Abstract
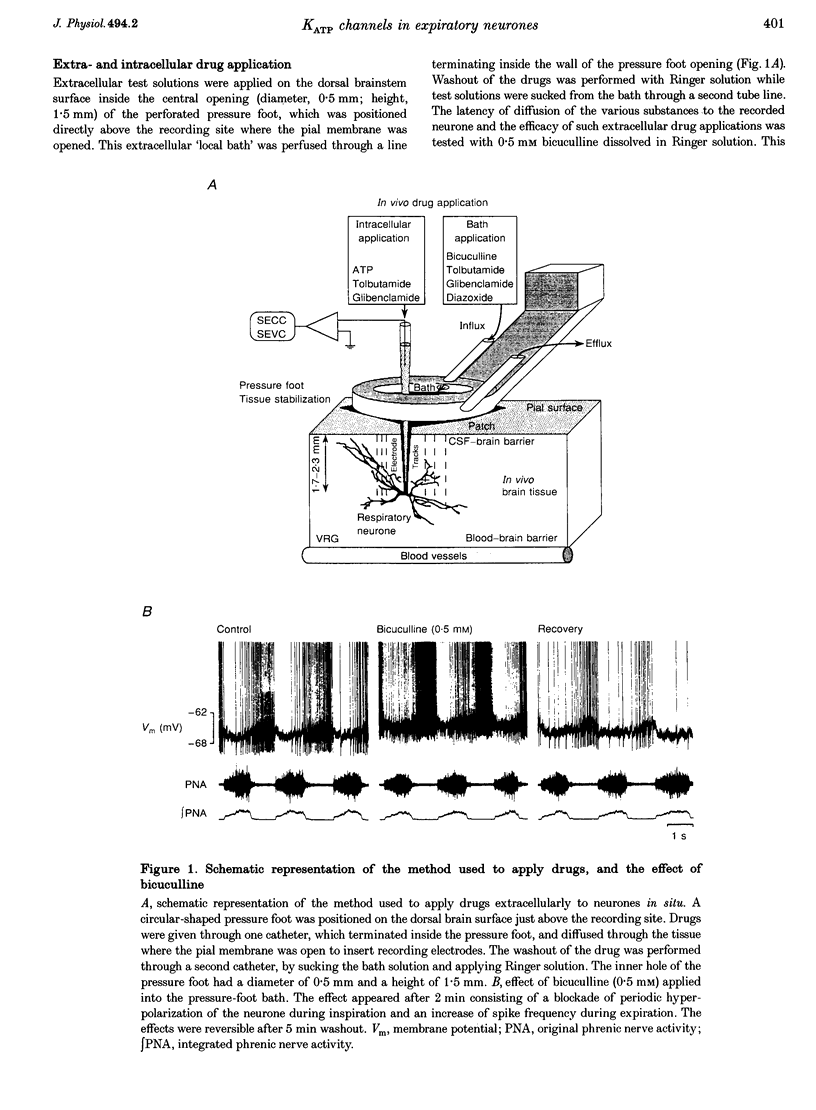
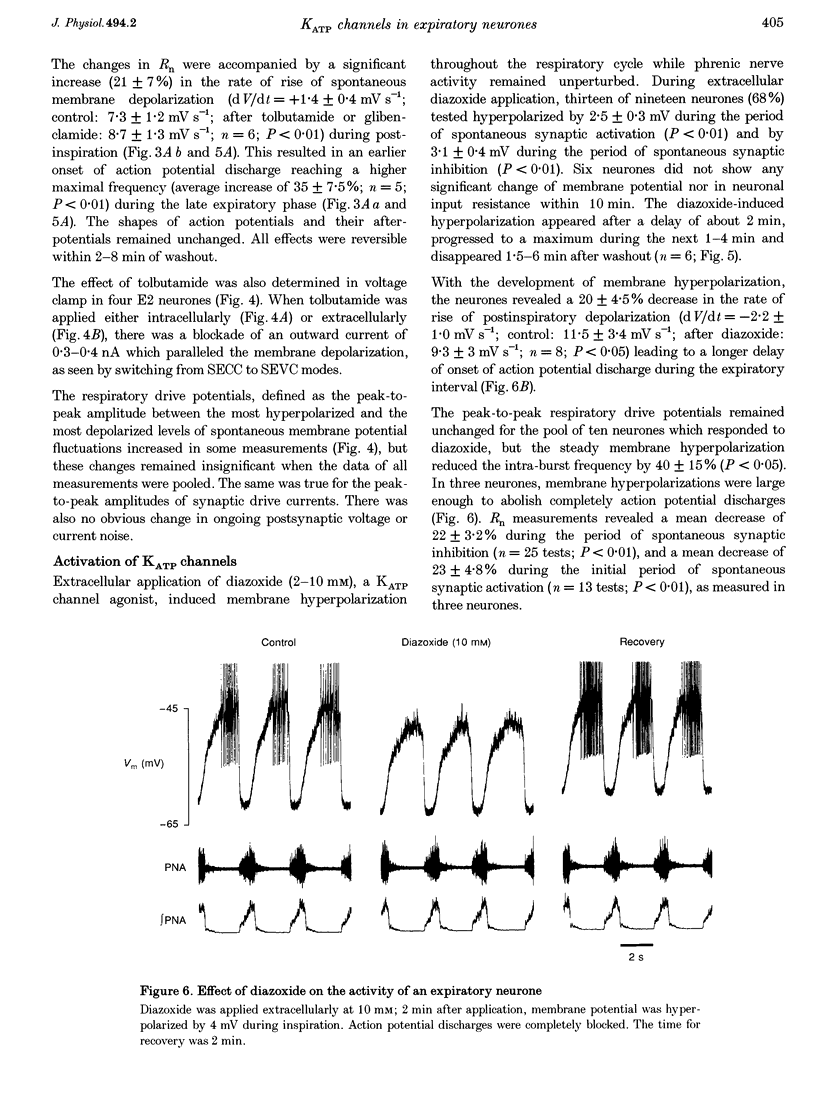
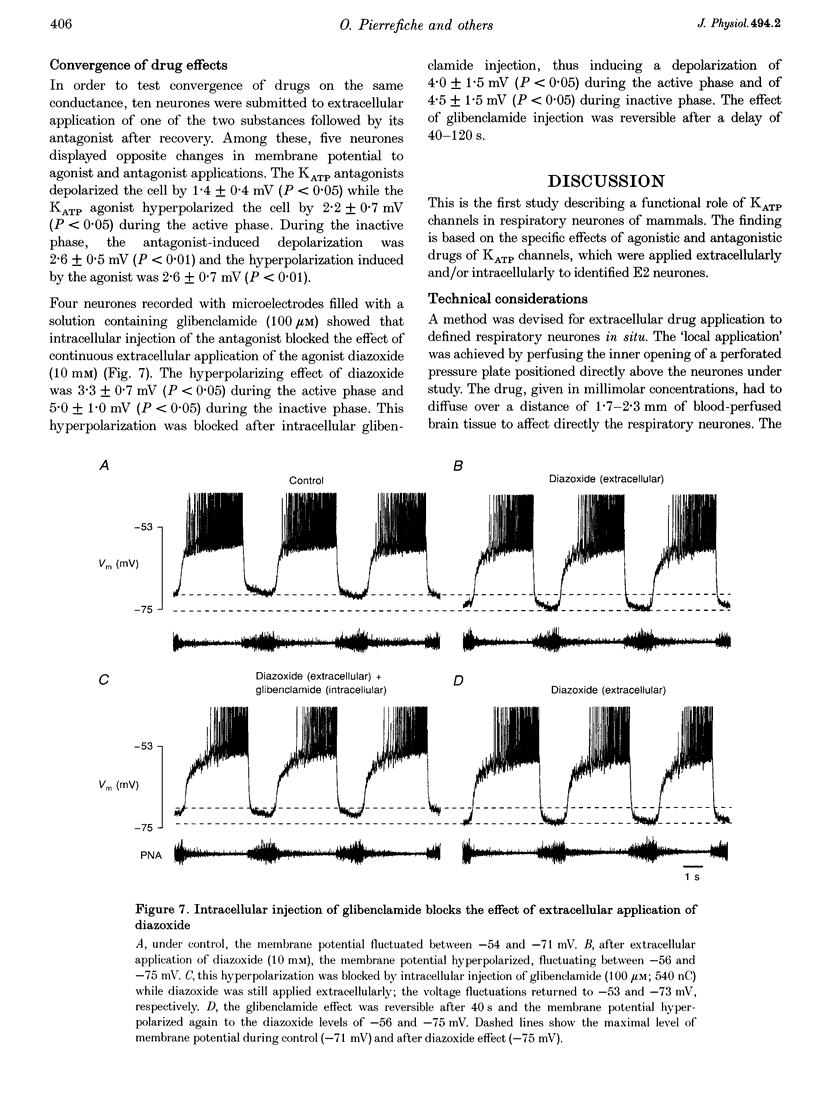
1. We analysed spontaneously active expiratory neurones (n = 48) of anaesthetized cats for the presence of ATP-sensitive K+ (KATP) channels. 2. Intracellular injection of ATP reversibly depolarized neurones during all phases of the respiratory cycle. During expiration, membrane potential depolarized by an average of 1.5 +/- 0.1 mV leading to a 25% increase of discharge frequency. During inspiration, ATP induced a 1.8 +/- 0.2 mV depolarization, which was accompanied by a maximum of 20% increase of input resistance (Rn). 3. Extracellular application of diazoxide, an agonist of KATP channels, resulted in reversible membrane hyperpolarization in 68% of neurones (n = 19). This hyperpolarization (2.5 mV during expiration and 3.1 mV during inspiration) was accompanied by a 22% decrease in Rn. 4. Extracellular application of tolbutamide and glibenclamide, two antagonists of KATP channels, evoked reversible depolarizations in 76% of neurones (n = 21). The depolarization was relatively constant throughout the respiratory cycle (1.4 mV during expiration and 2.3 mV during inspiration). Rn increased by 22%. 5. The same sulphonylureas also changed the steepness of membrane depolarization when neurones escaped spontaneous synaptic inhibition during postinspiration. Extracellularly applied tolbutamide and glibenclamide increased the steepness of depolarization by 21%, while diazoxide reduced it by 20%. 