Abstract
Background
Serum creatinine and cystatin C levels are influenced by different non-renal factors. The difference between the estimated glomerular filtration rates (eGFRdiff) based on these two markers helps assess cardiovascular risk factors unrelated to kidney function. However, its impact on post-ablation atrial fibrillation (AF) recurrence remains unknown.
Methods
From July 2017 to February 2023, we prospectively observed 989 consecutive AF patients who underwent radiofrequency ablation. The association between eGFRdiff and post-ablation AF recurrence was analyzed using Kaplan-Meier methods, adjusted Cox regression analysis, and restricted cubic spline (RCS) analysis.
Results
During a median follow-up period of 29 months, 326 cases of AF recurrence were detected. Participants were divided into three groups based on eGFRdiff: high (≥ −9.22), medium (−20.98 to −9.22), and low (≤ −20.98). Multivariable Cox proportional hazards models revealed that, compared to the medium eGFRdiff group, individuals in the low eGFRdiff group (hazard ratio [HR] 1.46, 95% confidence interval [CI] 1.08–1.94, p < 0.01) and the high eGFRdiff group (HR 1.69, 95% CI 1.27–2.27, p < 0.01) had a significantly increased risk of AF recurrence. RCS analysis demonstrated a U-shaped association between eGFRdiff and AF recurrence. Stratified analyses confirmed the robustness of the core findings across subgroups, except for females. Notably, the geriatric nutritional risk index and the derived neutrophil-to-lymphocyte ratio partially mediated the association between eGFRdiff and high AF recurrence by 5.7% and 10.7%, respectively.
Conclusion
eGFRdiff is related to individual nutritional and inflammatory statuses and can be used to predict the risk of AF recurrence. (Clinical trial registration number: ChiCTR-OIN-17013021).
Keywords: atrial fibrillation, estimated glomerular filtration rate, cystatin C, creatinine, recurrence
Plain Language Summary
This study investigated the association between eGFRdiff and AF recurrence after ablation. eGFRdiff is calculated from two different markers in the blood (creatinine and cystatin C), which reflect both kidney health and other body conditions like nutrition and inflammation. We followed 989 patients who had AF and underwent ablation from July 2017 to February 2023. We found that eGFRdiff was much higher or lower, the risk of AF recurrence increased, forming a U-shaped curve. This pattern was consistent in most groups of patients, except for women who showed a different trend. Our findings suggest that both nutritional status and inflammation play roles in AF recurrence, but their impacts differ based on eGFRdiff levels. Poor nutrition was more significant at lower eGFRdiff levels, while inflammation was more crucial at higher levels. Interestingly, inflammation seemed to have a stronger impact on women. These insights emphasize the need to monitor and manage patients’ nutritional and inflammatory status to reduce the risk of AF recurrence, especially considering sex-specific factors. Future larger studies are needed to confirm these results and further refine treatment strategies.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, contributing significantly to adverse events such as heart failure, stroke, and mortality.1 Catheter ablation has emerged as an effective treatment strategy for maintaining sinus rhythm in patients with symptomatic AF refractory to medical therapy.2 Despite advancements in ablation techniques, AF recurrence post-ablation remains a substantial clinical challenge, necessitating the identification of reliable predictors for recurrence.3,4
Serum creatinine and cystatin C are widely utilized biomarkers for estimating glomerular filtration rate, an essential indicator of renal function. However, these markers are influenced by different non-renal factors. Serum creatinine is affected by muscle mass, diet, and certain medications, whereas cystatin C levels are impacted by age, gender, inflammation, and thyroid function.5 Consequently, discrepancies between the estimated glomerular filtration rate derived from creatinine (eGFRcr) and cystatin C (eGFRcys) can provide insights into various physiological and pathological conditions beyond renal function. The difference between eGFR based on these two biomarkers (eGFRdiff) has been proposed as a novel metric to evaluate factors distinct from kidney function. There are often significant intra-individual differences between eGFRcys and eGFRcr. Malmgren et al5 highlighted the risk of potentially overlooking patients with severe kidney disorders if relying solely on eGFRcr.
The co-occurrence of AF and kidney injury is linked to a higher rate of AF recurrence.6 Several studies have demonstrated that eGFRcys has a stronger association with AF incidence compared to eGFRcr.7,8 Additionally, previous studies have shown an association between eGFRdiff and cardiovascular risk,9–11 which are important predictors of poor prognosis following AF ablation. Therefore, this study aims to investigating the association between eGFRdiff and AF recurrence after radiofrequency ablation, while also examining whether nutritional and inflammatory statuses mediate this relationship.
Methods
Study Population
We prospectively and consecutively enrolled 989 patients diagnosed with AF who were hospitalized at the Third People’s Hospital of Chengdu (Sichuan, China) and underwent radiofrequency ablation from July 2017 to February 2023. The exclusion criteria were as follows: (1) age under 18 years; (2) history of cardiac surgery; (3) presence of left atrial appendage thrombosis or other absolute contraindications to ablation; (4) AF secondary to reversible or pathological factors such as hyperthyroidism, acute alcoholism, electrolyte disturbances, drug effects or severe valvular heart disease; and (5) incomplete data on key variables, including cystatin C and creatinine levels. This study was approved by the ethics committees of the China Ethics Committee of Registering Clinical Trials (No. ChiECRCT-20170082) and conducted in strict accordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants.
Data Collection and Definitions
The pre-ablation characteristics, comprehensive medical histories, and imaging data of all patients were collected. Baseline serum samples were collected, and cystatin C and serum creatinine concentrations were measured before the ablation. The estimated glomerular filtration rate based on serum creatinine was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation with race coefficients.12 The estimated glomerular filtration rate based on cystatin C was calculated using the CKD-EPI 2012 equation.13 The primary indicator of interest was the baseline eGFRdiff, defined as eGFRcys minus eGFRcr.
Ablation Strategy
All patients received anticoagulation therapy prior to ablation, and left atrial thrombosis was excluded using transoesophageal echocardiography. Ablation procedures were performed under general anesthesia with the assistance of a three-dimensional imaging system (CARTO-3 system, Version 6) and continuous intraoperative monitoring of vital signs. Patients with paroxysmal AF underwent circumferential ablation around the pulmonary veins, with verification of conduction block between the pulmonary veins and the atrium. In patients with persistent AF, additional left atrial ablation beyond pulmonary vein isolation was permitted. Ablation of the cavotricuspid isthmus was conducted only in patients with documented typical atrial flutter.
