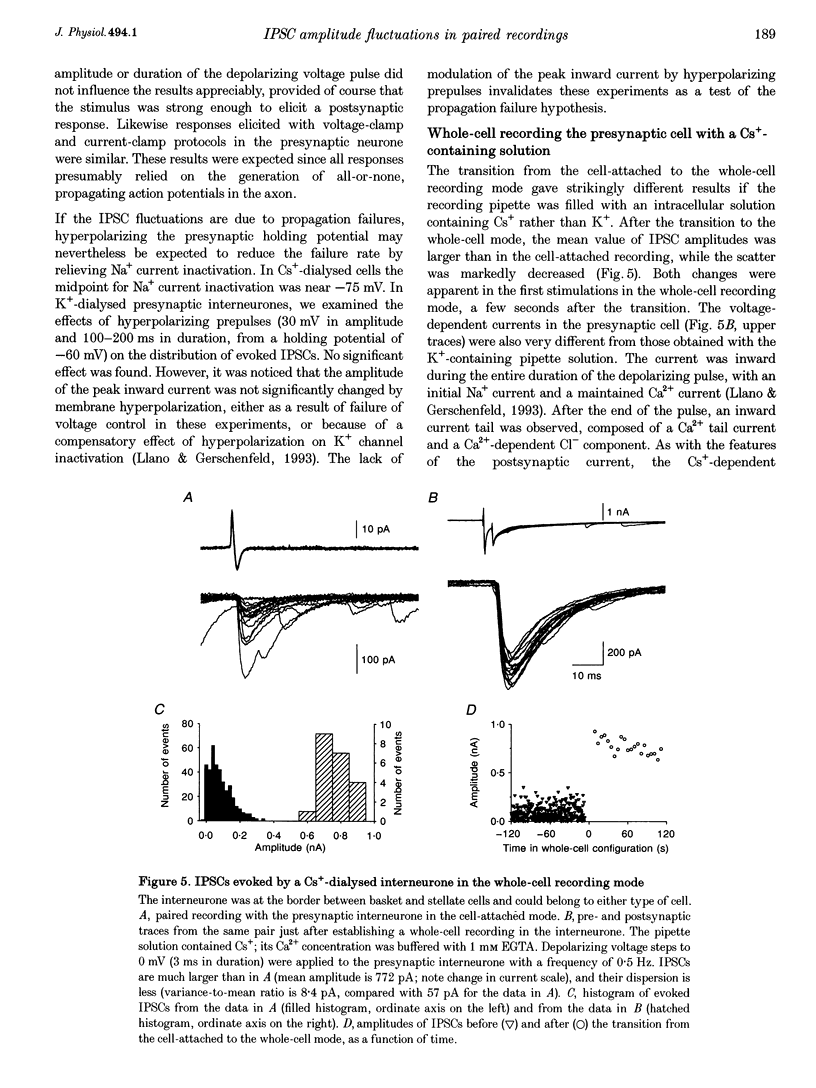
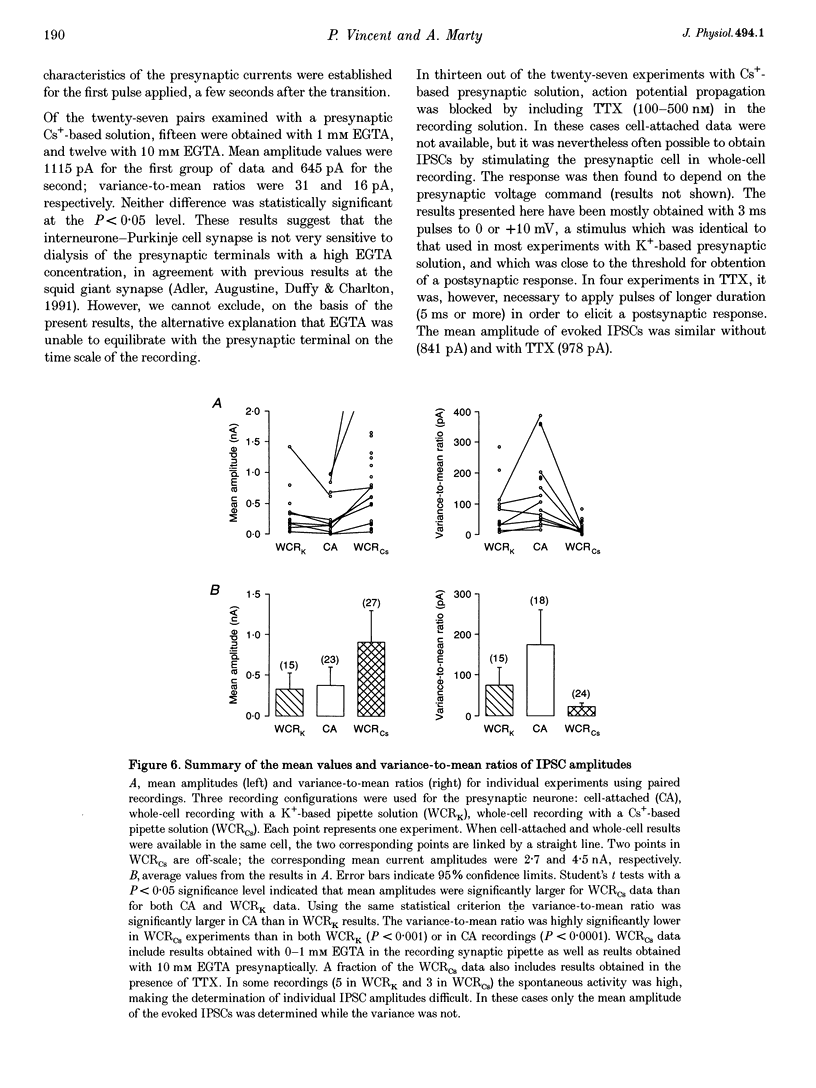
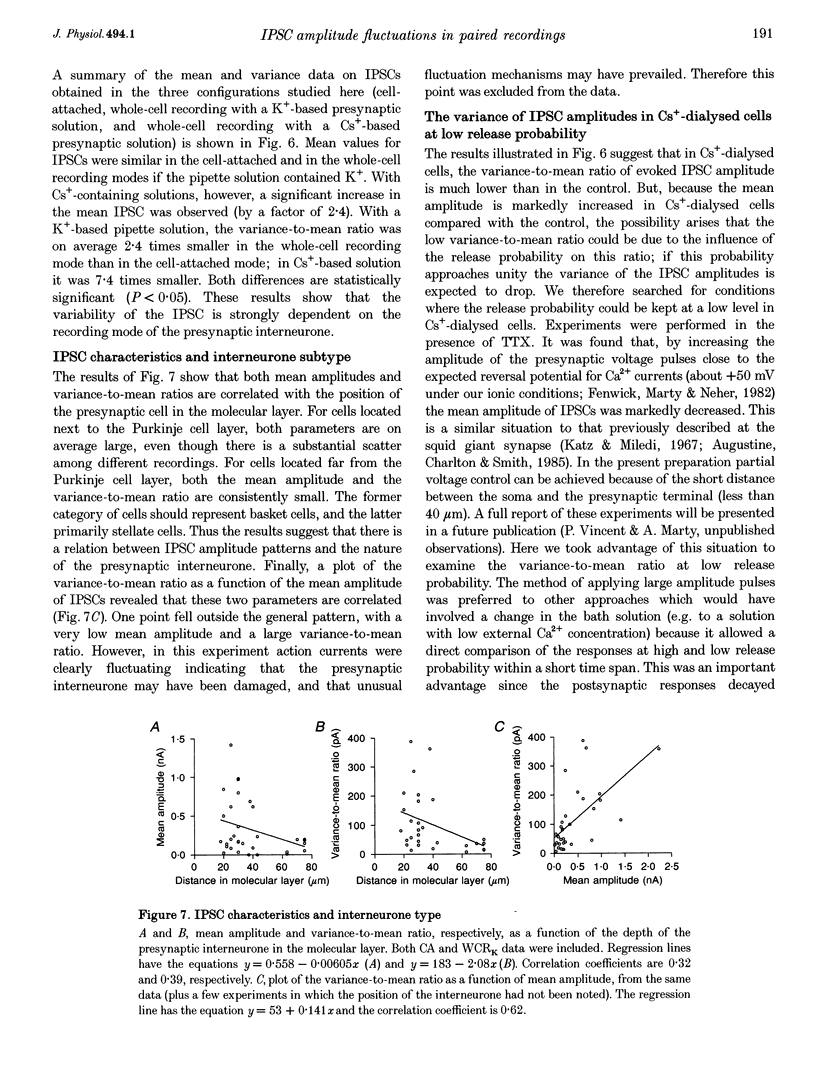
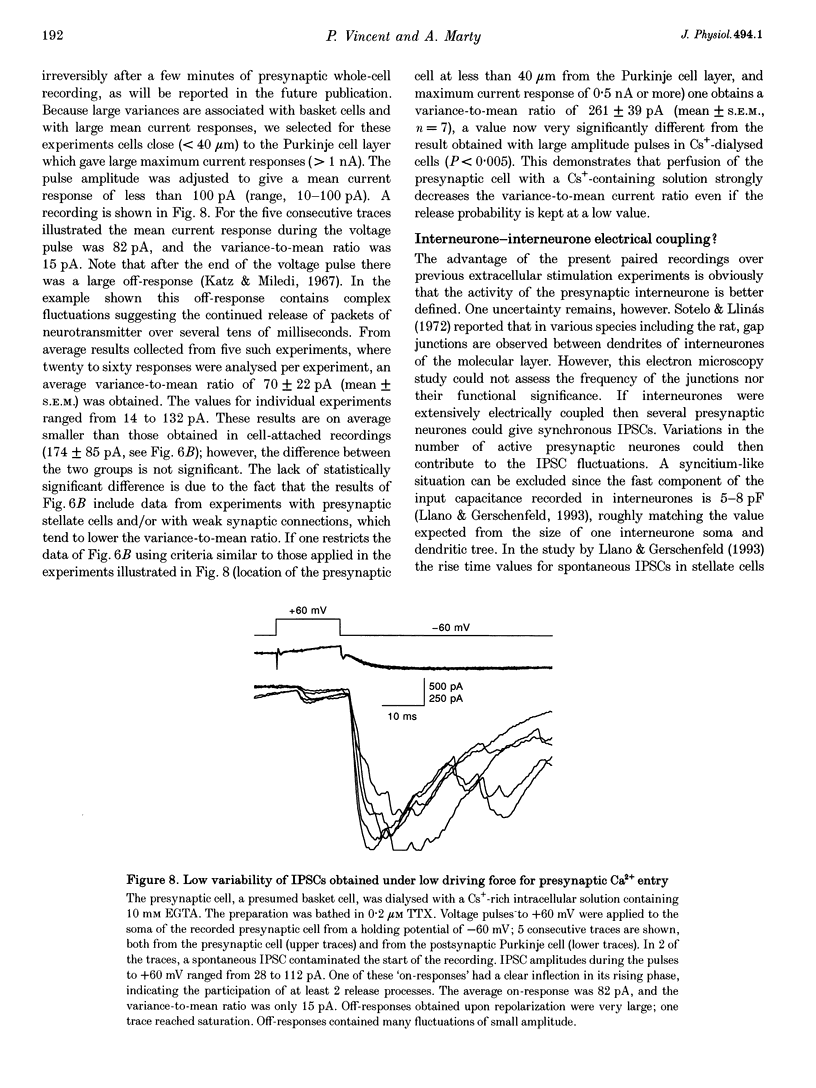
1. Paired patch-clamp recordings were performed in cerebellar slices to study IPSCs evoked by interneurones (stellate and basket cells) in Purkinje cells. 2. IPSCs were first examined while keeping the presynaptic cell in the cell-attached mode. In some of these experiments IPSCs had a remarkably broad amplitude distribution. Large variabilities were associated with large mean IPSC amplitudes and were preferentially obtained with presynaptic basket cells. Action currents recorded from the presynaptic interneurone in the cell-attached mode were reproducible indicating that the variability occurs downstream of action potential generation. 