Abstract
Purpose
Ocular cysticercosis, caused by Taenia solium larvae, presents significant public health challenges, especially in regions with poor sanitation. Traditional imaging techniques often fail to detect anterior segment cysticercosis accurately, necessitating the exploration of more advanced diagnostic modalities like Ultrasound Biomicroscopy (UBM).
Patients and Methods
A retrospective observational analysis was conducted on 18 eyes from 14 patients with cysticercosis involving the anterior segment. UBM imaging was performed using a Sonomed VuMax HD ultrasound biomicroscopy machine with a 50 MHz probe.
Results
In the study, 18 eyes from 18 patients were analyzed, revealing 12 cases of conjunctival cysticercosis, 4 of anterior chamber cysticercosis, and 2 of iris cysticercosis. Patients averaged 37.89 (± 16.2) years, with a male predominance. Conjunctival cysts appeared as clear masses, occasionally eliciting inflammatory reactions. Iris involvement caused thinning near the angle, while anterior chamber cysts could displace the lens, leading to iris bombe in some instances.
Conclusion
UBM emerges as a valuable diagnostic tool for anterior segment cysticercosis, providing detailed imaging superior to traditional methods. Its cost-effectiveness and accessibility make it particularly valuable, especially in resource-constrained settings. Future research should focus on validating UBM’s diagnostic accuracy and exploring its role in monitoring disease progression and treatment response.
Keywords: ultrasound biomicroscopy, cysticercosis, Taenia solium, anterior segment
Introduction
Cysticercosis, caused by the larval stage of the pork tapeworm Taenia solium, remains a significant public health concern, particularly in regions with poor sanitation and limited access to healthcare.1–3 This parasitic disease has a complex life cycle that involves humans as definitive hosts and pigs as intermediate hosts.3 The transmission to humans occurs through the ingestion of contaminated food or water containing the tapeworm’s eggs.3 Once ingested, the eggs hatch in the intestine, and the larvae migrate to various tissues, including the muscles, brain, and eyes, where they develop into cysticerci.3
While neurocysticercosis, involving the central nervous system, garners considerable attention due to its potential for severe neurological sequelae, ocular cysticercosis poses unique diagnostic and therapeutic challenges.3–6 The eye, with its complex anatomy and delicate structures, is particularly susceptible to inflammatory and degenerative changes induced by cysticercosis.3–6 The parasitic infection can affect different ocular structures, including the anterior segment, posterior segment, extraocular muscles, and the orbital cavity.4–6 The presence of cysticerci in the eye can lead to a range of symptoms, including visual disturbances, pain, and inflammation, which can significantly impair vision and quality of life.4–6 Given the potential severity of ocular cysticercosis, early and accurate diagnosis is crucial for timely intervention and management. Traditional imaging techniques like ultrasound B-scan, while effective in identifying ocular pathologies, often fall short in detecting anterior segment cysticercosis due to their limited resolution and penetration depth.7,8
Ultrasound Biomicroscopy (UBM) has emerged as a valuable diagnostic tool for evaluating anterior segment abnormalities, including cysticercosis.8,9 Unlike conventional ultrasound B-scan, which is limited in its ability to identify lesions in the anterior segment due to its lower resolution and depth penetration, UBM offers high-resolution imaging of the anterior segment structures with greater detail and accuracy.8,10 The principle of UBM is based on the use of high-frequency ultrasound waves (typically 35–50 MHz) to produce detailed cross-sectional images at 40 micron resolution of the anterior segment, allowing for the visualization of subtle anatomical changes and pathological features.7,10
The role of UBM in the detection of anterior segment cysticercosis is of paramount importance. Unlike neuroimaging modalities such as CT or MRI, which are primarily focused on central nervous system involvement, UBM provides a direct view of ocular structures, allowing for the identification of cysticercosis cysts in the anterior chamber comprising the conjunctiva, anterior chamber, and iris.7–10 Ultrasound is preferable to CT due to the avoidable radiation exposure of the eye lens and the better resolution compared to MRI. It allows for the precise localization, sizing, and characterization of cysticerci in the anterior segment, thereby facilitating targeted treatment planning and monitoring of the disease progression.9,10 Also, cysticercosis is seen as a cyst with scolex within it on UBM, which helps differentiate it from other entities such as simple cysts, dermoid, and solid tumors.9,10 This UBM morphology corresponds to the histopathology of the entity with the central scolex surrounded by the cystic wall.8–10 Additionally, it is a more cost-effective and practical imaging modality, especially in lower-middle income countries (LMICs) which are resource-constrained.