Assessment of Endpoints
All patients were prescribed antiarrhythmic drugs for 3 months unless contraindicated. Follow-up visits were scheduled at 3, 6, and 12 months post-procedure, conducted by outpatient physicians who were blinded to the study design. During these visits, patients underwent 24-hour Holter electrocardiogram monitoring and were asked about any arrhythmia-related symptoms. In subsequent phases, clinical visits were scheduled every 6 months, with annual 24-hour Holter monitoring. Patients who experienced symptoms suggestive of arrhythmia were advised to undergo 24-hour Holter monitoring at the nearest hospital. The primary endpoint was AF recurrence, defined as the occurrence of at least 30 seconds of atrial tachyarrhythmia detected on an electrocardiogram or Holter monitoring after a single procedure, excluding events occurring within the blanking period (the first 3 months post-procedure).
Statistical Analysis
The baseline characteristics are presented as the mean and standard deviation for continuous variables and as numbers and percentages for categorical variables across different subgroups stratified by tertiles of the eGFRdiff. Multivariate Cox proportional hazards regression models, adjusted for potential confounders, were used to estimate hazard ratios for AF recurrence associated with eGFRdiff. Restricted cubic spline curves based on Cox proportional hazards models were employed to examine the nonlinear or irregular shapes of the hazard functions, with knots between 3 and 7 selected as the lowest value for the Akaike information criterion. Kaplan–Meier curves for event-free patients in the different groups were plotted and compared using the Log rank test.
Given that eGFRdiff is largely determined by cystatin C and creatinine concentrations, and these variables are highly correlated with eGFRdiff, we adjusted for cystatin C and creatinine concentrations to better capture the associations between eGFRdiff and the risk of recurrence. Additionally, several subgroup analyses were conducted to explore whether the associations between eGFRdiff and the risk of recurrence remained consistent across patients with diverse demographic characteristics or comorbidities. Considering that inflammation and nutritional status can differently affect creatinine and cystatin C levels and are independent risk factors for AF recurrence, the Geriatric Nutritional Risk Index (GNRI) and derived Neutrophil-to-Lymphocyte Ratio (dNLR) were used for further mediation analysis to investigate whether these factors mediate the association between eGFRdiff and post-ablation recurrence risk.
Statistical analyses were performed using R (version 4.3.1; R Foundation, Vienna, Austria) and SPSS (version 29.0; IBM, Armonk, New York). A value of P less than 0.05 (two-tailed) was considered to indicate statistical significance.
Results
Baseline Characteristics of the Study Cohort
Table 1 shows the baseline characteristics of 989 participants. The mean participant age was 65.7 years at baseline; 49.2% (487/989) were female. During a median followup period of 29 months (interquartile range: 18 to 43 months), AF recurrence was detected in 326 participants (33.0%). Based on the tertiles of eGFRdiff, patients were divided into three groups: T1 (eGFRdiff ≤-20.98), T2 (−20.98< eGFRdiff ≤-9.22) and T3 (eGFRdiff>-9.22). Baseline characteristics of the study population by eGFRdiff tertiles are presented in Table 2. Patients in the T2 group were older, had a higher proportion of females, and exhibited higher left ventricular ejection fraction and APPLE scores. The APPLE score, a predictive model for AF recurrence, showed a decreasing trend across the three groups, indicating that the probability of AF recurrence, as inferred from common combined risk factors, gradually decreased.
Table 1.
Baseline Characteristics of the Study Cohort
| Parameters | All Patients (n=989) |
|---|---|
| Demographics | |
| Age, years | 65.7±10.6 |
| Female sex, n (%) | 487 (49.2) |
| Body-mass index, kg/m2 | 24.7±3.4 |
| Current Smoker, n (%) | 140 (14.2) |
| Alcohol consumption, n (%) | 125 (12.6) |
| Duration after AF diagnosis, days | 453 (90, 1556) |
| Persistent atrial fibrillation, n (%) | 450 (45.5) |
| Previous medical history, n (%) | |
| Hypertension | 548 (55.4) |
| Diabetes mellitus | 204 (20.6) |
| Vascular disease | 142 (14.4) |
| Heart failure | 165 (16.7) |
| Previous stroke | 69 (7.0) |
| Dyslipidemia | 199 (20.1) |
| Chronic obstructive pulmonary disease | 36 (3.6) |
| Laboratory data | |
| Fasting blood glucose, mmol/L | 5.5±1.5 |
| Brain natriuretic peptide, pg/mL | 111 (66, 235) |
| Creatinine, umol/L | 76.8±20.6 |
| Cystatin C, mg/L | 1.10±0.31 |
| Albumin, g/dl | 39.8±4.5 |
| Cholesterol, mmol/L | 4.21±1.02 |
| Triglyceride, mmol/L | 1.45±0.89 |
| Imaging | |
| Left atrial diameter, mm | 41.4±5.6 |
| Left ventricular end diastolic diameter, mm | 45.9±4.8 |
| Left ventricular ejection fraction, % | 59.3±5.6 |
| Medication at discharge, n (%) | |
| New oral anticoagulants | 814 (82.3) |
| Vitamin K antagonist oral anticoagulant | 138 (14.0) |
| Class III antiarrhythmic drugs | 686 (69.4) |
| Beta blocker | 158 (16.0) |
| Calcium channel blocker | 40 (4.0) |
| CHA2DS2-VASc score | 2.53±1.66 |
| APPLE score | 1.62±1.07 |
| eGFRCys | 71.5±20.2 |
| eGFRcr | 86.5±17.2 |
| eGFRdiff | −15.0±14.2 |
Notes: Vascular disease, denotes peripheral artery disease or coronary heart disease; APPLE score, calculated by age >65 years, Persistent atrial fibrillation, eGFRcr <60 mL/min/1.73 m2, Left atrial diameter ≥43mm, and Left ventricular ejection fraction <50%; eGFRdiff, eGFRcys-GFRcr. Data are presented as the mean ± SD, median (IQR), or n (%).
