Abstract
Background
Microvascular ultrasound imaging techniques such as Angio PLanewave UltraSensitive (Angio-PL.U.S.) have been used to detect microvascular blood flow in various organs and tissues. However, the advantage of Angio-PL.U.S. for assessing muscle microvascularity over other non-invasive imaging modalities has not been investigated. This cross-sectional study compared ultrafast Angio-PL.U.S. and conventional color Doppler flow imaging (CDFI) techniques for assessing intramuscular blood perfusion.
Methods
Forty-five older adults participated (age = 59.1 ± 7.6). The vascularity index (VI) was used to quantify intramuscular blood flow of the bilateral biceps brachii (BB) and medial gastrocnemius (MG). Intra-limb (difference in VI between CDFI and Angio-PL.U.S. techniques) and inter-limb differences [percent side-to-side differences (%SSD) in VI between dominant and non-dominant sides] were compared using Wilcoxon Signed Ranks and Mann-Whitney U tests, respectively. Associations between techniques were assessed using Spearman’s rho (ρ).
Results
No significant differences were observed between dominant and non-dominant BB (p ≥ 0.053) and MG (p ≥ 0.756) for both CDFI-VI and Angio-PL.U.S.-VI. Only VI measures for the non-dominant BB demonstrated significant intra-limb difference between techniques (p = 0.002). A significant %SSD between techniques was observed for BB (p = 0.022) but not MG (p = 0.225). Strong to very strong correlations were observed between CDFI-VI and Angio-PL.U.S.-VI across all muscles (ρ = 0.616–0.814, p ≤ 0.001).
Conclusion
Ultrafast Angio-PL.U.S. and conventional ultrasound imaging techniques were comparable when used in conjunction with the VI for quantifying resting intramuscular blood flow. Angio-PL.U.S. appeared to be more sensitive in detecting bilateral disparities in upper extremity muscles. However, further research is needed to validate these findings and investigate the potential clinical utility of this technique for characterizing disease progression in populations with global or unilateral musculoskeletal tissue alterations.
Keywords: (MeSH): Ultrasonography, Doppler, Color
Introduction
The use of Doppler ultrasound imaging for blood flow quantification is integral to cardiovascular disease and cancer assessment [1]. However, the performance of conventional color Doppler is limited by sampling rate, spatial region and resolution. In many contexts, microvascular imaging using ultrafast Doppler techniques can enhance assessment accuracy [2–10]. The parallel processing approach of ultrafast Doppler is a relatively recent innovation to address the inherent constrains associated with trade-offs between flow imaging within a large field of view and more detailed flow quantification for a single location [8]. Ultrafast compound Doppler imaging involves tilted planewave insonification and the summation of backscattered signals to improve acquisition frame rates and increase sensitivity during high and low velocity flow states over a large region of interest (ROI) [11].
Current ultrafast Doppler imaging modalities such as Angio PLanewave UltraSensitive (Angio-PL.U.S.) and Superb Microvascular Imaging (SMI) have been used as non-invasive alternatives to contrast-enhanced ultrasound for the assessment of microvascular blood flow in breast lesions [2, 3], thyroid nodules [4, 5], carotid atherosclerotic plaques [6, 7], renal cortices [8], and endoleaks following endovascular aneurism repair [9, 10]. Compared to conventional color Doppler flow imaging (CDFI), where blood flow velocity is mapped by superimposing a color-coded signal over grayscale ultrasound [12], Angio-PL.U.S. employs three-dimensional (3D) wall filtering with continuous ultrafast plane-wave sampling to generate a detailed display of low velocity flow states in small vessels [4]. Previous studies comparing SMI and CDFI have demonstrated relatively greater sensitivity in detecting malignant lesion blood flow within breast tissue [2] and thyroid nodules [5] using SMI. Similarly, Angio-PL.U.S. has also shown equivocal or greater sensitivity in detecting microvascularity, blood vessel branching, or slow flow states when compared to standard diagnostic imaging modalities [e.g., contrast-enhanced ultrasound (CEUS), computed tomography angiography] [10] and image interpretation guidelines (e.g., Thyroid Imaging Reporting And Data System) [4]. The spatio-temporal resolution of the Angio-PL.U.S. imaging mode in particular is enhanced through the combination of continuous 3D wall filtering and ultrafast image acquisition to optimize the detection of slow velocity flow while minimizing tissue motion [11]. Although SMI has been used to evaluate exercise-induced flow states in muscle [13], research involving the use of Angio-PL.U.S. for similar applications is lacking. The use of the Angio-PL.U.S. imaging technique in assessing muscle microvascular flow relative to other non-invasive blood flow imaging modalities also requires further study.
The vascularity index (VI) is a parameter derived from a Doppler ultrasound image post-processing technique which provides a semi-quantitative estimation of tissue blood perfusion. The VI has been used to estimate site-specific intramuscular blood perfusion following exercise interventions among healthy young adults [13] as well as clinical populations with plantar fasciitis [14] and unilateral limb impairment (i.e., hemiparesis) [15, 16]. The measurement was initially developed by Newman et al. (1997) as a means grading exercise-induced permutations in muscle tissue blood flow qualitatively [17]. Ying et al. (2009) subsequently adapted and refined this measure by developing a semi-quantitative approach to estimate tissue vascularity in the bilateral lobes of the thyroid gland using high-sensitivity power Doppler [12]. In the adapted method, VI within a given ROI is calculated as a ratio of color to total pixels. This semi-quantitative method has demonstrated a strong association with Newman’s qualitative grading scale approach when measuring the same construct in patients with plantar fasciitis (r = 0.70) [14].
