Abstract
Background
People of all ages are affected by epilepsy, a prevalent chronic brain illness that is primarily found in underdeveloped nations. It is very necessary to implement epilepsy self-management techniques to support individuals with epilepsy in order to impact outcomes related to epilepsy. The purpose of this 2-site randomised controlled experiment is to investigate this further, based on encouraging preliminary evidence.
Methods
A total of 188 adult people with epilepsy (PWE) attending the neurology clinics at Mulago and Mbarara hospitals and consent to participate in the study will be recruited. They will be randomised into intervention versus enhanced treatment control (eTAU) study groups. The intervention group will receive 12-week “intensive” educational sessions and a 12-week remotely accessed telephone follow-up stage. The controls will continue in their usual care supplemented by written materials on epilepsy in their preferred language and tailored to the reading level of most patients at the clinic. SMART-U consists of 2 main components: a 12-week “intensive” group format stage and a 12-week remotely accessed telephone follow-up stage. SMART-U will be assessed for acceptability, fidelity, and efficacy compared to eTAU. The primary study outcome is the mean change in cumulative past 24-week seizure frequency (24 weeks prior to the study baseline compared to the 24-week follow-up). Seizure frequency will be via self-report with corroboration by family/support system informants whenever possible. Participants will self-report the seizure frequency (numeric count) that they experienced between baseline and 13 weeks and again between 13 and 24 weeks, and the mean change from baseline to 24 weeks in QOL.
Discussion
The curriculum-guided Self-Management intervention for Reducing The epilepsy burden among Ugandans (SMART-U) program is anticipated to reduce the epilepsy burden seizure frequency and improve other health outcomes, including depression, functional status and health resource use.
Trial Registration Number (TRN)
Date of registration
November142023.
Keywords: self-management, epilepsy, targeted management, intervention
Introduction
Eighty per cent of the estimated 70 million people diagnosed with epilepsy globally reside in poor nations.1,2 Approximately 75% of epileptics in underdeveloped nations are thought to not receive the necessary care.1 In addition, stigma and prejudice against individuals with epilepsy and their families are common in their communities.1 Inadequate treatment and poor seizure control are common in Sub-Saharan Africa (SSA), which increases the number of years that patients and their families may lose to epilepsy and makes their condition worse. A patient’s low quality of life affects their accountability and frequently results in medication non-compliance, exposure to triggers for seizures, and worsening illness outcomes. Assessing the quality of life for epilepsy patients can help develop new seizure control strategies, reduce seizure frequency, and improve drug compliance. Identifying clinical and demographic factors affecting quality of life can improve patient accountability and outcomes.
Epilepsy self-management describes how a patient manages the medications and daily lifestyle adjustments linked to their condition, which significantly impacts the results associated with it.3,4 PWE receive care from a variety of sources, including family members, nurses, and medical professionals. With an estimated one neurologist for every 4 million people in East Africa, neurologists are scarce throughout sub-Saharan Africa, particularly in Uganda.5 There are just nine practising neurologists in Uganda’s urban districts; none practice in the country’s rural areas. Because of this, PWE care is provided in appalling conditions in both rural and urban areas. Utilisation of self-management approaches that may be provided by caregivers, nurses or other family members creates a rare opportunity to provide epilepsy care when healthcare services are difficult to come by and reduce the associated morbidity and mortality rates among this population. Self-management programs like SMART (Self-management for people with epilepsy and a history of negative health events) that were developed to support patients in coping with their chronic condition can improve Qol, reduce perceived stigma, and offer an opportunity to reduce epilepsy-associated mortality and morbidity.6,7
Although an increasing amount of research is in favour of using evidence-based strategies to help epileptics lessen their seizures and enhance their health,8–10 few studies have been conducted to demonstrate the efficacy of self-management programs for individuals with epilepsy, particularly in sub-Saharan Africa, where most treatments focus on raising awareness of the condition.11 As a result, we suggest employing a prospective design to assess the effectiveness of the SMART intervention for individuals with epilepsy over a 24-month period in PWE in Uganda.