Abbreviations: AF, atrial fibrillation; eGFRcys, estimated glomerular filtration rate based on cystatin C; eGFRcr, estimated glomerular filtration rate based on creatinine.
Table 2.
Baseline Characteristics of the Study Population by eGFRdiff Tertiles
| Parameters | T1 (n=329) | T2 (n=330) | T3 (n=330) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 66.5±10.7 | 67.3±9.6 | 63.4±11.0 | <0.001 |
| Female sex, n (%) | 138 (41.9) | 176 (53.3) | 173 (52.4) | 0.005 |
| Body-mass index, kg/m2 | 24.7±3.3 | 24.8±3.5 | 24.5±3.2 | 0.673 |
| Current Smoker, n (%) | 56 (17.0) | 39 (11.8) | 45 (13.6) | 0.151 |
| Alcohol consumption, n (%) | 51 (15.5) | 31 (9.3) | 43 (13.0) | 0.060 |
| Duration after AF diagnosis, days | 450 (92, 1609) | 435 (90, 1578) | 495 (92, 1470) | 0.935 |
| Persistent atrial fibrillation, n (%) | 166 (50.4) | 144 (43.6) | 140 (42.4) | 0.083 |
| Previous medical history, n (%) | ||||
| Hypertension | 198 (60.2) | 178 (53.9) | 172 (52.1) | 0.092 |
| Diabetes mellitus | 68 (20.7) | 66 (20.0) | 70 (21.2) | 0.928 |
| Vascular disease | 46 (14.0) | 51 (15.5) | 45 (13.6) | 0.779 |
| Heart failure | 68 (20.7) | 46 (13.9) | 51 (15.5) | 0.052 |
| Previous stroke | 25 (7.6) | 23 (7.0) | 21 (6.4) | 0.824 |
| Dyslipidemia | 66 (20.1) | 75 (22.7) | 58 (17.6) | 0.256 |
| Chronic obstructive pulmonary disease | 13 (4.0) | 16 (4.8) | 7 (2.1) | 0.162 |
| Laboratory data | ||||
| Fasting blood glucose, mmol/L | 5.4±1.2 | 5.6±1.7 | 5.6±1.4 | 0.231 |
| Brain natriuretic peptide, pg/mL | 160 (84, 311) | 108 (67, 237) | 108 (60, 210) | 0.391 |
| Creatinine, umol/L | 71.9±13.8 | 78.3±20.0 | 80.2±25.5 | <0.001 |
| Cystatin C, mg/L | 1.21±0.24 | 1.12±0.31 | 0.96±0.32 | <0.001 |
| Albumin, g/dl | 39.4±3.8 | 40.0±4.1 | 39.9±5.3 | 0.165 |
| Cholesterol, mmol/L | 4.16±1.02 | 4.31±1.06 | 4.17±0.99 | 0.105 |
| Triglyceride, mmol/L | 1.47±0.96 | 1.44±0.81 | 1.44±0.91 | 0.899 |
| Imaging | ||||
| Left atrial diameter, mm | 42.0±5.5 | 41.1±5.5 | 41.0±5.8 | 0.055 |
| Left ventricular end diastolic diameter, mm | 46.2±5.0 | 45.4±4.6 | 46.2±4.8 | 0.110 |
| Left ventricular ejection fraction, % | 58.8±5.9 | 60.0±5.0 | 59.3±5.6 | 0.020 |
| Medication at discharge, n (%) | ||||
| New oral anticoagulants | 264 (80.2) | 279 (84.5) | 271 (82.1) | 0.349 |
| Vitamin K antagonist oral anticoagulant | 49 (14.9) | 42 (12.7) | 47 (14.2) | 0.712 |
| Class III antiarrhythmic drugs | 232 (70.5) | 228 (69.1) | 226 (68.5) | 0.845 |
| Beta blocker | 51 (15.5) | 50 (15.2) | 57 (17.3) | 0.728 |
| Calcium channel blocker | 11 (3.3) | 13 (3.9) | 16 (4.8) | 0.614 |
| CHA2DS2-VASc score | 2.61±1.64 | 2.62±1.64 | 2.35±1.68 | 0.062 |
| APPLE score | 1.67±1.05 | 1.70±1.05 | 1.49±1.11 | 0.022 |
| eGFRCys | 61.1±13.8 | 68.5±16.8 | 84.7±21.5 | <0.001 |
| eGFRcr | 91.3±13.2 | 83.6±17.0 | 84.5±19.8 | <0.001 |
| eGFRdiff | −30.2±7.4 | −15.1±3.5 | 0.28±8.3 | <0.001 |
Notes: The eGFRdiff groups were stratified by the tertiles of the eGFRdiff: T1 (eGFRdiff ≤ −20.98), T2 (−20.98 < eGFRdiff ≤ −9.22) and T3 (eGFRdiff > −9.22). Vascular disease, denotes peripheral artery disease or coronary heart disease; APPLE score, calculated by age >65 years, Persistent AF, eGFRcr <60 mL/min/1.73 m2, Left atrial diameter ≥43mm, and Left ventricular ejection fraction <50%; eGFR ratio, eGFRdiff, eGFRcys-GFRcr. Data are presented as the mean ± SD, median (IQR), or n (%).
Abbreviations: AF, atrial fibrillation; eGFRcys, estimated glomerular filtration rate based on cystatin C; eGFRcr, estimated glomerular filtration rate based on creatinine.
