Abstract
Background and Aim:
Pelung chicken Gallus gallus gallus (Linnaeus, 1758) is a chicken endemic to Cianjur, West Java, Indonesia. This study aimed to determine the effects of zinc sulfate (ZnSO4) and synthetic testosterone supplementations for 56 days on testosterone hormone levels and breast muscle performance in Pelung chickens.
Materials and Methods:
This study used 12 Pelung chickens with three treatment groups (G) and four replications, namely, control (G0), ZnSO4 0.9 mg/kg (G1), and synthetic testosterone 3 mg/day (G2). Chickens were acclimatized for 7 days and then supplemented for 56 days. Drinking water and commercial standard feed were provided ad libitum. Blood was collected through the brachial vein for the analysis of testosterone levels using the enzyme-linked immunosorbent assay. The samples were collected every 14 days; on days 0, 14, 28, 42, and 56. Breast muscles were collected for texture analysis, and breast muscle preparations were made with hematoxylin-eosin staining to measure the fascicle area (FA), number of myofibers in one fascicle (NMOF), and myofiber area (MA). The collected data were analyzed using analysis of variance at a 95% confidence level with the help of Statistical Package for the Social Sciences v.29.0. Furthermore, principal component analysis (PCA) was performed with the help of Minitab v. 19.
Results:
Statistical analysis results on 56 days of testosterone level parameters showed that G2 was significantly different from all treatments (p < 0.05). The results of statistical analysis on Pelung chicken breast muscle performance, especially hardness, chewiness, FA, NMOF, and MA, were significantly different (p < 0.05) compared with the other treatments. The results of PCA showed that the testosterone level parameters were positively correlated with FA, NMOF, MA, hardness, and chewiness, whereas the fracture parameters were negatively correlated with all parameters except the springiness index and were significantly different between the G2 group and the other groups (p < 0.05).
Conclusion:
It can be concluded that supplementing synthetic testosterone 3 mg/day body weight for 56 days can improve testosterone levels and breast muscle performance, especially hardness, chewiness, FA, NMOF, and MA in Pelung chickens.
Keywords: muscle, Pelung chicken, performance, testosterone, texture
Introduction
Pelung chicken Gallus gallus gallus (Linnaeus, 1758) is a native Indonesian chicken originating from Cianjur, West Java, Indonesia. This chicken has the distinction of a long and distinctive voice [1, 2]. Furthermore, its body is tall and large, and it has a relatively high body weight (BW) [3]. Breast muscle performance is influenced by the hormone testosterone [4]. It controls voice, growth, muscle strength, spermatogenesis, and sex drive [3, 5–11]. Parameters for determining muscle performance include muscle strength, apart from measuring chest circumference, breast muscle weight, fascicle area (FA), myofiber area (MA), number of myofiber in one fascicle (NMOF), and the percentage of positive proliferating cell nuclear antigen can also be supported by texture parameters in Pelung chicken breast muscles by measuring hardness, tenderness, springiness index, chewiness, and fracture [4, 12]. Injection of anabolic hormones, such as testosterone, into male poultry, is often performed to improve their performance [13].
The testosterone in the body is naturally converted into estrogen or estradiol by aromatase enzyme, which causes challenges in maintaining stable high testosterone levels [14]. However, continuous injection of low-dose synthetic testosterone can cause downregulation mechanisms and drastically reduce testosterone levels [13]. During downregulation, testosterone receptors decrease, leading to decreased voice performance [13, 15]. In addition, it can cause atrophy of the seminiferous tubules in Pelung chickens [4]. According to Yuneldi et al. [9], administration of testosterone 0.1 mL a day for 35 days can increase testosterone levels but reduce testicular weight significantly compared with controls and those administered blood clamshell powder (Anadara granosa) as a natural aromatase blocker at a dose of 0.036 mg/40 g BW. However, zinc sulfate and synthetic testosterone supplementation on breast muscle performance, especially texture of breast muscle in Pelung chickens has not been investigated.
Therefore, this study aimed to evaluate the effects of administering zinc sulfate (ZnSO4) and synthetic testosterone supplementation for 56 days on testosterone levels and breast muscle performance in Pelung chickens.
Materials and Methods
Ethical approval
All research procedures were approved by the Ethics Committee of Integrated Testing and Research, Universitas Gadjah Mada (UGM) (Approval no. 00020/04/LPPT/V/2020).