In order to improve PWE care, seizures, quality of life, and mHealth assistance for those with poorly controlled epilepsy and low self-management abilities must all be considered. In Uganda, there aren not any popular epilepsy self-management techniques. Understudied residents in remote areas have likewise received less attention. Short messaging service (SMS), a commonly accessible mobile phone technology that has not been extensively used inside SSA, is used to support PWE,s involvement in self-management and seizure surveillance.
Methods
Study Design
This will be a prospective non-inferiority randomized clinical trial at Mulago and Mbarara hospitals. Participants will be adults who meet the inclusion criteria from the epilepsy clinics at Mulago and Mbarara who will be approached to participate in the study and enrolled.
Study Objectives
To assess the efficacy of SMART- U vs eTAU in a prospective randomised controlled trial.
We hypothesised that individuals randomised to SMART-U will have significantly improved QOL with fewer seizures and have greater improvement in depression and functional status compared to eTAU.
-
2.
To use short message service (SMS) delivered via mobile phone text to validate patients, self-reported seizure occurrence and push epilepsy self-management messaging in a practical/accessible format.
-
3.
To obtain input from stakeholders (patients, family and clinicians) guided by an Integrated Promotion Action on Research Implementation in Health Services (i-PARIHS) framework to help establish a sustainable infrastructure that will facilitate future scale-up of SMART in Uganda with epilepsy partners.
Study Setting
The study population will be drawn from neurology clinics where patients with epilepsy and epilepsy-related complications receive free treatment and supportive care at Mulago and Mbarara hospitals. Mulago and Mbarara hospitals are the only hospitals currently that run regular epilepsy clinics supported by neurologists in Uganda, and they tend to provide care for severe forms that are intractable This offers an opportunity to study this intervention.
At Mulago, patients attending the neurology clinic are referred from medical wards, while others are referred from surrounding health centres for specialised care and treatment for epilepsy. The neurology clinic runs on Wednesdays from 9 am −2 pm with an average attendance of about 5–8 patients with confirmed epilepsy. These patients are then given scheduled visits unless certain illnesses warrant an unscheduled visit. Severely ill patients were referred to the respective wards for admission and in-patient care. A total of about 20–30 patients with epilepsy are seen in the neurology clinic monthly.
Mbarara Regional Referral Hospital is a public regional referral hospital for the districts of Mbarara, Bushenyi, Ntungamo, Kiruhura, Ibanda, and Isingiro, with an official bed capacity of 600. It is located in Mbarara, a teaching hospital for the Mbarara University of Science and Technology. MRRH has neurology and psychiatry clinics staffed by physicians and nurses caring for PWE. A neurologist and medical officer run the clinic, which is equipped with an electroencephalogram and technicians and nurses for diagnosis and care. The clinic receives about 5 −10 PWE weekly and 30 PWE monthly.
Study Population
All adult PWE attending the neurology clinics at Mulago and Mbarara hospitals during the study period or their caregivers will be approached. All participants and their legal guardians will provide written informed consent. All consecutive patients who came either for follow-up or as new referrals with a confirmed diagnosis of epilepsy will be screened for inclusion in the study. We will include those who meet the following inclusion criteria: 1. participants will have a clinical diagnosis of epilepsy documented with at least two outpatient visits; 2. aged more than 18 years; 3. provide written informed consent by the study participant or immediate caregiver/legal guardian; 4. Ability to participate in study procedures, 5. having had at least one seizure in the past six months; 6. Owning a mobile phone is done either by the PWE or immediate caregiver.
We will exclude those 1. Diagnosed with dementia, 2. Participants who are pregnant (given the likely need for different and more intensive treatments among pregnant PWE, may affect their ability to participate in the SMART-U sessions regularly and those unable to participate in study procedures.
Sample size determination The estimated sample size for the study is 188 people with epilepsy, allowing for 20% attrition or loss by missing data, reducing the final sample to a minimum of N= 150 participants.