Despite the growing recognition of UBM’s utility in detecting anterior segment cysticercosis, there is a paucity of literature, specifically addressing its role in this context. Therefore, the purpose of this study was to evaluate the role of UBM in the detection and characterization of cysticercosis involving the anterior segment of the eye, including the conjunctiva, anterior chamber, and iris. By analyzing a series of cases with confirmed ocular cysticercosis, we aim to demonstrate the diagnostic capabilities of UBM and highlight its potential benefits in the management of this challenging condition.
Materials and Methods
The study represents a retrospective observational analysis of sequential cases featuring patients identified with cysticercosis in the intraocular anterior segment. Carried out at a specialized tertiary ophthalmic ultrasound imaging facility in Mumbai, India, the study encompassed data from January 2003 to December 2023. The investigation adhered to the principles set forth in the Declaration of Helsinki, and it received approval from the Harmony Ethics Research Committee. All participating patients provided written informed consent for both the ultrasound scanning procedures and the possible publication of research findings.
The ultrasound imaging was conducted using the Paradigm B-scan from 1993 to 2003 and the Sonomed VuMax HD ultrasound biomicroscopy machine from 2004 to 2023. The UBM machine is equipped with a 50 MHz probe, which is optimized for high-resolution imaging of superficial ocular structures. The probe’s resolution capability is 40 microns, ensuring detailed visualization of fine anatomical features. It is noteworthy that the depth of penetration of the 50 MHz probe is limited to approximately 4 mm, and thus, only the anterior segment of the eye up to the posterior capsule of the lens was visualized. Prior to imaging, a coupling agent, typically a gel, was applied to the ocular surface to facilitate optimal acoustic contact between the probe and the eye. The patient’s eye was then carefully positioned, and the probe was gently placed (in the coupling medium) to obtain real-time, cross-sectional images of the anterior segment. The acquired images exhibit a level of detail that closely resembles the view obtained when examining tissues under a low-power microscope. To maintain consistency and reliability in the data collection, all UBM scans were performed by a single imaging specialist (DB) with over 35 years of experience in ocular imaging. This ensured standardized imaging protocols and minimized variability in image acquisition and interpretation. Standardized imaging protocols use a cup with a coupling medium placed over the eyeball. The 50 Mhz probe is place within the coupling medium and rotated such that the area of interest is clearly seen.
Patient data including age and gender were collected. UBM images were analysed to identify the anatomical location and to characterize cysticercosis lesions in the conjunctiva, anterior chamber, and iris. Specific features such as cyst size, location, relationship with adjacent ocular structures, and presence of inflammatory reactions were documented.
Statistical analysis was performed to delineate demographic characteristics, including mean age and gender distribution, among the study cohort. Descriptive statistics were employed to summarize the clinical features of anterior segment cysticercosis, with emphasis on distinctive findings associated with conjunctival, anterior chamber, and iris involvement.
Results
Study Cohort
A total of 18 eyes from 18 patients were included in the study, comprising 12 eyes with conjunctival cysticercosis, 4 eyes with anterior chamber cysticercosis, and 2 eyes with iris cysticercosis. The mean age of the patients was 37.89 years with a standard deviation of 16.2 years. The male-to-female ratio was 11:7, reflecting a slightly higher prevalence among males.
Anatomical Distribution
Conjunctival Cysticercosis
Conjunctival cysticercosis had varied presentations on UBM. In a few cases, a thickened cyst wall was noted causing external pressure on the sclera, while few were thin walled associated with scleral thinning (Figure 1). The size of the scolex varied too, which few cases demonstrating large scolex with significantly thickened cyst wall (Figure 2), while few having a thin walled cyst with a small scolex (Figure 3). Occasionally, secondary inflammatory reactions were observed, leading to membrane formation in and around the cyst (Figures 3 and 4) or severe conjunctival reactions around the cyst (Figure 4).