In this study, we compared the use of CDFI and Angio-PL.U.S. techniques in conjunction with a VI post-processing technique for evaluating intramuscular blood perfusion in the upper and lower extremities of older adults. Inter-limb differences in VI between techniques were also compared.
Methods
Participants
A total of 45 community-dwelling adults were recruited through convenience sampling between January and March of 2019. Informed consent was obtained on the day of the initial functional assessment prior to data collection. The study was approved by the Human Subjects Ethics Sub-committee of the University on April 10, 2018 (HSEARS20171212003). All procedures were conducted in accordance with the Helsinki Declaration for human experiments and reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [18]. Inclusion criteria were as follows: (1) adults with no significant musculoskeletal impairment or pathology (e.g., muscular dystrophy), (2) no other serious contraindications for study participation, and (3) able to provide informed consent and comply with study procedures.
Procedures
Assessments were conducted in the university imaging laboratory. Each assessment session was approximately 1–1.5 h in duration and performed within the same room kept at an average temperature of 22 °C. Ultrasound measures were performed by the same operator with 5 years of musculoskeletal and vascular imaging experience. Prior to the ultrasound assessment, demographic information and medical history were obtained. Cognition was assessed using the 10-item Abbreviated Mental Test (AMT), with scores ≤ 6 indicating abnormal cognitive function [19]. The AMT has been validated among geriatric patients [19] and older adults in residential care homes [20]. General physical activity level was assessed using the Physical Activity Scale for the Elderly (PASE), with higher scores indicative of higher activity level. Scores were also interpreted according to tertile stratifications of general activity level described by Curcio et al. (2019) (i.e., sedentary: PASE = 0–40, light physical activity: PASE = 41–90, moderate to intense physical activity: PASE > 90) [21]. The scale has been previously validated using accelerometry [22], actigraph monitoring [23], and has demonstrated fair to moderate association with other clinically relevant measures of physical function in the elderly [24]. Body mass indices (BMI) of 18.5–24.9, 25.0-29.9, and ≥ 30 were considered healthy, overweight, or obese, respectively [25]. Upper and lower limb dominance were determined according to preferential use during daily activities (i.e., hand-writing, eating with cutlery) and by a ball kicking task, respectively [26].
Following the PASE, AMT and limb dominance assessments, participants rested on the examination table for approximately 10 min prior to the ultrasound assessment. The assessment sequence for each participant (i.e., dominant or non-dominant side, upper or lower limb) was randomized to minimize the order effect. Participants were instructed to remain as relaxed as possible. Surface electromyography was used to confirm passive muscle status (Bagnoli EMG system, Delsys Inc, Natick, Massachusetts, USA). An Aixplorer ultrasound unit (Aixplorer, Supersonic Imagine, Aix en-Provence, France), coupled with a linear array transducer (4–15 MHz, SuperLinear, 15 − 4, Vermon, France) was used to measure intramuscular blood flow of the bilateral biceps brachii (BB) and medial gastrocnemius (MG). Super-compound and high penetration modes were set at a medium frame rate to optimize image resolution and visualization of anatomical boarders. To facilitate transmission and reduce probe compression during measures, a 2 mm thick gel coupling layer served as the interface between the skin and probe. The ankle joint was maintained in a neutral plantar-dorsiflexion (0⁰) fixed by an anchored device with restricted eversion, inversion and rotation and the elbow joint was maintained in 60° of flexion with the shoulder abducted in 45° using a custom arm immobilization device. The proximal one third (i.e., 33%) of the total tibia length measured from the base of the distal Achilles tendon, at the level of the inferior boarder of the lateral malleolus up to the midline of the popliteal crease, served as the measurement site for the MG [27]. The measurement site for the BB muscle was the distal third (i.e., 66%) of the total humeral length between the coracoid process of the scapula and the crease of cubital fossa on the radial side [28]. The probe was placed on the BB or MG muscle in a transverse orientation.
The method for determining VI was adapted from a protocol described by Huang et al. (2020) [15]. Using the CDFI mode to map Doppler signal strength from flow movement, the ROI was placed within the borders of the muscle fascia and standardized according to frame rate (11 Hz), pulse repetition frequency (6 cm/s), greyscale (50%), and color gain range (70–85%). To detect low velocity flow states of small vessels within the ROI, the color gain was increased to 85% and gradually reduced until background color noise was suppressed or reached the lower gain limit (70%) for each participant. Three CDFI recordings, each approximately 5 s in duration, were captured. Using the same ROI, probe location and settings, three additional recordings were captured using the Angio-PL.U.S. imaging mode. Using a pre-defined system setting, the pulse repetition frequency scale was reduced to 4 cm/s in Angio-PL.U.S. imaging mode in order to optimize visualization of low velocity microvascular blood flow. Color gain was then kept the same for subsequent recordings performed on the opposing limb. Custom scripts were used for all image and data processing (Matlab software, version R2024a, Mathworks, Natick, Massachusetts, USA). These procedures are described in detail in previous studies [12, 16].