6. Antagonism of drugs was verified by simultaneous extra- and intracellular application of diazoxide and glibenclamide, respectively. 7. During voltage clamp at holding potential at -60 to -67 mV, intracellular or extracellular application of tolbutamide and glibenclamide blocked a persistent outward current. 8. We conclude that KATP channels are functional in expiratory neurones of adult cats and contribute to the control of excitability even during normoxia.
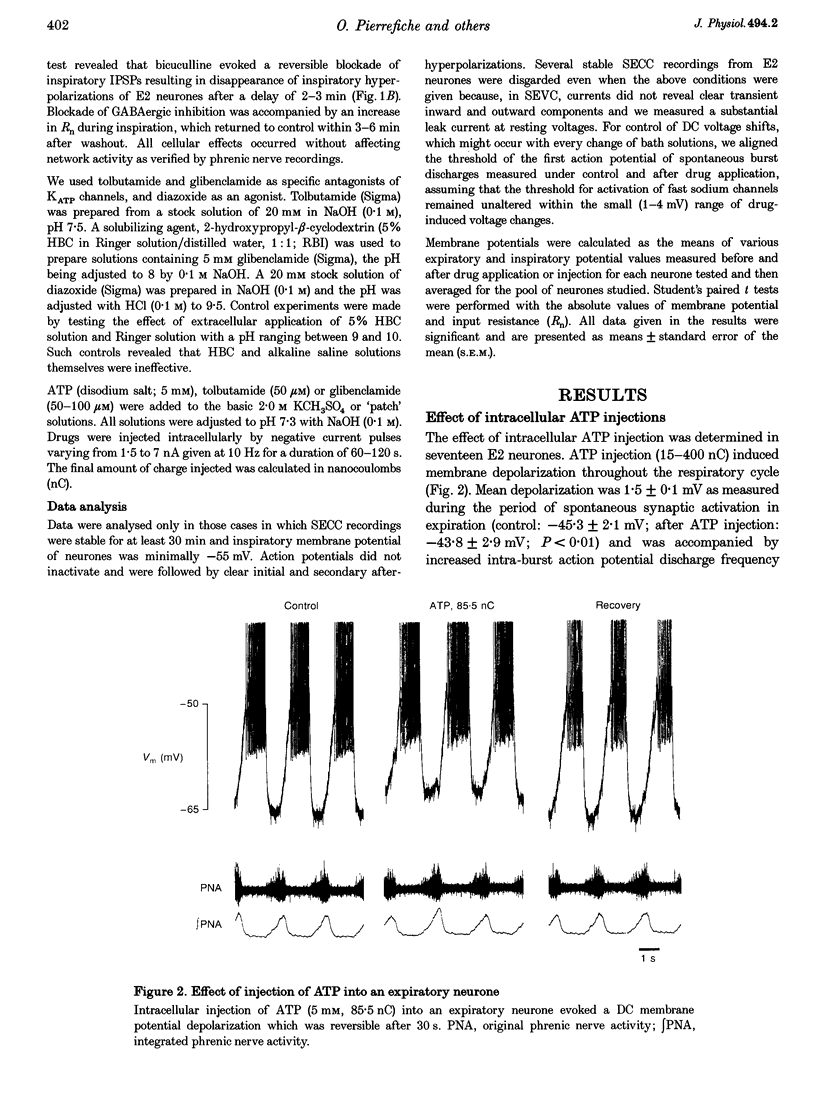
Full text
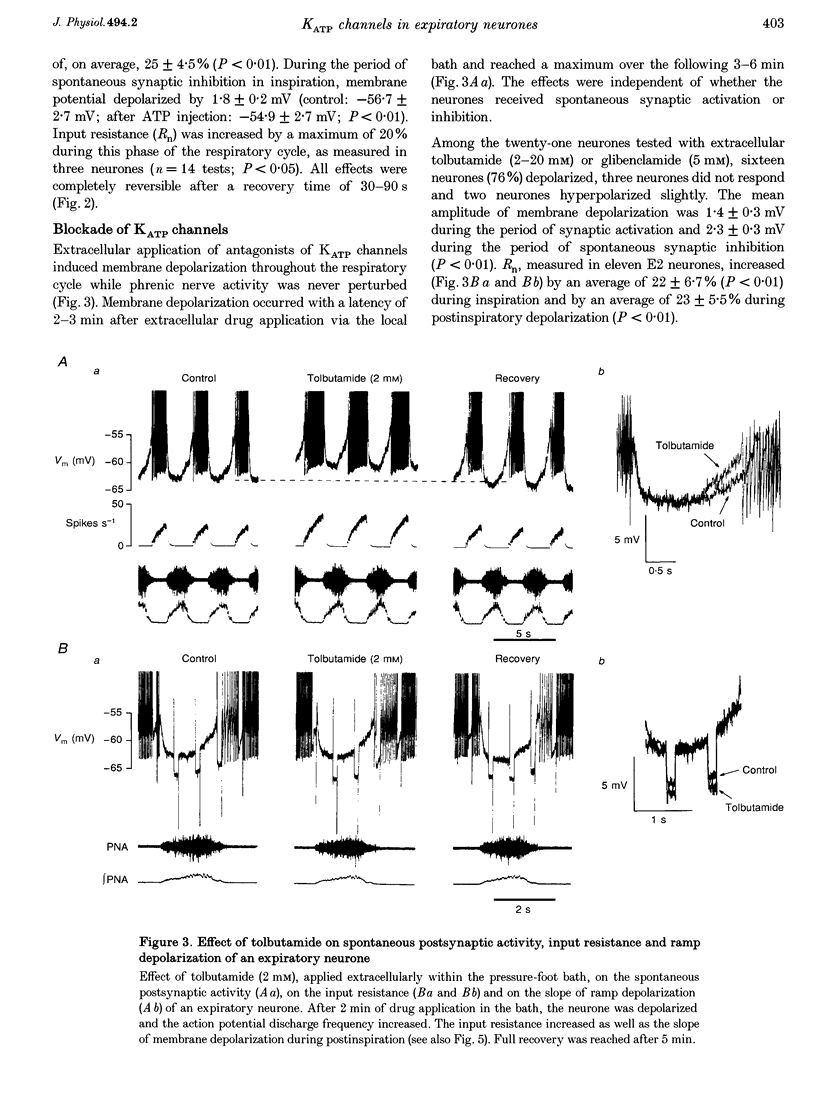
PDF

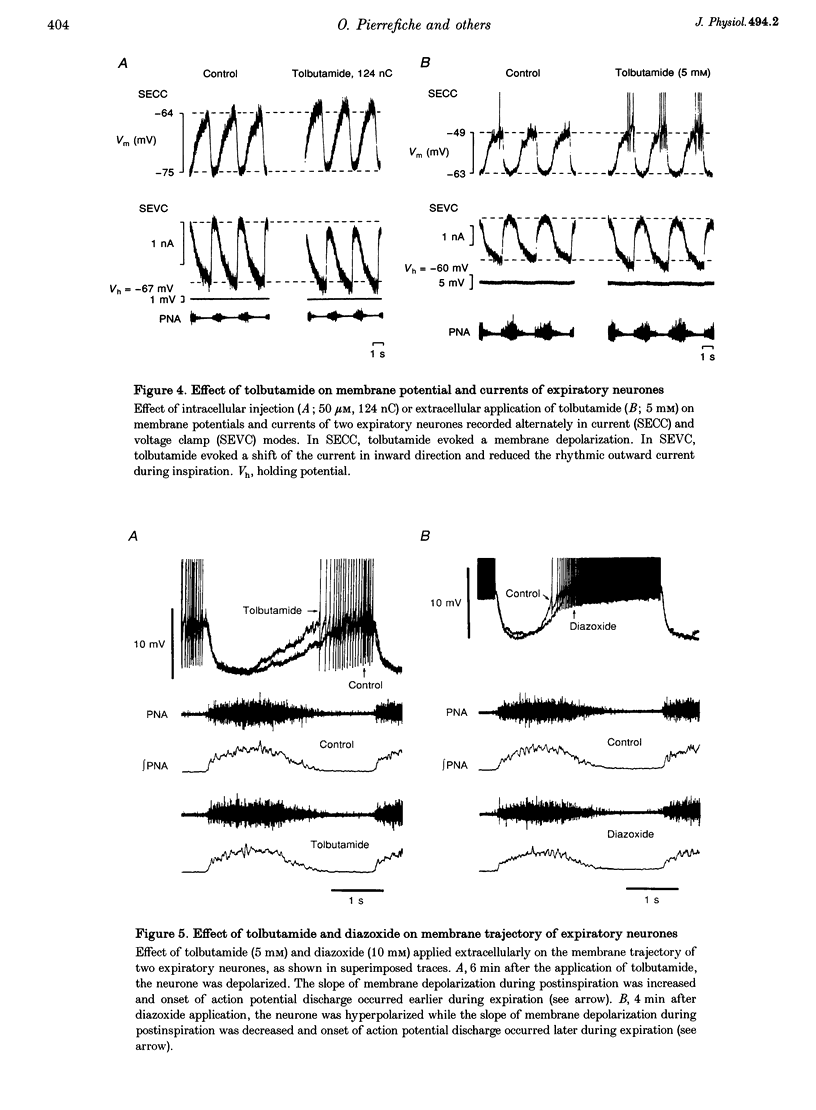



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., 4th, Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995 Apr 21;268(5209):423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990 Jan;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Bond C. T., Blair T. A., Adelman J. P. Cloning and functional expression of a rat heart KATP channel. Nature. 1994 Aug 11;370(6489):456–459. doi: 10.1038/370456a0. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Sturgess N. C., Trout N. J., Gardner N. J., Hales C. N. Adenosine-5'-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988 Aug;412(3):297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Mückenhoff K., Bellingham M. C., Okada Y., Scheid P., Richter D. W. Activity-related pH changes in respiratory neurones and glial cells of cats. Neuroreport. 1994 Dec 30;6(1):33–36. doi: 10.1097/00001756-199412300-00010. [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Völker A., Richter D. W. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. Neuroreport. 1994 Dec 30;6(1):165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Brockhaus J., Ballanyi K., Smith J. C., Richter D. W. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. J Physiol. 1993 Mar;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J., Richter D. W. The roles of K+ conductance in expiratory pattern generation in anaesthetized cats. J Physiol. 1994 Aug 15;479(Pt 1):127–138. doi: 10.1113/jphysiol.1994.sp020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crépel V., Krnjević K., Ben-Ari Y. Sulphonylureas reduce the slowly inactivating D-type outward current in rat hippocampal neurons. J Physiol. 1993 Jul;466:39–54. [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986 Nov 10;208(1):59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- Findlay I. Effects of pH upon the inhibition by sulphonylurea drugs of ATP-sensitive K+ channels in cardiac muscle. J Pharmacol Exp Ther. 1992 Jul;262(1):71–79. [PubMed] [Google Scholar]
- Finta E. P., Harms L., Sevcik J., Fischer H. D., Illes P. Effects of potassium channel openers and their antagonists on rat locus coeruleus neurones. Br J Pharmacol. 1993 Jun;109(2):308–315. doi: 10.1111/j.1476-5381.1993.tb13571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A., Remmers J. E., Connelly C., Takeda R. Effects of glycine and GABA on bulbar respiratory neurons of cat. J Neurophysiol. 1990 May;63(5):955–965. doi: 10.1152/jn.1990.63.5.955. [DOI] [PubMed] [Google Scholar]