Study period and location
The study was conducted from April to September 2022 at a Pelung chicken farm in Bantul, Yogyakarta. Texture profile analysis and hematoxylin-eosin (HE) staining of Pelung chicken breast muscle were conducted in the Faculty of Animal Science, Faculty of Agricultural Technology and Animal Development Structure Laboratory, Faculty of Biology, UGM. Testosterone level analysis was conducted at the Physiology Laboratory, Faculty of Veterinary Medicine, UGM, Indonesia.
Experimental design
Before treatment, Pelung chickens were allowed to acclimatize for 7 days, and their health was monitored daily. The chicken housings were individual-stage wood cages with slit bases (60 × 60 × 60 cm). Drinking water and commercial standard feed were provided ad libitum [16]. The study used 12 male Pelung chickens (the sample size was small as the chicken falls under the threatened species category) aged 40–56 weeks. The sample size was determined as described by Yuneldi et al. [4]. The general physiological performance of male Pelung chickens decreased from 40 to 56 weeks. Pelung chickens (BW ± 3 kg) were divided randomly into three treatment groups (G): G0 (control); G1 (ZnSO4 at 0.9 mg/kg BW); and G2 (synthetic testosterone 3 mg/day). Each treatment group contained four Pelung chickens. ZnSO4 was administered orally [3, 6, 9, 17] and testosterone was administered subcutaneously [9, 18]. The chickens were supplemented for 56 days.
Testosterone analysis
Testosterone hormone level analysis was carried out according to the procedure described by Yuneldi et al. [3]. Serum samples were examined for testosterone hormone levels using enzyme-linked immunosorbent assay (ELISA). Testosterone level analysis was performed according to the manufacturer’s instructions in the kit (Calbiotech®, California, USA) using competitive ELISA. Absorbance was analyzed using ELISA or a microplate reader (Zenix-320, Shenzhen, China) at an absorbance of 450 nm for 15 min.
Euthanasia and organ preparation
On the 56th day, chickens were slaughtered using the halal method. Breast muscles were collected and washed with 0.9% NaCl [19, 20]. The left breast muscle was analyzed for tenderness, and the right muscle was analyzed for hardness, springiness index, chewiness, and fracture. Furthermore, part of the right breast muscle was cut to a smaller size of approximately 1 × 1 cm and fixed for 18–24 h using 10% neutral buffer formalin solution before histological preparation [21, 22].
HE staining method
The process of making slides using the paraffin method, after cutting them to 5 μm, was performed and placed on a glass object. Furthermore, HE staining was performed (Merck, Darmstadt, Germany), and the slides were observed under a microscope (Leica DM 750, Germany) at a magnification of 10 × 10. The area of myofiber and fascicle was measured, and the NMOF was calculated. These measurements were performed using ImageJ software (Version 1.53 k, National Institute of Health, USA) [23].
Texture analysis of breast muscle
Pelung chicken breast muscle analysis with the parameter of tenderness (kg/cm2) was performed using a Warner-Bratzler (WB) device, and the parameters of hardness (N), springiness index, chewiness (N), and fracture (N) were tested using a Texture Analyzer TA1 (Ametek Lloyd Instruments Ltd., Fareham, UK). Tenderness analysis was continued by testing meat measuring 1.5 × 0.67 cm in length parallel to the muscle fibers. Changes in the scale of the WB tool indicate tenderness scores. The analysis of hardness (N), springiness index, chewiness (N), and fracture (N) requires a meat block with a minimum size of 1 × 1 × 1 cm. Operation of the Texture Analyzer and data obtained using Nexygenplus software (Ametek Lloyd Instrument Ltd.).
Statistical analysis
All parameters were statistically analyzed by one-way analysis of variance (ANOVA) using Statistical Package for Social Sciences v.29.0 (IBM, NY, USA) with a 95% confidence level (α = 0.05). The analysis was confirmed using Duncan’s test [4, 24]. Furthermore, principal component analysis (PCA) was applied. All the data were analyzed statistically using PCA in Minitab® statistical software v.19 (Minitab Inc., State College, PA, USA) to reduce dimensionality and identify correlations between all parameters. Two principal components (PC) with cumulative proportion values exceeding 70% were plotted in the graphic as loading plots of variables and score plots of objects.