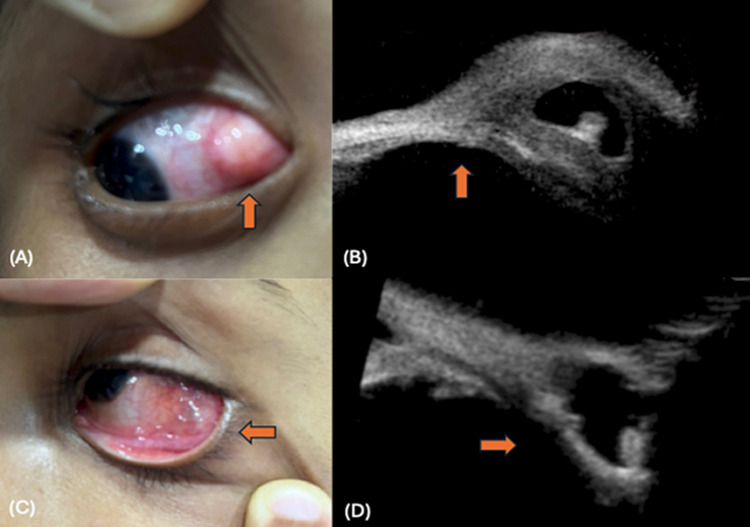
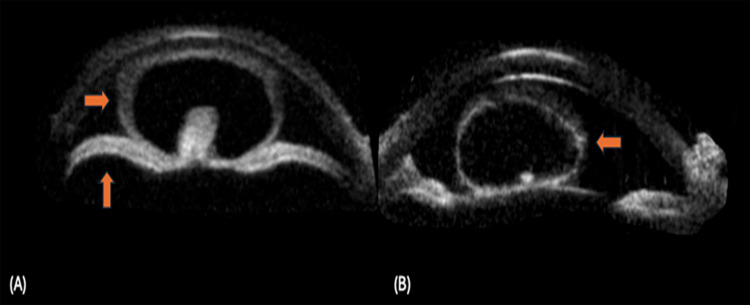
Figure 1.
Conjunctival cysticercosis: (A) thick walled cyst noted at the temporal fornix (up arrow), (B) UBM shows a thick walled cyst with scolex causing external pressure on the sclera (up arrow), (C) thin walled cyst with translucency noted (left arrow) in inferior fornix, (D) UBM shows a thin walled cyst with scolex leading to marked thinning of the sclera (right arrow).
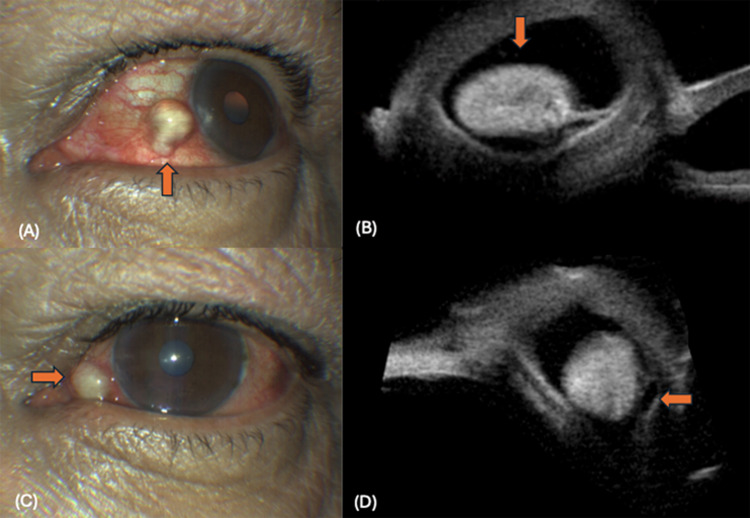
Figure 2.
Conjunctival cysticercosis: (A) Thick walled cyst noted at the nasal limbus (up arrow), (B) Thick walled cysticercosis noted with a large scolex (down arrow), (C) Cyst noted at the nasal fornix (right arrow), (D) Thick walled cyst noted with a large echogenic scolex (left arrow).