- Jiang C., Xia Y., Haddad G. G. Role of ATP-sensitive K+ channels during anoxia: major differences between rat (newborn and adult) and turtle neurons. J Physiol. 1992 Mar;448:599–612. doi: 10.1113/jphysiol.1992.sp019060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Koh D. S., Kampe K., Hermsteiner M., Vogel W. ATP-sensitive and Ca-activated K channels in vertebrate axons: novel links between metabolism and excitability. Pflugers Arch. 1991 Mar;418(1-2):68–73. doi: 10.1007/BF00370453. [DOI] [PubMed] [Google Scholar]
- Mifflin S., Richter D. W. The effects of QX-314 on medullary respiratory neurones. Brain Res. 1987 Sep 8;420(1):22–31. doi: 10.1016/0006-8993(87)90235-6. [DOI] [PubMed] [Google Scholar]
- Mourre C., Ben Ari Y., Bernardi H., Fosset M., Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res. 1989 May 1;486(1):159–164. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- Mourre C., Widmann C., Lazdunski M. Sulfonylurea binding sites associated with ATP-regulated K+ channels in the central nervous system: autoradiographic analysis of their distribution and ontogenesis, and of their localization in mutant mice cerebellum. Brain Res. 1990 Jun 11;519(1-2):29–43. doi: 10.1016/0006-8993(90)90057-i. [DOI] [PubMed] [Google Scholar]
- Panten U., Heipel C., Rosenberger F., Scheffer K., Zünkler B. J., Schwanstecher C. Tolbutamide-sensitivity of the adenosine 5'-triphosphate-dependent K+ channel in mouse pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):566–574. doi: 10.1007/BF00169047. [DOI] [PubMed] [Google Scholar]