Results
Testosterone hormone levels and breast muscle performance in Pelung chickens
The results of ANOVA for testosterone hormone levels showed that there was a significant difference (p < 0.05) in G2 compared with other treatments on days 28, 42, and 56 (Table-1). The results of the ANOVA statistical analysis of hardness and chewiness showed that the value for G2 was higher (p < 0.05) compared with the other treatments. However, the springiness index, fracture, and tenderness showed no significant differences between the treatments (p > 0.05) (Table-2). The FA, NMOF, and MA were significantly different (p < 0.05) in G2 compared with the other treatments (Table-3 and Figure-1).
Table-1.
Testosterone levels of male Pelung chickens aged 40–56 weeks after 56 days of supplementation (Average ± standard deviation).
| Group | 0 day (ng/mL) | 14 day (ng/mL) | 28 day (ng/mL) | 42 day (ng/mL) | 56 day (ng/mL) |
|---|---|---|---|---|---|
| G0 | 0.19 ± 0.09a | 0.25 ± 0.12b | 0.30 ± 0.05c | 0.38 ± 0.04b | 0.80 ± 0.07b |
| G1 | 0.44 ± 0.38a | 0.50 ± 0.28ab | 0.52 ± 0.15b | 0.65 ± 0.06b | 0.97 ± 0.10b |
| G2 | 0.74 ± 0.45a | 0.89 ± 0.49a | 1.47 ± 0.19a | 4.70 ± 0.41a | 9.94 ± 0.18a |
| p-value | 0.052 | 0.042 | <0.001 | <0.001 | <0.001 |
a–cMeans with different superscripts within the same column are significantly different (p < 0.05). G0=Control, G1=ZnSO4 0.9 mg/kg body weight, G2=Synthetic testosterone 3 mg/day. SD=Standard deviation
Table-2.
Breast muscle texture of male Pelung chickens aged 40–56 weeks after 56 days of supplementation (Average ± standard deviation).
| Group | Tenderness (kg/cm2) | Hardness (N) | Springiness index | Chewiness (N) | Fracture (N) |
|---|---|---|---|---|---|
| G0 | 4.06 ± 0.93a | 134.43 ± 12.10b | 0.667 ± 0.01a | 29.08 ± 13.39b | 2.08 ± 0.09a |
| G1 | 3.82 ± 1.49a | 139.77 ± 28.72b | 0.685 ± 0.03a | 31.13 ± 6.26b | 2.01 ± 0.13a |
| G2 | 4.17 ± 0.10a | 190.70 ± 32.42a | 0.687 ± 0.03a | 93.50 ± 20.98a | 2.04 ± 0.14a |
| p-value | 0.884 | 0.025 | 0.612 | <0.001 | 0.731 |
a–cMeans with different superscripts within the same column are significantly different (p < 0.05). G0=Control, G1=ZnSO4 0.9 mg/kg body weight, G2=Synthetic testosterone 3 mg/day. SD=Standard deviation
Table-3.
Myofibers in one fascicle of Pelung chickens aged 40–56 weeks after 56 days of supplementation (Average ± standard deviation).
| Group | Fascicle area (µm2) | NMOF | Myofiber area (µm2) |
|---|---|---|---|
| G0 | 210551.34 ± 13147.80c | 100.75 ± 2.09c | 1937.74 ± 56.90c |
| G1 | 279314.87 ± 14349.24b | 107.58 ± 2.10b | 2123.52 ± 83.75b |
| G2 | 390620.03 ± 7460.67a | 126.51 ± 3.42a | 3604.32 ± 286.58a |
| p-value | <0.001 | <0.001 | <0.001 |
a–cMeans with different superscripts within the same column are significantly different (p < 0.05). G0=Control, G1=ZnSO4 0.9 mg/kg body weight, G2=Synthetic testosterone 3 mg/day. SD=Standard deviation, NMOF=Number of myofibers in one fascicle
Figure-1.