3. The variability of IPSC amplitudes was observed independently of the instantaneous firing rate of the presynaptic cell, and could therefore not be ascribed to synaptic fatigue. 4. Highly variable IPSC amplitude were also obtained if the presynaptic interneurone was placed in whole-cell recording with a potassium-based intracellular solution. 5. If the recording pipette were filled with Cs+ ions a large increase in the mean of the IPSCs was observed within seconds after establishing the whole-cell recording. In Cs(+)-dialysed cells it was possible to modulate the mean IPSC amplitudes by altering the characteristics of the presynaptic stimulation. IPSC amplitudes obtained in response to presynaptic voltage pulses to 0 mV were large and had little scatter. IPSCs obtained in response to pulses near +60 mV, close to the reversal potential of presynaptic Ca2+ currents, had a much lower mean amplitude and also had little scatter. Thus presynaptic application of Cs+ ions both increases the mean amplitude and decreases the variability of the postsynaptic response. 6. To test whether interneurones could be coupled by electrical synapses, paired recordings were performed from neighbouring interneurones. No correlation was found between the firing patterns of such paired recordings, indicating that electrical coupling among presynaptic neurones is not responsible for large IPSC fluctuations as recorded in Purkinje cells. 7. Finally, IPSC fluctuations were investigated in paired recordings from two Purkinje cells. IPSCs corresponding to the activity of common interneurones were identified on the basis of temporal correlation. By plotting the amplitudes of such common IPSCs in one cell against those obtained simultaneously in the other cell, the pattern of IPSCs due to a single presynaptic neurone could be identified. These results show that fluctuations of IPSCs due to the same interneurone in one postsynaptic Purkinje cell are independent of those occurring in another Purkinje cell. 8. The results indicate that the major source of fluctuations is localized within the axonal arborization of presynaptic interneurones. The results with presynaptic Cs+ require that the fluctuations involve the concerted release of several presynaptic vesicles. Two possible mechanisms for such multiquantal events are discussed: fluctuations in presynaptic depolarization, and fluctuations in a regenerative Ca2+ amplification mechanism.