Sample size estimation
Sample size calculation was performed using G*Power software (version 3.1, Heinrich Heine Universitat, Dusseldorf, Germany) [29]. A previous study by Yang et al. (2019) comparing ultrafast Doppler (i.e., SMI) and contrast enhanced ultrasound techniques to assess neovascularization of unstable carotid artery plaques in elderly individuals demonstrated a strong, positive correlation between SMI level and contrast enhancement intensity (Spearman’s ρ = 0.737, p = 0.001) [6]. Assuming a more conservative correlation of 0.5 (medium effect size), a power of 0.90, and an alpha level of 0.05 (2-tailed), it was estimated that a minimum of 34 participants was required.
Statistical analysis
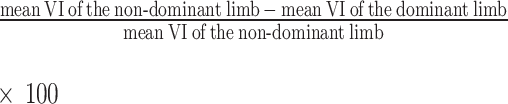
All statistical analyses were conducted using SPSS software (version 29.0, SPSS Inc., Armonk, New York, USA) at a significance level of p ≤ 0.05. Homogeneity of variance and normality were determined using Levene’s and Shapiro-Wilks tests, respectively. Paired t-tests were used to compare mean differences between dominant and non-dominant limbs (i.e., inter-limb comparisons), as well as between imaging techniques for the same limb and the percent side-to-side difference (%SSD) for upper and lower limb muscles (i.e., inter-technique comparisons). Pearson’s correlation coefficients were used to assess the strength of the relationship between imaging techniques. Correlation coefficients ranging from 0.0 to 0.2, 0.2–0.4, 0.4–0.6, 0.6–0.8, and 0.8-1.0 indicated very weak, weak, moderate, strong and very strong associations, respectively [30]. An equivalent non-parametric test [i.e., Spearman’s rho (ρ), Wilcoxon Signed Ranks] was used if homogeneity of variance and normality criteria were not met. To account for multiple comparisons and reduce the potential for Type I errors, the alpha threshold was adjusted using Bonferroni correction (i.e., p ≤ 0.05/5 = 0.01). The %SSD in VI for each imaging technique and extremity (upper and lower) was calculated using the following formula:
 |
Results
Participant characteristics
A summary of participant characteristics is provided in Table 1. A total of 45 participants completed the study (men = 31, women = 14, age = 59.1 ± 7.6). On average, participants reported moderate to high levels of general physical activity (PASE = 154.5 ± 83.3), had normal cognitive function (AMT = 9.9 ± 0.4), and were considered to have a healthy BMI (23.4 ± 2.7).
Table 1.
Participant characteristics
| N = 45 | % | |
|---|---|---|
| Demographics | ||
| Sex (men/women), n | 31 / 14 | 68.9 / 31.1 |
| Age (years) | 59.1 ± 7.6 | - |
| Height (cm) | 166.3 ± 8.6 | - |
| Weight (kg) | 64.6 ± 10.4 | - |
| Body mass index (kg/m2) | 23.4 ± 2.7 | - |
| Arm dominance (left/right/equivalent), n | 2 / 43 / 0 | 4.4 / 95.6 |
| Leg dominance (left/right/equivalent), n | 2 / 43 / 0 | 4.4 / 95.6 |
| Alcohol consumption, n | 17 | 37.8 |
| Drinks per day, n | 0.1 ± 0.2 | - |
| Tobacco use, n | 9 | 20.0 |
| Packs per day, n | 0.6 ± 0.3 | - |
| AMT | 9.9 ± 0.4 | |
| PASE | 154.5 ± 83.3 | - |
| Comorbidity | ||
| Total number of comorbidities, n | 0.5 ± 0.9 | - |
| Hypertension, n | 13 | 28.9 |
| Hyperlipidemia, n | 5 | 11.1 |
| Diabetes mellitus, n | 4 | 8.9 |
| Medication | ||
| Total number of medications, n | 0.8 ± 1.0 | - |
| Antihypertensive agents, n | 12 | 26.7 |
| Hypolipidemic agents, n | 5 | 11.1 |
| Hypoglycemic agents, n | 4 | 8.9 |
| Anticoagulants, n | 1 | 2.2 |
| PPI/ gastric agent, n | 2 | 4.4 |
Abbreviations: AMT = Abbreviated Mental Test, PASE = Physical Activity Scale for the Elderly, PPI = Proton Pump Inhibitor
Inter-limb comparisons
No significant differences were observed between dominant and non-dominant muscles of the upper (p ≥ 0.053) and lower extremities (p ≥ 0.756) for both CDFI-VI and Angio-PL.U.S.-VI. A comparative results summary is provided in Table 2.
Table 2.