Association Between the eGFRdiff and Post-Ablation AF Recurrence
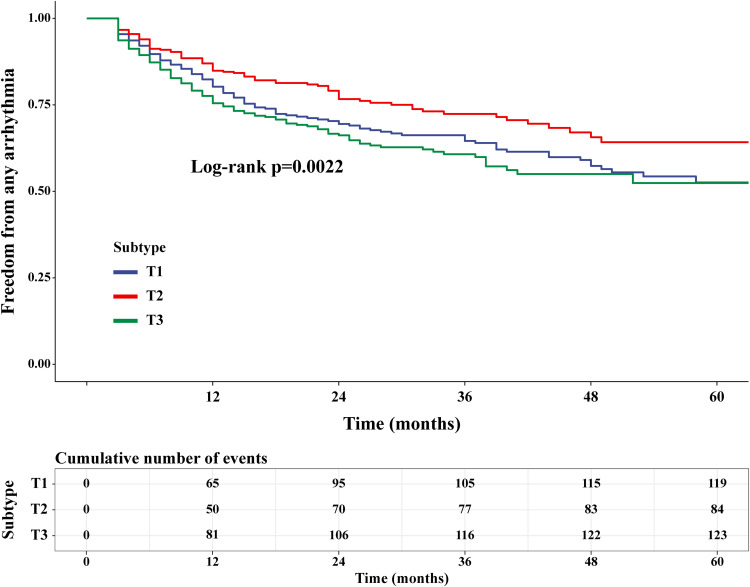
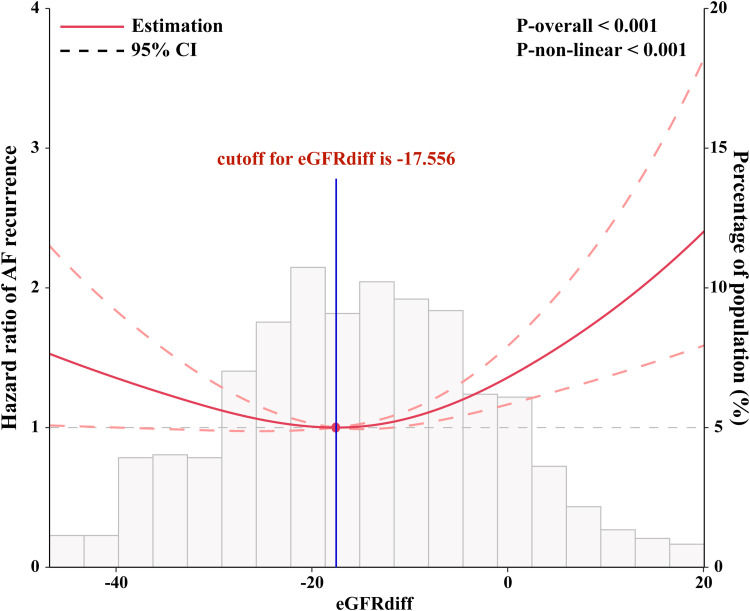
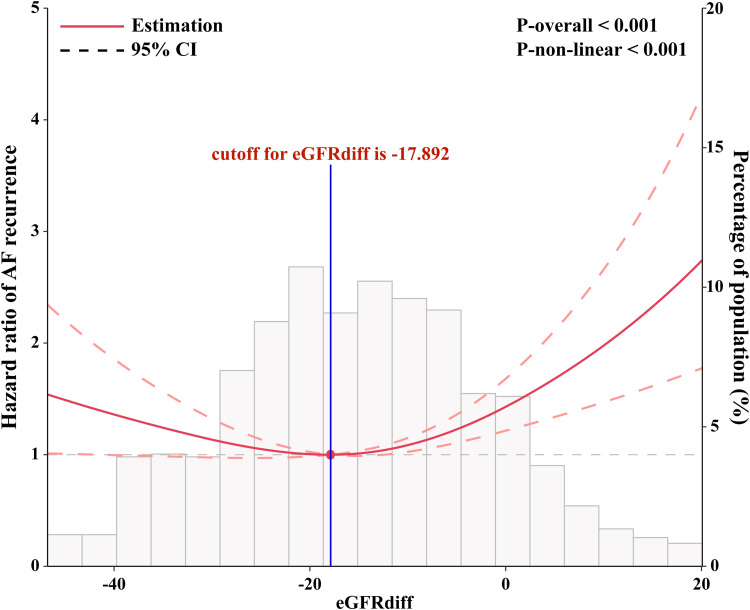
Interestingly, the Kaplan-Meier survival analysis results showed that the T2 group had a lower long-term AF recurrence rate compared to the T1 and T3 groups (Figure 1). Compared with T2 group, the multivariable adjusted hazard ratio for AF recurrence was 1.46 (95% confidence interval [CI] 1.08 to 1.94) for T1 group and 1.69 (95% CI 1.27 to 2.27) for T3 group (Table 3). Univariate Cox proportional hazards models demonstrated U-shaped associations between eGFRdiff and the risk of AF recurrence (P < 0.001) (Figure 2). After fully adjusting for potential confounders influencing AF recurrence, the results consistently showed a statistically significant U-shaped association between eGFRdiff and the risk of experiencing AF recurrence (P<0.001) (Figure 3).
Figure 1.
Kaplan-Meier curves for AF recurrence-free survival across different tertiles of eGFRdiff (T1, T2, and T3 groups). The Log rank test was used for comparison, showing that T2 group had a lower long-term AF recurrence rate compared to T1 and T3 groups.
Abbreviation: AF, atrial fibrillation.
Table 3.
Hazard Ratios for AF Recurrence by eGFRdiff Tertiles
| Tertiles of eGFRdiff | Recurrence, No./Total No. | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| T1 | 119/329 | 1.41 (1.07 to 1.87) | 1.48 (1.12 to 1.97) | 1.46 (1.09 to 1.95) | 1.46 (1.08 to 1.94) |
| T2 | 84/330 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| T3 | 123/330 | 1.61 (1.22 to 2.13) | 1.79 (1.34 to 2.38) | 1.72 (1.29 to 2.30) | 1.69 (1.27 to 2.27) |
Notes: The eGFRdiff groups were stratified by the tertiles of the eGFRdiff: T1 (eGFRdiff ≤ −20.98), T2 (−20.98 < eGFRdiff ≤ −9.22) and T3 (eGFRdiff > −9.22). Model 1: Cox proportional hazards model without adjustment; Model 2: Model 1 with adjustment for age, sex and body mass index; Model 3: Model 2 with additional adjustment for comorbidities (hypertension, diabetes, vascular disease, heart failure, previous stroke, dyslipidemia and chronic obstructive pulmonary disease); Model 4: Model 3 with additional adjustment for laboratory measurements (brain natriuretic peptide, fasting blood glucose, triglyceride and cholesterol), imaging parameters (left atrial diameter and ejection fraction), AF type, antiarrhythmic drugs, oral anticoagulants and duration after atrial fibrillation diagnosis. Vascular disease, denotes peripheral artery disease or coronary heart disease.