Full text
PDF

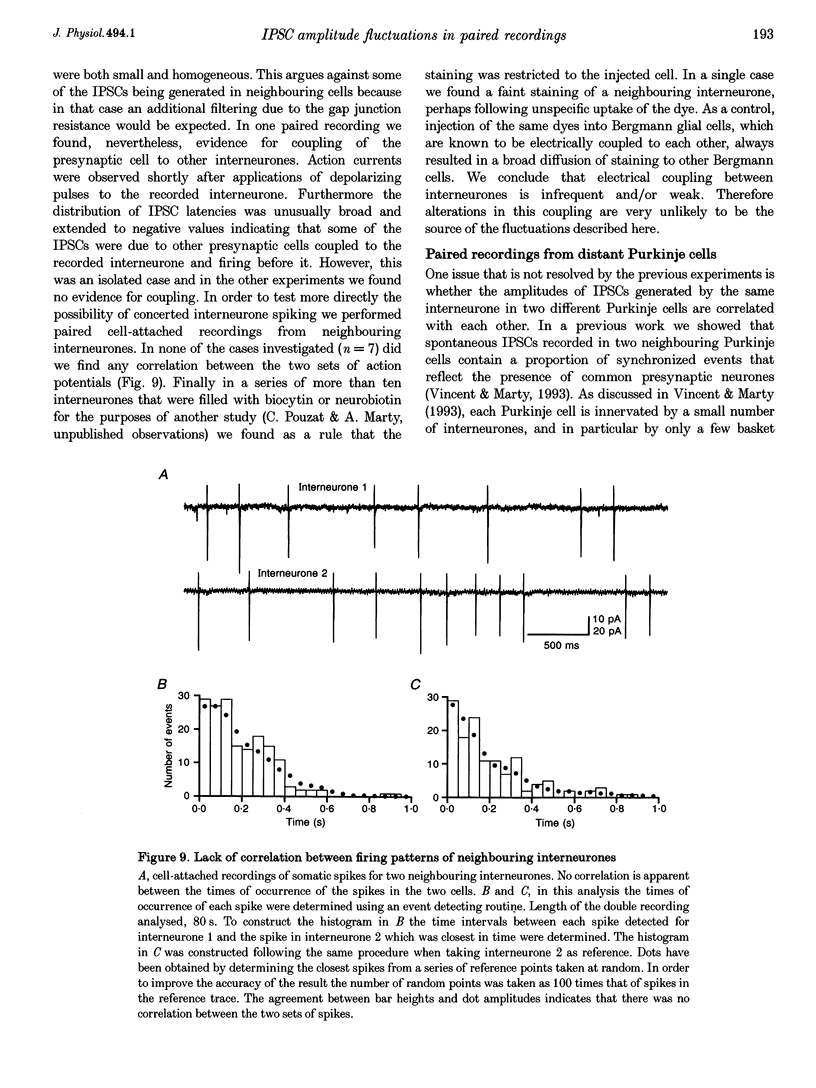
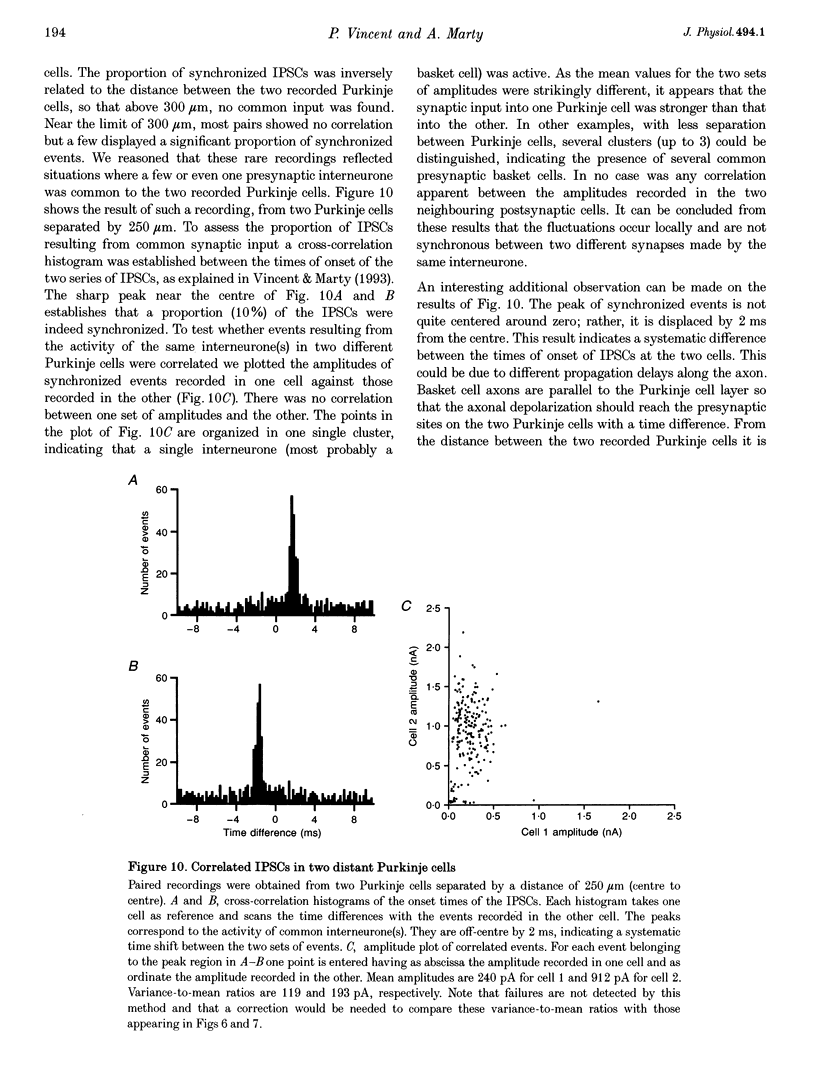





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Pitler T. A. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995 Aug;18(8):333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- Allen C., Stevens C. F. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. Intermittent conduction in the spinal cord. J Physiol. 1935 Aug 22;85(1):73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M. Quantal analysis of synaptic transmission in the central nervous system. Curr Opin Neurobiol. 1994 Jun;4(3):360–365. doi: 10.1016/0959-4388(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Borst J. G., Lodder J. C., Kits K. S. Large amplitude variability of GABAergic IPSCs in melanotropes from Xenopus laevis: evidence that quantal size differs between synapses. J Neurophysiol. 1994 Feb;71(2):639–655. doi: 10.1152/jn.1994.71.2.639. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Deschênes M., Landry P. Axonal branch diameter and spacing of nodes in the terminal arborization of identified thalamic and cortical neurons. Brain Res. 1980 Jun 9;191(2):538–544. doi: 10.1016/0006-8993(80)91302-5. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y., Stevens C. F. Two components of transmitter release at a central synapse. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Spira M. E., Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973 Dec 21;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Johansson S., Friedman W., Arhem P. Impulses and resting membrane properties of small cultured rat hippocampal neurons. J Physiol. 1992 Jan;445:129–140. doi: 10.1113/jphysiol.1992.sp018915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Adrenaline and failure of neuromuscular transmission. Nature. 1957 Oct 19;180(4590):814–815. doi: 10.1038/180814b0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Gerschenfeld H. M. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. J Physiol. 1993 Aug;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991 Apr;6(4):565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Llano I. Modulation of inhibitory synapses in the mammalian brain. Curr Opin Neurobiol. 1995 Jun;5(3):335–341. doi: 10.1016/0959-4388(95)80046-8. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J Physiol. 1989 Dec;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midtgaard J. Stellate cell inhibition of Purkinje cells in the turtle cerebellum in vitro. J Physiol. 1992 Nov;457:355–367. doi: 10.1113/jphysiol.1992.sp019382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R. Variation in strength of inhibitory synapses in the CA3 region of guinea-pig hippocampus in vitro. J Physiol. 1990 Dec;431:659–676. doi: 10.1113/jphysiol.1990.sp018353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972 Nov;35(6):903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., Konnerth A. GABA-mediated synaptic transmission in neuroendocrine cells: a patch-clamp study in a pituitary slice preparation. Pflugers Arch. 1992 Jul;421(4):364–373. doi: 10.1007/BF00374225. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Llinás R. Specialized membrane junctions between neurons in the vertebrate cerebellar cortex. J Cell Biol. 1972 May;53(2):271–289. doi: 10.1083/jcb.53.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W., Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994 Oct;74(4):899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Vincent P., Armstrong C. M., Marty A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J Physiol. 1992 Oct;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Marty A. Neighboring cerebellar Purkinje cells communicate via retrograde inhibition of common presynaptic interneurons. Neuron. 1993 Nov;11(5):885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]


