Abstract
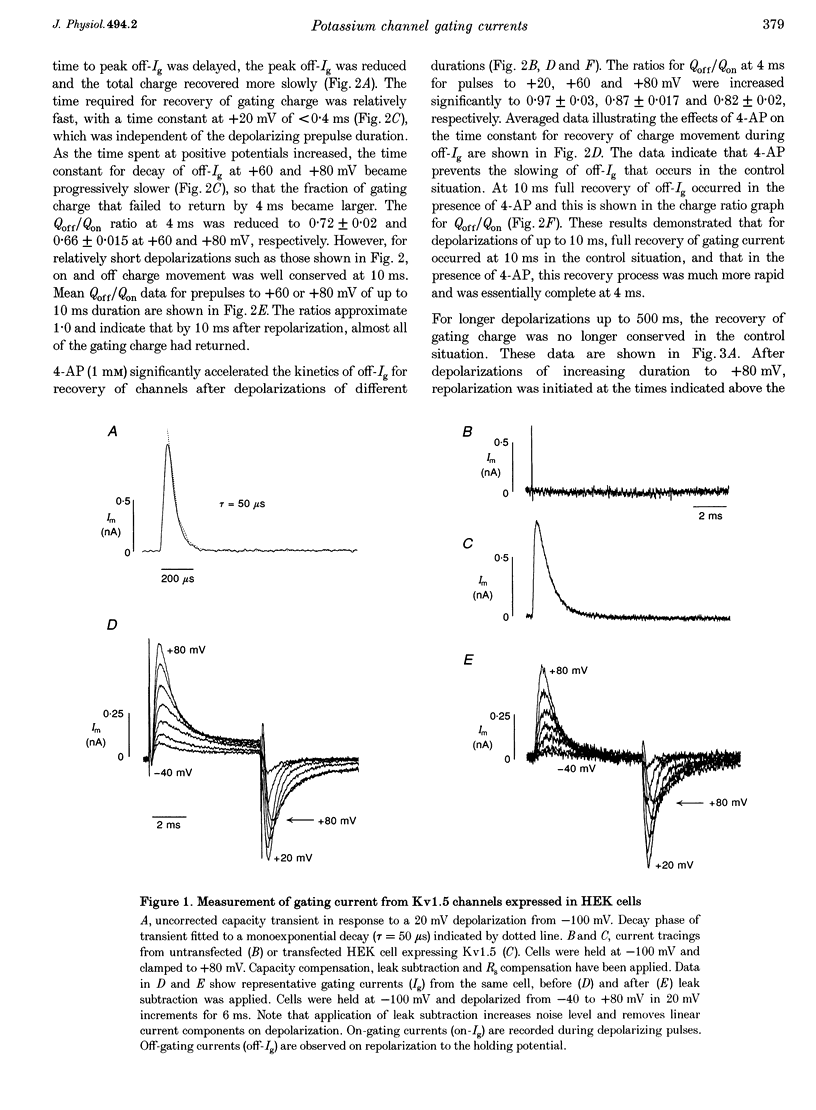
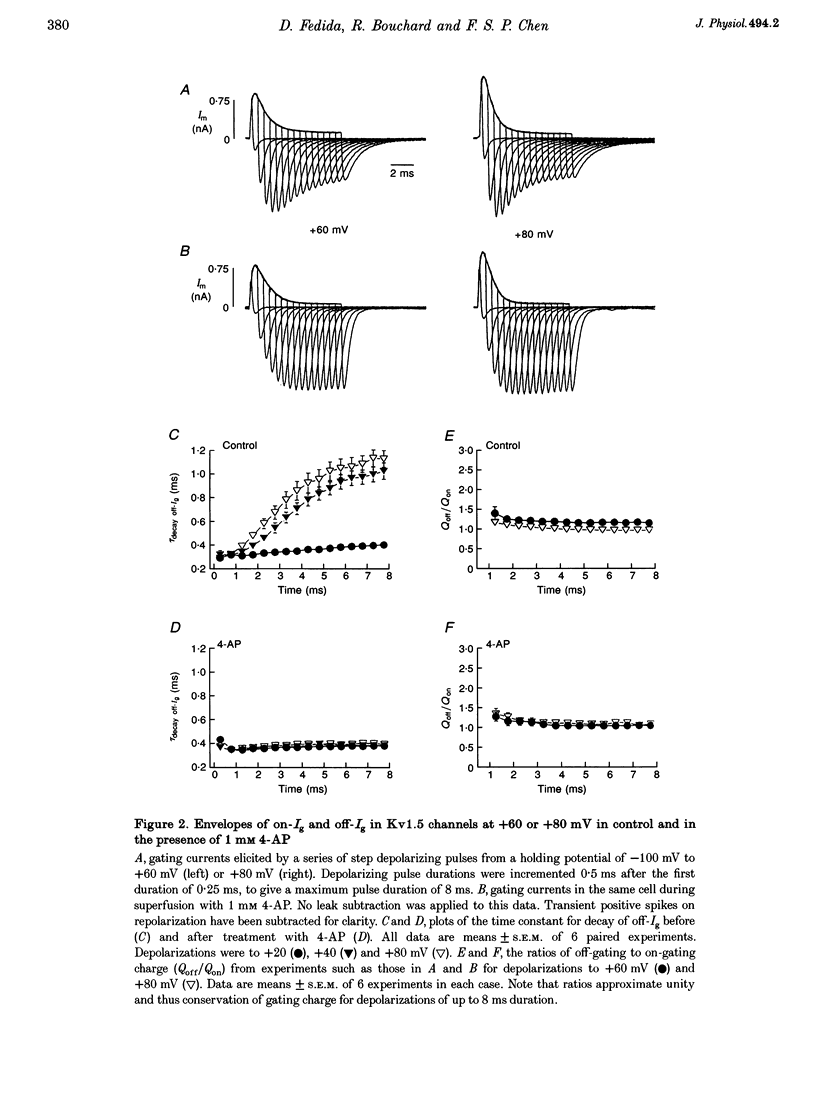
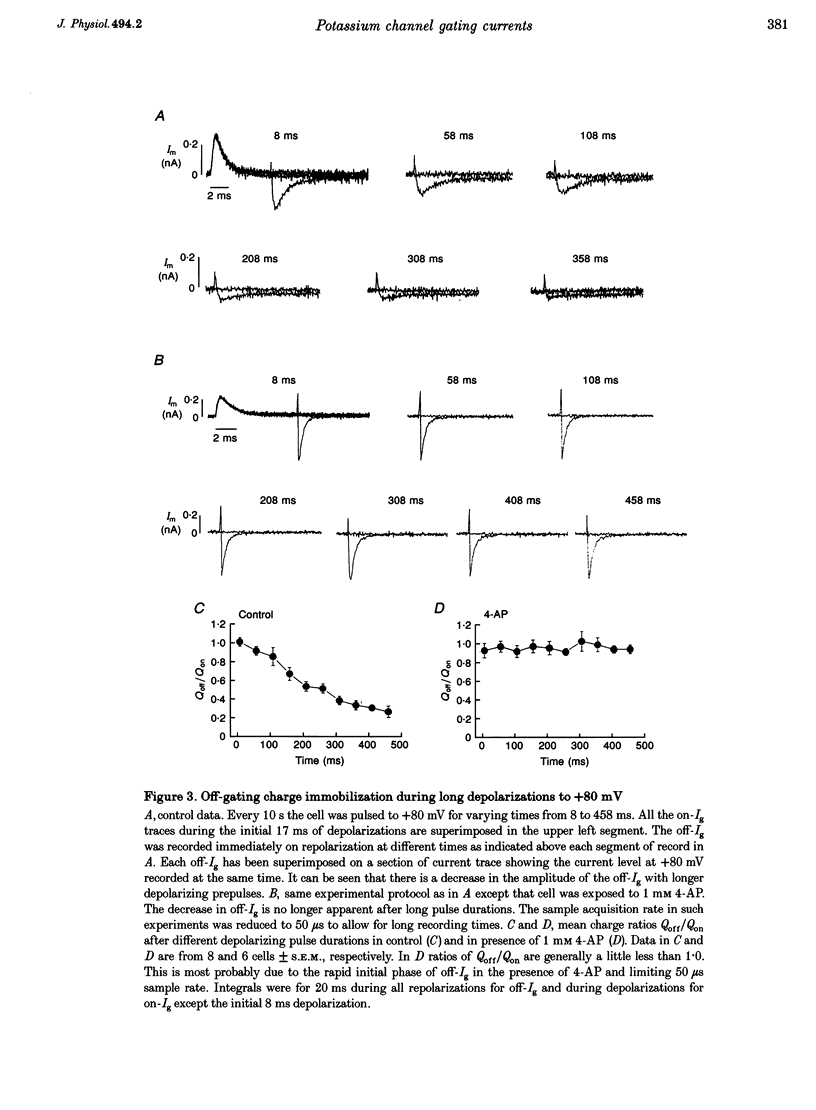
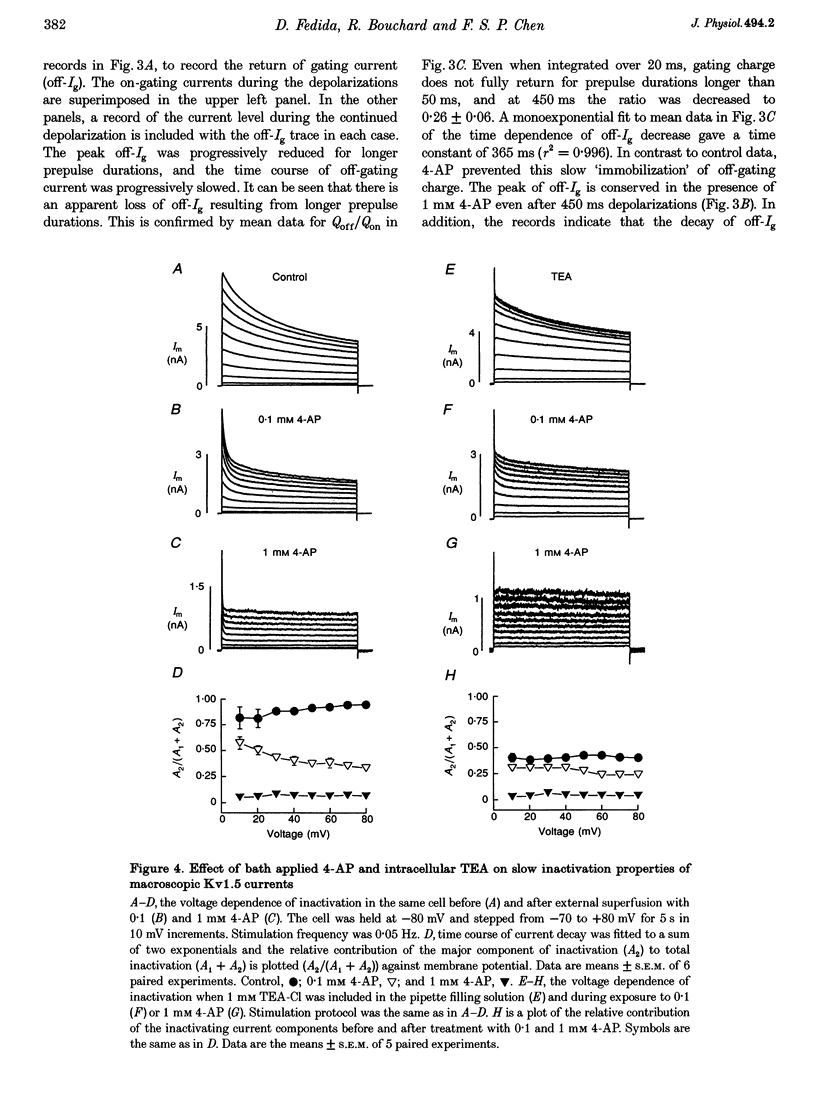
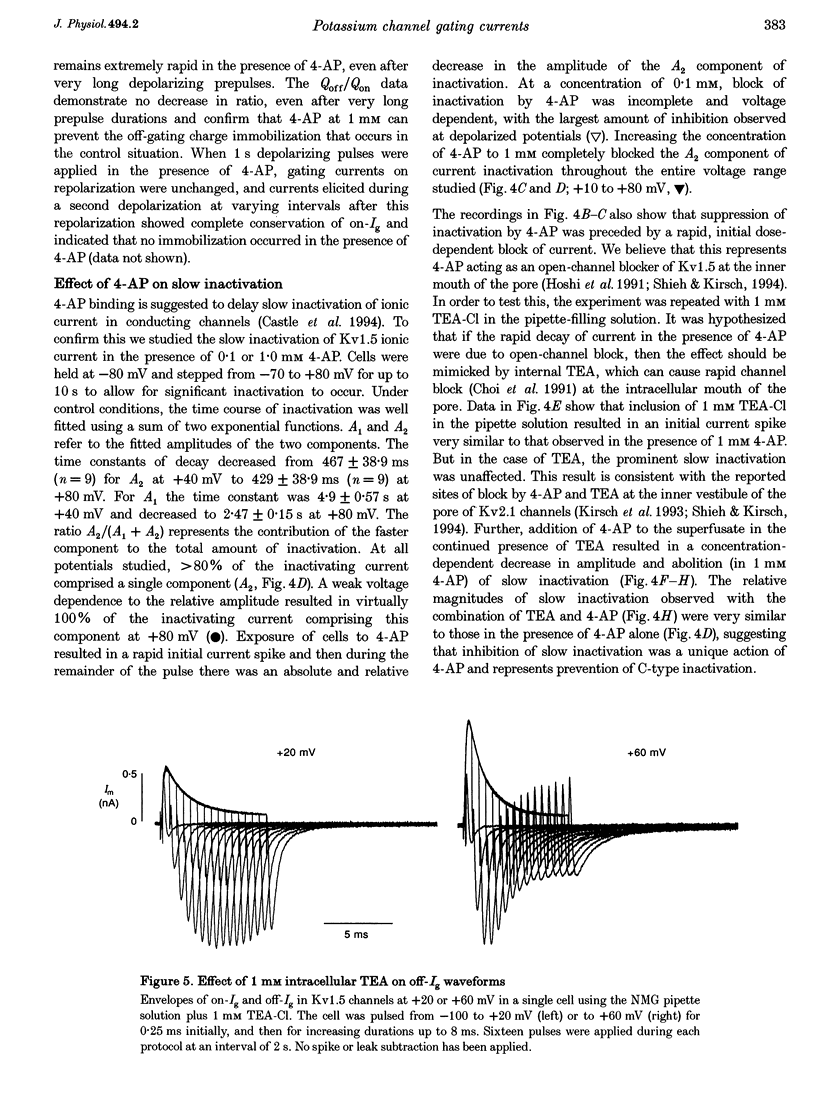
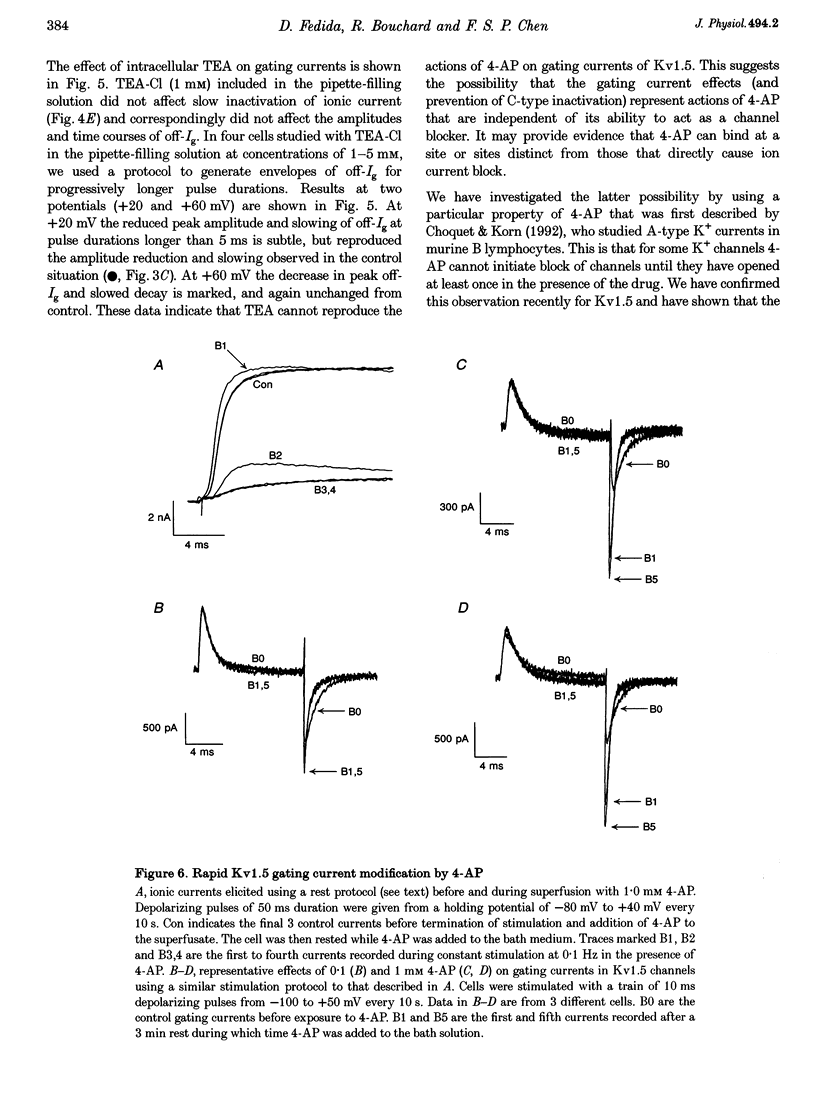
1. The relationship between ionic current inactivation and immobilization of 'off'-gating charge in human Kv1.5 channels expressed in human embryonic kidney (HEK293) cells was studied using 4-aminopyridine (4-AP) and tetraethylammonium chloride (TEA-Cl). 2. The charge transferred during short (< 10 ms) depolarizations (Q(on)) was conserved on repolarization (Q(off)) although peak off-gating current (off-Ig) was reduced and the time course prolonged (tau decay increased from 0.4 to > 1.2 ms). For +80 mV pulses longer than 50 ms, Q(off) at 20 ms was less than Q(on) (Q(off)/Q(on) ratio was 0.26 +/- 0.06 at 450 ms). We attribute this to a relative 'immobilization' of gating charge during long depolarizations. 3. 