Abbreviation: AF, atrial fibrillation.
Figure 2.
Restricted cubic spline plots based on univariate Cox proportional hazards models for the associations between the eGFRdiff and the risk of AF recurrence. The results are presented as hazard ratios (HRs, solid lines) and 95% confidence intervals (CIs, light red areas). The knots were set at the 10th, 50th, and 90th percentiles of the eGFRdiff. The histograms represent the distribution of the eGFRdiff in our study.
Abbreviation: AF, atrial fibrillation.
Figure 3.
Restricted cubic spline plots based on multivariate Cox proportional hazards models. The models were adjusted for sex, age, body mass index, AF type, duration from AF diagnosis to ablation, fasting blood glucose, triglyceride, cholesterol, brain natriuretic peptide, left ventricular ejection fraction, left atrial diameter, antiarrhythmic drugs, oral anticoagulants, high blood pressure, diabetes, and history of vascular disease, chronic obstructive pulmonary disease, congestive heart failure, stroke, and dyslipidaemia. The knots were set at the 10th, 50th, and 90th percentiles of the eGFRdiff.
Abbreviation: AF, atrial fibrillation.
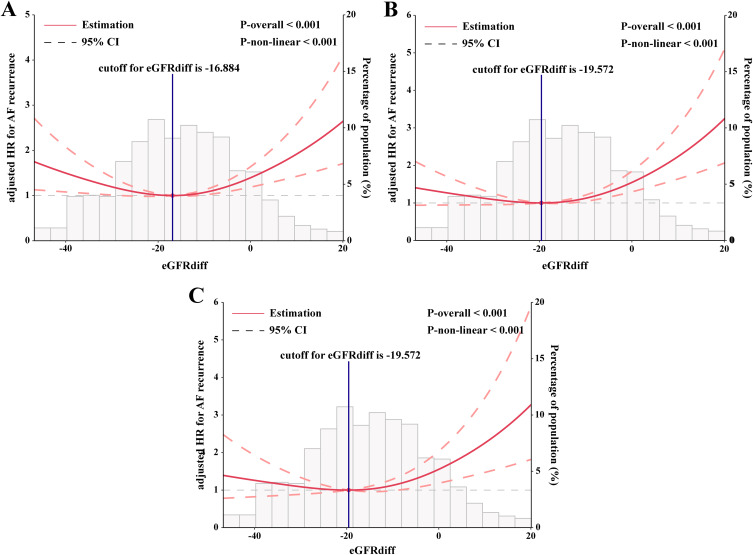
The inflection point (cut-off value) of the U-shaped curve indicating the lowest risk of AF recurrence was identified as an eGFRdiff of −17.892 after full adjustment for confounding factors. The curve indicated that the risk of AF recurrence was significantly reduced for individuals with an eGFRdiff in the lower range, and it increased thereafter. After full adjustment for all covariates, additional adjustments were made for creatinine and cystatin C. The eGFRdiff showed a consistent association with the risk of AF recurrence after further adjusting for creatinine (Figure 4A), cystatin C (Figure 4B), and both creatinine and cystatin C (Figure 4C).
Figure 4.
Association between eGFRdiff and AF recurrence after additional adjustment for (A) Creatinine, (B) Cystatin C and (C) Both creatinine and cystatin C. The results are presented as hazard ratios (HRs, solid lines) and 95% confidence intervals (CIs, light red areas). The knots were set at the 10th, 50th, and 90th percentiles of the eGFRdiff.
Abbreviation: AF, atrial fibrillation.
Association Between the eGFRdiff and Post-Ablation AF Recurrence in Various Subgroups
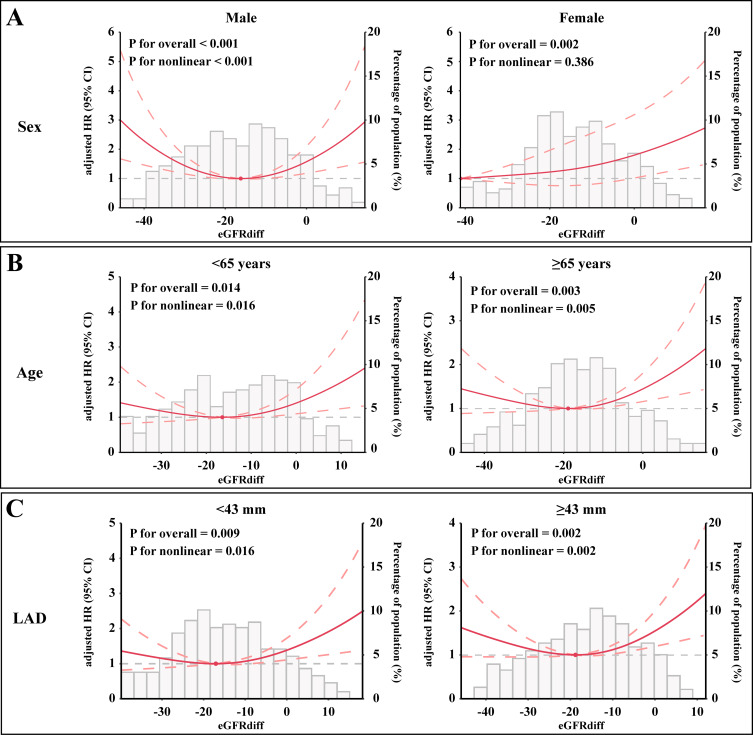
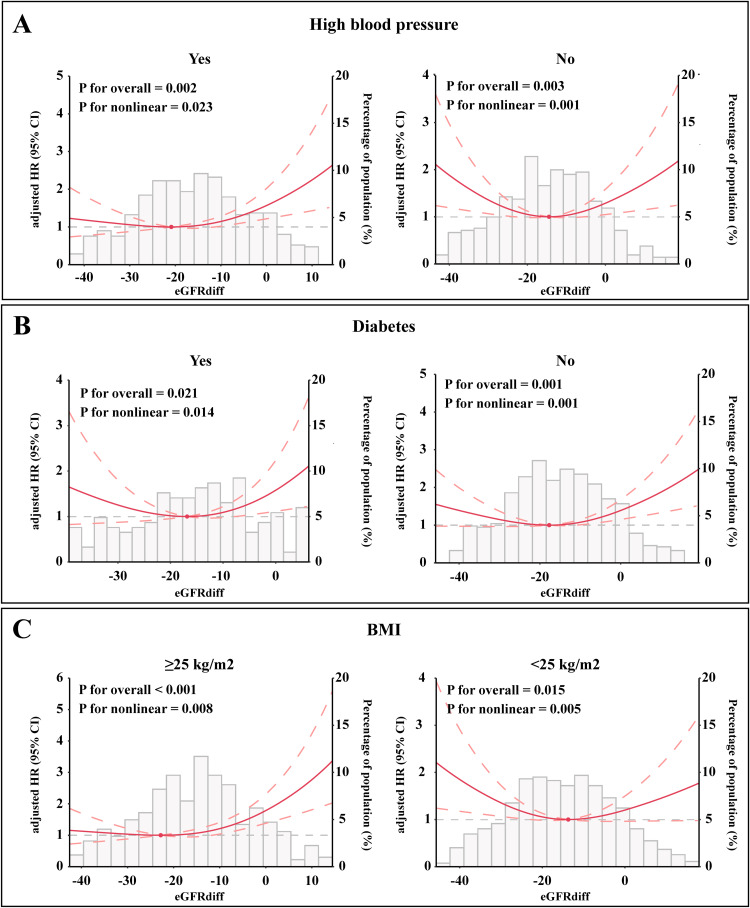
According to our subgroup analysis, a strong U-shaped association between eGFRdiff and the risk of experiencing AF recurrence was observed in men. In women, although eGFRdiff was significantly associated with the risk of experiencing AF recurrence, a positive linear association was found (Figure 5A). Additionally, U-shaped associations between eGFRdiff and the risk of AF recurrence were observed among participants aged ≥65 years, aged <65 years, with a left atrial diameter <43 mm, and with a left atrial