Based on a power analysis using G*Power 3.1.9.2121 for a 2 group by 3-time points (baseline, 13-week and 24-week follow-up), repeated measures analysis of variance (RMANOVA) with an alpha of 0.05, a power of. 80, for a sample of 150, we will be able to detect a small standardised effect size of 0.10 for the within-time effect, within the time-by-group interaction effect and a small to moderate standardised effect of 0.19 for the between-group effect.
Study Procedures
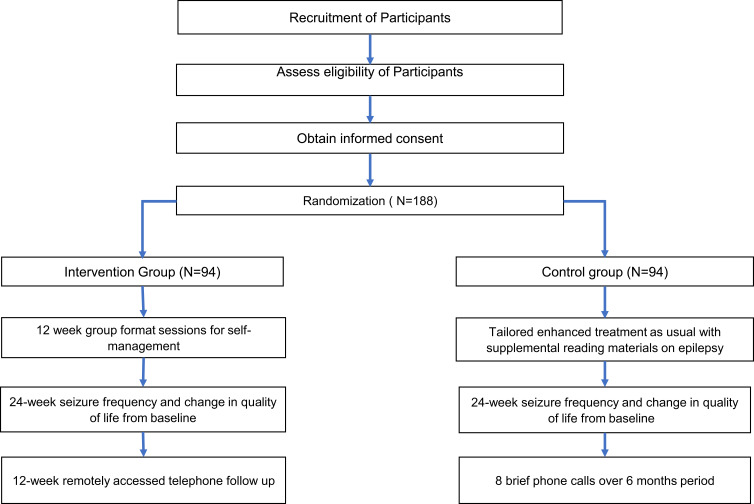
During the Phase 1 part of the study, we will refine the SMART -U intervention in terms of content and process with guidance from stakeholders (patients/family, clinicians, administrators) for the local context. We will establish a Stakeholder Advisory Board (SAB) to guide the modest refinement of the program to meet the diverse needs of stakeholders and integrate SMART-U into clinic workflows. In Phase 2 of the study, we will conduct a prospective 6-month randomised controlled trial (RCT) to evaluate the effects of standard medical care + SMART-U vs enhanced medical treatment as usual (ETAU). Figure 1 shows the flow diagram of the study progress through the phases of a parallel randomised trial of two groups (enhanced treatment as usual and SMART-U intervention). Baseline socio-demographic data and clinical factors like age of seizure onset, aetiology, comorbidities, drug tolerability, stigma and psychological well-being will be collected from the study participants.
Figure 1.
Flow diagram of the study progress through the phases of a parallel randomised trial of two groups (enhanced treatment as usual and SMART-U intervention.
Randomisation
Block srandomisation will be used to randomly assign participants in a 1:1 ratio to receive either SMART-U (N=94) or eTAU (N=94). To guarantee that there are an equal number of SMART and ETAU patients within stratum and that the distribution is balanced, block srandomisation will be carried out with a random block size of 4 to 8 consecutive patients. Non-study staff members from the CWRU Neurological and Behavioural Outcomes Center Biostatistical Core will use a computer to create the srandomisation list.
SMART-U Intervention
Written curricula that include an intervention manual, participant manual, slides, and handouts are used to operationalise SMART-U intervention. The subjects covered are listed in Table 1 and are usually taught over ten to twelve weeks.
Table 1.