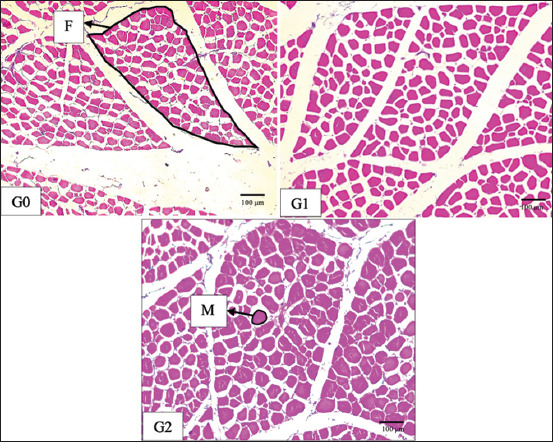
Histology of the breast muscle of male Pelung chickens aged 40–56 weeks after supplementation for 56 days. G0=Control, G1=ZnSO4 0.9 mg/kg body weight, G2=Synthetic testosterone 3 mg/day. Magnification 10 × 10. Hematoxylin-eosin staining. F=Fascicle, M=Myofiber, G=Group.
According to PC analysis, several parameters were clustered into one main cluster (Cluster I) and two other clusters (Cluster II and III). The main cluster (Cluster I) consisted of testosterone level, FA, MA, NMOF, hardness, and chewiness, with a highly positive correlation. Cluster II consisted of fracture and springiness, whereas Cluster III consisted of tenderness. Fracture was negatively correlated with Clusters I and III, which means that increasing the value in Clusters I and III will decrease the value of fracture. The score plot of the object revealed that G0, G1, and G2 were separated from each other, with G2 being plotted as having the highest score. Therefore, G2 generally obtained the highest value for almost all parameters compared to G0 and G1.
Discussion
Testosterone hormone levels in Pelung chickens at 56 days post-supplementation
This study showed that supplementing synthetic testosterone 3 mg/day increased testosterone hormone levels in Pelung chickens. This is because testosterone, which is injected continuously every day, accumulates in the blood, and testosterone levels in the blood continue to increase until the 56th day. This is in accordance with the findings of Yuneldi et al. [9], who reported that supplementing testosterone 0.1 mL/day for 35 days can increase testosterone levels in male-layer chickens. This is due to the direct intake of testosterone into the blood, which causes testosterone levels to increase.
However, continuous testosterone injections cause downregulation, such as a drastic reduction in testicular weight, compared with other treatments [9]. Therefore, injection of anabolic hormones such as testosterone into male poultry is not recommended for maintaining performance. Huang et al. [25] showed that continuous administration of testosterone can cause downregulation. Direct testosterone injection can induce the downregulation of these mechanisms [15, 26]. Yuneldi et al. [4] reported that the administration of testosterone at 3 mg/day for 56 days caused atrophy of the seminiferous tubules in Pelung chickens.
Performance of breast muscle in Pelung chickens at 56-day post-supplementation
The results showed a significant increase in hardness and chewiness in the breast muscles of Pelung chickens, but an increase did not follow this trend in springiness index, fracture, and tenderness. In contrast to the results of texture analysis of Bangkok chicken breast muscle, a combination of clam shell powder and milkfish bone powder increased the springiness index compared with hardness and tenderness [12]. The hardness and chewiness results increased in line with the fascicle, NMOF, and MAs, resulting in the G2 treatment, which was significantly different from the other treatment groups. These results are similar to those reported by Yuneldi et al. [4] in that the NMOF, MA, and fasciculus area experienced significant differences in treatment given synthetic testosterone. These results are interconnected in that the myofiber and FAs increase with the increasing hardness of NMOF. According to U-Chupaj et al. [27], muscle hardness is related to the number of myofiber in the muscle. High hardness is related to the density, number, and composition of muscle myofiber in chickens [28]. The neuromuscular system activates more myofiber, leading to stronger muscle contraction produced [29]. High hardness indicates a strong muscle structure and the number of myofiber [12].
Conclusion
Supplementing synthetic testosterone 3 mg/day BW for 56 days can increase testosterone hormone levels and breast muscle performance, especially in the hardness, chewiness, FA, NMOF, and MA of Pelung chickens. Therefore, future study is needed regarding synthetic testosterone residues in breast muscle.
Authors’ Contributions
PA, CMA, HTSS, and RFY: Planned and designed the study. PA, CMA, HTSS, and AYP: Supervised the study. PA, CMA, and RFY: Collected and analyzed the samples. PA, CMA, HTSS, RFY, and AYP: Drafted and revised the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
We express our gratitude to the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for funding (grant no. 1986/UN1/DITLIT/DIT-LIT/PT/2020).
Footnotes
We express our gratitude to the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for funding (grant no. 1986/UN1/DITLIT/DIT-LIT/PT/2020).