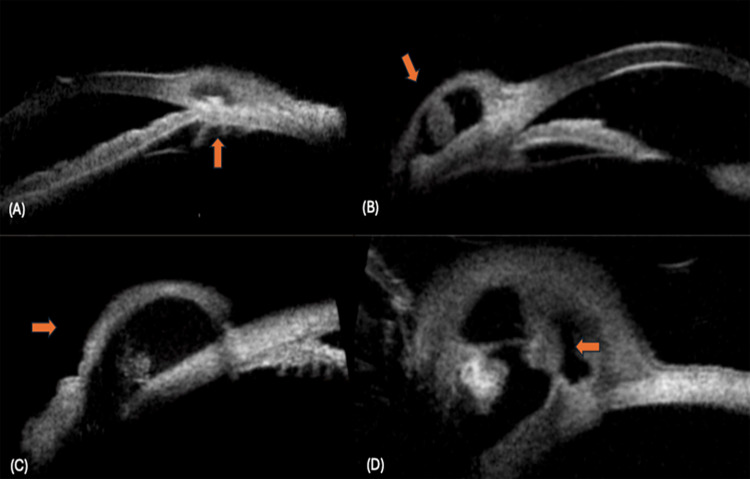
Figure 3.
Conjunctival cysticercosis: (A) Small thin walled cyst noted with a small scolex (up arrow), (B) A thick walled cyst with a large scolex (oblique arrow), (C) A thin walled cyst with a small scolex (right arrow), (D) Thick walled cyst with scolex and secondary inflammation causing septa within the cyst (left arrow).
Figure 4.
Conjunctival cysticercosis: (A) Thick walled cyst noted at limbus showing multiple hypoechoic dots around the scolex, suggestive of secondary inflammation (left arrow), (B) Cyst with scolex, not a very thick wall of the cyst (oblique arrow), due to severe conjunctival reaction around the cyst.
Iris Cysticercosis
Iris cysticercosis was typically seen near the angle region and led to marked thinning of the iris, potentially causing secondary angle closure. Extension of the cyst into the ciliary body was also noted in some cases (Figure 5).
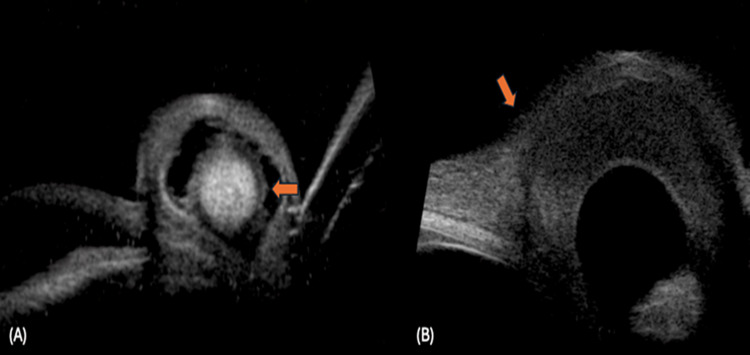
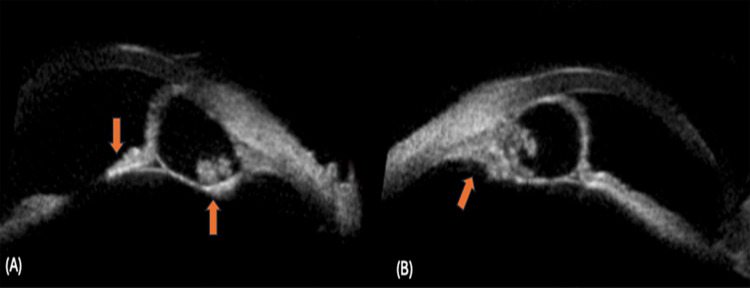
Figure 5.
Iris cysticercosis: (A) Shows a well-defined cyst with scolex (up arrow), the remaining iris is seen near the pupillary margin (down arrow), (B) Shows a iris cysticercosis with scolex extending into the ciliary body (oblique arrow).
Anterior Chamber Cysticercosis
Anterior chamber cysticercosis occupied the entire anterior chamber and could push the lens posteriorly, causing secondary iris bombe. Thin-walled cysts did not exert pressure on the lens or iris in some cases (Figure 6).
Figure 6.
Anterior chamber cysticercosis: (A) A thick walled anterior chamber cysticercosis with a scolex noted (right arrow), the cyst has pushed the lens posteriorly causing iris bombe (up arrow), (B) A thin walled cysticercosis noted with a small scolex (left arrow).