Topics Addressed During SMART-U Sessions
| Sessions | Activities/Topic |
|---|---|
| Session 1 | Orientation and introductions; Emphasise ground rules; Establishment of a therapeutic relationship; Facts and myths about epilepsy, and general epilepsy management principles |
| Session 2 | Relationship of epilepsy and stress; Stigma and “double stigma”; Strategies to cope with stigma; Introduction to personal goal setting |
| Session 3 | Treatments for epilepsy; Complications of epilepsy; Minimising epilepsy complications; The importance of daily routine and good sleep habits |
| Session 4 | Problem–solving skills and the IDEA approach (Identify the problem, define possible solutions, Evaluate the solutions, Act on the best solution); Talk with your healthcare providers; Role play of communication with care providers. |
| Session 5 | Nutrition for the best physical and emotional health; Substance abuse and its effects on epilepsy; Specific stress–management approaches |
| Session 6 | Effects of exercise and being outdoors on physical and emotional health; Medication routines; Prioritising medication side effects and discussing it with your clinician |
| Session 7 | Social support and using your available supports; Advocacy groups for epilepsy; A personal care plan to take care of the mind and the body |
| Session 8 | Normalising your life in spite of having a chronic but unpredictable condition; Self-management as a lifestyle; Acknowledgment of group process; Setting the stage for ongoing illness management and recovery. |
Nurses and peer educator dyads (PEDs), which are composed of patients and their care partners, collaborate to offer the intervention using the SMART-U self-management approach. The initial 30 to 60-minute SMART-U orientation session involves the patient and their care partner meeting with the PED and nurse. Eight weekly one-hour group sessions with six to eight patients and their caregivers come next. Every SMART-U participant is still being treated by their usual medical professionals.
Before the study begins, a 2-day in-person intensive training for the study nurse and Peer Educator Dyads (PED, consisting of the PWE and his or her care partner) will occur. Regular “refresher” sessions will then take place to go over the material, work through any issues, and answer any concerns that might come up. When research participants express any urgent or safety issues during the experiment, the study neurologists will be on hand to address them.
Using methods similar to those used in earlier research,9,12 the scientists will enrol up to eight Peer Educators with epilepsy—four from Mulago and four from Mbarara—to administer the SMART-U intervention. This self-management curriculum, which focuses on patient and family needs, problem-solving, and attention to emotional and role management, is co-led by nurses and Peer Educator dyads (PEDs), which are made up of a patient Peer Educator (PWE) and their family member. Peer educators provide relevant life experiences crucial for directing PWE,s self-care and self-management.
Initial Intervention (Stage 1)
Stage 1 comprises eight 45–60 minute in-person group sessions, with up to 10 PWE participants per group. A nurse and PED will work together to administer these sessions. PWE will be encouraged to attend the meetings with a family member who plays a significant role in their epilepsy self-management.
Stage 1 consists of 8 group-format, 45–60-minute in-person sessions (up to 10 PWE participants per group), which a nurse and the PED will collaboratively deliver. PWE will be encouraged to have an important family member in their epilepsy self-management attend the groups with them. SMART-U aims to encourage active self-management among PWE, with minimal participation from family members or care partners. The intervention will focus on interactive discussion and printed materials in local language. It addresses issues related to epilepsy, comorbidity, problem identification, and goal-setting, while behavioral modeling and group format address adherence, social support, and self-efficacy.
Ongoing Self-Management and Recovery (Stage 2)
After the Stage 1 group meetings, participants will have three quick (no more than 15 minutes) monthly maintenance sessions by phone or online, led by the SMART-U nurse. The topics of ongoing epilepsy self-management, such as medication adherence, will be covered in phone sessions. By updating providers on the status of the SMART-U program, the nurse will also act as a linking facilitator between the patient’s epilepsy care clinicians.
Control Arm-Enhanced Treatment as Usual (eTAU) Group
Those who were randomly assigned to eTAU will continue receiving their regular care in addition to written resources on epilepsy that are catered to the majority of clinic patients, reading levels and are available in their preferred language. The regular care PWE receive treatment from the epilepsy clinics at Mulago and Mbarara hospitals, which provide drug refills, address the attendant medical issues and prescriptions for comorbidities and make required referrals for specialised care. Anti-seizure medications are prescribed and provided depending on availability or purchased out of pocket by the patients to meet their required doses. Investigations are requested, such as an electroencephalogram, a Brain CT scan, a full hemogram, renal and liver tests, and serum drug level assessments, which are not readily available.
The nurse in eTAU will follow up with participants with eight short phone calls spaced out over six months (roughly every two weeks during months 1 and 2, then approximately monthly thereafter) to control for the same number of patient contacts as SMART-U.