diameter ≥43 mm (Figure 5B and C). We also examined the associations stratified by high blood pressure (Figure 6A), diabetes (Figure 6B), and BMI level (Figure 6C). A similar U-shaped or J-shaped association between eGFRdiff and the risk of AF recurrence was observed across all six subgroups. Notably, a stronger U-shaped association between eGFRdiff and the risk of AF recurrence was found in patients without high blood pressure and with a BMI <25 kg/m², compared to those with high blood pressure and a higher BMI.
Figure 5.
Subgroup analysis of the association between eGFRdiff and the risk of AF recurrence. A U-shaped association in (A) Men and participants (B) Aged both ≥65 years and <65 years, as well as in those with a (C) Left atrial diameter <43 mm and ≥43 mm, while a positive linear association was found in (A) women. The results are presented as hazard ratios (HRs, solid lines) and 95% confidence intervals (CIs, light red areas) after multivariate adjustment for all confounders.
Abbreviation: AF, atrial fibrillation.
Figure 6.
Subgroup analysis of the association between eGFRdiff and the risk of AF recurrence stratified by (A) High blood pressure status, (B) Diabetes status, and (C) BMI level. The results show a stronger U-shaped association in patients without high blood pressure and with a BMI <25 kg/m², while a J-shaped association is observed in those with high blood pressure and BMI ≥25 kg/m².
Abbreviations: AF, atrial fibrillation; BMI, Body Mass Index.
Mediation Analysis of eGFRdiff for Post-Ablation AF Recurrence
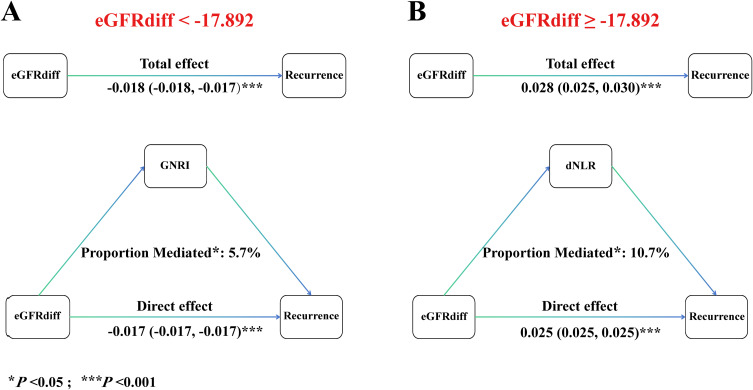
When eGFRdiff is in the lower range (< −17.892), mediation analyses revealed a total effect of GNRI on AF recurrence of −0.018 (95% CI, −0.018 to −0.017; P < 0.001), which includes a direct effect of 0.0085 (95% CI, −0.017 to −0.017; P < 0.001) (Figure 7A). GNRI exhibited a significant negative indirect effect on AF recurrence, with an estimated mediated effect proportion of 5.7% (95% CI, 1.7% to 10.0%; P < 0.05). Additionally, when eGFRdiff is in the higher range (≥ −17.892), the total effect of dNLR on AF recurrence was 0.028 (95% CI, 0.025 to 0.030; P < 0.001), and the direct effect was 0.025 (95% CI, 0.025 to 0.025; P < 0.001) (Figure 7B). dNLR significantly mediated the positive association between eGFRdiff and AF recurrence, with an estimated mediated effect proportion of 10.7% (95% CI, 2.1% to 20.0%; P < 0.05).
Figure 7.
Mediation analysis of GNRI and dNLR on the association between eGFRdiff and AF recurrence. (A) When eGFRdiff is in the lower range (< −17.892). GNRI exhibited a significant negative indirect effect on AF recurrence, (B) when eGFRdiff is in the higher range (≥ −17.892). dNLR significantly mediated the positive association between eGFRdiff and AF recurrence.
Abbreviations: AF, atrial fibrillation; GNRI, Geriatric Nutritional Risk Index; dNLR, derived Neutrophil-to-Lymphocyte Ratio.