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Asmara I.Y, Garnida D, Partasasmita R. Crowing characteristics of Pelung chickens at different age and body weight. Biodivers. J. Biol. Divers. 2020;21(9):4339–4344. [Google Scholar]
- 2.Asmara I.Y, Garnida D, Partasasmita R. Short communication:Duration and volume of crowing of Pelung chickens of West Java, Indonesia. Biodivers. J. Biol. Divers. 2020;21(2):748–752. [Google Scholar]
- 3.Yuneldi R.F, Astuti P, Saragih H.T.S, Airin C.M. Anadara granosa shell powder improves the metabolism, testosterone level, and sound frequency of Pelung Chickens. Vet. World. 2021;14(6):1564–1571. doi: 10.14202/vetworld.2021.1564-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuneldi R.F, Airin C.M, Saragih H.T, Sarmin S, Astuti P, Alimon A.R. Growth, pectoralis muscle performance, and testis of Pelung cockerels (Gallus gallus gallus [Linnaeus, 1758]) supplemented with blood clam shell powder (Anadara granosa [Linnaeus, 1758]) Vet. World. 2023;16(3):474–482. doi: 10.14202/vetworld.2023.474-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astuti P, Yusuf T.L, Hayes E, Maheshwari H, Sjahfirdi L, Sajuthi D. Diurnal patterns of testosterone and cortisol metabolites in fecal of Javan Gibbons (Hylobates moloch) in captivity [Pola diurnal metabolit testosteron dan kortisol di dalam feses Owa Jawa (Hylobates moloch) di Penangkaran] Hayati J. Biosci. 2006;13(2):69–72. [Google Scholar]
- 6.Astuti P, Airin C.M, Sarmin S, Nururrozi A, Harimurti S. Effect of shell as natural testosterone boosters in Sprague Dawley rats. Vet. World. 2019;12(10):1677–1681. doi: 10.14202/vetworld.2019.1677-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josiak K, Jankowska E.A, Piepoli M.F, Banasiak W, Ponikowski P. Skeletal myopathy in patients with chronic heart failure:Significance of anabolic-androgenic hormones. J. Cachexia Sarcopenia Muscle. 2014;5(4):287–296. doi: 10.1007/s13539-014-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko M.C, Frankl-Vilches C, Bakker A, Gahr M. The gene expression profile of the song control nucleus hvc shows sex specificity, hormone responsiveness, and species specificity among songbirds. Front. Neurosci. 2021;15:680530. doi: 10.3389/fnins.2021.680530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuneldi R.F, Airin C.M, Saragih H.T, Astuti P. Application of natural aromatase blocker towards the level of testosterone in rooster layer [Gallus gallus gallus (Linn., 1758)] Key Eng. Mater. 2021;884:252–255. [Google Scholar]
- 10.Yuneldi R.F, Airin C.M, Saragih H.T.S, Astuti P. Profile of Thyroid in Male Layer Chickens Given by Testosterone. IOP Conference Series:Earth and Environmental Science. 2021:1–5. [Google Scholar]
- 11.Yuneldi R.F, Airin C.M, Saragih H.T, Astuti P. Efficiency of Testosterone Administration on the Performance of Day Old Chick (DOC) Layer:Cockscomb Size, T3/T4 Ratio, and Histopathological Description of Bursa Fabricius. Vol. 12. Netherlands: Atlantis Press; 2021. pp. 35–39. [Google Scholar]
- 12.Sidiqi A.A.A, Airin C.M, Sarmin S, Astuti P. A combination of Anadara nodifera shell and milkfish thorns powder effectively promote springiness index, serum testosterone, and breast muscle testosterone in Bangkok rooster. Hayati J. Biosci. 2023;30(4):701–710. [Google Scholar]
- 13.Astuti P, Putra M.N.P, Shiddiq M.F.A, Yuneldi R.F, Airin C.M, Sarmin S. The potency of Anadara nodifera shell as natural testosterone booster for male canary (Seriunus canaria) HAYATI J. Biosci. 2022;29(1):107–113. [Google Scholar]
- 14.Cinar V, Polat Y, Baltaci A.K, Mogulkoc R. Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol. Trace Elem. Res. 2011;140(1):18–23. doi: 10.1007/s12011-010-8676-3. [DOI] [PubMed] [Google Scholar]