- Philipson L. H., Steiner D. F. Pas de deux or more: the sulfonylurea receptor and K+ channels. Science. 1995 Apr 21;268(5209):372–373. doi: 10.1126/science.7716539. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O., Champagnat J., Richter D. W. Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett. 1995 Jan 23;184(2):101–104. doi: 10.1016/0304-3940(94)11179-m. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Bischoff A., Anders K., Bellingham M., Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. J Physiol. 1991 Nov;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. W., Camerer H., Sonnhof U. Changes in extracellular potassium during the spontaneous activity of medullary respiratory neurones. Pflugers Arch. 1978 Sep 6;376(2):139–149. doi: 10.1007/BF00581577. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Champagnat J., Jacquin T., Benacka R. Calcium currents and calcium-dependent potassium currents in mammalian medullary respiratory neurones. J Physiol. 1993 Oct;470:23–33. doi: 10.1113/jphysiol.1993.sp019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Roeper J., Hainsworth A. H., Ashcroft F. M. Tolbutamide reverses membrane hyperpolarisation induced by activation of D2 receptors and GABAB receptors in isolated substantia nigra neurones. Pflugers Arch. 1990 Jun;416(4):473–475. doi: 10.1007/BF00370758. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Bellingham M. C., Richter D. W. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J Physiol. 