Discussion
In this study, we found a U-shaped association between preoperative eGFRdiff and post-ablation AF recurrence. Specifically, the recurrence rate was lowest when eGFRdiff was −17.892; higher or lower eGFRdiff values were associated with an increased risk of AF recurrence. This association was confirmed across multiple subgroup analyses, demonstrating stability except in women, where a positive linear relationship was observed. These findings also suggest that managing nutritional and inflammatory factors may be important when selecting and managing patients undergoing AF ablation.
Association Between eGFRdiff and AF Recurrence
Estimated GFR based on serum creatinine is often imprecise, prompting the consideration of cystatin C as a viable alternative. Subsequent studies demonstrated that formulas combining both biomarkers perform better than those using either marker alone.13,14 Scholars have noted that the estimated GFR difference shows significant predictive value in a variety of diseases, and has been used not only to assess renal function, but also as a marker of overall health and frailty.15–17 Based on these characteristics, it has attracted attention in the field of cardiovascular disease and has been shown to be associated with poor prognosis of heart failure,18 coronary atherosclerosis,19 and mortality.20 Recently, a large prospective cohort study suggested that the eGFRdiff value is associated with the risk of AF developing.21
Therefore, this study further explored the relationship between eGFRdiff and AF recurrence after radiofrequency ablation. The results showed that eGFRdiff was an independent predictor of AF recurrence and remained consistent even after adjusting for creatinine and cystatin C levels. eGFRdiff as the result of subtraction, factors that can affect eGFRcr or eGFRcys can affect eGFRdiff. Previous studies have reported that increasing age, decreased muscle mass, inflammation, and decreased physical activity can affect eGFRdiff.5,22 In conclusion, eGFRdiff, as a comprehensive health indicator reflecting the overall physiological and pathological status of patients, can be used as an independent predictor of AF recurrence.
Sex Differences in Association Between eGFRdiff and AF Recurrence
In this study, we identified significant sex differences in the association between eGFRdiff and the risk of AF recurrence. Specifically, a strong U-shaped association was observed in men, whereas a positive linear association was noted in women, with the overall population displaying a U-shaped trend. Several potential reasons may explain these findings. First, we observed that there is an inherent difference in eGFRdiff between male and female participants in our study. Second, physiological differences between the sexes could play a crucial role. For instance, some studies suggested that women are more susceptible to inflammation and fibrosis,23–25 which may further affect the sex-specific relationship between eGFRdiff and AF recurrence. Additionally, sex differences in muscle mass, fat distribution, and inflammatory response may have contributed to the different patterns observed between men and women. It is noteworthy that our population consisted mainly of older adults around 65 years of age with fewer comorbidities, some of whom were on medical therapy, and more complex patient populations may require further validation.
Influence of Nutritional and Inflammatory Status on AF Recurrence
Considering that eGFRdiff has been considered as a comprehensive indicator reflecting non-renal functions, its precise clinical significance has been challenging to define, necessitating an expansion of its clinical understanding. Based on prior literature, we introduced nutritional and inflammatory status into our analysis. Our study found that both inflammation and poor nutritional status are associated with a higher AF recurrence rate. Inflammation is widely acknowledged as the pathophysiological foundation for the initiation, progression, and maintenance of AF.26 It may enhance the ectopic activity associated with triggering,27 and has been shown to be associated with AF recurrence.28 Furui et al29 demonstrated that malnourished patients have a higher recurrence rate of AF after catheter ablation compared to those with normal nutritional status. Additionally, our prior research indicated that low hemoglobin levels, closely linked to poor nutritional status, can influence AF recurrence by inducing atrial remodeling.30
Moreover, our results suggest that the impact of nutritional status on AF recurrence is more pronounced when eGFRdiff is in the lower range, whereas inflammation may play a more critical role when eGFRdiff is in the higher range. In summary, mediation analysis establishes a link between eGFRdiff and the increased risk of AF recurrence, but this relationship should be interpreted with caution.
Limitations
First, as with any observational study, we cannot establish causality, and the predominantly single-center sample may limit the generalizability of our findings. Therefore, our results should be interpreted with caution within the context of our study population, and further validation in more diverse populations is necessary. Next, the eGFRdiff is derived from secondary calculations, and its clinical significance is limited, which may lead to interpretations that are not entirely robust. Finally, while mediation analysis suggests that eGFRdiff could be associated with inflammatory nutritional status, further biological testing is required to understand the pathophysiological changes in different populations.
Conclusion
This study identified eGFRdiff as an independent risk factor for AF recurrence after radiofrequency ablation. A U-shaped association was observed, indicating that both elevated and reduced eGFRdiff values were associated with a higher risk of AF recurrence. Additionally, the study suggested that eGFRdiff might contribute to AF recurrence by affecting inflammatory and nutritional status.
Funding Statement
This work was supported by Natural Science Foundation of China (Grant No. 82400390), Chengdu High-level Key Clinical Specialty Construction Project (Grant No. 2021YJ0215), and Chengdu famous doctor Liu Hanxiong studio (Grant No. 20240216).
Abbreviations
AF, atrial fibrillation; CI, confidence interval; dNLR, derived Neutrophil-to-Lymphocyte Ratio; GNRI, Geriatric Nutritional Risk Index; eGFRcr, estimated glomerular filtration rate based on creatinine; eGFRcys, estimated glomerular filtration rate based on cystatin C; eGFRdiff, the difference between the estimated glomerular filtration rates.
Data Sharing Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were approved by local ethics committees, and all patients gave informed consent. The privacy of the participants was covered, and all data were anonymized to maintain the confidentiality of the patient information.