- 15.Pomara C, Barone R, Marino Gammazza A, Sangiorgi C, Barone F, Pitruzzella A, Locorontondo N, Gaudio F.D, Salerno M, Maglietta F, Sarni A.L, Felice V.D, Cappello F, Turillazzi E. Effects of nandrolone stimulation on testosterone biosynthesis in leydig cells. J. Cell Physiol. 2016;231(6):1385–1391. doi: 10.1002/jcp.25272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elwahesh R.M, Ben-Elhaj K.M, Draid M.M. Relationship between body weight performance and plasma thyroid hormones in broiler hens. Int. J. Med. Res. Prof. 2016;2(6):6–10. [Google Scholar]
- 17.Astuti P, Airin C.M, Nurrurozi A, Aidi R, Hana A, Hadi S, Harimurti H. Potential Natural Aromatase Blockers on Enhance the Frequency and Sound Quality of Male Canaries. E3S Web Conference EDP Sciences. 2020:1–3. [Google Scholar]
- 18.Shah M, Zaneb H, Masood S, Khan R.U, Din S, Khan I, Tariq A. Ameliorative effect of zinc and multistrain probiotic on muscle and bone characteristics in broiler reared under cyclic heat stress. Pak. J. Zool. 2019;51(3):1041–1046. [Google Scholar]
- 19.Sitanggang E.N, Hasnudi H, Hamdan H. Diversity of qualitative trait and morphometrics between Kampung, Katai Birma, Bagon, and Magon chicken in Medan. J. Anim. Husb. 2016;3(2):167–189. [Google Scholar]
- 20.Setiawan H, Jingga M.E, Saragih H.T. The effect of cashew leaf extract on small intestine morphology and growth performance of Jawa Super chicken. Vet. World. 2018;11(8):1047–1054. doi: 10.14202/vetworld.2018.1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zielinska M.K, Sawosz E, Chwalibog A, Ostaszewska T, Kamaszewski M, Grodzik M, Skomiał J. Nano-nutrition of chicken embryos. Effect of gold and taurine nanoparticles on muscle development. J. Anim. Feed Sci. 2010;19(2):277–285. [Google Scholar]
- 22.Alward B.A, Balthazart J, Ball G.F. Dissociable effects on birdsong of androgen signaling in cortex-like brain regions of canaries. J. Neurosci. 2017:37–36. 8612–8624. doi: 10.1523/JNEUROSCI.3371-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saragih H.T, Muhamad A.A.K, Alfianto A, Viniwidihastuti F, Untari L.F, Lesmana I, Widyatmoko H, Rohmah Z. Effects of Spirogyra jaoensis as a dietary supplement on growth, pectoralis muscle performance, and small intestine morphology of broiler chickens. Vet. World. 2019;12(8):1233–1239. doi: 10.14202/vetworld.2019.1233-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Çilek S, Tekin M.E. The environmental factors affecting milk yield and fertility traits of Simmental cattle raised at the Kazova state farm and phenotypic correlations between these traits. Turk. J. Vet. Anim. Sci. 2005;29(4):987–993. [Google Scholar]
- 25.Huang C, Gu H, Zhang W, Herrmann J.L, Wang M. Testosterone-down-regulated Akt pathway during cardiac ischemia/reperfusion:A mechanism involving BAD, Bcl-2 and FOXO3a. J. Surg. Res. 2010;164(1):e1–e11. doi: 10.1016/j.jss.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andric S.A, Janjic M.M, Stojkov N.J, Kostic T.S. Testosterone-induced modulation of nitric oxide-cGMP signaling pathway and androgenesis in the rat Leydig cells. Biol. Reprod. 2010;83(3):434–442. doi: 10.1095/biolreprod.110.083626. [DOI] [PubMed] [Google Scholar]
- 27.U-Chupaj J, Malila Y, Gamonpilas C, Kijroongrojana K, Petracci M, Benjakul S, Visessanguan W. Differences in textural properties of cooked caponized and broiler chicken breast meat. Poult. Sci. 2017;96(7):2491–2500. doi: 10.3382/ps/pex006. [DOI] [PubMed] [Google Scholar]
- 28.Weng K, Huo W, Li Y, Zhang Y, Zhang Y, Chen G, Xu Q. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci. 2022;101(1):101537. doi: 10.1016/j.psj.2021.101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer W.J, Looney D.P. Underlying mechanisms and physiology of muscular power. Strength Cond. J. 2012;34(6):13–19. [Google Scholar]