Those who are randomised to eTAU will continue receiving their regular care in addition to written resources about epilepsy that are catered to the majority of clinic patients, reading levels and are available in their preferred language. During this session, patients can bring a family member who can help them read written materials if they are not very literate and who may also ask questions. The outpatient clinic, where patients receive their medical care, will be the location of this visit. The nurse in eTAU will then follow up with participants with a series of 8 short phone calls spaced out over 6 months (roughly every 2 weeks during months 1 and 2, then approximately monthly thereafter) in order to control for the same amount of patient contacts as SMART-U. The orientation visit materials will be reinforced by the content, and the nurse will be on hand to address any queries that may come up. The SMART-U and eTAU interventions will be administered by distinct nursing workers to reduce the possibility of contamination among the research arms.
SMS Seizure Monitoring and Support
Participants in the SMART-U and eTAU trials will use and receive SMSs to track and report the onset and duration of seizures during the 24-week trial. Every week, participants will get an automated survey over SMS inquiring if they have experienced a seizure during the previous week.
Research Variables and Tools
The study aims to measure the change in cumulative seizure frequency over the past 24-weeks, using self-report data and corroboration from family or support system informants. Participants will be trained using SMS to track seizure occurrence and validate self-reported data, with weekly text messages indicating seizure occurrence and duration. The Quality of Life in Epilepsy (QOLIE-31) questionnaire assesses a patient’s physical, social, mental, and emotional well-being and seizure control features, with higher scores indicating better QOL.
The study will use validated questionnaires to assess secondary outcomes like mental health comorbidity, stigma, and epilepsy self-management competency. The Hospital Anxiety and Depression scale (HADS) will evaluate the effects of SMART-U on depressive symptoms, while the SF-36 will assess functional health status using a validated tool for comparing disease burdens. Both tools have been validated in Uganda and other East African countries.
SMART-U targets patient attitudes and health practices, including epilepsy self-management competency, self-efficacy, social support, and stigma. The Epilepsy Self Efficacy Scale (ESES) measures self-efficacy, while the Multidimensional Scale of Perceived Social Support(MSPSS) measures social support and satisfaction. The Kilifi Stigma Scale of Epilepsy measures perceived stigma, with responses rated on a 3-point Likert scale. The ESES and MSPSS will be used to measure self-efficacy, competency, social support, and stigma to assess patients, attitudes and health practices.
Fidelity
At the conclusion of every eight-session group program, acceptance will be evaluated using a brief self-assessment questionnaire. Following Fraser, both quantitative and qualitative measures will be used to evaluate fidelity to the SMART-U intervention. At least 25% of the group sessions will be seen and scored by research personnel not involved in the intervention. Each facet of fidelity will be rated on a scale from 1 to 10. The number of text responses to the total number of texts sent will be used to determine whether texting is acceptable for both the SMART-U-U and eTAU groups.
Qualitative Evaluation
We will use In-depth interviews on perceived benefits vs burdens and barriers/facilitators to SMART-U, and eTAU implementation will be conducted at each of the 2 sites. Given the corrosive and persistent nature of stigma on QOL among PWE, input and recommendations on specific strategies regarding ways to potentially mitigate stigma in families and communities will be assessed. Informants will include 20 PWE from SMART-U and 20 PWE from eTAU (total N=40). We will conduct qualitative interviews to elicit participant perceptions of the intervention at 13 weeks (when SMART-U groups are completed) and again at 24 weeks. For qualitative interviews, this sample size is within the range of recommended sample sizes (ie, 20–50 individuals).
Study Outcomes
The main outcomes will be the mean change in cumulative past 24-week seizure frequency (24 weeks before the study baseline compared to the 24-week follow-up) and the mean change in quality of life (QOL) from baseline to 24 weeks. Using validated, sstandardised questionnaires, other secondary outcomes will be evaluated, such as stigma, mental health comorbidity, epilepsy self-management ability, and other pertinent characteristics.