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- 1.Kornej J, Börschel CS, Benjamin EJ, et al. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4–20. doi: 10.1161/CIRCRESAHA.120.316340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 3.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2):e004549. doi: 10.1161/JAHA.112.004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muston BT, Bilbrough J, Eranki A, et al. Mid-to-long-term recurrence of atrial fibrillation in surgical treatment vs. catheter ablation: a meta-analysis using aggregated survival data. Ann Cardiothorac Surg. 2024;13(1):18–30. doi: 10.21037/acs-2023-afm-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malmgren L, Öberg C, Den Bakker E, et al. The complexity of kidney disease and diagnosing it - cystatin C, selective glomerular hypofiltration syndromes and proteome regulation. J Intern Med. 2023;293(3):293–308. doi: 10.1111/joim.13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng H, Shantsila A, Xue Y, et al. Renal function and outcomes after catheter ablation of patients with atrial fibrillation: the Guangzhou atrial fibrillation ablation registry. Arch Cardiovasc Dis. 2019;112(6–7):420–429. doi: 10.1016/j.acvd.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Mcmanus DD, Corteville DC, Shlipak MG, et al. Relation of kidney function and albuminuria with atrial fibrillation (from the Heart and Soul Study). Am J Cardiol. 2009;104(11):1551–1555. doi: 10.1016/j.amjcard.2009.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Burgh AC, Geurts S, Ikram MA, et al. Bidirectional association between kidney function and atrial fibrillation: a population-based cohort study. J Am Heart Assoc. 2022;11(10):e025303. doi: 10.1161/JAHA.122.025303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz EF, Kukin M, Javed F, et al. Right ventricular dysfunction is a strong predictor of developing atrial fibrillation in acutely decompensated heart failure patients, ACAP-HF data analysis. J Card Fail. 2010;16(10):827–834. doi: 10.1016/j.cardfail.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Herou E, Dardashti A, Nozohoor S, et al. The mortality increase in cardiac surgery patients associated with shrunken pore syndrome correlates with the eGFR(cystatin C)/eGFR(creatinine)-ratio. Scand J Clin Lab Invest. 2019;79(3):167–173. doi: 10.1080/00365513.2019.1576101 [DOI] [PubMed] [Google Scholar]
- 11.Xhakollari L, Grubb A, Jujic A, et al. The Shrunken pore syndrome is associated with poor prognosis and lower quality of life in heart failure patients: the HARVEST-Malmö study. ESC Heart Fail. 2021;8(5):3577–3586. doi: 10.1002/ehf2.13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A IL, H SC, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang LW, Luo MQ, Zeng JL, et al. The association of intraindividual difference between cystatin- and creatinine-based estimated GFR and contrast-associated acute kidney injury. Clin Interv Aging. 2024;19:411–420. doi: 10.2147/CIA.S447042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potok OA, Ix JH, Shlipak MG, et al. The difference between cystatin C- and creatinine-based estimated GFR and associations with frailty and adverse outcomes: a cohort analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. 2020;76(6):765–774. doi: 10.1053/j.ajkd.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potok OA, Katz R, Bansal N, et al. The difference between cystatin C- and creatinine-based estimated GFR and incident frailty: an analysis of the Cardiovascular Health Study (CHS). Am J Kidney Dis. 2020;76(6):896–898. doi: 10.1053/j.ajkd.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen DC, Shlipak MG, Scherzer R, et al. Association of intra-individual differences in estimated GFR by creatinine versus cystatin C with incident heart failure. Am J Kidney Dis. 2022;80(6):762–72.e1. doi: 10.1053/j.ajkd.2022.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H, Park JT, Lee J, et al. The difference between cystatin C- and creatinine-based eGFR is associated with adverse cardiovascular outcome in patients with chronic kidney disease. Atherosclerosis. 2021;335:53–61. doi: 10.1016/j.atherosclerosis.2021.08.036 [DOI] [PubMed] [Google Scholar]
- 20.Chen DC, Shlipak MG, Scherzer R, et al. Association of intraindividual difference in estimated glomerular filtration rate by creatinine vs cystatin C and end-stage kidney disease and mortality. JAMA Network Open. 2022;5(2):e2148940. doi: 10.1001/jamanetworkopen.2021.48940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heo GY, Koh HB, Jung CY, et al. Difference between estimated GFR based on cystatin C versus creatinine and incident atrial fibrillation: a Cohort Study of the UK Biobank. Am J Kidney Dis. 2024;83(6):729–38.e1. doi: 10.1053/j.ajkd.2023.11.004 [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Li Y, Yang X, et al. Serum cystatin C as an inflammatory marker in exacerbated and convalescent COPD patients. Inflammation. 2016;39(2):625–631. doi: 10.1007/s10753-015-0287-x [DOI] [PubMed] [Google Scholar]
- 23.Huang W, Sun H, Xiong S, et al. Sex-related differences in left atrial substrate among patients with atrial fibrillation: evidence from high-density voltage mapping. Eur J Med Res. 2024;29(1):354. doi: 10.1186/s40001-024-01952-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu N, Liu W, Yang G, et al. Sex difference in atrial scar prevalence: what can we learn from the STABLE-SR-III trial? Heart Rhythm. 2024;21(7):1001–1007. doi: 10.1016/j.hrthm.2024.02.020 [DOI] [PubMed] [Google Scholar]
- 25.De With RR, Artola Arita V, Nguyen BO, et al. Different circulating biomarkers in women and men with paroxysmal atrial fibrillation: results from the AF-RISK and RACE V studies. Europace. 2022;24(2):193–201. doi: 10.1093/europace/euab179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nattel S, Heijman J, Zhou L, et al. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res. 2020;127(1):51–72. doi: 10.1161/CIRCRESAHA.120.316363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D, Li Y, Zhao Q. Effects of inflammatory cell death caused by catheter ablation on atrial fibrillation. J Inflamm Res. 2023;16:3491–3508. doi: 10.2147/JIR.S422002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W, Sun H, Tang Y, et al. Platelet-to-lymphocyte ratio improves the predictive ability of the risk score for atrial fibrillation recurrence after radiofrequency ablation. J Inflamm Res. 2023;16:6023–6038. doi: 10.2147/JIR.S440722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furui K, Morishima I, Morita Y, et al. Impact of preoperative nutritional status on the outcome of catheter ablation for atrial fibrillation. Circ J. 2022;86(2):268–276. doi: 10.1253/circj.CJ-21-0218 [DOI] [PubMed] [Google Scholar]
- 30.Huang W, Sun H, Luo Y, et al. Including hemoglobin levels and female sex provide the additional predictive value of the APPLE score for atrial fibrillation recurrence post-catheter ablation. Hellenic J Cardiol. 2023. doi: 10.1016/j.hjc.2023.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.