Inter-limb and inter-technique vascularity index comparisons
| CDFI | Angio-PL.U.S. | Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | %SSD | Mean ± SD | %SSD | p a | p b | p c | p d | |
| Biceps Brachii | ||||||||
| Non-Dominant VI | 0.55 ± 0.31 | -2.43 ± 43.81 | 0.46 ± 0.36 | -77.79 ± 129.57 | 0.167 | 0.053 | 0.022* | 0.002*† |
| Dominant VI | 0.51 ± 0.29 | 0.57 ± 0.35 | 0.170 | |||||
| Medial Gastrocnemius | ||||||||
| Non-Dominant VI | 0.99 ± 0.41 | -12.11 ± 52.19 | 0.93 ± 0.48 | -27.17 ± 80.09 | 0.950 | 0.756 | 0.225 | 0.312 |
| Dominant VI | 1.02 ± 0.46 | 0.97 ± 0.58 | 0.147 | |||||
|
* Statistically significant difference (p ≤ 0.05) † Statistically significant difference after adjusting for multiple comparisons (p ≤ 0.01) a Inter-limb comparisons for VI obtained using CDFI (Non-Dominant vs. Dominant) (Wilcoxon Signed Ranks) b Inter-limb comparisons for VI obtained using Angio-PL.U.S. (Non-Dominant vs. Dominant) (Wilcoxon Signed Ranks) c Percent side-to-side difference comparisons between imaging techniques (CDFI vs. Angio-PL.U.S.) (Wilcoxon Signed Ranks) d Intra-limb VI comparisons between imaging techniques (CDFI vs. Angio-PL.U.S.) (Wilcoxon Signed Ranks) Abbreviations: %SSD = Percent Side-to-Side Difference, Angio-PL.U.S. = Angio PLanewave UltraSensitive Imaging, CDFI = Color Doppler Flow Imaging, VI = Vascularity Index | ||||||||
Inter-technique comparisons
Only VI measures for the non-dominant BB demonstrated a significant intra-limb difference between imaging techniques (p = 0.002). For BB muscles, the %SSD in Angio-PL.U.S.-VI was significantly greater than %SSD in CDFI-VI (p = 0.022). No significant %SSD was observed between imaging techniques for MG muscles (p = 0.225) (Table 2). Visual comparisons of the VI for the BB and MG muscles using each imaging technique are provided in Figs. 1 and 2, respectively.
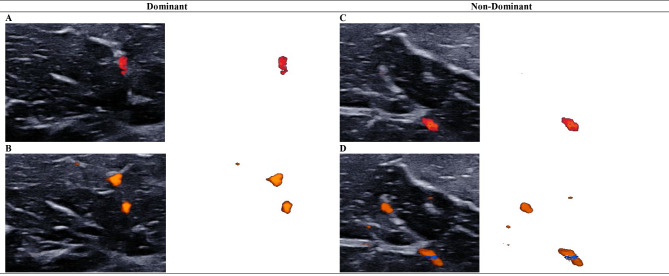
Fig. 1.
Comparing biceps brachii intramuscular blood perfusion between imaging techniques using the vascularity index
Intramuscular blood perfusion of the biceps brachii muscle was measured with color Doppler flow imaging (A, C) and Angio PLanewave UltraSensitive Imaging (B, D). Isolated color pixels were used to calculate the vascularity index for dominant (A, B) and non-dominant (C, D) biceps brachii muscles. This bilateral comparison of images obtained for a single participant indicate Angio PLanewave UltraSensitive Imaging was relatively more sensitive than color Doppler flow imaging in detecting intramuscular blood flow of the biceps brachii muscle
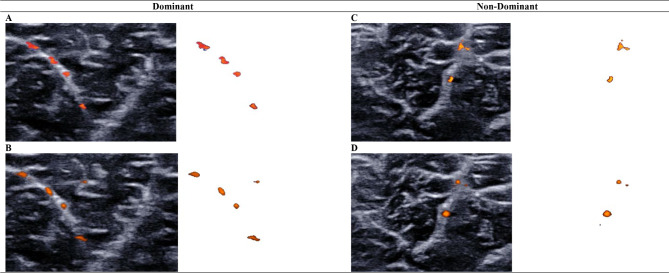
Fig. 2.
Comparing medial gastrocnemius intramuscular blood perfusion between imaging techniques using the vascularity index
Intramuscular blood perfusion of the medial gastrocnemius muscle was measured with color Doppler flow imaging (A, C) and Angio PLanewave UltraSensitive Imaging (B, D). Isolated color pixels were used to calculate the vascularity index for dominant (A, B) and non-dominant (C, D) medial gastrocnemius muscles. Color Doppler flow imaging and Angio PLanewave UltraSensitive Imaging demonstrated similar sensitivity in detecting intramuscular blood flow of the medial gastrocnemius muscle
Correlations
Correlations between the CDFI-VI and Angio-PL.U.S.-VI were positive and ranged from strong to very strong across all muscles (ρ = 0.616–0.814, p ≤ 0.001). Scatterplots depicting limb-specific correlations between imaging techniques are provided in Fig. 3.
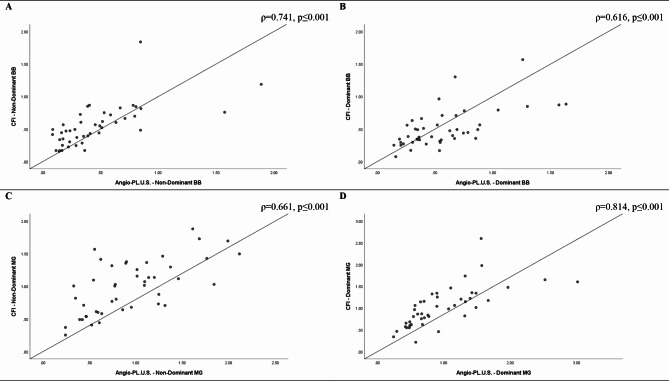
Fig. 3.