Study Data
Data will be collected through the use of study questionnaires. The study teams will be stationed at the outpatient neurology clinics; an interviewer will conduct the interviews and document the participants, responses on the paper questionnaire. The entry clerk will enter the collected data into a Microsoft Access database and double-enter it to guarantee accuracy. At the conclusion of each data-entering session, backup database files will be stored. Query scripts will be written into the database for quality control purposes, limiting inaccurate data entry and ensuring data entry into the relevant fields.
Data Storage
The study will utilize survey questionnaires and investigative results as its primary sources of data. All research materials will be stored in safe file cabinets within the College of Health Sciences offices at Makerere University. The security of every study document will fall under the purview of the primary investigator.
Quality Control
Every member of the research team will receive training on the goals of the project, how to communicate with study participants in an efficient manner, and how to gather high-quality data. Additional training tailored to the project’s tasks—such as administering surveys, conducting interviews, and filling out questionnaires—will be provided to the study team. Standard Operating Procedures (SOPs) will be prepared for every project activity, and each project team member will get a booklet containing all pertinent documentation.
Data Analysis and Data Management
Together with the study statistician and epidemiologist, the investigators at each study site will supervise the quality and input of the data as well as the quantitative data analytic processes. The CWRU statistics and data management staff will also offer data management consultation when necessary. Errors at the time of collection will be decreased by the meticulous creation of data-collecting forms and staff training on correctly filling and verifying them. Further data management procedures, both prior to and during database entry into RedCap, will detect possible issues or anomalous values and other mistakes on data collection forms. Regular data checks and form tracking will be the responsibility of the data management team. SAS 9.4 will be used to prepare analytical data sets.
Repeated Measure Analysis of Variance. The main outcomes of interest are the quality of life (QOL) and cumulative seizure frequency. To evaluate AIM 1, three distinct analytical approaches will be applied. Repeated measures analysis of variance (RMANOVA) will be utilised to investigate QOL and the secondary outcomes, while independent samples t-tests and linear regressions will be employed to evaluate cumulative seizure frequency. We will look at two distinct RMANOVA sets.
Qualitative Data Analysis Plan
Qualitative data analysis. Interview theme analysis will be conducted using accepted qualitative data analysis methods, including specialised software, calculation of inter-rater reliabilities, rater calibration, and systematic synthesis of findings. Additional methodologic detail is provided in the data analysis section.
Adverse Event Identification/Classification
Adverse occurrences will be noted by the study investigators and/or trained research assistants. Regardless of their level of severity, all adverse events will be continuously documented, examined, and reported to the IRB in compliance with local IRB regulations by the study principal investigators. Events that lead to any of the following are classified as serious adverse events (A.E.s): death; a life-threatening encounter; hospitalisation, either in-patient or outpatient; a persistent or significant disability or incapacity; or a congenital anomaly or birth defect (or an event that may require medical or surgical intervention to prevent one of the above-listed outcomes).
Seizures, as a defining hallmark of epilepsy, will be recorded if/when they occur but will not be considered either SAEs or A.E.s. All A.E.s, including all SAEs, will be reported to the IRB according to local IRB policy and to the Data Safety and Monitoring Board (DSMB). A summary report of all adverse events will be submitted annually in the report and at the end of the study.
Data and Safety Monitoring Board (DSMB) and Safety Review Plan
A DSMB for the study will be made up of individuals not on the study team. Every 12 months, they will, at the very least, examine and assess the collected data for participant safety, A.E.s., research conduct, and advancement. Ad hoc meetings may be convened to assess unexpected major adverse events or any other pertinent, urgent matters that may arise throughout the study. The DSMB will be composed of three non-study team members who have vast experience with federally funded research: two clinicians at the Uganda site with expertise in epilepsy, a faculty member/clinician at the US site with knowledge in epilepsy, and a biostatistician at the US site. When there are concerns that require immediate response, the DSMB will communicate and oversee them by Email or videoconference. When there are concerns that require immediate response, the DSMB will communicate and oversee them by Email or videoconference.
