Abstract
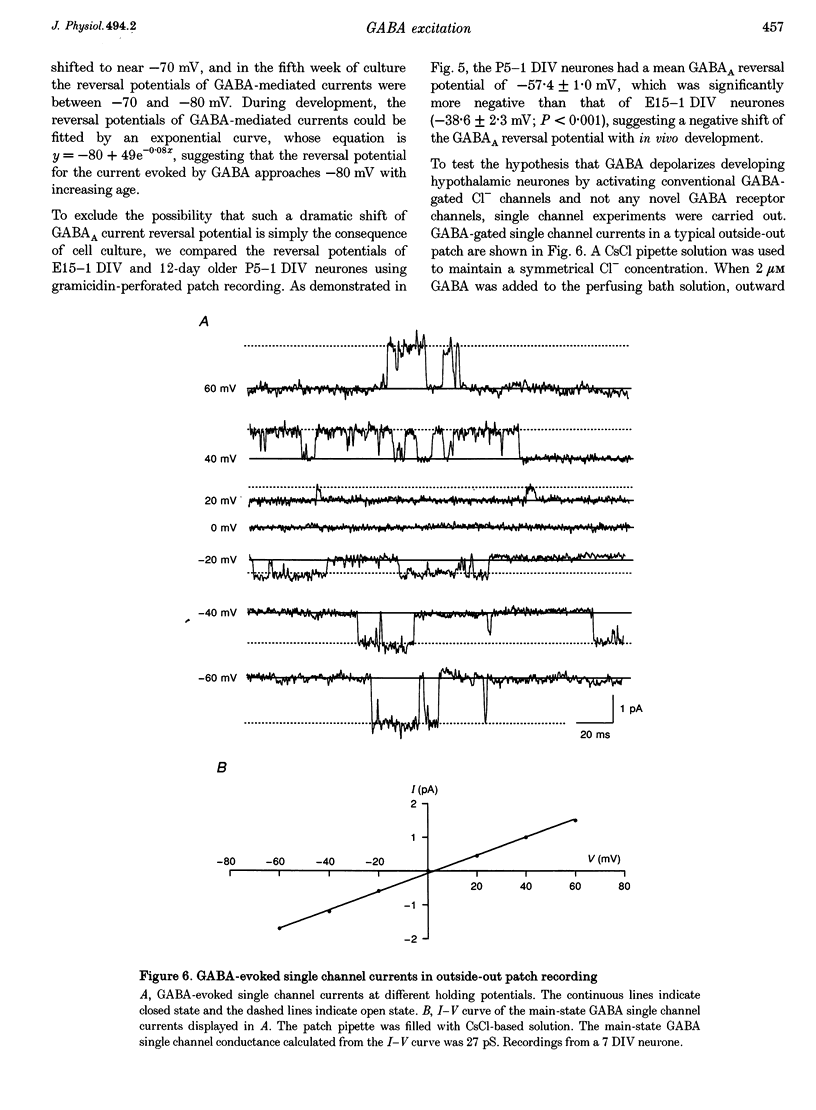
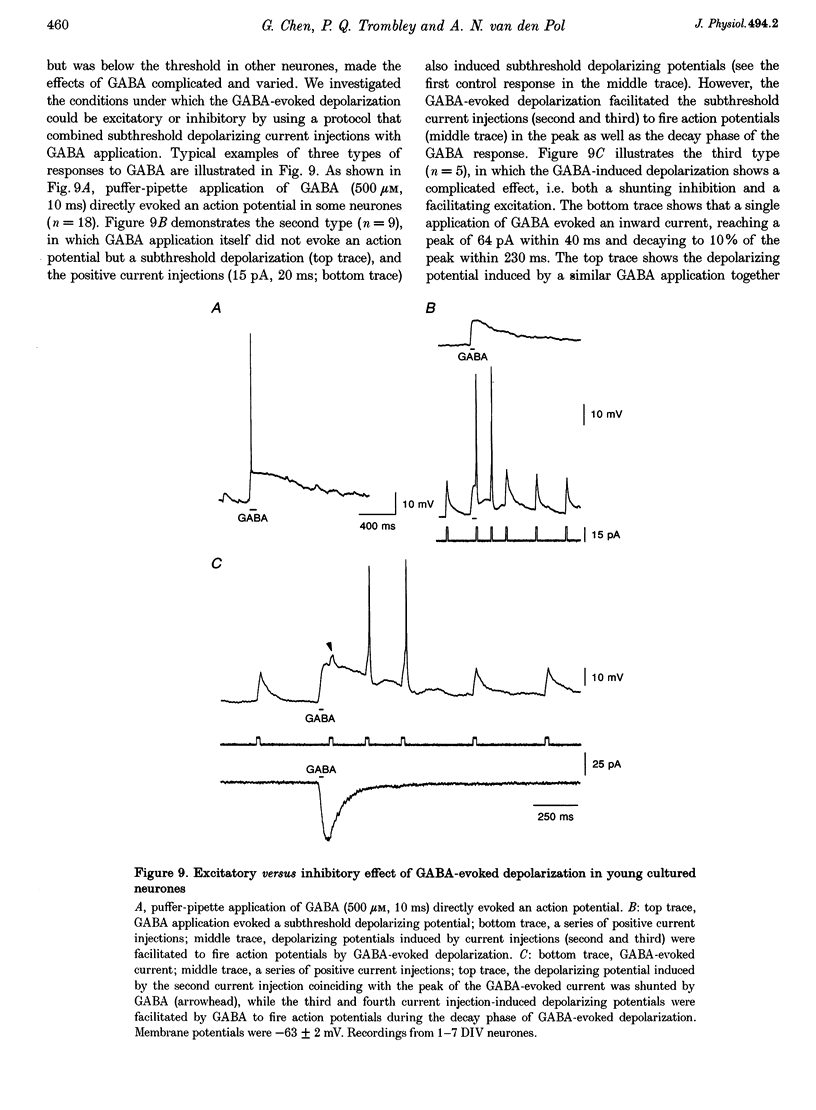
1. Gramicidin-perforated patch clamp recording was employed to study GABA-mediated responses in rat hypothalamic neurones (n = 102) with an intracellular Cl- concentration unaltered by the pipette solution. 2. In young cultures after 1-7 days in vitro (DIV), GABA induced depolarizing membrane potentials (+16.5 +/- 1.3 mV) that often surpassed the threshold for the firing of action potentials (-42 +/- 1 mV) and resulted in an increase in neuronal activity. The depolarizing responses to GABA in young cultures were dose dependent. The concentration of GABA necessary to evoke the half-maximal depolarization (EC50) was 2.8 microM. In contrast, GABA induced hyperpolarizing membrane potentials (-12.0 +/- 1.4 mV) and a decrease in neuronal activity in older neurones (20-33 DIV). Both the depolarization and the hyperpolarization induced by GABA were blocked by bicuculline, indicating a mediation by GABAA receptors. 3. The reversal potentials of the GABA-evoked currents were between -40 to -50 mV during the first week of culture, and shifted to below -70 mV after 3 weeks of culture. In parallel, neurones that were dissociated from older animals (postnatal day 5) had a more negative reversal potential for the GABA-evoked currents than cells from younger animals (embryonic day 15), suggesting that the negative shift of the reversal potential occurs both in vitro and in vivo. Our data suggest that the mechanism for GABA-induced depolarization is the depolarized Cl- reversal potential found in young but not older neurones. 4. Consistent with the depolarizing response to exogenous application of GABA, some spontaneous depolarizing postsynaptic potentials in young cultures were insensitive to AP5-CNQX, but were eliminated by bicuculline, indicating that synaptically released GABA mediated excitatory synaptic transmission in early development. 5. By combining a rapid computer-controlled delivery of GABA with subthreshold positive current injections into recorded neurones, we found in young cultures that the GABA-evoked depolarization could directly trigger action potentials, facilitate some depolarizing input to fire action potentials, and shunt other depolarizing input. Whether the GABA-induced depolarization is excitatory or inhibitory would be determined by the reversal potential of the GABA-evoked current, and the temporal relationship between GABA-evoked depolarizations and other excitatory events. 6. We conclude that the reversal potential of the GABA-evoked current shifts negatively from depolarizing to hyperpolarizing in developing hypothalamus. Consequently, GABA neurotransmission may serve both excitatory and inhibitory roles during early development.