Strength of association between imaging techniques
Strong positive correlations were observed between color Doppler flow imaging and Angio PLanewave UltraSensitive Imaging techniques for the non-dominant (A) and dominant biceps brachii (B), and the non-dominant (C) and dominant medial gastrocnemius muscles (D) (ρ = 0.616–0.814, p ≤ 0.001)
Discussion
This study compared the use of Angio-PL.U.S. and CDFI in conjunction with semi-quantitative estimates of muscle tissue blood perfusion (i.e., VI) in the extremities of older adults. Overall, our results suggest these imaging techniques were comparable for quantifying resting intramuscular blood flow in upper and lower extremity muscles. Compared to CDFI, the Angio-PL.U.S. technique demonstrated relatively greater sensitivity for detecting bilateral disparities in upper extremity intramuscular blood perfusion.
Inter-limb and inter-technique comparisons
For individual limbs, only the non-dominant arm showed a significant difference in VI between techniques suggesting that intramuscular blood flow was greater in CDFI mode than Angio-PL.U.S at this site (p = 0.002). This finding is in contrast to evidence reported in previous studies comparing two similar ultrasound imaging techniques (i.e., SMI and CDFI) [2, 31]. Although the tissues measured and ultrasound techniques differed from those used in the current study, compared to the ultrafast technique (i.e., SMI), conventional CDFI demonstrated lower sensitivity for detecting microvascular blood flow in breast lesions (RSMI=0.58, RCDFI=0.41, µ = 3.71, p < 0.001) [2] and thyroid nodules (p < 0.012) [31]. On the other hand, we found a significantly larger %SSD in Angio-PL.U.S.-VI of the BB muscles in comparison to CDFI-VI (p = 0.022). Additionally, inter-limb comparisons between dominant and non-dominant BB muscles for Angio-PL.U.S.-VI were also marginally insignificant (p = 0.053) compared to the inter-limb difference observed for CDFI-VI (p = 0.756). This suggests that although our study may have been underpowered for detecting differences in VI across all measurement sites, the Angio-PL.U.S. technique appeared to demonstrate relatively greater sensitivity in detecting bilateral disparities in upper extremity intramuscular blood perfusion. Moreover, the lack of difference in intramuscular blood flow between dominant and non-dominant MG muscles may be reflective of preferential usage patterns, which are likely to be more pronounced in upper extremity muscles (i.e., habitual usage of the dominant side arm and hand) compared to lower extremity muscles in healthy older adults. Among individuals with chronic stroke, learned disuse of the hemiparetic side may contribute substantially to inter-limb disparities in intramuscular blood perfusion assessed using conventional color Doppler for both upper (z=-4.09, r = 0.35) and lower extremity muscles (z=-4.42, r = 0.38) [16]. The potential trend observed in the present study suggests that Angio-PL.U.S. may demonstrate relatively greater sensitivity in detecting unilateral differences in muscle blood flow following neurological insult. Further research involving a larger and more diverse participant sample is needed to validate the current findings and investigate the potential application of this technique in clinical contexts.
Additional factors may also influence bilateral limb disparities in intramuscular blood perfusion. Previous research has demonstrated relatively greater arterial blood flow for dominant compared to non-dominant lower limbs [32]. However, inter-limb differences in VI for lower extremity muscles were less detectable at rest in our study than the difference in intramuscular blood flow response between limbs reported in previous studies of the passive MG and rectus femoris muscles following exercise [13, 15]. Intramuscular blood flow is also associated with muscle function [15], which declines precipitously with age [33]. Reductions in muscle blood flow due to impaired nitric oxide production and microvascular damage, though poorly studied, are considered predominant pathophysiologic mechanisms leading to age-related sarcopenia [34, 35]. A study by Ditroilo et al. (2010) showed greater functional asymmetry between dominant and non-dominant muscles of the upper extremity compared to the lower extremity [36]. This suggests age-related changes in upper extremity muscle function may contribute to inter-limb differences in intramuscular blood perfusion. Moreover, most of our participants were male (n = 31, 68.9%). This may be meaningful in the context of our study population given that the onset and rate of functional decline is relatively earlier and faster for men (i.e., age 50–60) than women (i.e., age 60–65) [36]. Sex-dependent changes in muscle function may also affect limb-specific blood flow. A recent study by Tafunai et al. (2021) showed significant differences in post-occlusion femoral artery blood flow between the dominant and non-dominant legs of males (230 ± 41 vs. 209 ± 37 cm/s, p = 0.009) but not females [32]. Additional studies involving sex-balanced cohorts are needed to examine the influence of these demographic factors (i.e., age, sex) on microvascular blood flow in muscle tissue.