1995 Mar 15;483(Pt 3):769–781. doi: 10.1113/jphysiol.1995.sp020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanstecher C., Panten U. Identification of an ATP-sensitive K+ channel in spiny neurons of rat caudate nucleus. Pflugers Arch. 1994 May;427(1-2):187–189. doi: 10.1007/BF00585961. [DOI] [PubMed] [Google Scholar]
- Schwanstecher M., Schwanstecher C., Dickel C., Chudziak F., Moshiri A., Panten U. Location of the sulphonylurea receptor at the cytoplasmic face of the beta-cell membrane. Br J Pharmacol. 1994 Nov;113(3):903–911. doi: 10.1111/j.1476-5381.1994.tb17078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S., Ballanyi K., Richter D. W. Spontaneous activation of KATP current in rat dorsal vagal neurones. Neuroreport. 1994 Jun 2;5(10):1285–1288. doi: 10.1097/00001756-199406020-00033. [DOI] [PubMed] [Google Scholar]
- Trippenbach T., Richter D. W., Acker H. Hypoxia and ion activities within the brain stem of newborn rabbits. J Appl Physiol (1985) 1990 Jun;68(6):2494–2503. doi: 10.1152/jappl.1990.68.6.2494. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Zini S., Roisin M. P., Langel U., Bartfai T., Ben-Ari Y. Galanin reduces release of endogenous excitatory amino acids in the rat hippocampus. Eur J Pharmacol. 1993 Mar 15;245(1):1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Lins S., Ohno-Shosaku T., Trube G., Panten U. Cytosolic ADP enhances the sensitivity to tolbutamide of ATP-dependent K+ channels from pancreatic B-cells. FEBS Lett. 1988 Nov 7;239(2):241–244. doi: 10.1016/0014-5793(88)80925-6. [DOI] [PubMed] [Google Scholar]