4-AP (0.1-1 mM) prevented slowing of off-Ig, allowing saturation of peak off-Ig. 4-AP also completely prevented immobilization of off-Ig after long depolarizations. In 1 mM 4-AP, off-Ig waveforms decayed rapidly and the charge ratio Q(off)/Q(on) remained at 1.0. 4. In addition to its effects on Ig, 1 mM 4-AP prevented the slow inactivation of ionic current seen during strong depolarizations. An initial block was caused by 4-AP or 1 mM intracellular TEA internally applied. However, only 4-AP prevented the slower, later development of C-type inactivation. 5. We suggest that slow current inactivation is accompanied by a gating charge immobilization in Kv1.5. 4-AP potently inhibits the changes in Q(off)/Q(on0, off-Ig, and ionic currents that underlie slow inactivation. Some actions of 4-AP appear independent of its properties as a blocker of open K+ channels, and are not mimicked by internal TEA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995 Oct;15(4):951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Perozo E., Papazian D. M., Stefani E. Molecular basis of gating charge immobilization in Shaker potassium channels. Science. 1991 Nov 1;254(5032):679–683. doi: 10.1126/science.1948047. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Perozo E., Stefani E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys J. 1994 Apr;66(4):1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard R., Fedida D. Closed- and open-state binding of 4-aminopyridine to the cloned human potassium channel Kv1.5. J Pharmacol Exp Ther. 1995 Nov;275(2):864–876. [PubMed] [Google Scholar]
- Castle N. A., Fadous S. R., Logothetis D. E., Wang G. K. 4-Aminopyridine binding and slow inactivation are mutually exclusive in rat Kv1.1 and Shaker potassium channels. Mol Pharmacol. 1994 Dec;46(6):1175–1181. [PubMed] [Google Scholar]
- Choi K. L., Aldrich R. W., Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D., Korn H. Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J Gen Physiol. 1992 Feb;99(2):217–240. doi: 10.1085/jgp.99.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Wible B., Wang Z., Fermini B., Faust F., Nattel S., Brown A. M. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res. 1993 Jul;73(1):210–216. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- Grissmer S., Cahalan M. TEA prevents inactivation while blocking open K+ channels in human T lymphocytes. Biophys J. 1989 Jan;55(1):203–206. doi: 10.1016/S0006-3495(89)82793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Korn S. J. Influence of permeating ions on potassium channel block by external tetraethylammonium. J Physiol. 1995 Jul 15;486(Pt 2):267–272. doi: 10.1113/jphysiol.1995.sp020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J Gen Physiol. 1993 Nov;102(5):797–816. doi: 10.1085/jgp.102.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Shieh C. C., Drewe J. A., Vener D. F., Brown A. M. Segmental exchanges define 4-aminopyridine binding and the inner mouth of K+ pores. Neuron. 1993 Sep;11(3):503–512. doi: 10.1016/0896-6273(93)90154-j. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Labarca P., Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. Am J Physiol. 1995 Mar;268(3 Pt 1):C535–C556. doi: 10.1152/ajpcell.1995.268.3.C535. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- McCormack K., Joiner W. J., Heinemann S. H. A characterization of the activating structural rearrangements in voltage-dependent Shaker K+ channels. Neuron. 1994 Feb;12(2):301–315. doi: 10.1016/0896-6273(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Ogielska E. M., Zagotta W. N., Hoshi T., Heinemann S. H., Haab J., Aldrich R. W. Cooperative subunit interactions in C-type inactivation of K channels. Biophys J. 1995 Dec;69(6):2449–2457. doi: 10.1016/S0006-3495(95)80114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G., Sheng Z., Deutsch C. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys J. 1995 Sep;69(3):896–903. doi: 10.1016/S0006-3495(95)79963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E., Papazian D. M., Stefani E., Bezanilla F. Gating currents in Shaker K+ channels. Implications for activation and inactivation models. Biophys J. 1992 Apr;62(1):160–171. doi: 10.1016/S0006-3495(92)81802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh C. C., Kirsch G. E. Mutational analysis of ion conduction and drug binding sites in the inner mouth of voltage-gated K+ channels. Biophys J. 1994 Dec;67(6):2316–2325. doi: 10.1016/S0006-3495(94)80718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Stocker M., Pongs O., Heinemann S. H. Gating currents of inactivating and non-inactivating potassium channels expressed in Xenopus oocytes. Pflugers Arch. 1991 May;418(4):423–429. doi: 10.1007/BF00550881. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Stefani E. Gating currents of the cloned delayed-rectifier K+ channel DRK1. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4758–4762. doi: 10.1073/pnas.90.10.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G., Sodickson D., Chen T. Y., Jurman M. E. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys J. 1994 Apr;66(4):1068–1075. doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]