Correlation between techniques
To the knowledge of the authors, this is the first study to evaluate site-specific associations between ultrafast and conventional ultrasound blood flow imaging techniques. The strong relationship we observed between VI values obtained using CDFI and Angio-PL.U.S. (ρ = 0.616–0.814) suggest these techniques were largely comparable in assessing intramuscular blood flow across measurement sites. Chen et al. (2013) reported a similar correlation magnitude between plantar fascia vascularity assessed using VI and Newman’s qualitative grading scale (r = 0.70) [14]. Baseline VI was also correlated with perceived pain intensity (r = 0.36, p = 0.02) prior to receiving extracorporeal shock wave therapy and explained approximately 49% of plantar fascial pain reduction reported after therapy [14]. Future studies are needed to examine the association between musculoskeletal tissue VI using microvascular ultrasound techniques and clinically relevant patient-reported outcomes (e.g., pain, fatigue).
Limitations
This study has several methodological limitations. As stated previously, comparisons may have been underpowered for detecting significant differences in intramuscular blood perfusion between imaging techniques across measurement sites. Enrolled participants were not sex-matched (i.e., men = 31, women = 14), and therefore not optimally balanced for conducting additional subgroup analyses. Future studies involving larger and more diverse sample sizes are needed moving forward.
General cognitive function was assessed using a cursory tool (i.e., AMT) due its brevity and clinical feasibility. As age-related changes in microvascular blood flow have been shown to affect neuronal function and plasticity [37, 38], cognition [39], and sympathetic skin response [40], additional research involving a more comprehensive battery of cognitive and sensorimotor assessments may be useful in elucidating their association with alterations in intramuscular blood flow.
Additionally, our participants had no history of musculoskeletal disease or pathology, hence, the comparative sensitivity of these ultrasound imaging techniques in detecting bilateral limb differences in intramuscular blood perfusion between more- and less-affected limbs was not assessed. Studies comparing the use of ultrafast and conventional Doppler ultrasound imaging techniques for evaluating microvascularity and slow flow states in clinical populations with global (e.g., Duchenne muscular dystrophy) or unilateral (e.g., stroke) [41] musculoskeletal tissue alterations are warranted. The scope of the current study is also limited to the investigation of blood flow in resting skeletal muscles of the upper and lower extremities. Additional studies evaluating the use of these techniques for characterizing blood flow changes during dynamic scenarios (e.g., cardiac tissue Doppler imaging for assessing myocardial mechanics and blood flow) are also needed moving forward.
Finally, ground truth and depth dependent sensitivity of microvascular blood flow measures using ultrafast and conventional Doppler techniques could not be determined due to resource limitations. We were also unable to assess measurement reliability due to the limited availability of the participants and personnel involved. Although moderate [intraclass correlation coefficient (ICC) = 0.73 to 0.74, 95% confidence interval (CI) = 0.31–0.90)] to excellent reliability [ICC = 0.928 to 0.932 (95%CI = 0.887–0.960)] has been reported previously for intramuscular blood perfusion measures using the CDFI-VI technique [15, 16], the reliability of measures using Angio-PL.U.S.-VI are unknown. Furthermore, the acquisition procedures used are highly operator dependent and the determination of the color scale range (i.e. 70-85%) in CDFI mode may introduce measurement bias. Additionally, only images captured at the upper range of the color flow spectrum were used to determine VI [16]. Other clinically relevant metrics by which to compare differences in blood flow direction, speed, and pattern overtime, were not assessed. More methodologically robust studies involving multiple operators, measurement sessions and the use of flow phantoms to simulate controlled microvascular blood flow patterns [42] are needed to determine measurement reproducibility, validity and facilitate future comparisons with other imaging modalities and metrics.
Conclusion
The results indicate that CDFI and Angio-PL.U.S. imaging techniques were comparable when used in conjunction with the VI for quantifying resting intramuscular blood flow in the upper and lower extremity muscles of older adults. Angio-PL.U.S. appeared to demonstrate relatively higher sensitivity in detecting bilateral differences, particularly in the biceps brachii muscles, but further investigation involving a larger cohort is required to confirm these results. The potential role of this ultrasound imaging technique for quantifying intramuscular blood perfusion in clinical populations with global or unilateral musculoskeletal tissue alterations will also require further study.
Acknowledgements
We would like to thank the study participants and Sik Cheung Siu for providing logistical support and technical assistance during the study.
Author contributions
Concept and design: TM, NC, MTCY, MYCP; Data curation: TM, NC, MTCY, MYCP; Formal analysis: TM, NC, MTCY, MYCP; Funding acquisition: MYCP; Investigation: TM, NC; Methodology: TM, NC, MTCY, MYCP; Project administration: TM, NC, MTCY, MYCP; Resources: MTCY, MYCP; Software: MTCY, MYCP; Supervision: MTCY, MYCP; Validation: TM, NC, MTCY, MYCP; Visualization: TM, NC, MTCY, MYCP; Writing-original draft: TM; Writing-review and editing: TM, NC, MTCY, MYCP; Final approval: TM, NC, MTCY, MYCP.
Funding
This study was substantially supported by a research grant provided to MYCP by the The Hong Kong Polytechnic University (1-ZVWT) and the General Research Fund (151031/18 M). TM was supported by a postgraduate research scholarship provided by the Department of Rehabilitation Sciences at The Hong Kong Polytechnic University (funding code: RL27).
Data availability
Study data are available from the corresponding author (MYCP) on reasonable request.