Discussion
Our study explored the utility of UBM in diagnosing and characterizing anterior segment cysticercosis. We examined 18 eyes from 18 patients with various manifestations of ocular cysticercosis. The majority presented with conjunctival cysticercosis, followed by anterior chamber and iris involvement. UBM demonstrated high-resolution imaging capabilities, allowing precise localization, sizing, and characterization of cysticerci in the anterior segment. This study underscores the significant potential of UBM as an effective diagnostic tool for anterior segment cysticercosis, aiding in targeted treatment planning and disease monitoring.
Cysticercosis is caused by the larval stage of Taenia solium, the pork tapeworm.1–3 Its life cycle involves humans as definitive hosts and pigs as intermediate hosts.3 Humans become infected by ingesting food or water contaminated with tapeworm eggs.3 Once ingested, the eggs hatch in the intestine, releasing larvae that migrate to various tissues, including muscles, brain, and eyes, where they form cysticerci.3 These cysticerci can cause a range of symptoms depending on their location, including neurological and ocular manifestations.3
Ocular cysticercosis poses unique diagnostic and therapeutic challenges due to the eye’s complex anatomy and susceptibility to inflammatory and degenerative changes induced by the parasite. The infection can affect various ocular structures, including the anterior and posterior segments, extraocular muscles, and the orbital cavity.4–6 Anterior segment involvement, particularly in the conjunctiva, anterior chamber, and iris, can lead to visual disturbances, pain, and inflammation, significantly impairing vision and quality of life.4–6 The clinical presentation varies depending on the location of the cysticercus. This study demonstrates the varied presentations of conjunctival cysticercosis on UBM, highlighting its complex impact on ocular structures. Cases with thickened cyst walls caused external pressure on the sclera, potentially leading to structural deformation, while thin-walled cysts were associated with scleral thinning, indicating different mechanical interactions. The size of the scolex also varied, with larger scolexes linked to significantly thickened cyst walls, and smaller scolexes found in thin-walled cysts. This suggests a possible correlation between the parasite size and host tissue response. Additionally, secondary inflammatory reactions, such as membrane formation around the cyst and severe conjunctival reactions, were observed, complicating management.11,12 Iris cysticercosis typically leads to marked thinning of the iris near the angle region, potentially causing secondary angle closure.4–6 Anterior chamber cysticercosis can occupy the entire anterior chamber, pushing the lens posteriorly and causing secondary complications like iris bombe.13–15 Early and accurate diagnosis of anterior segment cysticercosis is crucial for timely intervention and management. Traditional imaging techniques like ultrasound B-scan often fall short in detecting anterior segment lesions due to limited resolution and depth penetration.7,8 Thus, there is a need for high-resolution imaging modalities like UBM to accurately visualize and characterize cysticercosis in the anterior segment, facilitating targeted treatment and monitoring of disease progression.7,10
The pathogenesis of ocular cysticercosis involves the migration and encystation of Taenia solium larvae in ocular tissues.4–6 In the anterior segment, cysticerci can lead to inflammation, degeneration, and structural changes due to their presence and associated inflammatory responses.13–15 Conjunctival cysticercosis can cause external pressure on the sclera or marked thinning, leading to potential complications like membrane formation or severe conjunctival reactions.11,12 Iris cysticercosis results in thinning of the iris, potentially causing secondary angle closure or extending into the ciliary body.4–6,13 Anterior chamber cysticercosis can push the lens posteriorly, causing iris bombe or other secondary complications.14,15 The presence of cysticerci induces inflammatory reactions, altering the normal anatomy and function of the affected ocular structures. Understanding these pathogenic mechanisms is crucial for effective diagnosis, management, and treatment planning.