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979 Sep 27;281(5729):315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H., Ginty D. D., Greenberg M. E. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993 Apr 9;260(5105):181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Barbin G., Pollard H., Gaïarsa J. L., Ben-Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett. 1993 Apr 2;152(1-2):150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Tseeb V., Raggozzino D., Khazipov R., Gaiarsa J. L. gamma-Aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Trombley P. Q., van den Pol A. N. GABA receptors precede glutamate receptors in hypothalamic development; differential regulation by astrocytes. J Neurophysiol. 1995 Oct;74(4):1473–1484. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J. L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991 Dec;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Decavel C., Van den Pol A. N. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990 Dec 22;302(4):1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Ebihara S., Shirato K., Harata N., Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol. 1995 Apr 1;484(Pt 1):77–86. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Gaiarsa J. L., McLean H., Congar P., Leinekugel X., Khazipov R., Tseeb V., Ben-Ari Y. Postnatal maturation of gamma-aminobutyric acidA and B-mediated inhibition in the CA3 hippocampal region of the rat. J Neurobiol. 1995 Mar;26(3):339–349. doi: 10.1002/neu.480260306. [DOI] [PubMed] [Google Scholar]
- Grover L. M., Lambert N. A., Schwartzkroin P. A., Teyler T. J. Role of HCO3- ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J Neurophysiol. 1993 May;69(5):1541–1555. doi: 10.1152/jn.1993.69.5.1541. [DOI] [PubMed] [Google Scholar]
- Hales T. G., Sanderson M. J., Charles A. C. GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Neuroendocrinology. 1994 Mar;59(3):297–308. doi: 10.1159/000126671. [DOI] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Leinekugel X., Tseeb V., Ben-Ari Y., Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995 Sep 1;487(Pt 2):319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco J. J., Owens D. F., Heath M. J., Davis M. B., Kriegstein A. R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995 Dec;15(6):1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luhmann H. J., Prince D. A. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991 Feb;65(2):247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Kater S. B. Excitatory and inhibitory neurotransmitters in the generation and degeneration of hippocampal neuroarchitecture. Brain Res. 1989 Jan 30;478(2):337–348. doi: 10.1016/0006-8993(89)91514-x. [DOI] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994 Dec;17(12):517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Obata K. Transmitter sensitivities of some nerve and muscle cells in culture. Brain Res. 1974 Jun 14;73(1):71–88. doi: 10.1016/0006-8993(74)91008-7. [DOI] [PubMed] [Google Scholar]