Declarations
Ethics approval and consent to participate
Informed consent was obtained on the day of the initial functional assessment prior to data collection. The study was approved by the Human Subjects Ethics Sub-committee of the Hong Kong Polytechnic University on April 10, 2018 (HSEARS20171212003). All procedures were conducted in accordance with the Helsinki Declaration for human experiments and reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Szabo TL. Diagnostic ultrasound imaging: inside out, second edition, 2nd ed. edn. Waltham, MA; Kidlington, Oxford, UK: Academic Press; 2014.
- 2.Ma Y, Li G, Li J, Ren W-D. The diagnostic value of superb microvascular imaging (SMI) in detecting blood Flow signals of breast lesions: a preliminary Study comparing SMI to Color Doppler Flow Imaging. Med (Baltim). 2015;94(36):e1502–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo G, Feng J, Jin C, Gong X, Chen Y, Chen S, Wei Z, Xiong H, Lu J. A Novel Nomogram based on imaging biomarkers of Shear Wave Elastography, Angio Planewave Ultrasensitive Imaging, and Conventional Ultrasound for Preoperative Prediction of Malignancy in patients with breast lesions. Diagnostics (Basel) 2023, 13(3). [DOI] [PMC free article] [PubMed]
- 4.Chambara N, Liu SYW, Lo X, Ying M. Diagnostic value of AngioPLUS microvascular imaging in thyroid nodule diagnosis using quantitative and qualitative vascularity grading. Biomedicines 2022, 10(7). [DOI] [PMC free article] [PubMed]
- 5.Zhu YC, Zhang Y, Deng SH, Jiang Q. A prospective study to compare superb microvascular imaging with Grayscale Ultrasound and Color Doppler Flow Imaging of Vascular Distribution and morphology in thyroid nodules. Med Sci Monit. 2018;24:9223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D-B, Zhou J, Feng L, Xu R, Wang Y-C. Value of superb micro-vascular imaging in predicting ischemic stroke in patients with carotid atherosclerotic plaques. World J Clin Cases. 2019;7(7):839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oura K, Kato T, Ohba H, Terayama Y. Evaluation of Intraplaque Neovascularization using superb microvascular imaging and contrast-enhanced Ultrasonography. J Stroke Cerebrovasc Dis. 2018;27(9):2348–53. [DOI] [PubMed] [Google Scholar]
- 8.Correas J-M, Anglicheau D, Joly D, Gennisson J-L, Tanter M, Hélénon O. Ultrasound-based imaging methods of the kidney—recent developments. Kidney Int. 2016;90(6):1199–210. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel M, Tomczak J, Snoch-Ziółkiewicz M, Dzieciuchowicz Ł, Strauss E, Pawlaczyk K, Wojtusik D, Oszkinis G. Superb micro-vascular imaging (SMI): a Doppler ultrasound technique with potential to identify, classify, and follow up endoleaks in patients after endovascular aneurysm repair (EVAR). Abdom Radiol. 2018;43(12):3479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomczak J, Gabriel M, Snoch-Ziółkiewicz M, Dzieciuchowicz Ł, Strauss E, Pawlaczyk K. Angio PLanewave UltraSensitive Imaging (Angio PL.U.S.) As an innovative Doppler Ultrasound technique with a potential to follow up endoleaks after Endovascular Aneurysm Repair (EVAR). Ultrasound Med Biol. 2020;46(7):1707–14. [DOI] [PubMed] [Google Scholar]
- 11.Bercoff J, Montaldo G, Loupas T, Savery D, Mézière F, Fink M, Tanter M. Ultrafast compound doppler imaging: providing full blood flow characterization. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(1):134–47. [DOI] [PubMed] [Google Scholar]
- 12.Ying M, Ng DKS, Yung DMC, Lee EST. A semi-quantitative approach to compare high-sensitivity power Doppler sonography and conventional power Doppler sonography in the assessment of thyroid vascularity. Thyroid. 2009;19(11):1265–9. [DOI] [PubMed] [Google Scholar]
- 13.Caliskan E, Akkoc O, Bayramoglu Z, Gozubuyuk OB, Kural D, Azamat S, Adaletli I. Effects of static stretching duration on muscle stiffness and blood flow in the rectus femoris in adolescents. Med Ultrason. 2019;21(2):136–43. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Ho HM, Ying M, Fu SN. Correlation between computerised findings and Newman’s scaling on vascularity using power Doppler ultrasonography imaging and its predictive value in patients with plantar fasciitis. Br J Radiol. 2012;85(1015):925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang M, Miller T, Ying M, Pang MYC. Whole-body vibration modulates leg muscle reflex and blood perfusion among people with chronic stroke: a randomized controlled crossover trial. Sci Rep. 2020;10(1):1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller T, Ying MTC, Chung RCK, Pang MYC. Convergent validity and Test-Retest Reliability of Multimodal Ultrasonography and related clinical measures in people with chronic stroke. Arch Phys Med Rehabil. 2022;103(3):459–e472454. [DOI] [PubMed] [Google Scholar]
- 17.Newman JS, Adler RS, Rubin JM. Power Doppler sonography: use in measuring alterations in muscle blood volume after exercise. Am J Roentgenol. 1997;168(6):1525–30. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu L, Pei C, Ho M, Chan P. Validation of the Abbreviated Mental Test (Hong Kong version) in the elderly medical patient. 1995.