Ethics and Dissemination
The International Conference on Harmonization E6 Guideline for Good Clinical Practice,13 the Helsinki Declaration,14 and the NIH Human Subjects guidelines will all be followed in the conduct of the study. Leaders in the community will be consulted before any research in the suggested areas is done. Before beginning any study procedures, the research nurses will get potential participants, informed consent. The study participant will receive a copy of the permission form.
Analytical patient identification will be different from the patient’s clinic medical record number. Files connecting study numbers to patient identities will be stored in locked cabinets in the study staff offices. There will be no publication or presentation of particular patient information—only aggregate data that is unidentifiable. The UNCST, HS2944ES and the School of Medicine, Research and Ethics Committee (SOMREC) Mak-SOMREC-2023-648 have sauthorised protocol version 2, which was approved on September 29, 2023. The main findings of this research will be discussed at national conferences, and the results will be submitted for peer-reviewed publications.
Discussion
For adults with epilepsy, this is the first randomised controlled study of self-management education conducted in Uganda. The study intends to offer qualitative and quantitative evidence of the influence of this self-management intervention on the patient’s quality of life and seizure frequency. The intervention has been modified for use within the Ugandan healthcare delivery system.
Evidence-based self-management approaches can improve health outcomes, but quality research data are needed to guide clinical care. Self-management approaches, including work done by this study team, can improve health outcomes in PWE.15–17 However, given diverse needs across populations and cultures, there is no “one-size-fits-all” epilepsy self-management program. This calls for refining approaches such to make them culturally acceptable and feasible for LMICs. Partnering with community stakeholders to develop feasible and culturally acceptable interventions, performing rigorous testing of these interventions, and empowering PWE facilitates the potential scale-up of efficacious interventions in similar settings across SSA.
The above-described study procedures aim to assess the obstacles and enablers of epilepsy self-management while conducting a SMART effectiveness trial in both rural and urban settings.
The use of a lived experience and provider stakeholder group made up of individuals, as well as the integration of mobile services in Ugandan epilepsy care, are novel and significant aspects of the proposed project. This study method allows a focus on pragmatic epilepsy outcomes relevant to payers/policymakers and both PLWE and them, including hospitalisations, attempts at self-harm, stigma, depression, and seizure occurrence.
Acknowledgments
The authors would like to express their gratitude to everyone who helped create the suggested strategy, including those who have risk factors or epilepsy. The paper has been uploaded to The National Library of Medicine as a preprint: Self-management Intervention for Reducing Epilepsy Burden Among Adult Ugandans With Epilepsy (Smart-u), Randomised Clinical Trial Protocol - PubMed.
Funding Statement
Research reported in this publication was supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS129041 to Mark Kaddumukasa and Martha Sajatovic. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
DSMB, Data safety monitoring board; eTAU, Enhanced Treatment As Usual; PWE, People with epilepsy; QOL, Quality of life; SMART-U, Self-Management intervention for Reducing The epilepsy burden among Ugandans; SMS, Short messaging service; CWRU, Case Western Reserve University.
Data Sharing Statement
No datasets were generated or analyzed during the current study. All relevant data will be made available upon its completion.
Ethics Approval and Consent to Participate
The study was approved by the School of Medicine, Research and Ethics Committee (SOMREC) Mak-SOMREC-2023-648, and UNCST; HS2944ES. Written informed consent was obtained by the study participant or immediate caregiver/legal guardian. The International Conference on Harmonization E6 Guideline for Good Clinical Practice, the Helsinki Declaration, and the NIH Human Subjects guidelines will all be followed in the conduct of the study.
Authors, Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work”.