Ultrasound has been widely utilized in the diagnosis of ocular cysticercosis, with traditional B-scan ultrasound being the most commonly used modality.4–6 However, its effectiveness in detecting anterior segment cysticercosis is limited due to its lower resolution and depth penetration.7,8 UBM has emerged as a valuable diagnostic tool for evaluating anterior segment abnormalities, offering high-resolution imaging of the anterior segment structures with greater detail and accuracy.8,9 UBM is a high-resolution imaging modality that utilizes high-frequency ultrasound waves (typically 35–50 MHz) to produce detailed cross-sectional images of the anterior segment of the eye, including the conjunctiva, anterior chamber, iris, and lens.7–10 Unlike traditional ultrasound B-scan, which is limited by its lower resolution and depth penetration, UBM offers superior imaging capabilities, allowing for the visualization of subtle anatomical changes and pathological features with greater detail and accuracy.7–10
The principle of UBM is based on the reflection of ultrasound waves at tissue interfaces, producing high-resolution images that closely resemble the view obtained when examining tissues under a low-power microscope.16,17 This enables the precise localization, sizing, and characterization of various ocular abnormalities, including cysticercosis, tumors, inflammatory conditions, and structural anomalies, facilitating accurate diagnosis and targeted treatment planning.8,16 Also, unlike CT and MRI, which primarily focus on central nervous system involvement and require the use of contrast agents, UBM provides a direct view of ocular structures without the need for ionizing radiation or contrast administration, making it safer and more convenient for patients.10,18 Furthermore, UBM’s real-time imaging capabilities enable dynamic assessment of cysticercosis lesions, facilitating monitoring of disease progression, treatment response, and postoperative outcomes.16,17 This is particularly beneficial in cases requiring surgical intervention, as UBM can provide valuable insights regarding the extent and location of lesions, aiding in surgical planning, intraoperative guidance, and postoperative management.
UBM’s role in ocular disorders extends beyond cysticercosis, encompassing a wide range of anterior segment pathologies, such as glaucoma, cataract, corneal diseases, and anterior segment tumors.8,10,16,17 It provides valuable information regarding the anatomical configuration, integrity, and relationship of ocular structures, aiding in the differential diagnosis, staging, and management of various ocular disorders. Despite the growing recognition of UBM’s utility in detecting and characterizing anterior segment lesions, there is a paucity of literature specifically addressing its role in the context of cysticercosis. There is a single case report illustrating the cysticerci lesion on UBM.9 Future studies should focus on evaluating the diagnostic accuracy, reliability, and reproducibility of UBM in detecting and characterizing anterior segment cysticercosis, as well as comparing its performance with other imaging modalities, such as MRI and CT, to establish its superiority and cost-effectiveness in this setting.
UBM offers significant cost advantages over CT and MRI, especially in lower-middle-income countries (LMICs) with limited resources. Its non-invasive, real-time imaging capability eliminates the need for ionizing radiation or contrast agents, reducing overall imaging costs.10,18 Moreover, UBM’s portability and accessibility make it a practical and cost-effective alternative to more expensive and less accessible imaging modalities like CT and MRI in LMICs. By providing high-resolution imaging of anterior segment cysticercosis, UBM facilitates early and accurate diagnosis, enabling timely intervention and management, thereby reducing long-term healthcare costs associated with advanced disease complications. Future research should focus on evaluating the diagnostic accuracy, reliability, and reproducibility of UBM in detecting and characterizing anterior segment cysticercosis, as well as comparing its performance with other imaging modalities, such as MRI and CT, to establish its superiority and cost-effectiveness in this setting. Additionally, studies exploring the role of UBM in monitoring disease progression, treatment response, and postoperative outcomes, as well as assessing its impact on patient management and quality of life, are warranted.
Our study has a few limitations, including its retrospective design, small sample size, and potential selection bias. The study was conducted at a specialized tertiary ophthalmic ultrasound imaging facility, which may limit the generalizability of the findings to other settings and populations. Furthermore, the depth of penetration of the 50 MHz probe used in UBM imaging is limited to approximately 4 mm, which may restrict visualization of deeper anterior segment structures and cysticercosis lesions. Despite its limitations, our study has several strengths, including its comprehensive evaluation of UBM’s diagnostic capabilities in detecting and characterizing anterior segment cysticercosis. The study employed a standardized imaging protocol, performed by a single imaging specialist with over 35 years of experience in ocular imaging, ensuring consistency, reliability, and accuracy in data collection and interpretation. The high-resolution images obtained allowed for precise localization, sizing, and characterization of cysticerci, facilitating targeted treatment planning and monitoring of disease progression.