- Obrietan K., van den Pol A. N. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995 Jul;15(7 Pt 1):5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanović S. S., Shefner S. A. gamma-Aminobutyric acid responses in rat locus coeruleus neurones in vitro: a current-clamp and voltage-clamp study. J Physiol. 1990 Feb;421:151–170. doi: 10.1113/jphysiol.1990.sp017938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling D. B., Kyrozis A., Wang J., MacDermott A. B. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994 May 1;476(3):411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. D., Brien J. F. Ontogeny of glutamate and gamma-aminobutyric acid release in the hippocampus of the guinea pig. J Dev Physiol. 1992 Nov;18(5):243–252. [PubMed] [Google Scholar]
- Schreurs B. G., Sanchez-Andres J. V., Alkon D. L. GABA-induced responses in Purkinje cell dendrites of the rabbit cerebellar slice. Brain Res. 1992 Nov 27;597(1):99–107. doi: 10.1016/0006-8993(92)91510-l. [DOI] [PubMed] [Google Scholar]
- Serafini R., Valeyev A. Y., Barker J. L., Poulter M. O. Depolarizing GABA-activated Cl- channels in embryonic rat spinal and olfactory bulb cells. J Physiol. 1995 Oct 15;488(Pt 2):371–386. doi: 10.1113/jphysiol.1995.sp020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K. J., Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992 Jul;68(1):197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Soldo B. L., Proctor W. R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995 Aug 18;269(5226):977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Taube J. S. Electrophysiological properties of neurons in the rat subiculum in vitro. Exp Brain Res. 1993;96(2):304–318. doi: 10.1007/BF00227110. [DOI] [PubMed] [Google Scholar]
- Wu W. L., Ziskind-Conhaim L., Sweet M. A. Early development of glycine- and GABA-mediated synapses in rat spinal cord. J Neurosci. 1992 Oct;12(10):3935–3945. doi: 10.1523/JNEUROSCI.12-10-03935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R., Katz L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991 Mar;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Zhang S. J., Jackson M. B. GABA-activated chloride channels in secretory nerve endings. Science. 1993 Jan 22;259(5094):531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]