Disclosure
Professor Martha Sajatovic reports grants from US National Institute of Neurological Disorders and Stroke, during the conduct of the study; grants from Neurelis, Intra-Cellular, Merck, Otsuka, Alkermes, International Society for Bipolar Disorders (ISBD), personal fees from Alkermes, Otsuka, Lundbeck, Janssen, Teva, Publication royalties from Springer Press, Johns Hopkins University Press, Oxford Press, UpToDate, Compensation for preparation of continuing medical education activities from American Physician’s Institute (CMEtoGo), Psychopharmacology Institute, American Epilepsy Society, Clinical Care Options, outside the submitted work. The authors declare that they have no other competing interests.
References
- 1.Epilepsy. Available from: http://www.who.int/mental_health/neurology/epilepsy/en/. Accessed November 19, 2024.
- 2.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–890. doi: 10.1111/j.1528-1167.2009.02481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsten H, Nystrom L, Forsgren L. Mortality risk in an adult cohort with a newly diagnosed unprovoked epileptic seizure: a population-based study. Epilepsia. 2000;41(11):1469–1473. doi: 10.1111/j.1528-1157.2000.tb00124.x [DOI] [PubMed] [Google Scholar]
- 4.Forsgren L, Hauser WA, Olafsson E, Sander JW, Sillanpaa M, Tomson T. Mortality of epilepsy in developed countries: a review. Epilepsia. 2005;46(Suppl 11):18–27. doi: 10.1111/j.1528-1167.2005.00403.x [DOI] [PubMed] [Google Scholar]
- 5.Neligan A, Bell GS, Shorvon SD, Sander JW. Temporal trends in the mortality of people with epilepsy: a review. Epilepsia. 2010;51(11):2241–2246. doi: 10.1111/j.1528-1167.2010.02711.x [DOI] [PubMed] [Google Scholar]
- 6.Ngugi AK, Bottomley C, Kleinschmidt I, et al. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12(3):253–263. doi: 10.1016/S1474-4422(13)70003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baskind R, Birbeck GL. Epilepsy-associated stigma in sub-Saharan Africa: the social landscape of a disease. Epilepsy Behav. 2005;7(1):68–73. doi: 10.1016/j.yebeh.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 8.WHO. Epilepsy in the WHO African region: bridging the gap? In: Epilepsy in the African Region. Geneva: WHO; 2004:5–14. [Google Scholar]
- 9.Ovuga E, Kipp W, Mungherera M, Kasoro S. Epilepsy and retarded growth in a hyperendemic focus of onchocerciasis in rural western Uganda. East Afr Med J. 1992;69(10):554–556. [PubMed] [Google Scholar]
- 10.Watts AE. A model for managing epilepsy in a rural community in Africa. BMJ. 1989;298(6676):805–807. doi: 10.1136/bmj.298.6676.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mushi D, Hunter E, Mtuya C, Mshana G, Aris E, Walker R. Social-cultural aspects of epilepsy in Kilimanjaro Region, Tanzania: knowledge and experience among patients and carers. Epilepsy Behav. 2011;20(2):338–343. doi: 10.1016/j.yebeh.2010.11.016 [DOI] [PubMed] [Google Scholar]
- 12.Mbuba CK, Ngugi AK, Newton CR, Carter JAJE. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49(9):1491–1503. doi: 10.1111/j.1528-1167.2008.01693.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guideline IHT. E6: Note for Guidance on Good Clinical Practice. (PMP/ICH/135/95). European Medicines Agency; 2002. [Google Scholar]
- 14.Jama WMAJ. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 15.Ozuna J, Kelly P, Towne A, Hixson J. Self-management in epilepsy care: untapped opportunities. Fed Pract. 2018;35(Suppl 3):S10. [PMC free article] [PubMed] [Google Scholar]
- 16.Helmers SL, Kobau R, Sajatovic M, et al. Self-management in epilepsy: why and how you should incorporate self-management in your practice. Epilepsy Behav. 2017;68:220–224. doi: 10.1016/j.yebeh.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajatovic M, Jobst BC, Shegog R, et al. The managing epilepsy well network:: advancing epilepsy self-management. Am J Prev Med. 2017;52(3 Suppl 3):S241–S245. doi: 10.1016/j.amepre.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study. All relevant data will be made available upon its completion.