- 20.Lam SC, Wong Y-y, Woo J. Reliability and validity of the abbreviated mental test (Hong Kong version) in residential care homes. J Am Geriatr Soc. 2010;58(11):2255–7. [DOI] [PubMed] [Google Scholar]
- 21.Curcio F, Liguori I, Cellulare M, Sasso G, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Physical activity scale for the Elderly (PASE) score is related to Sarcopenia in Noninstitutionalized older adults. J Geriatr Phys Ther. 2019;42(3):130–5. [DOI] [PubMed] [Google Scholar]
- 22.Washburn RA, Ficker JL. Physical activity scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39(4):336. [PubMed] [Google Scholar]
- 23.Dinger MK, Oman F, Taylor EL, Vesely SK, Able J. Stability and convergent validity of the physical activity scale for the Elderly (PASE). J Sports Med Phys Fitness. 2004;44(2):186. [PubMed] [Google Scholar]
- 24.Ngai SP, Cheung RT, Lam PL, Chiu JK, Fung EY. Validation and reliability of the physical activity scale for the Elderly in Chinese population. J Rehabil Med. 2012;44(5):462–5. [DOI] [PubMed] [Google Scholar]
- 25.Barba C, Cavalli-Sforza T, Cutter J, Darnton-Hill I, Deurenberg P, Deurenberg-Yap M, Gill T, James P, Ko G, Miu AH, et al. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (British Edition). 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman M, Schrader J, Applegate T, Koceja D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects. J Athl Train. 1998;33(4):319–22. [PMC free article] [PubMed] [Google Scholar]
- 27.Mathevon L, Michel F, Aubry S, Testa R, Lapole T, Arnaudeau LF, Fernandez B, Parratte B, Calmels P. Two-dimensional and shear wave elastography ultrasound: a reliable method to analyse spastic muscles? Muscle Nerve. 2018;57(2):222–8. [DOI] [PubMed] [Google Scholar]
- 28.Wu CH, Ho YC, Hsiao MY, Chen WS, Wang TG. Evaluation of Post-stroke Spastic muscle stiffness using Shear Wave Ultrasound Elastography. Ultrasound Med Biol 2017, 43(6):1105–11. [DOI] [PubMed]
- 29.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 30.Portney LG. Foundations of clinical research: applications to practice, Third edition. edn. Philadelphia, PA: F.A. Davis Company; 2015.
- 31.Machado P, Segal S, Lyshchik A, Forsberg F. A Novel Microvascular Flow technique: initial results in thyroids. Ultrasound Q 2016, 32(1). [DOI] [PubMed]
- 32.Tafuna’i ND, Hunter I, Johnson AW, Fellingham GW, Vehrs PR. Differences in femoral artery occlusion pressure between Sexes and Dominant and non-dominant legs. In: Medicina 57; 2021. [DOI] [PMC free article] [PubMed]
- 33.Ticinesi A, Meschi T, Narici MV, Lauretani F, Maggio M. Muscle Ultrasound and Sarcopenia in older individuals: a clinical perspective. J Am Med Dir Assoc. 2017;18(4):290–300. [DOI] [PubMed] [Google Scholar]
- 34.Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of Sarcopenia: facts, numbers, and epidemiology—update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45(10):2288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ditroilo M, Forte R, Benelli P, Gambarara D, De vito G. Effects of age and limb dominance on upper and lower limb muscle function in healthy males and females aged 40–80 years. J Sports Sci. 2010;28(6):667–77. [DOI] [PubMed] [Google Scholar]
- 37.Ogrin R, Darzins P, Khalil Z. Age-related changes in Microvascular Blood Flow and Transcutaneous Oxygen Tension under basal and stimulated conditions. Journals Gerontology: Ser A. 2005;60(2):200–6. [DOI] [PubMed] [Google Scholar]
- 38.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2(2):149–68. [DOI] [PubMed] [Google Scholar]
- 39.Heringa SM, Bouvy WH, van den Berg E, Moll AC, Kappelle LJ, Biessels GJ. Associations between retinal microvascular changes and dementia, cognitive functioning, and Brain Imaging abnormalities: a systematic review. J Cereb Blood Flow Metabolism. 2013;33(7):983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naver H, Blomstrand C, Ekholm S, Jensen C, Karlsson T, Wallin BG. Autonomic and thermal sensory symptoms and Dysfunction after Stroke. Stroke. 1995;26(8):1379–85. [DOI] [PubMed] [Google Scholar]
- 41.Miller T, Ying M, Sau Lan Tsang C, Huang M, Pang MYC. Reliability and validity of Ultrasound Elastography for evaluating muscle stiffness in neurological populations: a systematic review and Meta-analysis. Phys Ther. 2021;101(1):pzaa188. [DOI] [PubMed] [Google Scholar]
- 42.Kamphuis ME, Greuter MJW, Slart R, Slump CH. Quantitative imaging: systematic review of perfusion/flow phantoms. Eur Radiol Exp. 2020;4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study data are available from the corresponding author (MYCP) on reasonable request.