Conclusion
UBM emerges as a valuable diagnostic tool for anterior segment cysticercosis, offering high-resolution imaging that surpasses traditional ultrasound B-scan limitations. UBM enables precise localization, sizing, and characterization of cysticerci in the anterior segment, facilitating targeted treatment planning and monitoring of disease progression. Future research should focus on validating UBM’s diagnostic accuracy and reliability in detecting and characterizing anterior segment cysticercosis, comparing it with other imaging modalities, as well as exploring its role in monitoring disease progression and treatment response.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.White AC Jr. Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu Rev Med. 2000;51:187–206. doi: 10.1146/annurev.med.51.1.187 [DOI] [PubMed] [Google Scholar]
- 2.Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol. 2011;7:584–594. doi: 10.1038/nrneurol.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon MA, Winskill P, Harrison WE, Basáñez MG. Taenia solium taeniasis/cysticercosis: from parasite biology and immunology to diagnosis and control. Adv Parasitol. 2021;112:133–217. doi: 10.1016/bs.apar.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 4.Pujari A, Bhaskaran K, Modaboyina S, et al. Cysticercosis in ophthalmology. Surv Ophthalmol. 2022;67(2):544–569. doi: 10.1016/j.survophthal.2021.07.002 [DOI] [PubMed] [Google Scholar]
- 5.Dhiman R, Devi S, Duraipandi K, et al. Cysticercosis of the eye. Int J Ophthalmol. 2017;10:1319–1324. doi: 10.18240/ijo.2017.08.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhary A, Bansal R, Singh K, Singh V. Ocular cysticercosis--a profile. Trop Doct. 2003;33:185–188. doi: 10.1177/004947550303300327 [DOI] [PubMed] [Google Scholar]
- 7.Bhatt V, Bhatt D, Barot R, Sheth J. Ultrasound biomicroscopy for zonular evaluation in eyes with ocular trauma. Clin Ophthalmol. 2021;15:3285–3291. doi: 10.2147/OPTH.S323349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heur M, Jeng BH. Ultrasonography of the anterior segment. Ultrasound Clin. 2008;3:201–206. doi: 10.1016/j.cult.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreoli MT, Farooq AV, Mieler WF. Asymptomatic intraocular mass. JAMA Ophthalmol. 2016;134:105–106. doi: 10.1001/jamaophthalmol.2015.2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman DJ, Silverman RH, Rondeau MJ, Lloyd HO, Daly S. Explaining the current role of high frequency ultrasound in ophthalmic diagnosis (ophthalmic ultrasound). Expert Rev Ophthalmol. 2006;1:63–76. doi: 10.1586/17469899.1.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh M, Sharma M, Chaurasia S, et al. Ophthalmic presentations and long-term outcomes of subconjunctival and atypical orbital myocysticercosis. Indian J Ophthalmol. 2021;69:2782–2787. doi: 10.4103/ijo.IJO_568_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi R. Subconj unctival cysticercosis. J Indian Med Assoc. 2009;107:177. [PubMed] [Google Scholar]
- 13.Mathur RN, Abraham L. Cysticercosis of the eye. A case of plastic iridocyclitis due to cysticercus cyst in the anterior chamber. Arch Ophthalmol. 1962;67:562–563. doi: 10.1001/archopht.1962.00960020562007 [DOI] [PubMed] [Google Scholar]
- 14.Chandra A, Singh MK, Singh VP, Rai AK, Chakraborty S, Maurya OP. A live cysticercosis in anterior chamber leading to glaucoma secondary to pupilary block. J Glaucoma. 2007;16:271–273. doi: 10.1097/IJG.0b013e31802d6dc2 [DOI] [PubMed] [Google Scholar]
- 15.Mahendradas P, Biswas J, Khetan V. Fibrinous anterior uveitis due to cysticercus cellulosae. Ocul Immunol Inflamm. 2007;15:451–454. doi: 10.1080/09273940701798454 [DOI] [PubMed] [Google Scholar]
- 16.He M, Wang D, Jiang Y. Overview of ultrasound biomicroscopy. J Curr Glaucoma Pract. 2012;6(1):25–53. doi: 10.5005/jp-journals-10008-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman RH. High-resolution ultrasound imaging of the eye - a review. Clin Exp Ophthalmol. 2009;37:54–67. doi: 10.1111/j.1442-9071.2008.01892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rath S, Honavar SG, Naik M, et al. Orbital cysticercosis: clinical manifestations, diagnosis, management, and outcome. Ophthalmology. 2010;117:600–605.e1. doi: 10.1016/j.ophtha.2009.07.030 [DOI] [PubMed] [Google Scholar]